Abstract
Melanoma is the leading cause of fatal skin cancer, and in the past few decades, there has been an increase in the incidence of and mortality from metastatic melanoma. Until recently, the therapeutic options for treatment of metastatic melanoma were limited. The approval of ipilimumab (an anti-CTLA-4 antibody) and vemurafenib (mutant B-RAFV600E kinase inhibitor) by the Federal Drug Administration has led to a new era in melanoma treatment, and additional promising drugs and drug combinations are currently being investigated. As the choices of treatment for melanoma have expanded, the need to identify predictive biomarkers to tailor treatment strategies to individual tumor or immune system characteristics has become necessary. Such strategies have the potential of maximizing antitumor effect while minimizing toxicity and improving clinical benefit. In this article, we review the currently approved targeted therapies in melanoma and discuss the future of personalized therapy for this disease.
Keywords: vemurafenib, mutations, inhibitors, tumors
Introduction
In the United States, melanoma is the fifth-leading cancer in men and the seventh in women. In recent years, the incidence of melanoma has increased, and despite improvements in awareness and early detection, the mortality from metastatic melanoma is on the rise, particularly in elderly patients.1 The prognosis of patients with metastatic melanoma remains extremely poor, with the median survival ranging from 8 to 18 months after diagnosis.2 Until 2011, only two therapies (dacarbazine, [Bayer– Hospira Inc. Lake Forest, IL (DTIC)] and high-dose interleukin-2 [Prometheus labs, San Diego, CA (IL-2)]) had been approved for metastatic melanoma by the Federal Drug Administration (FDA); both of these agents have not been shown to improve overall survival.3,4 Two recently approved therapies, ipilimumab (Bristol-Myers Squibb, Princeton, NJ) (anti-CTLA-4 antibody, an immune therapy) and vemurafenib (Genentech, South San Francisco, CA) (B-RAFV600E kinase inhibitor), have shown a survival benefit in large randomized clinical trials. A large number of therapeutic agents currently in clinical development are likely to expand the therapeutic options for melanoma.
Personalizing immune therapies
The response rates (RRs) for the currently available immune therapies (IL-2 and ipilimumab) are low, while experimental agents have been associated with higher RRs in small nonrandomized trials.3,5–7 The objective RR for high-dose IL-2 for selected patients with a good performance status is approximately 15%, including durable, long-term complete responses (CRs) in approximately 5% of all treated patients.3,6 Similarly lower RRs are seen with ipilimumab (∼15%), while RRs to investigational agents such as anti-PD-1 (Bristol-Myers Squibb, Princeton, NJ) appear to be somewhat higher (∼30%).5 Adoptive cell therapy is a treatment that uses T cells harvested from tumor-infiltrating lymphocytes (TILs) from resected tumors or peripheral blood activated and expanded. These tumor antigen-specific lymphocytes are expanded ex vivo and reinfused after partial or complete myeloablation.8 Adoptive cell therapy results in higher RRs (approximately 50%) in highly selected patients in Phase I trials; however, this modality of therapy is available only at select centers, and larger/randomized studies are difficult to conduct.8
Despite the low RR for immune therapies, clinical experience indicates that small subsets of patients achieve apparent durable benefit, and can potentially be cured. Thus identifying predictive markers could have a tremendous impact on personalizing immunotherapy in melanoma by enabling selection of treatment for patients who are more likely to respond. However, due to the complexity of interactions between tumors and the immune system that affect antitumor responses, discovery of such biomarkers has been particularly challenging.
Predicting response to ipilimumab
Ipilimumab is the first immunotherapeutic agent to improve overall survival in metastatic melanoma, as demonstrated in two randomized Phase III clinical trials that led to its approval by the FDA in March 2011.9,10 Although ipilimumab is associated with longer overall survival, the RR (CR and partial response [PR]) was only 10%–15%, and the rate of CR, PR, and stable disease (SD) in combination was approximately 30%. Given that the majority of patients do not respond to this drug, and given the associated toxicities, incorporation of predictive biomarkers could potentially improve the therapeutic ratio of ipilimumab and are the subject of intensive research.11
Expression of FOXP3 and indoleamine 2,3-dioxygenase (IDO) in the tumor microenvironment has been shown to be associated with clinical activity in patients treated with ipilimumab.12 Among 82 patients treated with ipilimumab, pretreatment samples stained for FOXP3 and IDO revealed higher RRs for patients whose tumors expressed high levels of IDO (n = 35) and FOXP3 (n = 33); for high and low expression, RRs for IDO and FOXP3 were 40% vs 11% (P = 0.014) and 50% vs 10% (P = 0.012), respectively. Analysis of the TILs within the tumor tissues showed that the increased density of the TILs 3 weeks after the start of treatment was associated with improved clinical benefit (P = 0.005).13
Recently, Yuan et al showed that high titers of pretreatment anti-NY-ESO-1 antibodies can predict response to ipilimumab.14 NY-ESO-1 seropositivity predicted improved clinical benefit, defined as the combination of CR, PR, and SD (P = 0.02). Furthermore, analysis of NY-ESO-1-specific CD4+ and CD8+ T-cell responses by intracellular multicytokine staining revealed that patients with pretreatment anti-NY-ESO-1 antibodies who developed CD8+ T-cell responses were more likely to respond to the drug treatment (10 of 13; 77%) than those with undetectable CD8+ T-cell responses (one of seven; 14%; P = 0.02, relative risk = 5.4), and were more likely to live longer (P = 0.01). However, an attempt to reproduce these results was unsuccessful; a retrospective analysis of patients treated with ipilimumab at the Surgery Branch of the National Institutes of Health failed to show a correlation between pretreatment or posttreatment seropositivity to NY-ESO-1 and response to ipilimumab (P = 1.0 and P = 0.7, respectively).15 Since the results for these two retrospective reports are conflicting, further studies with larger patient cohorts are required to test the validity of NY-ESO-1 antibodies as predictive biomarkers of response to ipilimumab.
Ongoing studies are investigating additional biomarkers in melanoma patients receiving ipilimumab.16 A large intergroup study (NCT01489423) is investigating various blood and tissue biomarkers, such as circulating immune effector cells (T, B, NK, and NK-T cells), circulating plasmacytoid dendritic cells, myeloid dendritic cells, and melanoma-associated antigen-specific T cells as predictors of the response to ipilimumab.17
Predicting response to anti-PD-1 antibodies
Antiprogrammed death 1 (PD-1) antibodies have shown promising results in early Phase clinical trials. PD-1, a receptor expressed on T cells, is an important negative immune checkpoint molecule that inhibits activation of cytotoxic T lymphocytes. Tumor cells and stromal cells can express PD-1 ligands PD-L1 (B7-H1) and PD-L2 (B7-DC), and thus suppress T-cell activation.18,19 In preclinical studies, inhibition of the interaction between PD-1 and PD-L1 has been shown to enhance T-cell responses and antitumor activity.20,21
In a Phase I/II clinical trial with the anti-PD-1 antibody BMS-936558, a response rate of 28% was seen among melanoma patients at doses ranging from 0.1 to 10.0 mg/kg intravenously.18 Stable disease lasting 24 weeks or more was observed in additional patients. BMS-936558 was well tolerated overall, but was associated with immune-related adverse events, including pneumonitis, vitiligo, colitis, hepatitis, hypophysitis, and thyroiditis.18,19 There were three drug-related deaths (1%) due to pneumonitis.18 The expression of PD-L1 by immunohistochemistry on pretreatment tumor samples of 42 patients was associated with a greater likelihood of response to treatment. Nine of 25 patients with PD-L1–positive tumors responded, while no responses were seen among the 17 patients whose tumors did not express PD-L1 (P = 0.006), suggesting that PD-L1 in tumor tissue could serve as a predictive biomarker.18 Prospective validation is needed. In addition, studies of antibodies to PL-L1 are ongoing (see www.clinicaltrials.gov).
Melanocyte drug targets
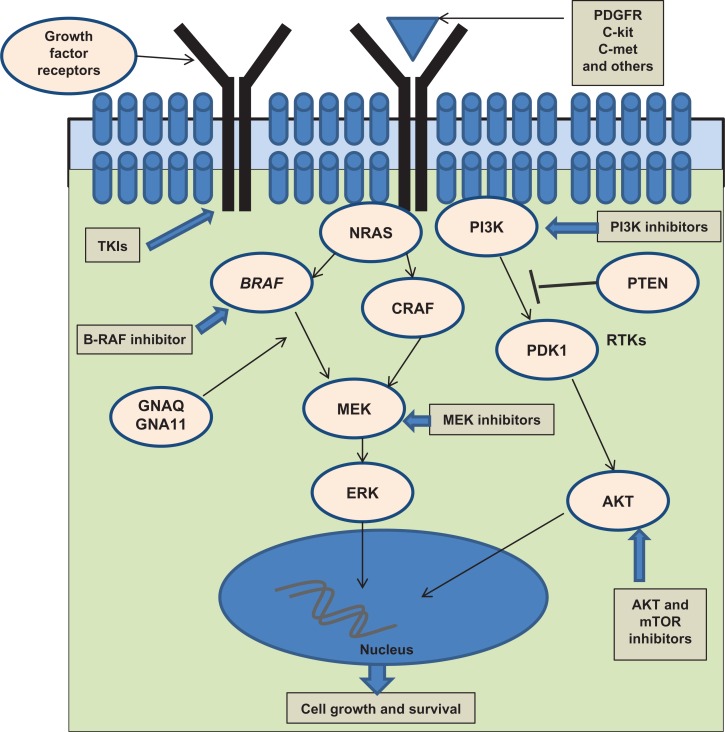
The MAPK pathway in melanoma (Figure 1)
Figure 1.
Cell-signaling pathways in melanoma.
The growing understanding of the biology and pathogenesis of melanoma has resulted in a path of development of targeted therapies, which has led to initial improvement in the care of subsets of patients with advanced melanoma. One of the most significant discoveries in the field of melanoma in recent years was the elucidation of the role of mitogen-activated protein kinase (MAPK) pathway, particularly the roles of mutant B-RAF and N-RAS.
The RAS-RAF-MAPK signaling pathway is activated in the vast majority of melanomas. In nonmalignant cells, the binding of growth factor receptors (such as epidermal growth factor receptor, c-Met, and c-KIT) to their corresponding ligand activates this intracellular kinase cascade. The MAPK pathway is activated in human melanomas either due to increased growth factor signaling or by genetic alterations in RAS and RAF proteins.22 MAPK regulates the activities of several transcription factors, such as C-Myc, CREB, and C-Fos. By altering the levels and activity of transcription factors, MAPK leads to altered transcription of genes that are fundamental for cell division and survival. The activation of B-RAF and the downstream signaling is also associated with enhanced NFκB promoter activity.23 Inhibition of B-RAF signaling has been shown to decrease NFκB promoter activity associated with cell survival, invasiveness, and angiogenesis.23,24
Vemurafenib (PLX4032 [Genentech, South San Francisco, CA], Zelboraf), a B-RAFV600E inhibitor with increased selectivity for mutant B-RAFV600E, was recently approved by the FDA for treatment of unresectable melanoma harboring B-RAFV600E mutations. Dabrafenib (GlaxoSmithKline, Brentford, UK, GSK2118436), another specific inhibitor of mutant B-RAFV600E kinase, has shown significant clinical efficacy in Phase II trials, and a Phase III clinical trial has completed accrual (NCT01245062). Additional molecules targeting the MAPK kinase pathway, including MEK inhibitors (trametinib [GlaxoSmithKline, Brentford, UK], MEK162 [Array Biopharma, Boulder, CO], TAK-733 [Millennium Pharmaceuticals, Inc., Cambridge, MA]), combinations of RAF and MEK inhibitors, pan-RAF inhibitors (such as RAF-265), and others, have shown promising results in preclinical studies and are being investigated in clinical trials. These molecules, alone and in combination with additional inhibitors of the MAPK pathway or parallel pathways, hold the promise of expanding the therapeutic options for melanoma patients, and provide the first tools for personalized therapy for patients whose tumors harbor MAPK pathway-activating mutations.
Mutations in B-RAF
Mutations in the B-RAF gene are found early in the development of melanoma, and can also be seen in benign nevi. The most common mutation of B-RAF (∼80%) in melanoma is V600E and it involves substitution of valine to glutamic acid.25,26 Less common mutations are the V600K (∼20%) and the V600D or V600R (∼3%).27 B-RAF mutations are found in various categories of melanocytic growth, including melanocytic nevi (70%–80%), vertical growth-phase melanoma (40%–50%), and metastatic melanoma (40%–50%).28 The precise role of B-RAF mutations in oncogenesis is unclear, but resultant constitutive activation of the MAPK pathway causes cellular growth and vascular development in melanoma tumors.29
Mutations in N-RAS
The RAS family members are G proteins, which serve as critical mediators in the transduction of growth signals into the cell. N-RAS mutations have been identified in 15%–20% of cutaneous melanoma and are presumed to be one of the important drivers of oncogenesis.30 A somatic mutation in the N-RAS gene causes constitutive activation of the N-RAS protein, which leads to the successive activation of downstream serine/threonine kinases, which promote cell cycle progression, cellular transformation, and enhanced cell survival.30 The overexpression of growth factor receptors, such as epidermal growth factor receptor, c-Met, platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor, and c-KIT is also implicated as a mechanism of cellular growth and transformation in RAS-driven melanomas.30,31 The most important downstream mediators of activated RAS are the serine/threonine kinases B-RAF and C-RAF, which are activated following RAS binding.
The most frequent mutations in N-RAS affect exon 2 (codon 60 and 61) and exon 1 (codon 12 and 13). Substitutions at positions 60 and 61 accounted for 82.4% of N-RAS mutations, most frequently a glutamine to arginine/lysine/leucine substitution at position 61 (Q61R/K/L). Other frequent mutations (17.6%) affect G12 and G13 on exon 1.32–34
Impact of B-RAF and N-RAS mutations on clinicopathological profile, prognosis, and response to therapy
B-RAF and N-RAS mutations appear to be biologically different than wild-type (WT) tumors and clearly have an impact on the clinical course. In early stage melanoma, N-RAS mutations are associated with thicker tumors; 75% of N-RAS mutant melanomas are >1 mm in depth, while 40% of B-RAF and 34% of WT primary melanomas are thicker than 1 mm. N-RAS mutations were also associated with increased proliferation rates, with 75% having >1 mitosis/mm2, versus 40% for B-RAF mutations and 55% for WT.33 The majority of early stage melanomas with B-RAF mutations are the superficial spreading subtype (88%), and B-RAF mutations are more likely to be found in body areas with intermittent sun exposure compared to chronic or no sun exposure (P = 0.02).35,36
N-RAS mutation status has been identified as an independent predictor of shorter survival in patients with metastatic melanoma (N-RAS vs WT, 8.2 vs 15.1 months; P = 0.004).32 The risk of brain metastasis at the time of diagnosis of stage IV disease was also noted to be significantly higher in B-RAF mutant (24%) and N-RAS mutant (23%) patients compared with WT patients (12%). However, patients with B-RAF mutant melanoma treated with specific B-RAF inhibitors have an improved survival compared with N-RAS and tumors that are WT for both.32,36 N-RAS mutation might be predictive of improved response to high-dose IL-2: a recent retrospective analysis showed differences in response to IL-2 in melanoma patients based on the mutation status (N-RAS 47%, B-RAF 23%, WT 12%; P = 0.05).37 Patients with N-RAS mutations had nonstatistically significant longer overall survival (5.3 vs 2.4 years; P = 0.30) and progression-free survival (214 vs 70 days; P = 0.13).
Other MAPK pathway mutations
GNAQ and GNA11 mutations in uveal melanoma
Activating somatic mutations in either of the homologous G-protein GNAQ or GNA11 genes lead to activation of MAPK kinase pathway in uveal melanoma. Mutations affecting Q209 in GNA11 were seen in 32% of primary uveal melanomas and in 57% of uveal melanoma metastasis.38,39 The Q209 mutation in GNAQ was seen in 45% of the uveal melanoma and 22% of the metastatic tumors. Transduction of mutated GNA11 in murine models showed enhanced growth and metastasis in xenografts and also revealed activation of the MAPK pathway.40 The molecules mediating cross-talk between these mutant proteins and MAPK pathway remains elusive. Data derived from a limited number of patients suggest that these mutations do not have prognostic significance.40
KIT mutations in acral lentiginous and mucosal melanoma
C-KIT is a transmembrane receptor that belongs to the receptor tyrosine kinase family of proteins and is normally expressed on a restricted set of cell types, including melanocytes and interstitial cells of Cajal.41,42 The endogenous ligand for c-KIT is stem cell factor. Binding of stem cell factor to c-KIT results in activation of several intracellular signaling pathways involved in cell proliferation, invasion, metastasis, and inhibition of apoptosis.43 Several activating mutations in c-KIT have been described that result in ligand-independent constitutive activity of the tyrosine kinase.
Activating c-KIT mutations are commonly found in gastrointestinal stromal tumors, which are thought to originate from interstitial cells of Cajal.43 In addition, c-KIT mutations are frequently present in three subpopulations of melanoma: mucosal (15%–27%), acral (9%–23%), and less frequently in sun-damaged cutaneous melanomas (0%–16%).44–46
Molecular-targeted therapy in melanoma
B-RAF-targeted therapy in melanoma
Development of drugs targeting mutant B-RAFV600E is a major advance in personalized therapy for melanoma. The two agents that have demonstrated the most dramatic clinical benefit in melanoma are vemurafenib (PLX4032) and dabrafenib (GSK2118436).
Vemurafenib
A parental compound of vemurafenib, PLX4720, a 7-azaindole derivative, was discovered by using a structure-guided discovery approach, and therefore it preferentially inhibits the constitutively active mutant B-RAFV600E compared with other kinases, and potent cytotoxic effects are also exclusive to cells bearing the V600-mutated allele.47 In a multicenter, Phase I, dose-escalation trial of PLX4032, the maximum-tolerated oral dose (960 mg twice daily) was established.48 In the expansion cohort, which consisted of patients with melanomas harboring B-RAFV600E mutations, 26 of the 32 patients had objective responses (81%), with a CR in two patients and a PR in 24 patients.48 B-RAF in Melanoma 2 (BRIM-2) was a Phase II study that enrolled 132 patients with previously treated stage IV melanoma with B-RAFV600E mutations, and demonstrated an RR of 53%, SD in 29%, median progression-free survival (PFS) of 6.7 months, and an OS at 6 and 12 months of 77% and 58%, respectively.49
The efficacy of vemurafenib was confirmed in a Phase III trial, BRIM-3, that compared vemurafenib to DTIC in 675 previously untreated patients, whose tumors harbored B-RAFV600E mutations.50 At interim analysis, the vemurafenib arm was associated with a significantly improved RR (48% vs 4%, P < 0.001), median PFS (5.3 vs 1.6 months, hazard ratio 0.26, 95% CI: 0.20–0.33; P < 0.001), and an improved OS at 6-month follow-up (84% vs 64%, hazard ratio for death 0.37, 95% CI: 0.26–0.55; P < 0.001).
Dabrafenib
This is a highly potent, selective ATP-competitive B-RAF inhibitor. In the preliminary results of a Phase I/II study, dabrafenib was shown to be very active in patients with metastatic melanoma, and a safe oral dose of 150 mg twice daily was established.51 Among the 57 melanoma patients with B-RAF mutations, a response rate of 27% was noted, including ten of 16 at the planned Phase II dose. A Phase III trial comparing dabrafenib with DTIC has completed accrual and final results are pending (NCT01227889). Dabrafenib also was noted to have activity against small, previously untreated brain metastases, and currently a Phase I/II trial is investigating its efficacy in central nervous system metastasis (NCT01266967).
Toxicities of selective B-RAF inhibitors
Overall, both vemurafenib and dabrafenib are well tolerated. Common adverse events associated with vemurafenib are arthralgia, rash, fatigue, alopecia, skin side effects, nausea, and diarrhea. Vemurafenib is associated with unexpected cutaneous toxicities that include squamous cell carcinomas of the skin (12%), keratoacanthomas (8%), and photosensitivity (30%). The reported incidence of non-melanoma skin cancer was lower (∼2%) with dabrafenib in the Phase I/II trials. Molecular studies indicate that these lesions are due to paradoxical activation of the MAPK pathway that bypasses the inhibition of B-RAF. Furthermore, the short latency period until the development of these skin lesions is consistent with the presence of preexisting RAS mutations in the skin that become oncogenic when subjected to B-RAF inhibition.52
Testing for B-RAF mutations in tumor samples
Mutations in B-RAF in tumor samples can be detected by a number of molecular methods. The Cobas 4800 B-RAFV600 Mutation Test (LabCorp, Burlington, NC) is a PCR-based diagnostic test developed by Roche Molecular Systems (Pleasanton, CA) and was used to detect V600E mutations in tumor samples for patient selection for the BRIM-2 and BRIM-3 clinical trials.53 It was approved by the FDA as a companion diagnostic for vemurafenib.
There are various methods of sequencing DNA, and traditional direct (Sanger or dideoxy) sequencing is a commonly used method for mutation testing in clinical laboratories. However, the Sanger sequencing method, which uses sequence-specific termination of a DNA synthesis reaction using modified nucleotide substrates, suffers from limited sensitivity for detecting mutations that are present in low percentages in a tumor tissue.54
The 454 method (massively parallel pyrosequencing method) was used as a gold standard to evaluate the discordances between polymerase chain reaction (Cobas) and Sanger results. The Cobas test was shown to be superior in detecting B-RAFV600E mutations to Sanger sequencing.55 However, the sensitivity of this test to detect V600K (10%–20% of all B-RAF) is only 70%, and additional testing might be needed to identify V600K mutations to avoid exclusion of patients who might benefit from B-RAF-targeting drugs.53 Detection of B-RAF mutation by immunohistochemistry using mutation-specific antibodies has shown remarkable specificity when confirmed by direct sequencing methods. This was studied on paraffin-embedded tissue samples of brain metastasis of various primary cancers (50% of which were melanoma) and hairy cell leukemia, and future application of this test on a larger melanoma sample cohort is warranted to confirm the validity of this test.56,57
Targeting c-KIT in melanoma
Imatinib (Novartis, NY, NY) a multikinase inhibitor of c-KIT, PDGFR-α and -β, and Bcr-Abl, was successfully used in the treatment of chronic myelogenous leukemia and gastrointestinal stromal tumors.58,59 Given that melanoma cells express several protein tyrosine kinases, studies were initiated to assess the activity of imatinib in melanoma.60 Two trials failed to demonstrate clinical activity of imatinib in unselected melanoma patients.61,62 Importantly however, subsequent analysis of available melanoma cell lines showed no c-KIT mutations,61 and none of the analyzed melanomas demonstrated strong staining for C-KIT.62 In another study, imatinib treatment of patients whose melanomas expressed at least one of the target protein tyrosine kinases showed a PR in only one out of 21 patients. Interestingly, despite strong c-KIT staining by immunohistochemistry, c-KIT mutation, copy number variation, and gene amplification could not be identified. An alternative splice variant was found in exon 15, the significance of which was unclear.63 In contrast, case reports were subsequently published showing substantial responses to imatinib in melanomas harboring c-KIT mutations.64–67 This led to the initiation of several Phase II trials in which metastatic melanoma patients, with either amplification or mutation of c-KIT in their tumors, were treated with imatinib. A Phase II trial by Guo et al in 2011 showed a promising 6-month PFS rate of 36.6% and a 1-year overall survival of 51.0%. Partial responses were seen in 23% and tumor shrinkage in 41.9% of the 43 patients. Most of the responses were seen in tumors with c-KIT mutations in exons 11 and 13.68 In a second study of imatinib in patients with c-KIT mutations and/or amplifications, complete durable responses were seen in two out of 25 patients, while four patients achieved PRs (two durable), and prolonged stable disease (≥6 months) was achieved in two patients. Similar to the previous study, most responders were found to have c-KIT mutations in exon 11 or exon 13.44
Although most published case reports and Phase II studies used imatinib as a c-KIT inhibitor, clinical responses in patients with c-KIT mutant melanomas to treatment with other small molecule kinase inhibitors including dasatinib (Bristol-Myers Squibb, Princeton, NJ), sorafenib (Bayer Healthcare Pharmaceuticals, Inc, Leverkusen Germany and Onyx Pharm, Inc, South San Francisco, CA) nilotinib (Novartis, NY, NY), and sunitinib (Pfizer, NY, NY) have been reported.69–73
In summary, these studies indicate that treatment of melanoma with c-KIT inhibitors is only effective in the small subset of melanomas that harbor c-KIT mutations; melanoma tumors with strong c-KIT expression without mutation or amplification of the gene do not appear to respond to c-KIT inhibitors. Further characterization of the c-KIT mutations in relation to response to c-KIT inhibitors is needed, since early evidence suggests that mutations in exons 11 and 13 (ie, L576P and K642E) are more likely to respond to therapy.
MEK inhibitors in melanoma
MEK is an attractive therapeutic target since MEK inhibitors have shown significant antiproliferative activity in preclinical melanoma studies.74 Currently, a number of active molecules targeting MEK are under investigation, and have shown promising results in preclinical and clinical trials.
GSK1120212 (GlaxoSmithKline, Brentford, UK) is a potent and selective allosteric inhibitor of MEK1/2. A Phase I/II clinical trial including 20 evaluable patients with a B-RAF mutant melanoma treated with 2 mg of daily oral dose of GSK1120212 (the recommended Phase II dose) showed an RR of 40% and SD in 18%. The therapy was well tolerated.75 The most common adverse events were an acneiform rash (85%), diarrhea (48%), fatigue (37%), nausea (20%), and vomiting (24%). The ocular toxicities, an uncommon class effect, include central serous retinopathy and retinal vein occlusion, seen in three and one patient out of 162, respectively. A two-arm, open-label, randomized Phase III study comparing single-agent GSK1120212 to chemotherapy (either DTIC or paclitaxel [Bristol-Myers Squibb, Princeton, NJ]) in patients with unresectable melanoma harboring V600E mutations has recently been completed and results are awaited (NCT01245062).
Selumetinib (AZD6244, Array BioPharma, Boulder, CO), a selective MEK inhibitor, was compared with temozolamide in a randomized Phase II study for metastatic or locally advanced melanoma. At 100 mg twice-daily oral dose, there was no significant difference noted between two arms in terms of the primary end point, PFS. In a subset of patients with tumors harboring B-RAFV600E mutations (45 patients out of 200), five patients had an objective response (11%).76 Several other MEK inhibitors are currently being investigated in early clinical trials.
Combinations of B-RAF and MEK inhibitors
The majority of the patients treated with B-RAF inhibitors eventually develop disease progression. For example, the median PFS for vemurafenib in the BRIM-3 trial was 5.5 months. Various mechanisms of acquired resistance to vemurafenib have been described. Resistance to B-RAF inhibition can be mediated by several different mechanisms that restore ERK activation. This may occur upstream (new N-RAS mutations, up regulation of C-RAF, and upregulation of receptor tyrosine kinases, PDGFRB, ERBB2), or downstream (such as new activating MEK mutations or activation of serine/threonine MAPK kinases [COT] or in parallel signaling pathways, particularly the PI3K-AKT pathway).77–80 Consequently, MEK inhibition in addition to B-RAF inhibition can potentially overcome the resistance mediated by mechanisms upstream of B-RAF. A Phase I/II clinical trial combining GSK436 (GlaxoSmithKline, Brentford, UK) (B-RAF inhibitor) and GSK212 (GlaxoSmithKline, Brentford, UK) (MEK inhibitor) demonstrated tolerability and dramatic clinical activity. In the cohort treated with the maximum tolerated dose combination (GSK436 200 mg daily and GSK212 1.5 mg daily, both given orally), of a total of 19 patients, the RR was 74% (CR in four and PR in ten patients), and SD was seen in five patients.81 Interestingly, the B-RAF and MEK inhibitor combination appears to be associated with a lower incidence of skin toxicities: fewer rashes were seen (25%) and only one case of cutaneous squamous cell carcinoma was seen in the whole Phase I/II cohort (109 patients).
Another combination of a B-RAF inhibitor (RAF265 [Chiron Corp, Emeryville, CA]) and an MEK inhibitor (MEK162) is currently being tested in advanced solid tumors harboring RAS and B-RAFV600E mutations (NCT01352273).
PTEN loss and use of PI3K inhibitors in melanoma
Constitutive activation of the AKT/PI3K pathway has been implicated in many preclinical and clinical melanoma studies.23 AKT has been shown to be overexpressed in nearly 60% of all melanomas.82 Significant decreases in PTEN expression occur in 43% of melanoma tumors and are associated with aggressive tumor behavior.83 ERK-dependent upregulation of c-Jun leads to suppression of PTEN expression and concomitant activation of the PI3K/AKT/mTOR pathway. Therefore, targeting the PI3K pathway appears to be a promising approach in melanoma. A Phase I clinical trial combining PI3K inhibitors with MEK inhibitors (PI3K inhibitor BAY80-6946 [Bayer Healthcare Pharmaceuticals, Leverkusen, Germany] [intravenous] and MEK inhibitor BAY86-9766 [Bayer Healthcare Pharmaceuticals, Leverkusen, Germany] [oral]; NCT01392521) is currently enrolling patients. It remains to be determined if loss of PTEN correlates with activity of PI3K and whether it can be used as a predictive biomarker of PI3K treatment.
Current status and future directions for personalized therapy for metastatic melanoma
At present, vemurafenib is the only approved therapy that specifically targets an aberrant molecule in melanocytes. However the field is rapidly expanding, and many newer molecules are likely to be approved in the near future. B-RAF mutation testing is now routinely done in patients with newly diagnosed metastatic melanoma. However, the decision about starting treatment with a B-RAF inhibitor is based on the individual clinical scenario, and many patients whose tumors harbor V600E mutations may initiate treatment with other therapies such as ipilimumab or IL-2. Vemurafenib can be helpful for patients who are symptomatic and require a rapid response. Immune-based therapies are associated with less frequent and sometimes slower responses; however, these responses are often durable and can result in prolonged survival. Predictive biomarkers for immune-based therapies are still lacking. Furthermore, biomarkers predictive of a short duration of response to melanocyte-targeted therapies are similarly lacking and are the subject of intense research. Although progress has been made in personalized therapy for melanoma in recent years, the next decade will likely bring additional therapies with improved companion diagnostics.
Footnotes
Disclosure
Harriet Kluger has served as a consultant to Genentech. The other authors have no conflicts of interest to declare for this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 4.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 5.Sznol M, Powderly D, Smith DC, et al. Safety and antitumor activity of biweekly MDX-1106 (Anti-PD-1, BMS-936558/ONO-4538) in patients with advanced refractory malignancies [abstract 2506] J Clin Oncol. 2010;28(Suppl):15s. [Google Scholar]
- 6.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–S14. [PubMed] [Google Scholar]
- 7.Besser MJ, Treves AJ, Itzhaki O, et al. Adoptive cell therapy for metastatic melanoma patients: pre-clinical development at the Sheba Medical Center. Isr Med Assoc J. 2006;8(3):164–168. [PubMed] [Google Scholar]
- 8.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 11.Sznol M. Molecular markers of response to treatment for melanoma. Cancer J. 2011;17(2):127–133. doi: 10.1097/PPO.0b013e318212dd5a. [DOI] [PubMed] [Google Scholar]
- 12.Hamid O, Tsuchihashi Z, Alaparthy S, Galbraith S, Berman D. Association of baseline and on-study tumor biopsy markers with clinical activity in patients (pts) with advanced melanoma treated with ipilimumab [abstract 9008] J Clin Oncol. 2009;27(Suppl):15S. [Google Scholar]
- 13.Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108(40):16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goff SL, Robbins PF, El-Gamil M, Rosenberg SA. No correlation between clinical response to CTLA-4 blockade and presence of NY-ESO-1 antibody in patients with metastatic melanoma. J Immunother. 2009;32(8):884–885. doi: 10.1097/CJI.0b013e3181affbf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarhini AA, Edington H, Butterfield LH, et al. Neoadjuvant ipilimumab in patients with stage IIIB/C melanoma: immunogenicity and biomarker analysis [abstract 8536] J Clin Oncol. 2011;29(Suppl):15S. [Google Scholar]
- 17.Hodi FS. Tissue and blood biomarkers from patients with stage iii or stage iv melanoma treated with ipilimumab with or without sargramostim. Available from: http://clinicaltrials.gov/ct2/show/NCT01489423. Accessed July 14, 2012.
- 18.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63(4):756–759. [PubMed] [Google Scholar]
- 23.Dhawan P, Richmond A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J Biol Chem. 2002;277(10):7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Suresh Kumar KG, Yu D, et al. Oncogenic BRAF regulates beta-Trcp expression and NF-kappaB activity in human melanoma cells. Oncogene. 2007;26(13):1954–1958. doi: 10.1038/sj.onc.1209994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62(23):6997–7000. [PubMed] [Google Scholar]
- 26.Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23(3):529–545. ix. doi: 10.1016/j.hoc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Rubinstein JC, Sznol M, Pavlick AC, et al. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010;8:67. doi: 10.1186/1479-5876-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollock PM, Cohen-Solal K, Sood R, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34(1):108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65(6):2412–2421. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 30.Furge KA, Kiewlich D, Le P, et al. Suppression of Ras-mediated tumorigenicity and metastasis through inhibition of the Met receptor tyrosine kinase. Proc Natl Acad Sci U S A. 2001;98(19):10722–10727. doi: 10.1073/pnas.191067898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bardeesy N, Kim M, Xu J, et al. Role of epidermal growth factor receptor signaling in RAS-driven melanoma. Mol Cell Biol. 2005;25(10):4176–4188. doi: 10.1128/MCB.25.10.4176-4188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakob JA, Bassett RL, Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. doi: 10.1002/cncr.26724. Epub December 16, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devitt B, Liu W, Salemi R, et al. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment Cell Melanoma Res. 2011;24(4):666–672. doi: 10.1111/j.1755-148X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 34.Monsel G, Ortonne N, Bagot M, Bensussan A, Dumaz N. c-Kit mutants require hypoxia-inducible factor 1alpha to transform melanocytes. Oncogene. 2010;29(2):227–236. doi: 10.1038/onc.2009.320. [DOI] [PubMed] [Google Scholar]
- 35.Poynter JN, Elder JT, Fullen DR, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16(4):267–273. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- 36.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 37.Joseph RW, Sullivan RJ, Harrell R, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother. 2012;35(1):66–72. doi: 10.1097/CJI.0b013e3182372636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363(23):2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Besaratinia A, Pfeifer GP. Uveal melanoma and GNA11 mutations: a new piece added to the puzzle. Pigment Cell Melanoma Res. 2011;24(1):18–20. doi: 10.1111/j.1755-148X.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 40.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yarden Y, Kuang WJ, Yang-Feng T, et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNiece IK, Briddell RA. Stem cell factor. J Leukoc Biol. 1995;58(1):14–22. doi: 10.1002/jlb.58.1.14. [DOI] [PubMed] [Google Scholar]
- 43.Lux ML, Rubin BP, Biase TL, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol. 2000;156(3):791–795. doi: 10.1016/S0002-9440(10)64946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 46.Garrido MC, Bastian BC. KIT as a therapeutic target in melanoma. J Invest Dermatol. 2010;130(1):20–27. doi: 10.1038/jid.2009.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105(8):3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. New Engl J Med. 2010;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. New Engl J Med. 2012;366(8):707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kefford R, Arkenau H, Brown MP, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors [abstract 8503] J Clin Oncol. 2010;28(Suppl):15s. [Google Scholar]
- 52.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. New Engl J Med. 2012;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halait H, Demartin K, Shah S, et al. Analytical performance of a real-time PCR-based assay for V600 mutations in the BRAF gene, used as the companion diagnostic test for the novel BRAF inhibitor vemurafenib in metastatic melanoma. Diagn Mol Pathol. 2012;21(1):1–8. doi: 10.1097/PDM.0b013e31823b216f. [DOI] [PubMed] [Google Scholar]
- 54.Tan YH, Liu Y, Eu KW, et al. Detection of BRAF V600E mutation by pyrosequencing. Pathology. 2008;40(3):295–298. doi: 10.1080/00313020801911512. [DOI] [PubMed] [Google Scholar]
- 55.Anderson S, Bloom KJ, Vallera DU, et al. Multisite analytic performance studies of a real-time polymerase chain reaction assay for the detection of BRAF V600E mutations in formalin-fixed paraffin-embedded tissue specimens of malignant melanoma. Arch Pathol Lab Med. doi: 10.5858/arpa.2011-0505-OA. Epub February 14, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Capper D, Berghoff AS, Magerle M, et al. Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol. 2012;123(2):223–233. doi: 10.1007/s00401-011-0887-y. [DOI] [PubMed] [Google Scholar]
- 57.Andrulis M, Penzel R, Weichert W, von Deimling A, Capper D. Application of a BRAF V600E mutation-specific antibody for the diagnosis of hairy cell leukemia. Am J Surg Pathol. doi: 10.1097/PAS.0b013e3182549b50. Epub April 22, 2012. [DOI] [PubMed] [Google Scholar]
- 58.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. New Engl J Med. 2002;347(7):472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 59.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 60.Shen SS, Zhang PS, Eton O, Prieto VG. Analysis of protein tyrosine kinase expression in melanocytic lesions by tissue array. J Cutan Pathol. 2003;30(9):539–547. doi: 10.1034/j.1600-0560.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 61.Ugurel S, Hildenbrand R, Zimpfer A, et al. Lack of clinical efficacy of imatinib in metastatic melanoma. Br J Cancer. 2005;92(8):1398–1405. doi: 10.1038/sj.bjc.6602529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wyman K, Atkins MB, Prieto V, et al. Multicenter phase II trial of high-dose imatinib mesylate in metastatic melanoma: significant toxicity with no clinical efficacy. Cancer. 2006;106(9):2005–2011. doi: 10.1002/cncr.21834. [DOI] [PubMed] [Google Scholar]
- 63.Kim KB, Eton O, Davis DW, et al. Phase II trial of imatinib mesylate in patients with metastatic melanoma. Br J Cancer. 2008 Sep 2;99(5):734–740. doi: 10.1038/sj.bjc.6604482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hodi FS, Friedlander P, Corless CL, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008 Apr 20;26(12):2046–2051. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- 65.Lutzky J, Bauer J, Bastian BC. Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res. 2008 Aug;21(4):492–493. doi: 10.1111/j.1755-148X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 66.Satzger I, Kuttler U, Volker B, Schenck F, Kapp A, Gutzmer R. Anal mucosal melanoma with KIT-activating mutation and response to imatinib therapy – case report and review of the literature. Dermatology. 2010;220(1):77–81. doi: 10.1159/000265558. [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi M, Harada K, Ando N, Kawamura T, Shibagaki N, Shimada S. Marked response to imatinib mesylate in metastatic acral lentiginous melanoma on the thumb. Clin Exp Dermatol. 2011 Mar;36(2):174–177. doi: 10.1111/j.1365-2230.2010.03885.x. [DOI] [PubMed] [Google Scholar]
- 68.Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011 Jul 20;29(21):2904–2909. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]
- 69.Cho JH, Kim KM, Kwon M, Kim JH, Lee J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Invest New Drugs. 2011 Nov 9; doi: 10.1007/s10637-011-9763-9. [DOI] [PubMed] [Google Scholar]
- 70.Kluger HM, Dudek AZ, McCann C, et al. A phase 2 trial of dasatinib in advanced melanoma. Cancer. 2011 May 15;117(10):2202–2208. doi: 10.1002/cncr.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minor DR, Kashani-Sabet M, Garrido M, O'Day SJ, Hamid O, Bastian BC. Sunitinib therapy for melanoma patients with KIT mutations. Clin Cancer Res. 2012 Mar 1;18(5):1457–1463. doi: 10.1158/1078-0432.CCR-11-1987. [DOI] [PubMed] [Google Scholar]
- 72.Quintas-Cardama A, Lazar AJ, Woodman SE, Kim K, Ross M, Hwu P. Complete response of stage IV anal mucosal melanoma expressing KIT Val560Asp to the multikinase inhibitor sorafenib. Nat Clin Pract Oncol. 2008 Dec;5(12):737–740. doi: 10.1038/ncponc1251. [DOI] [PubMed] [Google Scholar]
- 73.Woodman SE, Trent JC, Stemke-Hale K, et al. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther. 2009 Aug;8(8):2079–2085. doi: 10.1158/1535-7163.MCT-09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang XD, Borrow JM, Zhang XY, Nguyen T, Hersey P. Activation of ERK1/2 protects melanoma cells from TRAIL-induced apoptosis by inhibiting Smac/DIABLO release from mitochondria. Oncogene. 2003 May 15;22(19):2869–2881. doi: 10.1038/sj.onc.1206427. [DOI] [PubMed] [Google Scholar]
- 75.Infante JR, Nallapareddy S, Gordon MS, et al. Messersmith. Safety and efficacy results from the first-in-human study of the oral MEK 1/2 inhibitor GSK1120212. Paper presented at: ASCO annual meeting 2010. [Google Scholar]
- 76.Dummer R, Chapman PB, Sosman JA, et al. AZD6244 (ARRY-142886) vs temozolomide (TMZ) in patients (pts) with advanced melanoma: An open-label, randomized, multicenter, phase II study. Paper presented at: ASCO annual meeting 2008; Chicago, IL, USA. [Google Scholar]
- 77.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010 Dec 16;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010 Dec 16;468(7326):968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Montagut C, Sharma SV, Shioda T, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Research. 2008 Jun 15;68(12):4853–4861. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H, Daouti S, Li WH, et al. Identification of the MEK1(F129L) activating mutation as a potential mechanism of acquired resistance to MEK inhibition in human cancers carrying the B-RafV600E mutation. Cancer research. 2011 Aug 15;71(16):5535–5545. doi: 10.1158/0008-5472.CAN-10-4351. [DOI] [PubMed] [Google Scholar]
- 81.Infante JR, Lawrence DP, Weber JS, et al. Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436). Paper presented at: ASCO annual meeting 2011; Chicago, IL, USA. [Google Scholar]
- 82.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer research. 2004 Oct 1;64(19):7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 83.Mikhail M, Velazquez E, Shapiro R, et al. PTEN expression in melanoma: relationship with patient survival, Bcl-2 expression, and proliferation. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2005 Jul 15;11(14):5153–5157. doi: 10.1158/1078-0432.CCR-05-0397. [DOI] [PubMed] [Google Scholar]