Abstract
Purpose.
The mode of development of the human hyaloid vascular system (HVS) remains unclear. Early studies suggested that these blood vessels formed by vasculogenesis, while the current concept seems to favor angiogenesis as the mode of development. We examined embryonic and fetal human HVS using a variety of techniques to gain new insights into formation of this vasculature.
Methods.
Embryonic and fetal human eyes from 5.5 to 12 weeks gestation (WG) were prepared for immunohistochemical analysis or for light and electron microscopy. Immunolabeling of sections with a panel of antibodies directed at growth factors, transcription factors, and hematopoietic stem cell markers was employed.
Results.
Light microscopic examination revealed free blood islands (BI) in the embryonic vitreous cavity (5.5–7 WG). Giemsa stain revealed that BI were aggregates of mesenchymal cells and primitive nucleated erythroblasts. Free cells were also observed. Immunolabeling demonstrated that BI were composed of mesenchymal cells that expressed hemangioblast markers (CD31, CD34, C-kit, CXCR4, Runx1, and VEGFR2), erythroblasts that expressed embryonic hemoglobin (Hb-ε), and cells that expressed both. Few cells were proliferating as determined by lack of Ki67 antigen. As development progressed (12 WG), blood vessels became more mature structurally with pericyte investment and basement membrane formation. Concomitantly, Hb-ε and CXCR4 expression was down-regulated and von Willebrand factor expression was increased with the formation of Weibel-Palade bodies.
Conclusions.
Our results support the view that the human HVS, like the choriocapillaris, develops by hemo-vasculogenesis, the process by which vasculogenesis, erythropoiesis, and hematopoiesis occur simultaneously from common precursors, hemangioblasts.
Human embryonic hyaloid vasculature develops by hemo-vasculogenesis, the process by which blood vessels and blood cells develop simultaneously from common precursors, hemangioblasts.
Introduction
The hyaloid vascular system (HVS) is a transient network of blood vessels that nourishes the immature lens and avascular inner retina of the developing embryonic and fetal eye. It is composed of the hyaloid artery (HYA), vasa hyaloidea propria (VHP), tunica vasculosa lentis (TVL), and pupillary membrane (PM).1 Once the lens has formed and no longer requires oxygen and nutrients, this system undergoes spontaneous regression beginning around 14 weeks gestation (WG). This process is thought to involve programmed cell death2 and is concurrent with the appearance of the first retinal blood vessels. The failure of this vasculature to spontaneously involute, can manifest itself in a condition originally described by Reese as persistent hyperplastic primary vitreous3 and later termed persistent fetal vasculature syndrome by Goldberg.4 Failure of complete regression of these blood vessels results in contraction and opacification of the primary vitreous, retrolental forms of leukokoria, preretinal membranes, defects in iris, retina, and optic nerve and tractional retinal detachment.4 The mechanisms by which these vessels fail to completely regress remain poorly understood.
The HVS begins to develop around 4 WG in the human embryo and reaches its height of development around the 12th WG.1 The currently held dogma for development of this vascular system in the human embryo is as follows: the HYA passes through the embryonic fissure and branches by angiogenesis within the cavity of the primary optic vesicle.5 Behind the lens vesicle, some of the branches make contact with the posterior side of the developing lens, while others follow the margin of the cup and form anastomoses with confluent sinuses to form an annular vessel. The arborization of the HYA forms a dense capillary network around the posterior lens capsule (TVL) and surrounding the lens equator. Capillary branches then develop throughout the vitreous (VHP).
However, earlier studies by Mann and others observed that the future vitreous space contained cells that originated from the mesenchyme surrounding the outer surface of the optic cup around 4 WG.1,6 These investigators found that undifferentiated presumptive vascular primary mesenchymal cells entered the future vitreous space through the annular opening between the lens vesicle and the rim of the optic cup and through the open embryonic fissure. Very few of the cells from the primitive streak mesenchyme were in mitosis. Most of these cells appeared to form walls of small blood vessels, which gave rise to both the TVL and the hyaloid vessel system.1 At the rim of the optic cup the primary mesenchymal cells differentiated partly to become elongated, prevascular cells, that often appeared to be spherical hemangioblasts. The walls of the new capillaries were formed by one layer of prevascular cells that eventually developed into the endothelium of the hyaloid vessels. The process was similar at the embryonic fissure, where the hyaloid artery seemed to form piece by piece in situ by differentiation of the mesenchymal cells to prevascular and endothelial cells of the vessel wall, or to hemangioblasts and blood cells inside the developing vessel.
Therefore, the mode of development of the human HVS still remains somewhat controversial with the early work of Mann1 and subsequent studies of Balazs6 indicating a hemo-vasculogenic mechanism of development, and the current more widely accepted view supporting an angiogenic mode of development. While most of the recent studies have focused on the mechanisms involved in the involution of this vasculature because of its failure to do so in persistent fetal vasculature syndrome, very little is know about the molecules and processes involved in its formation. In this study, we examined embryonic and fetal human eyes with a variety of techniques to determine the morphological, immunohistochemical, and ultrastructural features of HVS development. Our results support the view that the human HVS forms by the in situ differentiation of hematopoietic stem cells (hemangioblasts) that give rise to both endothelial cells and blood cells. This is similar to the mode of development we have reported previously for choriocapillaris.7
Materials and Methods
Human fetal eyes, ranging in age from 5.5 to 12 WG (Table 1), were obtained from Advanced Bioscience Resources, Inc. (Alameda, CA) and Stem-Express (Placerville, CA). Utilization of this human tissue was in accordance with the Declaration of Helsinki with approval of the Joint Committee on Clinical Investigations at Johns Hopkins University School of Medicine. The gestational age of each embryo or fetus was determined by the fetal foot size and sonography of the fetus in utero.
Table 1. .
Age (Weeks Gestation) of Specimens
|
Age (Weeks Gestation) |
No. of Eyes |
Tissue Preparation |
| 5.5 | 2 | 1 JB-4, 1 Cryo |
| 6 | 3 | Cryo |
| 6.5 | 4 | 1 JB-4, 2 Cryo, 1 TEM |
| 7 | 2 | Cryo |
| 8 | 3 | 2 Cryo, 1 TEM |
| 9 | 2 | Cryo |
| 10 | 3 | Cryo |
| 12 | 4 | 1 JB-4, 2 Cryo, 1 TEM |
Streptavidin Alkaline Phosphatase (APase) Immunohistochemistry
APase immunohistochemistry was performed on cryopreserved tissue sections using a nitroblue tetrazolium (NBT) system as described previously.8 The sections of human fetal eyes were incubated overnight at 4°C with primary antibodies (Table 2). After washing in Tris-buffered saline (TBS), sections were incubated for 30 minutes at room temperature with the appropriate biotinylated secondary antibodies diluted 1:500 (Kirkegaard and Perry, Gaithersburg, MD). Finally, sections were incubated with streptavidin APase (1:500; Kirkegaard and Perry), and APase activity was developed with a 5-bromo-4-chloro-3-indoyl phosphate (BCIP)-NBT kit (Vector Laboratories, Inc., Burlington, CA), yielding a blue reaction product.
Table 2. .
Antibody Sources
|
Antibody |
Source |
City, State |
Catalog No. |
| Rb-α-alpha SMA | Abcam | Cambridge, MA | ab5694 |
| Rb-α-CD117 | Thermo Fisher | Waltham, MA | RB9038-P0 |
| Rb-α-vWf | Dako | Carpinteria, CA | A0082 |
| Rb-α-SCF | Assay Design (Enzo) | Farmingdale, NY | 915-005 |
| Rb-α-CXCR4 | Novus | Littleton, CO | NLS1380 |
| Rb-α-RUNX1 | Abcam | Cambridge, MA | ab61753 |
| Ms-α-Ki67 | Zymed | San Francisco, CA | 18-0192Z |
| Ms-α-CD31 | Dako | Carpinteria, CA | M0823 |
| Ms-α-CD34 | Covance Res Prod | Princeton, NJ | SIG-3326-1000 |
| Ms-α-CD39 | Ancell | Bayport, MN | 188-020 |
| M-α-Flk-1 | Imclone | Bridgewater, NJ | 6.64 |
| Gt-α-SDF-1 | Santa Cruz Biotech | Santa Cruz, CA | SC-6193 |
| Gt-α-Hgb Epsilon | Fitzgerald Industries | Acton, MA | CR8008M1F |
Immunofluorescence
Immunofluorescence and confocal microscopy were used for double labeling as described previously.7 Briefly, cryopreserved tissue sections were permeabilized with absolute methanol at −20°C and blocked with 2% normal goat serum in TBS (pH 7.4 with 1% BSA). After washing in TBS, the sections were incubated for 2 hours at room temperature with a mixture of one of the primary antibody cocktails in Table 3. After they were washed in TBS, sections were incubated for 30 minutes at room temperature with a cocktail of secondary antibodies (Table 3) containing 4′,6-diamino-2-phenyl-indole (DAPI). Finally, sections were coverslipped with mounting medium (Vector Laboratories, Inc.) and imaged with a Zeiss Meta 510 or Zeiss 710 confocal microscope (Carl Zeiss, Thornwood, NY).
Table 3. .
Primary and Secondary Antibody Combinations
|
Primary Antibody Combination |
Secondary Antibody Combination |
| Rb-α-alpha SMA + Ms-α-CD31 | Gt-α-Rb CY3 + Gt-α-Ms CY5 |
| Rb-α-CD117 (c-Kit) + Ms-α-CD34 | Gt-α-Rb CY3 + Gt-α-Ms CY5 |
| Rb-α-VEGFAb-1 + Ms-α-CD34 | Gt-α-Rb CY3 + Gt-α-Ms CY5 |
| Rb-α-CXCR4 + Ms-α-CD31 | Gt-α-Rb CY3 + Gt-α-Ms CY5 |
| Rb-α-RUNX1/AML1 + Ms-α-CD34 | Gt-α-Rb CY3 + Gt-α-Ms CY5 |
| Ms-α-Ki67 + Rb-α-vWf | Gt-α-Rb CY3 + Gt-α-Ms CY5 |
| Ms-α- Flk-1 + Gt-α-Hgb Epsilon* | Rb-α-Ms CY3 |
| Ms-α-CD31 + Gt-α-Hgb Epsilon* | Rb-α-Ms CY3 |
Direct Tag FITC.
Double Labeling of Epsilon Hemoglobin (Hb-ε) with Hemangioblast and Vascular Markers
Mouse anti–Flk-1 and mouse anti-CD31 were used as a hemangioblast and an endothelial cell marker, respectively. We have shown previously that in blood islands (BI) of embryonic choroid (up to 10 WG), CD31 is coexpressed with Hb-ε in hemangioblasts. For double labeling of Hb-ε and cell markers, sections were additionally postfixed at room temperature overnight with 2% paraformaldehyde in TBS before starting the immunofluorescence staining technique as reported previously.7 After washing in TBS, the sections were processed as previously stated. The sections were incubated for 2 hours at room temperature with the primary antibodies, 30 minutes at room temperature with the secondary antibodies conjugated with CY3 or CY5 (1:500; Jackson Immuno Research, West Grove, PA), and then incubated for 2 hours at room temperature with goat anti–Hb-ε FITC (1:500; Fitzgerald Biochem, Acton, MA). Finally, sections were washed in TBS and mounted in DAPI counterstaining medium (Vector Laboratories, Inc.).
Whole Eyes for Serial Sectioning
JB-4 (glycol methacrylate; Polysciences, Warrington, PA) embedded whole eyes (5.5, 7, and 12 WG) were fixed in 25% Karnovsky's glutaraldehyde/paraformaldehyde fixative at 4°C and then washed in 0.1 M cacodylate buffer with 5% sucrose. Eyes were embedded in JB-4, serially sectioned, and stained with Wright's Giemsa as previously described.7
Transmission Electron Microscopy (TEM)
Tissue was fixed within 1 hour of harvest. After primary fixation overnight in 2.5% glutaraldehyde/2% paraformaldehyde in cacodylate buffer, the tissue was washed in 0.1 M phosphate buffer and postfixed in 1% osmium tetroxide in 0.05 M cacodylate buffer for 90 minutes. After washing in 0.05 M cacodylate buffer, it was dehydrated in 50%, 70%, 80%, 95%, and 100% ethanol (ETOH) and then stained with 1% uranyl acetate in 100% ETOH. The tissue was placed in propylene oxide twice for 15 minutes each time, and then was kept overnight in 1:1 propylene oxide to resin mixture. The tissue was then infiltrated in 100% LX112 resin (Ladd Research Industry, Burlington, VT) for 4 to 6 hours under vacuum and finally embedded in a final change of 100% LX112 resin and polymerized at 60°C for 36 to 48 hours. Ultrathin sections were cut with a Leica Ultramicrotome UCT (Leica Microsystems, Wetzlar, Germany), stained with uranyl acetate and lead citrate and analyzed with a H7600 transmission electron microscope (Hitachi, Tokyo, Japan).
Results
Light Microscopy
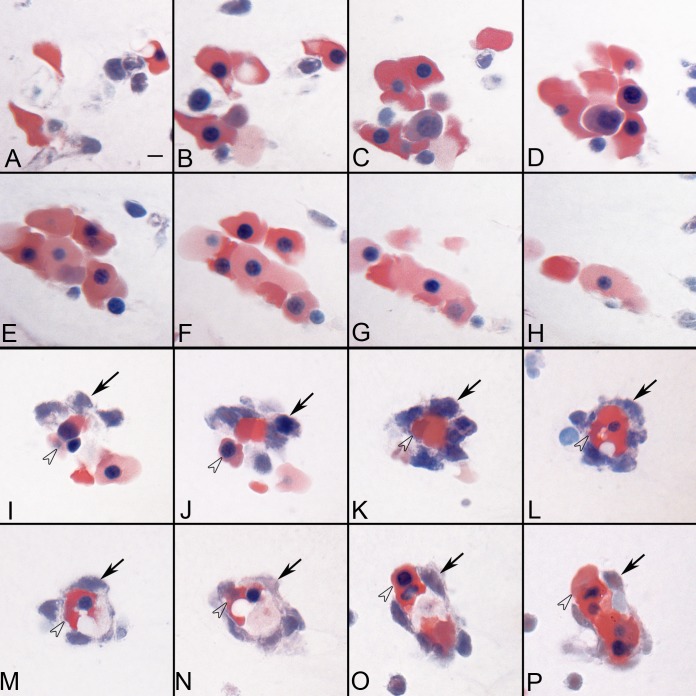
The hyaloid artery at 5.5 WG in serial sections was present in the optic stalk but had not entered the vitreous cavity (Fig. 1A). It was approximately 10 μm in diameter and was composed of a single layer of cells with its lumen containing tightly packed erythroblasts (Fig. 1B). However, the artery was not continuous but rather segmented when eyes were serial sectioned, similar to the observations of Balazs and associates.6 BI formations were present in the vitreous space near the lens vesicle and at the annular opening between the lens vesicle and the rim of the optic cup. With Wright-Giemsa staining, they generally consisted of two cells types and typically formed aggregates. Some aggregates consisted almost entirely of erythroblasts with highly acidophilic cytoplasm (Figs. 2A–H), while others consisted of a central core of erythroblasts surrounded loosely by basophilic mesenchyme (Figs. 2I–P).
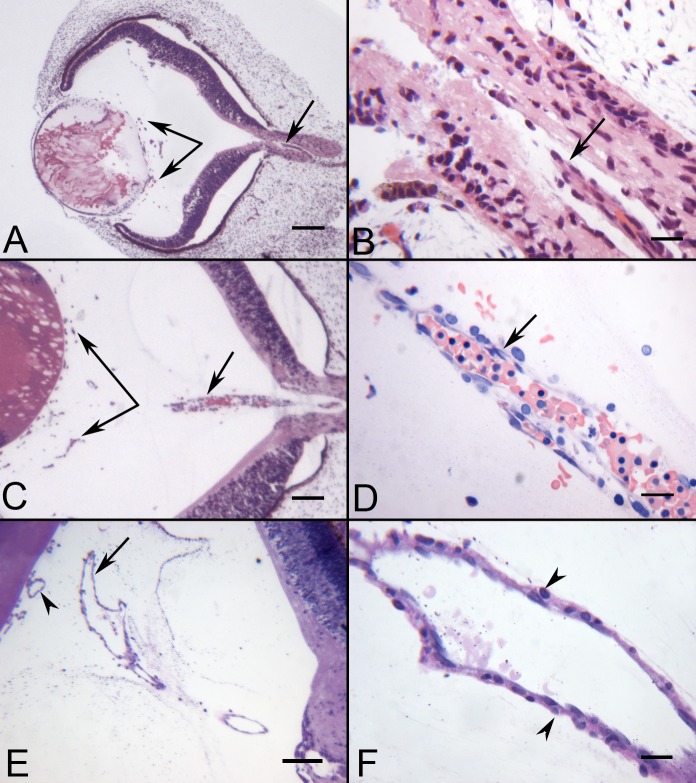
Figure 1. .
JB-4 sections of whole eyes at 5.5, 7, and 12 WG showing the hyaloid artery and blood vessels in vitreous. At 5.5 WG, the hyaloid artery was present in the optic stalk (arrow in A, B), but had not penetrated the vitreous cavity. Blood islands (BIs) were formed in vitreous near the lens vesicle (paired arrows in A). At 7 WG, the hyaloid artery was present in the vitreous and contained packed erythroblasts (arrow in C, D). Blood vessels were present in the vitreous near the lens and on its surface (paired arrows in C). By 12 WG, the hyaloid artery was fully formed and had several major branches near the posterior surface of the lens (arrow in E). Smooth muscle cells were present on the outer wall of the hyaloid artery (arrowheads in F). (A–C) Hematoxylin-eosin stain. (D) Wrights-Giemsa stain. (E, F) Periodic acid-Schiff and hematoxylin stain. Scale bars = 100 μm in (A, C, E) and 20 μm in (B, D, F).
Figure 2 .
Serial sections showing two types of BI-like structures in the vitreous at 5.5 WG. Some of the BIs consisted of aggregates of erythroblasts (A–H), while others (I–P) were composed of basophilic mesenchyme (arrows) surrounding a core of highly acidophilic erythroblasts (arrowheads). Wrights-Giemsa stain, Scale bar in A = 10 μm for all.
The hyaloid artery at 7 WG had invaded the vitreous space and was composed of multiple layers of cells surrounding packed erythroblasts (Figs. 1C, 1D). The BI in the vitreous space near the lens vesicle appeared more differentiated with lumens. They were composed of an outer layer of primitive vascular precursors, angioblasts, surrounding an inner core of erythroblasts. Few definitive pericytes were present; however, there were numerous loosely arranged perivascular cells, which made contact with, but did not fully invest, the primitive capillary wall. Free mesenchymal cells and erythroblasts were also observed at the annular opening between the lens vesicle and the rim of the optic cup.
By 10 WG, the hyaloid artery had extended farther through the vitreous and toward the lens where it branched several times to anastomose with capillaries that had formed in the vitreous and on the posterior surface of the lens vesicle (data not shown). It had a diameter of 25 μm at its greatest width. The capillaries were more developed with well differentiated endothelium and pericytes. The lumen appeared more open, and fewer erythroblasts were observed in and around the blood vessels.
By 12 WG, the hyaloid artery extended to the lens and had several major branchings (Figs. 1E, 1F). It was 50 μm in diameter at its greatest width and had one to two layers of smooth muscle cells mostly near its base. Some of these cells were in active mitosis. At the base, near its emergence from the optic nerve head, presumed astrocytes invested its outer surface. Capillaries were fully differentiated with well developed endothelial cells and pericytes.
Ultrastructure
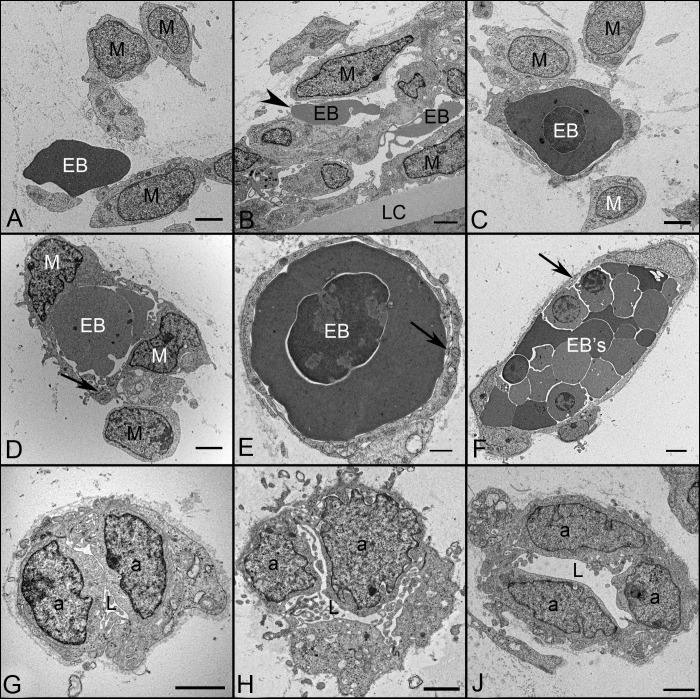
TEM demonstrated that early blood islands (BIs) observed at 6 WG consisted of two general types. Some were composed of aggregates of mesenchymal cells (indistinct chromatin) and erythroblasts (dense nucleus and cytoplasm) that were either free floating or surrounded by primitive endothelial progenitors (Figs. 3A–F). These erythroblasts had electron dense cytoplasm characteristic of highly hemoglobinized cells. In some islands, mesenchymal cells had long processes wrapping around erythroblasts (Figs. 3D–F), while others were composed of aggregates of angioblasts (called vasoformative mesoderm by Mann1), with little to no luminal space (Figs. 3G–J). Intercellular junctions between angioblastic aggregates were common, but not all were the typical tight junctions of mature endothelium. Although some loosely arranged angioblasts were present on the outer wall of these aggregates, it was not possible to distinguish endothelial cells from pericytes based on their ultrastructural characteristics (not shown). Weibel-Palade bodies were rare, and little basement membrane material was present surrounding the aggregates' outer or perivascular surface.
Figure 3. .
Ultrastructural features of BIs (A–F) and angioblastic aggregates (G–I) in the vitreous of a 6.5 WG embryonic eye showing mesenchymal cells (M), erythroblasts (EB), angioblasts (A), and early lumen formation (L). BIs (A–C) were composed of loosely arranged mesenchymal cells with free erythroblasts (A), incompletely enveloped erythroblasts (B), and erythroblasts completely enveloped by mesenchymal cells (C). In more mature structures (D–F), the central core of erythroblasts were completely surrounded by mesenchymal cells connected by junctions (arrows). Angioblastic aggregates (G–H) initially formed cords with slit-like lumen and finally primordial capillaries with lumen (J). There was a paucity of basement membrane material surrounding these BI and primitive capillaries. Scale bars = 2 μm in all.
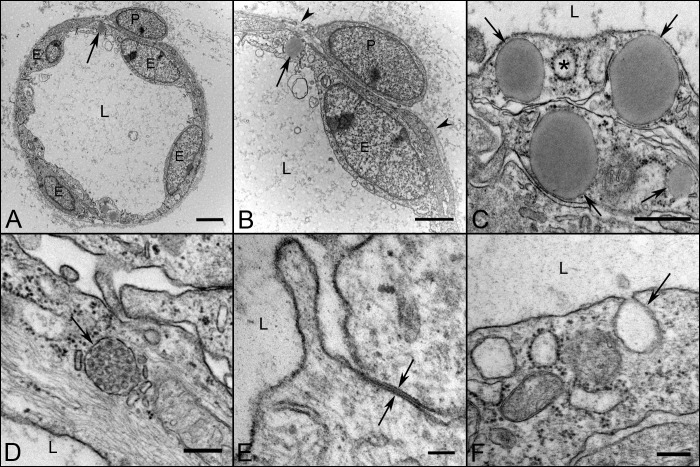
Blood vessels were more adult-like in structure by 12 WG with well differentiated endothelial cells and pericytes, open lumens, and a continuous basement membrane (Fig. 4). Tight junctions were seen between endothelial cells (Fig. 4E) and their cytoplasm contained numerous Weible-Palde bodies (Fig. 4D). Mitochondria were abundant as was endoplasmic reticulum. Occasional coated pits and caveolae were also observed (Figs. 4C, 4F). Most notable was the appearance of lipid droplets in endothelial cells at this age (Fig. 4C).
Figure 4. .
Ultrastructural appearance of capillaries and their cellular components at 12 WG. Capillaries (A, B) have well differentiated endothelium (E) and pericytes (P). The number of endothelial cells profile per capillary far outnumbered the perictytes. The lumen were fully open (L in all) and there was a continuous thin basement membrane present (arrowheads in B). Endothelial cells (C–F) contained lipid droplets (arrows in A–C), and coated pits (asterisk in C). Weibel-Palade bodies (arrow in D) were frequently observed at this age, as were tight junctions (opposing arrows in E) and caveolae (arrow in F). Scale bars = 2 μm in A, B, 500 nm in C, 250 nm in D, F, and 100 nm in E.
Embryonic Hemoglobin Expression Occurs Simultaneously with VEGFR2 and Endothelial Cell Markers
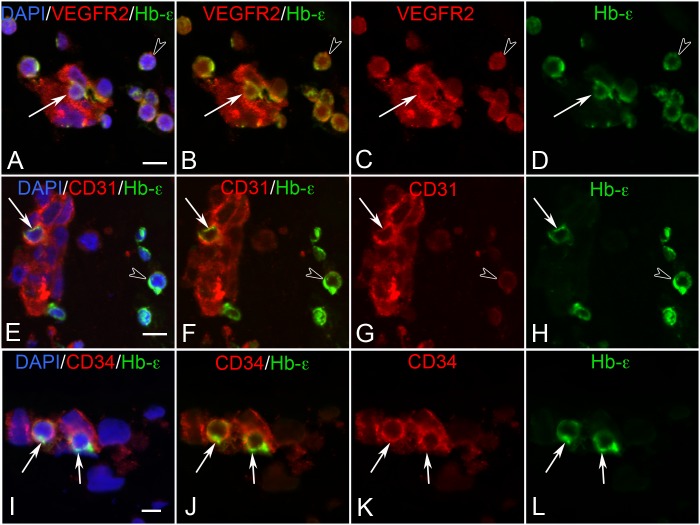
Both BI cells and free cells in 7 WG eyes were immunoreactive for VEGFR2, and to a lesser degree with CD31 (Fig. 5). CD34 was only associated with cells within aggregates. Of particular interest was the fact that some of these BI cells had a dual hematoendothelial phenotype (Fig. 5). They expressed both endothelial cell markers (CD31) and Hb-ε. As development progressed (10 WG), fewer cells were immunoreactive for Hb-ε. By 12 WG, few if any cells within blood vessels or in the vitreous expressed Hb-ε. However, VEGFR2 and CD31 and CD34 were expressed by the endothelium throughout the developmental period. A summary of the immunohistochemical results for all antibodies is shown in Table 4.
Figure 5. .
Double-labeling for VEGFR2 (A–D), CD31 (E–H), and CD34 (I–L) with Hb-ε and DAPI in BIs of the vitreous at 6 WG. VEGFR2 and Hb-ε were coexpressed in free erythroblasts (arrowhead in A–D) and in some cells within BI (arrow in A–D). All cells of the BI were VEGFR2+. In contrast, CD31/Hb-ε was coexpressed in select cells of BI (arrow in E–H) while free Hb-ε+ erythroblasts were only weakly immunoreactive for CD31 (arrowhead in E–H). CD34/Hb-ε was coexpressed in some scattered cells in BIs (arrows in I–L), while free Hb-ε+ erythroblasts were CD34− (not shown). Scale bar in A, E, I = 10 μm.
Table 4. .
Changes in Expression of Markers with Age
|
Antibody |
7 WG |
12 WG |
||
|
Erythroblasts |
Mesenchyme |
Endothelium |
Pericyte/SMC |
|
| CD31 | + | +++ | +++ | – |
| CD34 | +/– | ++ | +++ | – |
| CD39 | +/– | ++ | +++ | +++ |
| C-kIT | +++ | +++ | + | + |
| CXCR4 | +++ | +++ | + † | + † |
| Flk-1 | +++ | +++ | +++ | +++ |
| Hgb Epsilon | +++ | +++ * | ||
| Ki67 | – | – | +/– | + |
| NG2 | – | + | +++ | +++ |
| RUNX1/AML1 | +/– | +/– | +++ | ++ |
| αSMA | – | – | – | ++ ‡ |
| SCF | +++ | +++ | + | + |
| SDF-1 | +++ | +++ | ++ | + |
| vWf | – | +/– | ++ | – |
Some cells.
Decreases with age.
Only on hyaloid artery.
c-Kit and Stem Cell Factor (SCF)
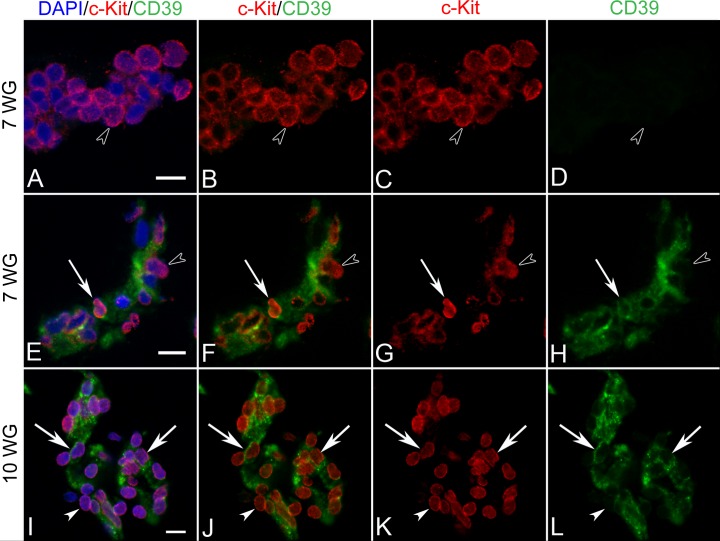
In 7 WG eyes, all erythroblastic aggregates (Figs. 6A–D) and cells of BIs (Figs. 6E–H) expressed c-Kit, with most having a diffuse cytoplasmic or membrane staining. There were, however, a few cells within BI that displayed nuclear labeling (Figs. 6E–H, arrow). Cells on the inner aspect of BI, primitive endothelium, coexpressed CD39 (CD39+) while those on the outer surface, presumed primitive pericytes, were CD39−. SCF, the ligand for c-Kit, was found in the developing lens and diffusely throughout the vitreous (Figs. 7A, 7B). c-Kit had a similar distribution but was more cellular in localization (Figs. 6B, 6D). As development progressed (10 WG), all blood vessel cells expressed c-Kit (Figs. 6I–L). This was regardless of whether they were luminal or abluminal in position. Only the endothelium coexpressed CD39. SCF and c-Kit immunoreactivities both decreased by 12 WG when blood vessels were mature (See Supplementary Material and Supplementary Fig. S1, http://www.iovs.org/content/53/13/7912/suppl/DC1).
Figure 6. .
Double-labeling for c-Kit and CD39 in BIs of vitreous at 7 WG (A–H) and blood vessels at 10 WG (I–L). At 7 WG, free erythroblasts and aggregates (arrowhead in A–D) expressed c-kit in their cytoplasm or membrane, but did not express CD39. All mesenchymal cells in BI were double-labeled with c-Kit/CD39 and some select cells showed nuclear c-Kit immunoreactivity (arrow in E–H). Free erythroblasts outside BI were CD39− (arrowhead in E–H). At 10 WG, all cells of blood vessels were c-Kit/CD39+ (I–L) with c-Kit being expressed exclusively in nuclei (arrows). A similar pattern of staining was observed at 12 WG (not shown). Scale bars in A, E, I = 10 μm.
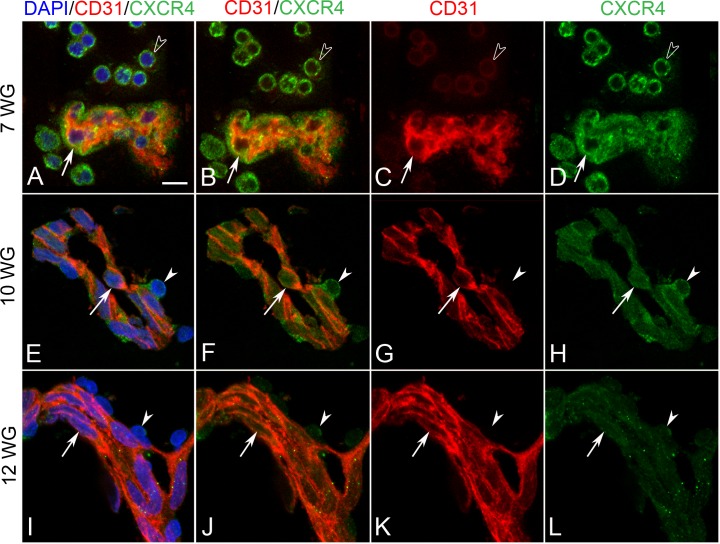
Figure 7. .
Double-labeling for CXCR4 and CD31 in BIs of vitreous at 7 WG (A–D) and blood vessels at 10 WG (E–H) and 12 WG (I–L). At 7 WG, free erythroblasts were CXCR4+/CD31+/− (arrowheads in A–D) while BI cells (arrow in A–D) were CXCR4+/CD31+. At 10WG, CD31+ endothelial cells (arrow in E–H) and CD31− pericytes (arrowhead in E–H) of blood vessels were CXCR4 immunoreactive. At 12 WG, endothelial cells (arrow in I–L) and pericytes (arrowhead in I–L) had weak CXCR4 immunoreactivity. Scale bar in A = 10 μm for all.
SDF-1/CXCR4
Diffuse SDF-1 immunoreactivity was found in the developing lens, within the vitreous and around blood islands of 7 WG eyes (See Supplementary Material and Supplementary Figs. S2A, S2C, http://www.iovs.org/content/53/13/7912/suppl/DC1). CXCR4, the receptor for SDF-1, was seen in both free cells in vitreous and in BI (See Supplementary Material and Supplementary Figs. S2B, S2D, S2F, http://www.iovs.org/content/53/13/7912/suppl/DC1). The cells within the islands were generally CXCR4+/CD31+ (Figs. 7A–D). As development progressed (10 WG), SDF-1 immunoreactivity decreased in the vitreous and was localized primarily in blood vessels (not shown). CXCR4 expression was observed in both endothelial cells and pericytes of blood vessels (Figs. 7E–H). At 12 WG, CXCR4 expression had dramatically decreased (See Supplementary Material and Supplementary Fig. S2, http://www.iovs.org/content/53/13/7912/suppl/DC1) and was only weakly associated with endothelial cells and pericytes (Figs. 7I–L). SDF-1 immunoreactivity was associated with blood vessels at 12 WG; however, it was weak in vitreous (See Supplementary Material and Supplementary Fig. S2, http://www.iovs.org/content/53/13/7912/suppl/DC1).
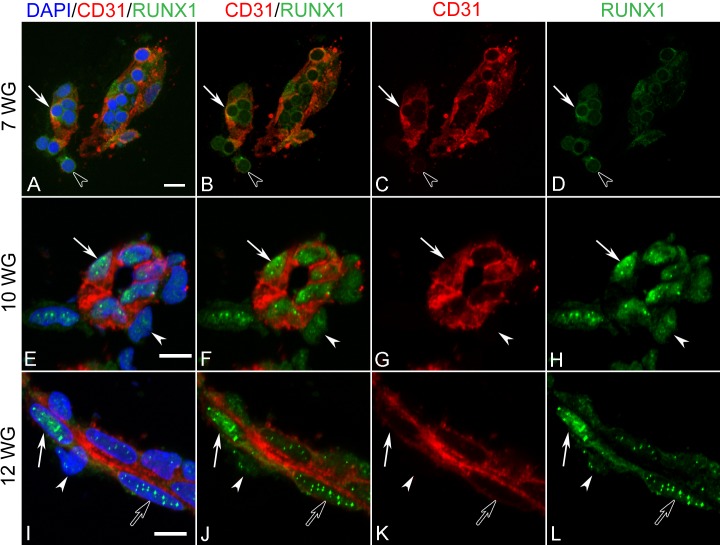
Runx1
Runx1 expression was weak and present in cytoplasm or on the cell surface at 7 WG in early BI cells and free cells in vitreous (Figs. 8A–D). By 10 WG, Runx1 expression was up-regulated and exhibited an almost exclusive nuclear localization in cells associated with developing blood vessels (Figs. 8E–H). This was regardless of whether cells were in a luminal or abluminal position (i.e., developing endothelial cells or pericytes). Moreover, there was a distinct subnuclear staining pattern observed in some cells that became more apparent by 12 WG. While endothelial cells in these more mature blood vessels appeared to have the greatest expression of Runx1, pericytes also demonstrated weak nuclear and subnuclear localization (Figs. 8I–L).
Figure 8. .
Double-labeling for Runx1 and CD31 in BIs of vitreous at 7 WG (A–D) and blood vessels at 10 WG (E–H) and 12 WG (I–L). At 7 WG, free erythroblasts (arrowhead in A–D) and cells in BI (arrow in A–D) expressed weak Runx1 and CD31. Runx-1 immunoreactivity appeared cytoplasmic or membrane associated. At 10 WG, both pericytes (arrowhead in E–H) and endothelial cells (arrow in E–H) of blood vessels were immunoreactive for Runx1 with CD31 being expressed exclusively in endothelial cells (arrows). Runx1 was primarily nuclear and in some cells was associated with subnuclear compartments. A similar Runx1 staining pattern of nuclei and subnuclear compartments was observed at 12 WG with pericytes having the weakest Runx1 immunoreactivity (arrowhead in I–L). Some endothelial cells had Runx-1 immunoreactivity exclusively in subnuclear compartments (open arrow in I–L), while others had diffuse nuclear and subnuclear localization. Scale bars in A, E, I = 10 μm.
Neuron-Glial 2 (NG-2) and Alpha-Smooth Muscle Actin (αSMA)
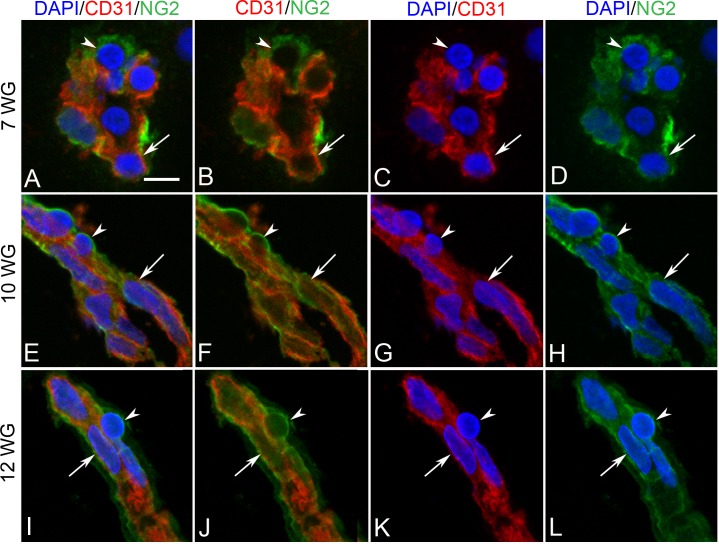
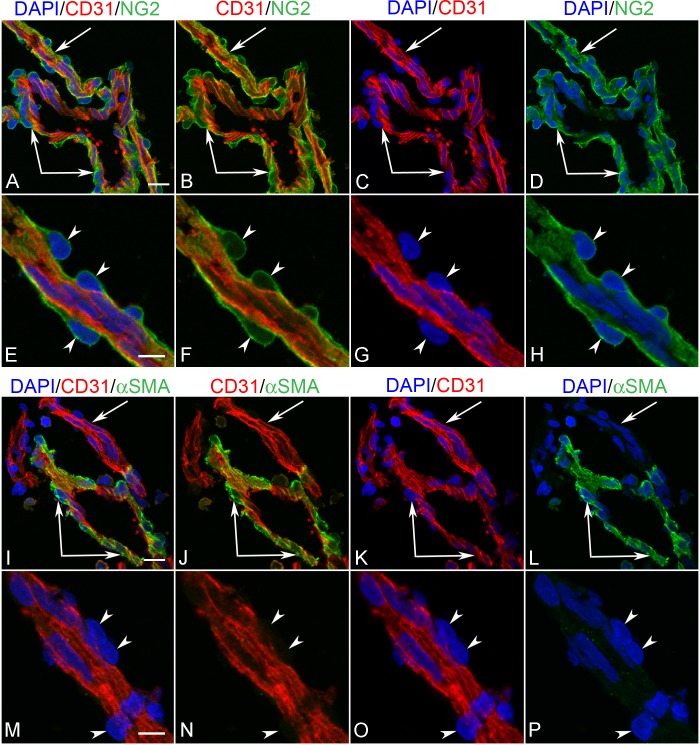
NG-2 was weakly expressed in BI at 7 WG. No free cells in the vitreous were NG-2 immunoreactive. Some of the cells in the inner portion of the blood islands coexpressed CD31, while those on the outer wall were CD31− (Figs. 9A–D). No αSMA immunoreactivity was observed in any blood vessels of the 7 WG eye (data not shown). As development progressed (10 WG), abluminal cells (primitive pericytes) became more immunoreactive for NG-2, while those in a luminal position (primitive endothelium) coexpressed NG-2 and CD31 (Figs. 9E–H). At the base of the hyaloid artery, where it emerged from the optic nerve head, NG-2 was exclusively associated with perivascular cells and not coexpressed with CD31+ endothelial cells (Figs. 10A–H). αSMA was expressed only by perivascular and medial cells on the main trunk of the hyaloid artery (Figs. 10I–L) and not in its smaller branches (Figs. 10M–P).
Figure 9. .
Double-labeling for NG-2 and CD31 in BIs of vitreous at 7 WG (A–D) and blood vessels at 10 WG (E–H) and 12 WG (I–L). At 7 WG some BI cells were CD31+/NG-2+ (arrow in A–D) and others were CD31−/NG-2+ (arrowhead in A–D). At 10 and 12 WG, CD31+ endothelial cells (arrow in E–H) and CD31− pericytes (arrowhead in E–H) of blood vessels were immunoreactive for NG-2. Scale bar in A = 10 μm for all.
Figure 10. .
Hyaloid artery (paired arrows) and one of its branches (arrow) in a 12 WG eye comparing double labeling with CD31+NG2 (A–H) and CD31+αSMA (I–P). NG2 immunoreactivity is found exclusively in all pericytes (arrowheads in E–H) while αSMA was only associated with abluminal cells of the main hyaloid artery. Pericytes were not immunoreactive for αSMA on smaller vessels. Scale bar in A, I = 10 μm, and in E,M = 5 μm.
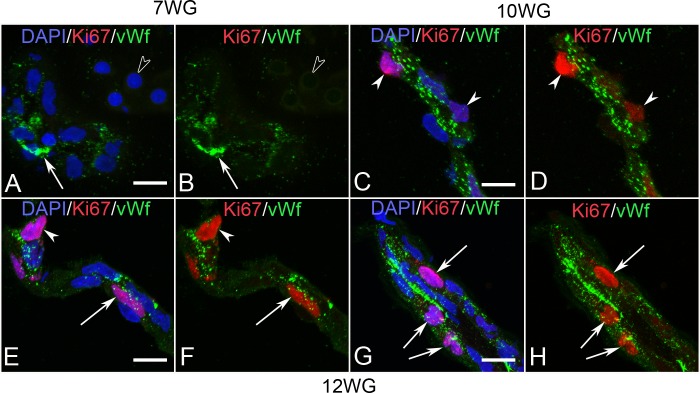
von Willebrands factor (vWf) and Ki67
In 7 WG eyes, vWf was expressed only in a few cells within BI formations (Fig. 11A). Few, if any, cells in aggregates or free cells in vitreous were actively proliferating (Fig. 11B). Out of 236 cell nuclei counted in three sections and double-labeled with vWf/Ki67 and counterstained with DAPI, none was found to be immunoreactive for Ki67. At 10 WG, vWf was upregulated in endothelial cells of blood vessels compared with 7 WG (Fig. 11C). Ki67 immunostaining was observed in a few cells of the blood vessels and was mostly associated with pericytes (Fig. 11D). Out of 189 total cell nuclei counted, 9% were Ki67+. In 12 WG eyes, vWf expression was prominent in endothelial cells of all blood vessels (Figs.11E–H) and likely reflected the increase in Weible-Palade bodies seen with electron microscopy (Fig. 4D). The number of proliferating vascular cells (mostly endothelial) was similar at 12 WG to the number observed at 10 WG (7.5%).
Figure 11. .
Double-labeling for Ki67 and vWf in BIs of vitreous at 7 WG (A, B) and blood vessels at 10 WG (C, D) and 12 WG (E–H). At 7 WG, free erythroblasts were vWf− (open arrowhead in A, B) while only some BI cells were vWf+ (arrow). No Ki67 labeling was observed in nuclei of cells within the vitreous at this age. At 10 WG, vWf immunoreactivity had increased in endothelial cells and occasional Ki67 labeled nuclei were observed. These cells were primarily in a pericyte position on the outer capillary wall (arrowheads in C, D). By 12 WG, occasional endothelial cells (arrow) and pericytes (arrowhead) of capillaries (E, F) were proliferating as were endothelial cells in the hyaloid artery (arrows in G, H). Scale bar in A, C, E, F = 10 μm.
Discussion
Progenitors for blood and endothelial cells form aggregates in embryonic human vitreous. This is the earliest morphological sign of differentiation of extraembryonic mesoderm.9 Different terms have been used to describe these aggregates, including BI, angioblasts, and hemangioblasts. The existence of the hemangioblast was proposed by Sabin10,11 and later by Murray.12 They are credited with the concept that both the endothelial and hematopoietic lineages are derived from a common precursor, the “hemangioblast,” in the BI of the yolk sac. Murray described the newly formed lateral and posterior mesodermal cell migration toward the yolk sac, where they will differentiate to endothelial cells and to hematopoietic cells of the BI. Sabin10 studied their migration under the light microscope and could distinguish the hemangioblastic aggregates from the remaining mesodermal cells by their increased bifringence. The peripheral cells of these aggregates subsequently flatten and differentiate to endothelial cells, while the centrally located cells differentiate to hematopoietic cells.
Today, the existence of a hemangioblast is clearly recognized and it is described as a multipotent precursor cell that can differentiate into both hematopoietic and endothelial cells. With the advent of the molecular genetics, studies utilizing targeted gene mutations have discovered shared genes important for both endothelial and hematopoietic development.13–17 The most direct evidence for the existence of the hemangioblast to date has come from studies using a model system based on the in vitro differentiation of human embryonic stem cells (hESC). When induced to differentiate in culture, hESCs form colonies known as embryoid bodies that contain derivatives of the three primary germ cell layers. Hematopoietic development within the EBs has been shown to be efficient, highly reproducible, and to follow a pattern similar to that found in the yolk sac.18,19 In addition, Sequeira Lopez et al., have observed intraembryonic BI-like formations in mouse embryo and called the simultaneous formation of blood vessels and blood cells “in situ hemo-vasculogenesis.”20 The labeling of the same cells with Hb-ε, endothelial cell (CD31), and/or hematopoietic (CD34) markers demonstrates that hemangioblasts are present in embryonic human vitreous and are the origin of the fetal vasculature in vitreous.
The elegant histologic studies of embryonic and fetal human ocular development by Mann1 nearly a century ago clearly described the presence of large numbers of vasoformative mesoderm at the gap between the edge of the optic cup and the equator of the lens in the human embryo as the initial event in the formation of the hyaloid vascular system. She described free cells associated with early blood vessels beginning around the 10-mm stage or 4.5 WG before the embryonic fissure closes. Balazs described the first step in vasogenesis of the hyaloid vessels as the aggregation of undifferentiated mesenchymal cells at the rim of the optic cup and embryonic fissure.6 He observed that the surface cells of these prevascular cell aggregates elongated and developed into the endothelial cells of the vessel wall and that this induced a gradual rounding up of the cells in the lumen, which differentiated to hemangioblasts and eventually immature blood cells. Our histologic findings at 5.5 WG are in complete agreement with the observations of Mann and Balazs at this age. We clearly show BI in the vitreous space near the lens vesicle and at the annular opening between the lens vesicle and the rim of the optic cup in the 5.5 WG embryonic eye prior to the hyaloid artery being present in the vitreous cavity. These BI consisted of two cells types: erythroblasts and basophilic mesenchymal cells. They typically formed aggregates with some being almost entirely erythroblasts with highly acidophilic cytoplasm and others having of a central core of erythroblasts surrounded loosely by basophilic mesenchyme. Our observations with Ki67 immunolabeling support Mann's observations that very few of these invading mesenchymal precursors proliferate during early hyaloid vascular development.
The current study demonstrates a series of markers for hemangioblasts. In the embryonic vitreous, c-Kit immunoreactivity was observed in cells of BI and in free erythroblasts, while SCF (the ligand) was coexpressed in the BI cells and diffusely within the primary vitreous surrounding these formations. C-Kit (CD117) is a type III receptor tyrosine kinase that belongs to the family of platelet-derived growth factor receptors (PDGFRs).21 Activation of c-Kit leads to the activation of numerous transcription factors regulating cell differentiation, proliferation, chemotaxis, and cell adhesion.22 c-Kit is essential in regulating the development of stem cells, germ cells, and melanocytes.23 Stem cell factor, a c-Kit ligand, dose-dependently promotes survival, migration, and capillary tube formation of human umbilical vein endothelial cells.24
We also observed prominent SDF-1 (CXCL12) in the BI and diffuse immunoreactivity in the forming primary vitreous of the embryonic eye. The SDF-1 in vitreous may have diffused from the developing lens where expression was high. SDF-1 is a member of a large family of structurally related chemoattractive cytokines. Its primary receptor is CXCR4, a hepta helical receptor coupled to heterotrimeric guanosine triphosphate (GTP) binding proteins. CXCR4 was present in hemangioblasts, erythroblasts, and all cells in aggregates. Studies of mutant mice with targeted gene disruption have revealed that CXCL12-CXCR4 signaling is essential for hematopoiesis and colonization of bone marrow by hematopoietic progenitors, including hESCs, during ontogeny as well as cardiovascular formation and neurogenesis.25–31 Cell migration is an essential element of embryogenesis because cell location in the embryo is a key determinant of cell fate. The process of tissue patterning is largely orchestrated by extracellular gradients of morphogenetic proteins (morphogens), which are diffusible substances like SCF and SDF-1. Among the chemokine receptors in the mouse embryo, the CXCR4 transcript is the most abundantly detected.32 Moreover, studies using human embryonic stem cells in an in vitro assay system have demonstrated a morphogenic role for SDF-1/CXCR4 in human embryonic vasculogenesis.33 In the embryonic and fetal human retina, we have described a gradient of SDF-1 in the inner retina that correlates temporally and spatially with CXCR4 expression in the pool of progenitors that give rise to retinal angioblasts.34
There was low expression of Runx1 in the cytoplasm of cells in early BI of vitreous. This was somewhat of a surprise given its known role in hematopoietic differentiation. The transcription factor Runx1/AML1 is a master regulator of hematopoietic development whose expression and function are associated with the establishment and maintenance of hematopoietic cells; therefore, one could hypothesize that it would be present in the hemangioblasts of embryonic vitreous.35 However, Runx1−/− mouse embryos develop normal BI and progress through the yolk sac phase of hematopoiesis but die between embryonic day 11 (E11) and E12.5.36 Depletion of Runx1 in zebra fish with antisense morpholino oligonucleotides abrogates the development of both blood cells and vessels, as demonstrated by loss of circulation, incomplete development of vasculature and the accumulation of immature hematopoietic precursors.37
VEGF-A is a multifunctional cytokine that plays a prominent role in normal vascular biology by regulating numerous cellular responses of endothelial precursors including proliferation, differentiation, migration, and apoptosis.38–42 In addition, a role for VEGF-A in hematopoietic differentiation has been identified.43–45 Among the VEGF receptors, VEGFR2 appears as the earliest endothelial lineage marker, as determined by in situ hybridization studies on mouse and quail embryos.46–51 Moreover, all primitive hematopoietic and endothelial progenitors arise within the mouse yolk sac BI from precursors that all appear to express VEGFR2.17 VEGFR2 immunoreactivity was present in all cells of BI of the embryonic HVS including those apparently committed to hematopoietic lineage (epsilon hemoglobin+) or endothelial lineage (CD31+ and CD34+) (Fig. 5). Moreover, some of these cells coexpress epsilon hemoglobin and endothelial markers suggesting a dual hematoendothelial phenotype, as in hemo-vasculogenesis, during this embryonic period. We observed a similar pattern of staining and coexpression of these markers in developing choriocapillaris of the embryonic human eye.7 Taken together these observations suggest that VEGF signaling through VEGFR2 is common in early ocular hemangioblasts during blood vessel formation by hemo-vasculogenesis. Our previous study demonstrated the anti-angiogenic form of VEGF165b was not detectable in BI of vitreous but the angiogenic VEGF165 was present.52
NG-2 immunoreactivity, a marker for pericytes, was observed in early BI cells, and some of these cells coexpressed the endothelial marker CD31. Moreover, in our TEM analysis, it was impossible to distinguish endothelial cells or pericytes in these formations based on their ultrastructural characteristics. Few of the cells in early BI had Weibel-Palade bodies, and this was reflected in the weak expression of vWf associated with these structures. Penfold et al. also observed that abluminal and luminal progenitors were similar in the development of the retinal vasculature from angioblasts53; however, this vasculogenic event is much later in development (12–14 WG)34,54 and no hematopoietic nor erythropoietic cells arise from the aggregates, as occurs in hemo-vasculogeneeis in vitreous and choriocapillaris.7 Additionally, cells of embryonic vitreous BI, regardless of their position on the forming vessel (lumenal or ablumenal), expressed several other common markers (VEGFR2, c-Kit, CXCR4, and Runx1). It is believed by some that endothelial cells and pericytes share a common precursor and that their position on the forming vessel wall determines their fate.55–57 Here we show that cells of early blood islands coexpressed not only hematopoietic and endothelial cell markers (CD31+/Hb-ε+), but also coexpressed endothelial cell and pericyte markers (CD31+/NG2+). Taken together, our data suggest that the mesenchyme invading the vitreous of the embryonic eye represents a population of hemangioblasts that is capable of producing blood cells, endothelial cells, and pericytes, which all contribute to formation of this vascular system. Another cell type that associates with the hyaloid blood vessels is the hyalocyte, which is considered the resident macrophage of the vitreous.6 It is quite possible that hyalocytes, which contribute to the normal involution of these blood vessels, are also derived from these progenitors.
As development progressed and blood vessels appeared stabilized, both c-Kit and SCF immunoreactivity decreased in BI cells and little to no immunoreactivity was found in vitreous. Initial expression of c-Kit appeared to be restricted to the cytoplasm in most cells, but as blood vessels matured, the staining pattern became primarily nuclear. However, using APase immunohistochemistry, the staining pattern in the HVS was not nuclear. We have previously reported nuclear c-Kit in progenitors of fetal retina,34 and others have described nuclear c-Kit in the adrenal gland.58 Recent studies have raised doubt about the validity of nuclear c-Kit in tumors due to cross-reaction with a DNA binding protein.59 It is unclear whether this was the case in the current study, but, nonetheless, this does not undermine the decrease in expression we observed with continued development.
SDF-1 immunoreactivity remained strong in the formed vasculature while CXCR4 immunoreactivity decreased dramatically. Because CXCR4 is apparently down-regulated after vessel stabilization, the role SDF-1 plays in the maintenance of these vessels is unclear. Perhaps SDF-1 expression in these mature blood vessels is related to monocyte recruitment and adhesion, another potential function of endothelial SDF-1.60 Monocytes/macrophages are known to initiate cell death and to participate in the natural involution of the HVS.2,61–63
Primitive endothelial cells and pericytes in the formed blood vessels both expressed nuclear Runx1 protein. Recent studies have shown that shear stress is capable of up-regulating expression of Runx1.64 Moreover, nitric oxide (NO), which is also dependent on blood flow, appears to be a key regulator of Runx1 expression.65 We previously report endothelial (eNOS) and neuronal (nNOS) nitric oxide synthase coexpression in BI and blood vessels of the embryonic and fetal human hyaloid vasculature. These NOS isoforms were weak in the early BI (7 WG) and increased with age (10 WG).66 Taken together, the coincident up-regulation of NOS and Runx1 between 7 and 10 WG may reflect establishment of flow in the hyaloid vasculature and the effects of shear stress on expression of these molecules. The staining pattern varied from a diffuse nuclear to a distinct punctate labeling of subnuclear sites. It is known that Runx1 proteins localize within the nucleus to punctate foci involved in transcriptional control and associate with the subnuclear scaffold designated as the nuclear matrix.67–70
Hyaloid blood vessels are generally composed of two interacting cell types. Endothelial cells form the inner lining of the vessel wall, and pericytes or mural cells envelop the outer vessel wall. An exception is the hyaloid artery and its main branches, which have several layers of smooth muscle cells in the wall. NG-2, a chondroitin sulfate proteoglycan, and α-SMA are molecular markers that are present in pericytes, albeit not exclusively, and are commonly used for their detection. In this study, we only observed α-SMA on cells of the hyaloid artery and its major branches during the period studied.
In summary, we observed BI in the vitreous of embryonic human eyes. Cells in these structures were primitive erythroblasts and angioblasts that formed aggregates and expressed hematopoietic stem cell markers, VEGFR2, c-Kit, and CXCR4. The ligands for these molecules, VEGF165,52 SCF, and SDF-1,34 are present at high levels in lens and inner retina as well as diffusely in the vitreous during BI formation; therefore, these ligands probably function in the homing of these cells into the vitreous space near lens and retina. Some cells in BI co-expressed embryonic hemoglobin and endothelial cell markers reflecting a dual hematoendothelial phonotype. Taken together, our observations support the view that BI in the embryonic eye originate from a common precursor, namely the hemangioblast, and that the fetal vasculature in vitreous assembles by hemo-vasculogenesis.
Supplementary Material
Acknowledgments
The authors thank Morton F. Goldberg, MD, for his critical evaluation of the manuscript.
Footnotes
Supported by grants from the National Institutes of Health EY-01765 (Wilmer) and RO1-EY09357 (GAL), Research to Prevent Blindness (Wilmer), the Altsheler Durell Foundation, and a Research to Prevent Blindness Senior Scientific Investigator award (GAL).
Disclosure: D.S. McLeod, None; T. Hasegawa, None; T. Baba, None; R. Grebe, None; I. Galtier d'Auriac, None; C. Merges, None; M. Edwards, None; G.A. Lutty, None
References
- 1.Mann IC. The development of the human eye. Cambridge: University Press; 1928 [Google Scholar]
- 2.Zhu M, Madigan MC, van Driel D, et al. The human hyaloid system: cell death and vascular regression. Exp Eye Res. 2000;70:767–776 [DOI] [PubMed] [Google Scholar]
- 3.Reese AB. Persistent hyperplastic primary vitreous. Am J Ophthalmol. 1955;40:317–331 [DOI] [PubMed] [Google Scholar]
- 4.Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 1997;124:587–626 [DOI] [PubMed] [Google Scholar]
- 5.Jakobiec FA. Ocular anatomy, embryology, and teratology. Philadelphia: Harper and Row Publishers, Inc.; 1982 [Google Scholar]
- 6.Balazs EA, Toth LZ, Ozanics V. Cytological studies on the developing vitreous as related to the hyaloid vessel system. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;213:71–85 [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa T, McLeod DS, Bhutto IA, et al. The embryonic human choriocapillaris develops by hemo-vasculogenesis. Dev Dyn. 2007;236:2089–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhutto IA, Kim SY, McLeod DS, et al. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:1544–1552 [DOI] [PubMed] [Google Scholar]
- 9.Sheng G. Primitive and definitive erythropoiesis in the yolk sac: a bird's eye view. Int J Dev Biol. 2010;54:1033–1043 [DOI] [PubMed] [Google Scholar]
- 10.Sabin FR. Origin and development of the primitive vessels of the chick and pig. Carnegie Contrib Embryol. 1917;6:61–124 [Google Scholar]
- 11.Sabin F. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living. J Hematother Stem Cell Res. 2002;11:5–7 [DOI] [PubMed] [Google Scholar]
- 12.Murray PD. The development in vitro of the blood of early chick embryo. Proc R Soc Lon. 1932;11:497–521 [Google Scholar]
- 13.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732 [DOI] [PubMed] [Google Scholar]
- 14.Choi K. Hemangioblast development and regulation. Biochem Cell Biol. 1998;76:947–956 [PubMed] [Google Scholar]
- 15.Eichmann A, Corbel C, Nataf V, Vaigot P, Breant C, Le Douarin NM. Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor receptor 2. Proc Natl Acad Sci U S A. 1997;94:5141–5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gering M, Rodaway AR, Gottgens B, Patient RK, Green AR. The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 1998;17:4029–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66 [DOI] [PubMed] [Google Scholar]
- 18.Zambidis ET, Sinka L, Tavian M, et al. Emergence of human angiohematopoietic cells in normal development and from cultured embryonic stem cells. Ann N Y Acad Sci. 2007;1106:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zambidis ET, Park TS, Yu W, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112:3601–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sequeira Lopez ML, Chernavvsky DR, Nomasa T, Wall L, Yanagisawa M, Gomez RA. The embryo makes red blood cell progenitors in every tissue simultaneously with blood vessel morphogenesis. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1126–R1137 [DOI] [PubMed] [Google Scholar]
- 21.Renkonen S, Hayry V, Heikkila P, et al. Stem cell-related proteins C-KIT, C-MYC and BMI-1 in juvenile nasopharyngeal angiofibroma–do they have a role? Virchows Arch. 2011;458:189–195 [DOI] [PubMed] [Google Scholar]
- 22.Edling CE, Hallberg B. c-Kit–a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol. 2007;39:1995–1998 [DOI] [PubMed] [Google Scholar]
- 23.Miettinen M, Lasota JKIT. (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–220 [DOI] [PubMed] [Google Scholar]
- 24.Matsui J, Wakabayashi T, Asada M, Yoshimatsu K, Okada M. Stem cell factor/c-kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J Biol Chem. 2004;279:18600–18607 [DOI] [PubMed] [Google Scholar]
- 25.Ara T, Itoi M, Kawabata K, et al. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170:4649–4655 [DOI] [PubMed] [Google Scholar]
- 26.Ara T, Nakamura Y, Egawa T, et al. Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1). Proc Natl Acad Sci U S A. 2003;100:5319–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagasawa T, Nakajima T, Tachibana K, et al. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci U S A. 1996;93:14726–14729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stumm RK, Zhou C, Ara T, et al. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988 [DOI] [PubMed] [Google Scholar]
- 30.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594 [DOI] [PubMed] [Google Scholar]
- 31.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599 [DOI] [PubMed] [Google Scholar]
- 32.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–456 [DOI] [PubMed] [Google Scholar]
- 33.Chen T, Bai H, Shao Y, et al. Stromal cell-derived factor-1/CXCR4 signaling modifies the capillary-like organization of human embryonic stem cell-derived endothelium in vitro. Stem Cells. 2007;25:392–401 [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa T, McLeod DS, Prow T, Merges C, Grebe R, Lutty GA. Vascular precursors in developing human retina. Invest Ophthalmol Vis Sci. 2008;49:2178–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sroczynska P, Lancrin C, Kouskoff V, Lacaud G. The differential activities of Runx1 promoters define milestones during embryonic hematopoiesis. Blood. 2009;114:5279–5289 [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A. 1996;93:3444–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalev-Zylinska ML, Horsfield JA, Flores MV, et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129:2015–2030 [DOI] [PubMed] [Google Scholar]
- 38.Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532 [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676 [DOI] [PubMed] [Google Scholar]
- 40.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312 [DOI] [PubMed] [Google Scholar]
- 41.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309 [DOI] [PubMed] [Google Scholar]
- 42.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379 [DOI] [PubMed] [Google Scholar]
- 43.Broxmeyer HE, Cooper S, Li ZH, et al. Myeloid progenitor cell regulatory effects of vascular endothelial cell growth factor. Int J Hematol. 1995;62:203–215 [DOI] [PubMed] [Google Scholar]
- 44.Kennedy M, Firpo M, Choi K, et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493 [DOI] [PubMed] [Google Scholar]
- 45.Liang D, Chang JR, Chin AJ, et al. The role of vascular endothelial growth factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in zebrafish development. Mech Dev. 2001;108:29–43 [DOI] [PubMed] [Google Scholar]
- 46.Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203:80–92 [DOI] [PubMed] [Google Scholar]
- 47.Eichmann A, Marcelle C, Breant C, Le Douarin NM. Two molecules related to the VEGF receptor are expressed in early endothelial cells during avian embryonic development. Mech Dev. 1993;42:33–48 [DOI] [PubMed] [Google Scholar]
- 48.Flamme I, Breier G, Risau W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol. 1995;169:699–712 [DOI] [PubMed] [Google Scholar]
- 49.Millauer B, Witzigmann-Voos S, Schnuerch H, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846 [DOI] [PubMed] [Google Scholar]
- 50.Wilting J, Eichmann A, Christ B. Expression of the avian VEGF receptor homologues Quek1 and Quek2 in blood-vascular and lymphatic endothelial and non-endothelial cells during quail embryonic development. Cell Tissue Res. 1997;288:207–223 [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. Flk-1, an flt-rated receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498 [DOI] [PubMed] [Google Scholar]
- 52.Baba T, McLeod DM, Edwards MM, et al. VEGF165b in the developing vasculatures of the fetal human eye. Dev Dyn. 2012;241:595–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penfold PL, Provis JM, Madigan MC, van Driel D, Billson FA. Angiogenesis in normal human retinal development: the involvement of astrocytes and macrophages. Graefes Arch Clin Exp Ophthalmol. 1990;228:255–263 [DOI] [PubMed] [Google Scholar]
- 54.McLeod DS, Hasegawa T, Prow T, Merges C, Lutty G. The initial fetal human retinal vasculature develops by vasculogenesis. Dev Dyn. 2006;235:3336–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashton N. Oxygen and the growth and development of retinal vessels. Am J Ophthalmol. 1966;62:412–435 [DOI] [PubMed] [Google Scholar]
- 56.Lutty GA, Merges C, Grebe R, Prow T, McLeod DS. Canine retinal angioblasts are multipotent. Exp Eye Res. 2006;83:183–193 [DOI] [PubMed] [Google Scholar]
- 57.Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96 [DOI] [PubMed] [Google Scholar]
- 58.Matsuda R, Takahashi T, Nakamura S, et al. Expression of the c-kit protein in human solid tumors and in corresponding fetal and adult normal tissues. Am J Pathol. 1993;142:339–346 [PMC free article] [PubMed] [Google Scholar]
- 59.Horvai AE, Li L, Xu Z, Kramer MJ, Jablons DM, Treseler PA. c-Kit is not expressed in malignant mesothelioma. Mod Pathol. 2003;16:818–822 [DOI] [PubMed] [Google Scholar]
- 60.Takahashi K, Shimokado K, Yoshida M. SDF-1-induced adhesion of monocytes to vascular endothelium is modulated by azelnidipine via protein kinase C inhibition. Eur J Pharmacol. 2006;552:162–169 [DOI] [PubMed] [Google Scholar]
- 61.Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462 [DOI] [PubMed] [Google Scholar]
- 62.Lang RA, Lustig M, Francois F, Sellinger M, Plesken H. Apoptosis during macrophage-dependent ocular tissue remodeling. Development. 1994;120:3395–3403 [DOI] [PubMed] [Google Scholar]
- 63.Mitchell CA, Risau W, Drexler HC. Regression of vessels in the tunica vasculosa lentis is initiated by coordinated endothelial apoptosis: a role for vascular endothelial growth factor as a survival factor for endothelium. Dev Dyn. 1998;213:322–333 [DOI] [PubMed] [Google Scholar]
- 64.Adamo L, Naveiras O, Wenzel PL, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.North TE, Goessling W, Peeters M, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McLeod DS, Baba T, Bhutto IA, Lutty GA. Co-expression of endothelial and neuronal nitric oxide synthases in the developing vasculatures of the human fetal eye. Graefes Arch Clin Exp Ophthalmol. 2012;839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNeil S, Zeng C, Harrington KS, et al. The t(8;21) chromosomal translocation in acute myelogenous leukemia modifies intranuclear targeting of the AML1/CBFalpha2 transcription factor. Proc Natl Acad Sci U S A. 1999;96:14882–14887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stein GS, van Wijnen AJ, Stein JL, et al. Intranuclear trafficking of transcription factors: implications for biological control. J Cell Sci. 2000;113;2527–2533 [DOI] [PubMed] [Google Scholar]
- 69.Tang L, Guo B, Javed A, et al. Crystal structure of the nuclear matrix targeting signal of the transcription factor acute myelogenous leukemia-1/polyoma enhancer-binding protein 2alphaB/core binding factor alpha2. J Biol Chem. 1999;274:33580–33586 [DOI] [PubMed] [Google Scholar]
- 70.Zeng C, McNeil S, Pockwinse S, et al. Intranuclear targeting of AML/CBFalpha regulatory factors to nuclear matrix-associated transcriptional domains. Proc Natl Acad Sci U S A. 1998;95:1585–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.