Abstract
Purpose.
Although hyperglycemia is implicated in retinal vascular dysfunction associated with the development of diabetic retinopathy, the temporal influence of hyperglycemia on retinal arteriolar reactivity remains unclear. Development of a large animal model of diabetes relevant to the human retina for evaluation of vascular function is also lacking. Herein, we examined nitric oxide (NO)-mediated dilation and endothelin-1 (ET-1)-induced constriction in retinal arterioles at various time periods in a porcine model of type 1 diabetes.
Methods.
Retinal arterioles were isolated from streptozocin-induced diabetic pigs (2, 6, and 12 weeks of hyperglycemia, 427 ± 23 mg/dL) and age-matched control pigs (73 ± 4 mg/dL), and then cannulated and pressurized for vasoreactivity study using videomicroscopic techniques.
Results.
Retinal arterioles isolated from control and diabetic pigs developed comparable levels of myogenic tone. The endothelium-dependent NO-mediated vasodilations to bradykinin and stepwise increases in luminal flow were significantly reduced within 2 weeks of hyperglycemia. The inhibitory effect was comparable following 6 and 12 weeks of hyperglycemia. However, the endothelium-independent vasodilation to sodium nitroprusside was unaffected. Constriction of retinal arterioles to ET-1 was unaltered at all time periods of hyperglycemia.
Conclusions.
Our findings provide the first direct evidence for selective impairment of endothelium-dependent NO-mediated dilation of retinal arterioles within 2 weeks of hyperglycemia in a pig model of diabetes. By contrast, the ability of arteriolar smooth muscle to dilate to NO donor or contract to ET-1 was unaffected throughout the study period. This endothelial vasodilator dysfunction during early diabetes may contribute to development of retinopathy with chronic hyperglycemia.
In a type 1 diabetes model in the pig, endothelium-dependent nitric oxide-mediated dilation, but not smooth muscle-dependent nitroprusside-induced dilation or endothelin-1–induced constriction, of retinal arterioles in vitro was impaired within 2 weeks of diabetes and no progression after 6 and 12 weeks.
Introduction
Retinopathy is a major complication of diabetes mellitus and a leading cause of sight-threatening eye disease in adults worldwide.1 Diabetic retinopathy affects the microcirculation in the retina where a progression of histological and pathophysiological changes leads to visual impairment and blindness.2 Elevated level of blood glucose or hyperglycemia, a hallmark of diabetes, is associated with reduced retinal blood flow in early stages of diabetic retinopathy in experimental3–12 and human diabetes.13–15 These studies suggest that dysfunction of resistance arterioles, the major site for flow regulation, may contribute to the retinal damage. However, the temporal influence of hyperglycemia on vasomotor function of retinal arterioles remains unclear. In addition, the development of an animal model of diabetes relevant to the human retinal microcirculation for evaluation of arteriolar function is lacking.
To address these clinically important issues, in the present study we developed streptozocin (STZ)-induced type 1 diabetes in the pig, an animal model that we have shown to resemble human in retinal vasomotor regulation.16 The retinal microcirculation is relatively unique in that it lacks direct innervation, and thus the basal tone of retinal arterioles and retinal blood flow are governed by a mechanism linked with changes in local hemodynamics17,18 and released autocrine/paracrine factors19 as a function of oxygen supply and tissue metabolism. In response to local stimuli, the vascular endothelium produces and releases vasoactive factors such as vasodilator nitric oxide (NO)20 and vasoconstrictor endothelin-1 (ET-1).21 However, vascular dysfunction in terms of a diminished release or activity of NO and augmented release or activity of ET-1 has been purported as a key event in the development of diabetic retinopathy based on in vivo evidence.22–25 The direct impact of type 1 diabetes on the function of endothelium and the ability of smooth muscle to respond to NO and ET-1 in retinal arterioles remains unknown. Therefore, we utilized an isolated vessel approach in vitro, which excludes neural-glial and humoral influences, to examine endothelium-dependent NO-mediated dilations to flow/shear stress16 and endogenous agent bradykinin,16,26 as well as vasodilation to NO donor sodium nitroprusside and vasoconstriction to ET-1 in retinal arterioles isolated from normoglycemic and diabetic pigs.
Methods
Porcine Diabetes Model
All animal procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Scott & White Institutional Animal Care and Use Committee. Diabetes was induced in domestic male pigs (8–12 weeks old, 9–11 kg) purchased from Real Farms (San Antonio, TX) by selective ablation of pancreatic β cells with intravenous injection of STZ (150 mg/kg, Zanosar; Teva Parenteral Medicines, Irvine, CA) via an ear vein. The control group was intravenously injected with saline instead of STZ. Based on earlier studies with slight modification,27,28 a special diet was required prior to injection with STZ to induce sustained hyperglycemia in pigs. Pigs were fasted for approximately 12 hours before feeding with a commercial pig diet containing ammonium chloride (20 g/3 c pig feed mixed with strawberry Boost [Nestlé HealthCare Nutrition, Florham Park, NJ], or other palatable food items to increase intake) the day prior to STZ injection, producing temporary systemic acidosis (pH ≈ 7.10–7.20) and thus enhancing the effect of STZ. The pH was checked prior to STZ injection to confirm acidosis. The pigs were maintained for a period of 2, 6, or 12 weeks. Following STZ or saline (control) injection, the animals were allowed free access to water and fed with syrup and/or Boost mixed with pig feed to prevent temporary hypoglycemia for 24 hours after STZ injection. The animals were allowed free access to water and commercial diet thereafter. The general condition, body weight, and the level of blood glucose were closely monitored in all pigs, and only those that developed sustained hyperglycemia with a fasting blood glucose level between 250 and 540 mg/dL were included in the study. Fasting blood glucose levels were obtained each day in the morning using a Contour glucometer (Bayer HealthCare, Mishawaka, IN). The blood pH was also measured to monitor whether the animals developed metabolic acidosis (normal range of 7.35–7.45). Our studies showed that sustained blood glucose levels greater than 540 mg/dL led to systemic acidosis, and the acidotic animals were excluded from further study. The retinal circulation was viewed with a fundus camera to determine clinical evidence of diabetic changes in the retina. Following the 2-, 6-, and 12-week time periods, pigs were sedated with Telazol (4.4 mg/kg, i.m.; TW Medical Veterinary Supply, Austin, TX) and anesthetized with 2% to 5% isoflurane. Heparin (1000 U/kg; Sagent Pharmaceuticals, Schaumburg, IL) was administered into the marginal ear vein to prevent clotting. The eyes were enucleated and immediately placed in a moist chamber on ice.
Isolation and Cannulation of Microvessels
The techniques used for identification, isolation, cannulation, pressurization, and visualization of the retinal vasculature have been described elsewhere.29 In brief, the isolated retinal arterioles (∼60–80 μm in situ) were cannulated with a pair of glass micropipettes and pressurized to 55 cm H2O intraluminal pressure without flow by two independent pressure reservoir systems. Vasomotor activity of isolated vessels was continuously recorded using videomicroscopic techniques throughout the experiments. Arterioles with side branches and leaks were excluded from further study and all arterioles used developed basal tone.
Study of Vasomotor Function
To study the vasodilator function mediated by endothelial NO, we utilized bradykinin and luminal flow/shear stress because these dilations are solely dependent on endothelial released NO in both porcine and human retinal arterioles as demonstrated in our recent reports.16,30 The endothelium-independent vasodilator sodium nitroprusside16 and vasoconstrictor ET-116,31,32 were used to test vascular smooth muscle function. Cannulated, pressurized arterioles were bathed in physiological saline solution (PSS)-albumin (0.1%; USB, Cleveland, OH) at 36°C to 37°C. After vessels developed stable basal tone (∼60 minutes), concentration-dependent responses to bradykinin, sodium nitroprusside, and ET-1 (BaChem, Bubendorf, Switzerland) were established. Vessels were exposed to each concentration of agonist for 4 to 5 minutes until a stable diameter was maintained. Vascular response to increased flow was studied under constant intraluminal pressure using dual-reservoir techniques as described previously.16 In brief, the luminal flow was produced by simultaneously moving the pressure reservoirs in opposite directions of the same magnitude, which generates a pressure gradient (ΔP; range from 10 to 60 cm H2O) across the length of the vessel without changing intraluminal pressure. We have previously demonstrated that the luminal flow is increased linearly with increasing ΔP and the range of mean volumetric flows for ΔP between 0 and 60 cm H2O is 0 to 34.8 nL/sec (0–2.1 μL/min),33 corresponding to the range reported in retinal arterioles in vivo.34
Chemicals
Drugs and chemicals used in the vasomotor function study were obtained from Sigma-Aldrich (St. Louis, MO) except when specifically stated otherwise. ET-1 was dissolved in water, whereas bradykinin and sodium nitroprusside were dissolved in PSS. Subsequent concentrations of ET-1 were diluted in PSS.
Data Analysis
At the end of each functional experiment, the vessel was relaxed with 0.1 mM sodium nitroprusside in ethylenediaminetetraacetic acid (EDTA, 1 mM)-Ca2+-free PSS to obtain its maximum diameter at 55 cm H2O intraluminal pressure.29 Diameter changes in response to vasodilator agonists and luminal flow were normalized to this maximum vasodilation and expressed as percent maximum dilation. The reductions in diameter in response to ET-1 were normalized to the resting diameter and expressed as percent resting diameter.16 The median effective concentration (EC50) value for the vasodilator responses was calculated using GraphPad Prism software (GraphPad Software, La Jolla, CA). Data are reported as mean ± SEM and n value represents the number of animals (two to three vessels per pig) studied. Student's t-test or ANOVA followed by Bonferroni multiple-range test was used to determine the significance of experimental interventions, as appropriate. A value of P < 0.05 was considered significant.
Results
Animal Model
Following STZ injection, blood glucose in pigs elevated from 74 ± 4 mg/dL (4 ± 1 mM) to 427 ± 23 mg/dL (24 ± 1 mM, 2–12 weeks after STZ injection, n = 31). Pigs injected with saline (control) had unaltered blood glucose levels: 74 ± 5 mg/dL (4 ± 1 mM) vs. 73 ± 4 mg/dL (4 ± 1 mM) after saline injection (2 to 12 weeks, n = 23). The body weight gain was less in diabetic pigs (before saline injection: 10 ± 1 kg; 2 weeks after STZ: 12 ± 1 kg; 6 weeks after STZ: 16 ± 2 kg; 12 weeks after STZ: 23 ± 3 kg) than in control pigs (before saline injection: 11 ± 1 kg; 2 weeks after saline: 17 ± 1 kg; 6 weeks after saline: 24 ± 3 kg; 12 weeks after saline: 36 ± 4 kg). An additional sign of diabetes was the development of cataracts in pigs within 6 weeks after STZ injection (n = 10). There were no overt signs of morphological abnormalities in the retinal circulation at 12 weeks of diabetes.
Vasodilation to Bradykinin
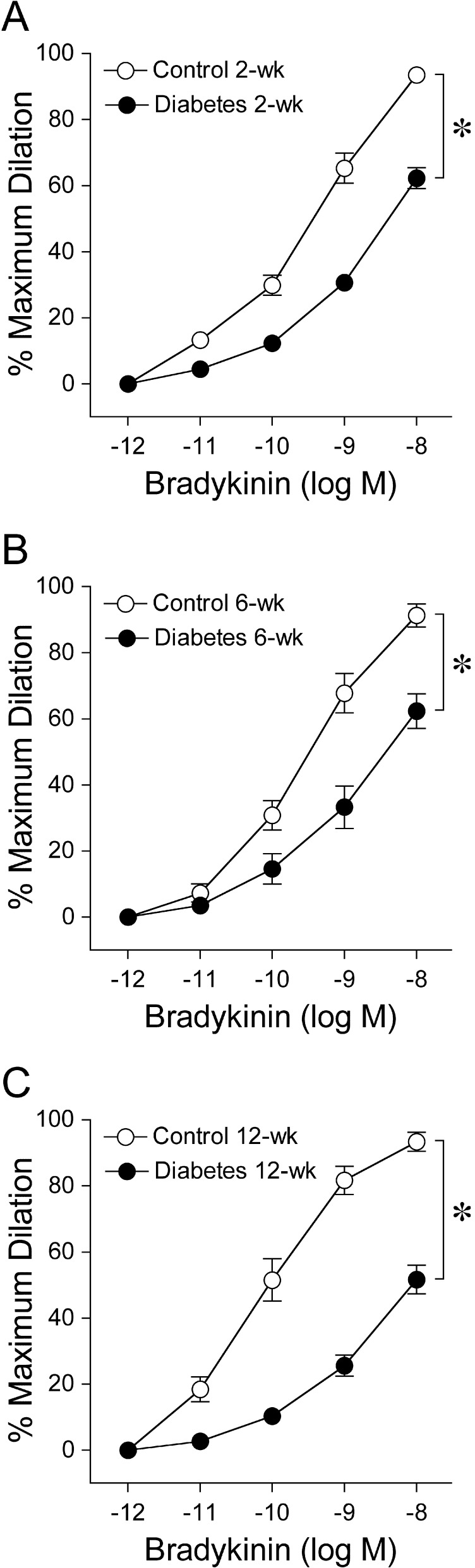
After developing basal tone, 49% ± 2% of maximum diameter (94 ± 2 μm), retinal arterioles from control pigs dilated concentration dependently to bradykinin (Fig. 1A). As early as 2 weeks of diabetes, retinal arteriolar dilation to bradykinin was significantly reduced (Fig. 1A) while basal tone (54% ± 2% of maximum diameter, 91 ± 2 μm) was not altered (P = 0.06). Bradykinin exhibited greater potency in 2-week control vessels than in vessels from age-matched diabetic animals (2-week control EC50 = 0.37 nM vs. 2-week diabetes EC50 = 1.3 nM, P < 0.05). The vasodilation to bradykinin was diminished in a similar manner following a longer duration of 6 weeks (6-week control EC50 = 0.25 nM vs. 6-week diabetes EC50 = 1.0 nM, P < 0.05; Fig. 1B) and 12-week diabetes (12-week control EC50 = 77 pM vs. 12-week diabetes EC50 = 1.3 nM, P < 0.05; Fig. 1C). Basal tone was also unaffected following 6-week (6-week control 50% ± 5% vs. 6-week diabetes 58% ± 3% of maximum diameter, P = 0.19) and 12-week diabetes (12-week control 64% ± 2% vs. 12-week diabetes 65% ± 2% of maximum diameter, P = 0.85).
Figure 1. .
Vasodilator response of isolated and pressurized porcine retinal arterioles to bradykinin. Concentration-dependent vasodilation to bradykinin was significantly reduced in a similar manner following all three time periods of diabetes mellitus: (A) 2 weeks (control = 15 pigs; diabetes = 17 pigs), (B) 6 weeks (control = 4 pigs; diabetes = 5 pigs), and (C) 12 weeks (control = 4 pigs; diabetes = 9 pigs). *P < 0.05 versus control.
Vasodilation to Sodium Nitroprusside
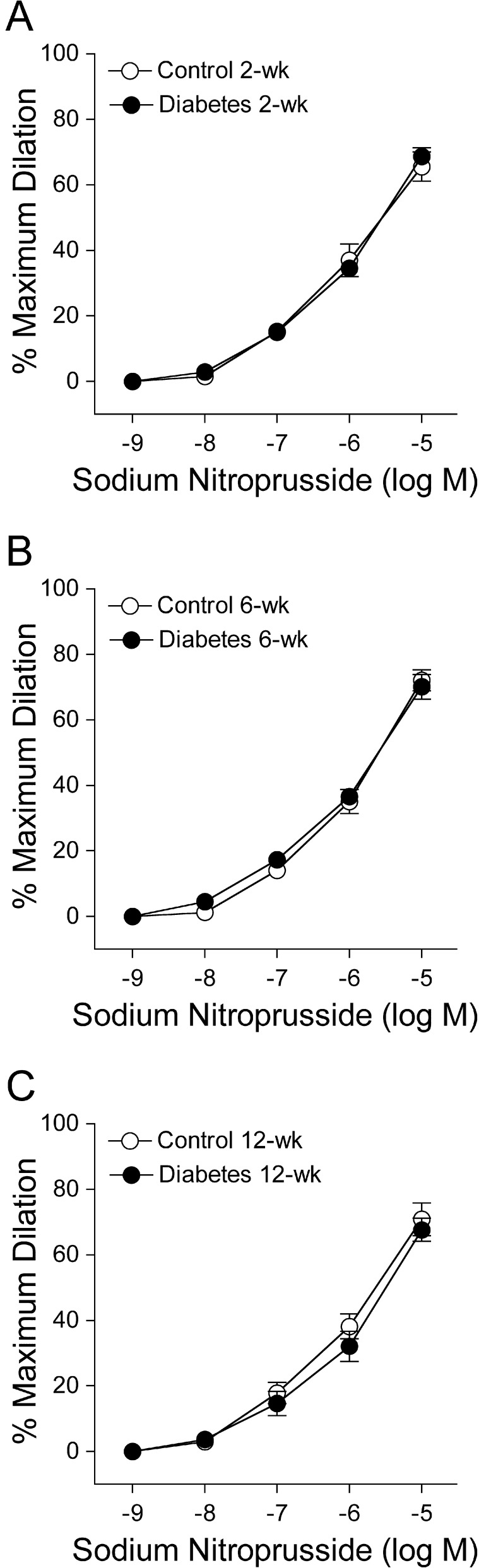
Both control and diabetic retinal arterioles dilated in a comparable manner to endothelium-independent NO donor sodium nitroprusside at all three time periods with maximum dilation of approximately 70% at 10 μM for all groups of vessels (Fig. 2).
Figure 2. .
Vasodilator response of isolated and pressurized porcine retinal arterioles to sodium nitroprusside. Concentration-dependent vasodilation to sodium nitroprusside was unaltered following all three time periods of diabetes mellitus: (A) 2 weeks (control and diabetes = 7 pigs each), (B) 6 weeks (control and diabetes = 4 pigs each), and (C) 12 weeks (control = 4 pigs; diabetes = 8 pigs).
Vasodilation to Increased Flow
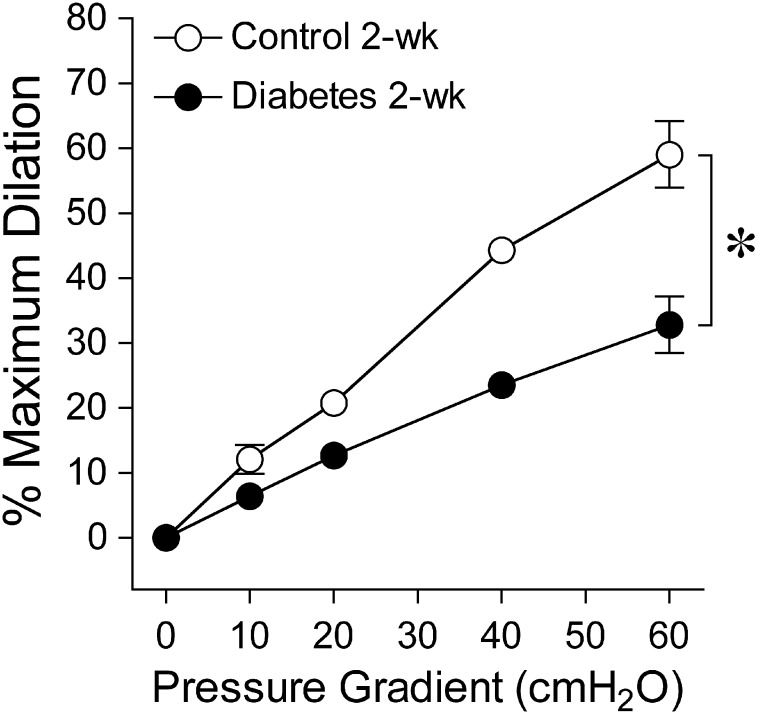
Figure 3 displays graded vasodilation of retinal arterioles when the pressure gradient, and thus luminal flow, was increased in a stepwise manner. Under control conditions, the highest flow elicited 59% ± 5% of maximum dilation, but following 2-week diabetes the response was reduced to 33% ± 4%.
Figure 3. .
Vasodilator response of isolated and pressurized porcine retinal arterioles to increased flow. Dilation of retinal arterioles to a stepwise increase in pressure gradient (i.e., flow/shear stress) was significantly reduced following 2 weeks of diabetes mellitus (control and diabetes = 5 pigs each). *P < 0.05 versus control.
Vasoconstriction to ET-1
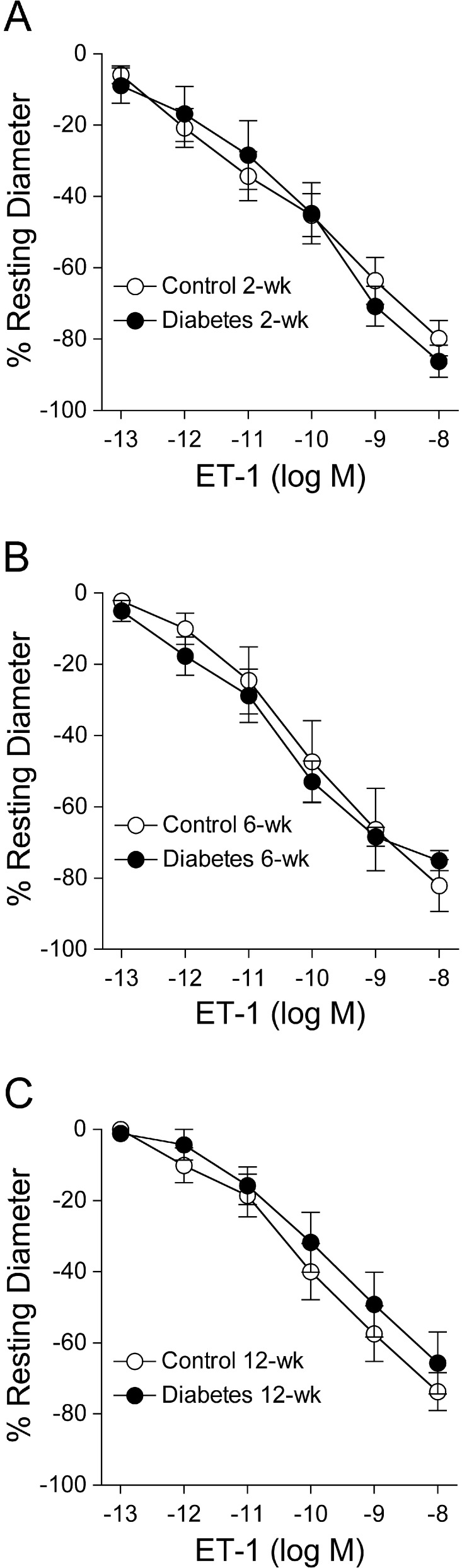
Both control and diabetic retinal arterioles constricted in a comparable manner to ET-1 at all three time periods with maximum constriction of approximately 70% to 90% of resting diameter at 10 nM for all groups of vessels (Fig. 4).
Figure 4. .
Vasoconstrictor response of isolated and pressurized retinal arterioles to ET-1. Concentration-dependent vasoconstriction to ET-1 was unaltered following all three time periods of diabetes mellitus: (A) 2 weeks (control and diabetes = 6 pigs each), (B) 6 weeks (control and diabetes = 3 pigs each), and (C) 12 weeks (control and diabetes = 4 pigs each).
Discussion
Our current findings provide the first direct evidence of endothelial vasodilator dysfunction in retinal arterioles during the early onset of diabetes. Hyperglycemia was associated with selective impairment of endothelium-dependent NO-mediated dilation of retinal arterioles to bradykinin and to increases in flow/shear stress within 2 weeks in a type 1 diabetic pig model. Comparable detrimental effect on endothelial vasodilator function was maintained for at least 3 months. On the other hand, the sustained hyperglycemia for up to 3 months did not influence the ability of the retinal arterioles to dilate and constrict in response to NO donor sodium nitroprusside and ET-1, respectively.
A fundamental understanding of the nature of the initial events contributing to vasomotor dysfunction of retinal arterioles is essential for identifying key vascular cell targets to improve retinal blood flow in patients with diabetic retinopathy.19,35 Several clinical studies have shown a reduction of retinal vasodilator function and retinal blood flow in patients with diabetes.13,36–38 These studies were unable to directly measure the diameter of the retinal arterioles but surmised that these resistance vessels in the microcirculation were impaired by diabetes and contributed to the diminished retinal blood flow. More recent studies by Harris and colleagues using intravital microscopic imaging of the retinal microcirculation have provided the first evidence that the resting diameter of retinal arterioles is smaller in the early stages of diabetes in mice and rats and correlates with reduced retinal blood flow.4–9,12 Furthermore, the ability of retinal arterioles to react to endothelium-dependent vasodilators in vivo has been shown to be reduced following acute exposure (3 hours) to hyperglycemia in cats39 and more sustained hyperglycemia in rats.40–44 To build on these studies and assess whether early diabetes has a direct impact on endothelial and smooth muscle vasomotor function, we utilized an isolated vessel approach in the current study to eliminate confounding effects from neurohumoral and local hemodynamic factors. Moreover, our recent in vitro studies disclose similarities in the vasoreactivity and its underlying signaling mechanisms between human and porcine retinal arterioles.16 Therefore, we developed an STZ-induced type-1 diabetes model in the pig to study the vasomotor function. The diabetic pigs maintained consistent elevation of plasma glucose levels nearly 5- to 6-fold greater than those in control pigs. Cataract development, another complication of diabetes, was evident within approximately 6 weeks of hyperglycemia. Structural changes or hemorrhage in the retina were not apparent with fundus imaging following all time periods studied. Collectively, our recent and current data strongly support the clinical relevance in using the pig model to assess endothelial and smooth muscle vasomotor function under normal and diabetic conditions.
Clinical evidence indicates that NO produced from NO synthase can influence retinal vascular tone and regulate retinal blood flow in humans.45–48 Local stimulation of metabolic activity in the retina with diffuse flickering light has been shown to increase retinal artery diameter48,49 and retinal blood flow49 in healthy human subjects, which is reduced by NO synthase blockade48 and in type 1 diabetic patients.37,50,51 Our recent studies provide the first direct evidence for a prominent vascular contribution of NO derived from NO synthase activation in the dilation of human and porcine retinal arterioles to bradykinin and flow/shear stress,16 two endogenous local regulators of retinal arteriolar tone.18,52–54 A direct negative impact of diabetes on bradykinin-induced and flow-mediated dilations of porcine retinal arterioles was evident in the current study within 2 weeks of hyperglycemia. Diminished dilation of retinal arterioles to bradykinin was comparable following 6 and 12 weeks, suggesting a sustained inhibitory mechanism once it is initiated and the importance of future studies to identify the specific mechanistic process causing the early vascular dysfunction. Because endothelium-independent vasodilation to NO donor sodium nitroprusside was unaltered up to 12 weeks following diabetes, the ability of the smooth muscle to relax in response to NO remained intact and the detrimental effect of diabetes was selective for the impairment of NO synthesis or release from the endothelium. Notably, the relatively rapid onset of endothelial dysfunction within 2 weeks of diabetes in the pig model is consistent with early studies showing diminished retinal blood flow at baseline44,55 and following intravitreal administration of acetylcholine in 2-week diabetic rats.44 Interestingly, in our previous human study,16 we found that retinal arterioles isolated from a patient with diabetic retinopathy exhibited diminished vasodilation to bradykinin and to increased shear stress (unpublished data), a phenomenon identical to that obtained from the diabetic pigs observed in the present study. It appears that retinal arterioles from pigs and humans exhibit similar vasomotor behavior either in physiology or pathophysiology.
The mechanical influence of an increase in shear stress due to luminal flow elicits endothelium-dependent NO-mediated dilation in both human and porcine retinal arterioles.16 This flow-mediated vasodilator response is considered to contribute to local flow regulation by recruiting blood flow to the tissue when metabolic demand is increased (e.g., functional hyperemia) or oxygen supply to the tissue is inadequate (e.g., reactive hyperemia and hypoxia).56 Flow-mediated dilation of the brachial artery via ultrasound measurement following transient forearm ischemia has been widely used as an index for clinical assessment of endothelial function57,58 and a diminished response has been reported in type 1 diabetic patients.59–61 Interestingly, reduced flow-mediated dilation of the brachial artery was evident in adolescents within 1 month to 5 years following diagnosis of type 1 diabetes.59 Although physiological corroboration of this vascular phenomenon in the human retinal microcirculation is lacking, evidence from Nagaoka and colleagues suggests that hypoxia18 and acute elevation of blood pressure54 in cats elicit flow/shear-induced dilation of retinal arterioles. Our present results showing the impairment of flow-mediated dilation of retinal arterioles following 2 weeks of diabetes are consistent with the clinical report of early endothelial dysfunction in the peripheral circulation59 and provide direct support for deleterious action of diabetes at the level of resistance vessels.
An imbalance of NO and ET-1 levels and/or activity has been implicated in pathophysiological conditions such as diabetic retinopathy,62 potentially causing vessel spasm (focal arteriolar constriction) and leading to the reduction of blood flow and tissue ischemia in the human retina. ET-1, which is produced primarily by vascular endothelial cells via the endothelin-converting enzyme (ECE-1), has been shown hitherto to be the most potent endogenous vasoconstrictor.63 Human and porcine retinal arterioles constrict to ET-1 in a comparable manner in vitro.16 Our current findings do not support a change in the ability of retinal arteriolar smooth muscle to respond to ET-1 following the early 3-month onset of diabetes in pigs. However, these results do not exclude whether diabetes increased synthesis of ET-1 within the neural or vascular retina. This consideration is corroborated by elevated ET-1 levels in the vitreous of patients with diabetic retinopathy.64,65 Since intravitreal treatment with pharmacological blockade of ECE-1-derived ET-1 in vivo has been shown to improve retinal blood flow in early diabetes in rats,66 increased ECE-1 activity/expression in diabetic retinal arterioles could contribute to vasomotor dysfunction. Moreover, administration of an ET-1 receptor antagonist to the drinking water has been shown to improve retinal blood flow in mice with type 1 diabetes.8 However, the direct evidence for a functional role of local ECE-1 in the retinal arterioles during early diabetes remains unclear. Nevertheless, our present study does not support the idea that the adverse effect of ET-1 observed in the retinal circulation in vivo is a result of enhanced smooth muscle contraction to ET-1. Future studies will examine the impact of diabetes on ET-1 levels in the vitreous and the functional activity of arteriolar ECE-1, which is supported by our recent report characterizing the endothelin system in the retina with greater expression of ECE-1 in retinal arterioles than in neural retina tissue.31
In summary, we have established a type 1 diabetic pig model and found that 2-week diabetes is sufficient to selectively impair retinal endothelial NO-mediated function, with no progression for an additional 2 to 3 months. All durations of diabetes had no impact on smooth muscle-dependent nitroprusside-induced vasodilation or ET-1-induced vasoconstriction. The current findings provide the framework for future studies designed to identify the mechanisms contributing to endothelial vasodilator dysfunction of retinal arterioles in a large animal model of type 1 diabetes relevant to the human retinal microcirculation.
Acknowledgments
We are grateful to Jennifer Lane, Rebecca Blackwood, Christina Du, Angie Hitt, and the animal facility staff for their technical assistance with animal care.
Footnotes
Supported by grants from the Scott & White Research Foundation (TWH), Retina Research Foundation (TWH, LK), NIH NEI R01EY018420 (TWH), the Scott & White Research Foundation Ophthalmic Vascular Research Program (LK), and the Kruse Chair Endowment Fund (LK).
Disclosure: T.W. Hein, None; L.B. Potts, None; W. Xu, None; J.Z. Yuen, None; L. Kuo, None
References
- 1.Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol. 2012;57:347–370 [DOI] [PubMed] [Google Scholar]
- 2.Durham JT, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011;11:253–264 [DOI] [PubMed] [Google Scholar]
- 3.Clermont AC, Brittis M, Shiba T, McGovern T, King GL, Bursell SE. Normalization of retinal blood flow in diabetic rats with primary intervention using insulin pumps. Invest Ophthalmol Vis Sci. 1994;35:981–990 [PubMed] [Google Scholar]
- 4.Lee S, Harris NR. Losartan and ozagrel reverse retinal arteriolar constriction in non-obese diabetic mice. Microcirculation. 2008;15:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Morgan GA, Harris NR. Ozagrel reverses streptozotocin-induced constriction of arterioles in rat retina. Microvasc Res. 2008;76:217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright WS, Harris NR. Ozagrel attenuates early streptozotocin-induced constriction of arterioles in the mouse retina. Exp Eye Res. 2008;86:528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright WS, Messina JE, Harris NR. Attenuation of diabetes-induced retinal vasoconstriction by a thromboxane receptor antagonist. Exp Eye Res. 2009;88:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Yadav AS, Leskova W, Harris NR. Attenuation of streptozotocin-induced microvascular changes in the mouse retina with the endothelin receptor A antagonist atrasentan. Exp Eye Res. 2010;91:670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Yadav AS, Leskova W, Harris NR. Inhibition of 20-HETE attenuates diabetes-induced decreases in retinal hemodynamics. Exp Eye Res. 2011;93:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higashi S, Clermont AC, Dhir V, Bursell SE. Reversibility of retinal flow abnormalities is disease-duration dependent in diabetic rats. Diabetes. 1998;47:653–659 [DOI] [PubMed] [Google Scholar]
- 11.Takagi C, King GL, Clermont AC, Cummins DR, Takagi H, Bursell SE. Reversal of abnormal retinal hemodynamics in diabetic rats by acarbose, an alpha-glucosidase inhibitor. Curr Eye Res. 1995;14:741–749 [DOI] [PubMed] [Google Scholar]
- 12.Yadav AS, Harris NR. Effect of tempol on diabetes-induced decreases in retinal blood flow in the mouse. Curr Eye Res. 2011;36:456–461 [DOI] [PubMed] [Google Scholar]
- 13.Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996;37:886–897 [PubMed] [Google Scholar]
- 14.Clermont AC, Aiello LP, Mori F, Aiello LM, Bursell SE. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Am J Ophthalmol. 1997;124:433–446 [DOI] [PubMed] [Google Scholar]
- 15.Kawagishi T, Nishizawa Y, Emoto M, et al. Impaired retinal artery blood flow in IDDM patients before clinical manifestations of diabetic retinopathy. Diabetes Care. 1995;18:1544–1549 [DOI] [PubMed] [Google Scholar]
- 16.Hein TW, Rosa RH Jr, Yuan Z, Roberts E, Kuo L. Divergent roles of nitric oxide and rho kinase in vasomotor regulation of human retinal arterioles. Invest Ophthalmol Vis Sci. 2010;51:1583–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaya S, Kolodjaschna J, Berisha F, Schmetterer L, Garhofer G. Comparison of the autoregulatory mechanisms between central retinal artery and posterior ciliary arteries after thigh cuff deflation in healthy subjects. Microvasc Res. 2011;82:269–273 [DOI] [PubMed] [Google Scholar]
- 18.Nagaoka T, Sakamoto T, Mori F, Sato E, Yoshida A. The effect of nitric oxide on retinal blood flow during hypoxia in cats. Invest Ophthalmol Vis Sci. 2002;43:3037–3044 [PubMed] [Google Scholar]
- 19.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330 [DOI] [PubMed] [Google Scholar]
- 20.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18 [DOI] [PubMed] [Google Scholar]
- 21.Thorin E, Webb DJ. Endothelium-derived endothelin-1. Pflugers Arch. 2010;459:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trepels T, Zeiher AM, Fichtlscherer S. The endothelium and inflammation. Endothelium. 2006;13:423–429 [DOI] [PubMed] [Google Scholar]
- 23.Toda N, Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007;26:205–238 [DOI] [PubMed] [Google Scholar]
- 24.Agapitov AV, Haynes WG. Role of endothelin in cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2002;3:1–15 [DOI] [PubMed] [Google Scholar]
- 25.Lam HC, Lee JK, Lu CC, Chu CH, Chuang MJ, Wang MC. Role of endothelin in diabetic retinopathy. Curr Vasc Pharmacol. 2003;1:243–250 [DOI] [PubMed] [Google Scholar]
- 26.Jeppesen P, Aalkjaer C, Bek T. Bradykinin relaxation in small porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2002;43:1891–1896 [PubMed] [Google Scholar]
- 27.Korompai FL, Ustinova E, Taulman AC, Yuan SY. Ammonium chloride potentiation of streptozotocin-induced diabetes in juvenile pigs. Horm Metab Res. 2000;32:256–258 [DOI] [PubMed] [Google Scholar]
- 28.Yuan SY, Ustinova EE, Wu MH, et al. Protein kinase C activation contributes to microvascular barrier dysfunction in the heart at early stages of diabetes. Circ Res. 2000;87:412–417 [DOI] [PubMed] [Google Scholar]
- 29.Hein TW, Yuan Z, Rosa RH Jr, Kuo L. Requisite roles of A2A receptors, nitric oxide, and KATP channels in retinal arteriolar dilation in response to adenosine. Invest Ophthalmol Vis Sci. 2005;46:2113–2119 [DOI] [PubMed] [Google Scholar]
- 30.Nagaoka T, Kuo L, Ren Y, Yoshida A, Hein TW. C-reactive protein inhibits endothelium-dependent nitric oxide-mediated dilation of retinal arterioles via enhanced superoxide production. Invest Ophthalmol Vis Sci. 2008;49:2053–2060 [DOI] [PubMed] [Google Scholar]
- 31.Hein TW, Ren Y, Yuan Z, et al. Functional and molecular characterization of the endothelin system in retinal arterioles. Invest Ophthalmol Vis Sci. 2009;50:3329–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potts LB, Ren Y, Lu G, et al. Constriction of retinal arterioles to endothelin-1: requisite role of rho kinase independent of protein kinase C and L-type calcium channels. Invest Ophthalmol Vis Sci. 2012;53:2904–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol. 1990;259:H1063–H1070 [DOI] [PubMed] [Google Scholar]
- 34.Riva CE, Grunwald JE, Sinclair SH, Petrig BL. Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci. 1985;26:1124–1132 [PubMed] [Google Scholar]
- 35.Gardiner TA, Archer DB, Curtis TM, Stitt AW. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation. 2007;14:25–38 [DOI] [PubMed] [Google Scholar]
- 36.Bek T, Hajari J, Jeppesen P. Interaction between flicker-induced vasodilatation and pressure autoregulation in early retinopathy of type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2008;246:763–769 [DOI] [PubMed] [Google Scholar]
- 37.Garhofer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004;88:887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandecka A, Dawczynski J, Blum M, et al. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care. 2007;30:3048–3052 [DOI] [PubMed] [Google Scholar]
- 39.Sogawa K, Nagaoka T, Izumi N, Nakabayashi S, Yoshida A. Acute hyperglycemia-induced endothelial dysfunction in retinal arterioles in cats. Invest Ophthalmol Vis Sci. 2010;51:2648–2655 [DOI] [PubMed] [Google Scholar]
- 40.Mori A, Saigo O, Hanada M, Nakahara T, Ishii K. Hyperglycemia accelerates impairment of vasodilator responses to acetylcholine of retinal blood vessels in rats. J Pharmacol Sci. 2009;110:160–168 [DOI] [PubMed] [Google Scholar]
- 41.Mori A, Saigo O, Sakamoto K, Nakahara T, Ishii K. Hyperglycemia impairs acetylcholine-induced vasodilation of retinal arterioles through polyol pathway-independent mechanisms in rats. J Pharmacol Sci. 2010;112:336–342 [DOI] [PubMed] [Google Scholar]
- 42.Nakazawa T, Kaneko Y, Mori A, et al. Attenuation of nitric oxide- and prostaglandin-independent vasodilation of retinal arterioles induced by acetylcholine in streptozotocin-treated rats. Vasc Pharmacol. 2007;46:153–159 [DOI] [PubMed] [Google Scholar]
- 43.Nakazawa T, Mori A, Saito M, Sakamoto K, Nakahara T, Ishii K. Vasodilator effects of adenosine on retinal arterioles in streptozotocin-induced diabetic rats. Naunyn-Schmiedebergs Arch Pharmacol. 2008;376:423–430 [DOI] [PubMed] [Google Scholar]
- 44.Horio N, Clermont AC, Abiko A, et al. Angiotensin AT1 receptor antagonism normalizes retinal blood flow and acetylcholine-induced vasodilatation in normotensive diabetic rats. Diabetologia. 2004;47:113–123 [DOI] [PubMed] [Google Scholar]
- 45.Michelson G, Warntges S, Harazny J, Oehmer S, Delles C, Schmieder RE. Effect of NOS inhibition on retinal arterial and capillary circulation in early arterial hypertension. Retina. 2006;26:437–444 [DOI] [PubMed] [Google Scholar]
- 46.Delles C, Michelson G, Harazny J, Oehmer S, Hilgers KF, Schmieder RE. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke. 2004;35:1289–1293 [DOI] [PubMed] [Google Scholar]
- 47.Polak K, Dorner G, Kiss B, et al. Evaluation of the Zeiss retinal vessel analyser. Br J Ophthalmol. 2000;84:1285–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorner GT, Garhofer G, Kiss B, et al. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol. 2003;285:H631–H636 [DOI] [PubMed] [Google Scholar]
- 49.Garhofer G, Zawinka C, Resch H, Huemer KH, Dorner GT, Schmetterer L. Diffuse luminance flicker increases blood flow in major retinal arteries and veins. Vision Res. 2004;44:833–838 [DOI] [PubMed] [Google Scholar]
- 50.Hammer M, Heller T, Jentsch S, et al. Retinal vessel oxygen saturation under flicker light stimulation in patients with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53:4063–4068 [DOI] [PubMed] [Google Scholar]
- 51.Lecleire-Collet A, Audo I, Aout M, et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2011;52:2861–2867 [DOI] [PubMed] [Google Scholar]
- 52.Ma JX, Song Q, Hatcher HC, Crouch RK, Chao L, Chao J. Expression and cellular localization of the kallikrein-kinin system in human ocular tissues. Exp Eye Res. 1996;63:19–26 [DOI] [PubMed] [Google Scholar]
- 53.Webb JG. The kallikrein/kinin system in ocular function. J Ocul Pharmacol. 2011;27:539–543 [DOI] [PubMed] [Google Scholar]
- 54.Nakabayashi S, Nagaoka T, Tani T, et al. Retinal arteriolar responses to acute severe elevation in systemic blood pressure in cats: role of endothelium-derived factors. Exp Eye Res. 2012;103:63–70 [DOI] [PubMed] [Google Scholar]
- 55.Bursell SE, Takagi C, Clermont AC, et al. Specific retinal diacylglycerol and protein kinase C beta isoform modulation mimics abnormal retinal hemodynamics in diabetic rats. Invest Ophthalmol Vis Sci. 1997;38:2711–2720 [PubMed] [Google Scholar]
- 56.Davis MJ, Hill M, Kuo L. Local regulation of blood flow. In: Tuma RF, Duran WN, Ley K.eds Handbook of Physiology; Section 2: The Cardiovascular System; Microcirculation. 2nd ed. Bethesda, MD: The American Physiological Society and Elsevier; 2008:159–284 [Google Scholar]
- 57.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115 [DOI] [PubMed] [Google Scholar]
- 58.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996;270:H1435–H1440 [DOI] [PubMed] [Google Scholar]
- 59.Ce GV, Rohde LE, da Silva AM, Punales MK, de Castro AC, Bertoluci MC. Endothelial dysfunction is related to poor glycemic control in adolescents with type 1 diabetes under 5 years of disease: evidence of metabolic memory. J Clin Endocrinol Metab. 2011;96:1493–1499 [DOI] [PubMed] [Google Scholar]
- 60.Ceriello A, Esposito K, Ihnat M, Thorpe J, Giugliano D. Long-term glycemic control influences the long-lasting effect of hyperglycemia on endothelial function in type 1 diabetes. J Clin Enocrinol Metab. 2009;94:2751–2756 [DOI] [PubMed] [Google Scholar]
- 61.Pemp B, Weigert G, Karl K, et al. Correlation of flicker-induced and flow-mediated vasodilatation in patients with endothelial dysfunction and healthy volunteers. Diabetes Care. 2009;32:1536–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haefliger IO, Flammer J, Beny JL, Luscher TF. Endothelium-dependent vasoactive modulation in the ophthalmic circulation. Prog Retin Eye Res. 2001;20:209–225 [DOI] [PubMed] [Google Scholar]
- 63.Yanagisawa M, Kurihara H, Kimura S, et al. A novel vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415 [DOI] [PubMed] [Google Scholar]
- 64.Oku H, Kida T, Sugiyama T, Hamada J, Sato B, Ikeda T. Possible involvement of endothelin-1 and nitric oxide in the pathogenesis of proliferative diabetic retinopathy. Retina. 2001;21:647–651 [DOI] [PubMed] [Google Scholar]
- 65.Roldan-Pallares M, Rollin R, Mediero A, et al. Immunoreactive ET-1 in the vitreous humor and epiretinal membranes of patients with proliferative vitreoretinopathy. Mol Vis. 2005;11:461–471 [PubMed] [Google Scholar]
- 66.Takagi C, Bursell SE, Lin YW, et al. Regulation of retinal hemodynamics in diabetic rats by increased expression and action of endothelin-1. Invest Ophthalmol Vis Sci. 1996;37:2504–2518 [PubMed] [Google Scholar]