Abstract
When facing an immune response, viruses can either attempt to elude them or confront them. A new report demonstrates that a lymphocytic choriomeningitis virus (LCMV) strain can suppress immune responses by targeting both development and activation of DCs. Ironically, type I IFN released in response to LCMV infection contributes to the blockade of DC development. The discovery of these immunosuppressive mechanisms provides new perspectives for the therapy of chronic infections associated with immunosuppression.
Viruses have evolved multiple strategies to counteract host immune responses. Noncytopathic lymphocytic choriomeningitis virus (LCMV) employs several of these strategies to successfully infect mice. Initial immunosurveillance of LCMV infection is mediated by CTLs. However, this response may lead to selection of LCMV variants that carry mutations in the relevant CTL epitopes and, therefore, can elude cytotoxic responses (1, 2). Antibody responses are also essential for long-term protection. However, LCMV variants can evade humoral responses with point mutations that encode novel amino acids distorting the envelope glycoprotein epitope recognized by neutralizing antibodies (3).
DCs as targets of LCMV immunosuppression
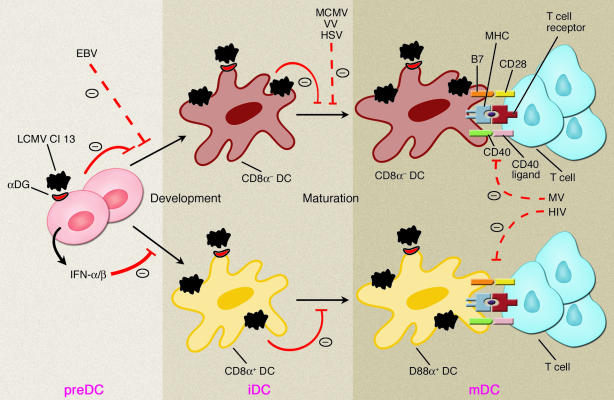
Remarkably, LCMV not only eludes specific immune surveillance, but can also actively suppress immune responses. How is this accomplished? In this issue of the JCI, Sevilla and colleagues elucidate the mechanism used by an immunosuppressive LCMV variant known as LCMV clone 13 (Cl 13) (4). This viral variant was originally isolated from the lymphoid tissues of neonatal mice with a persistent LCMV infection induced by the nonimmunosuppressive wild-type LCMV, Armstrong 53b (ARM) (5). Initial studies indicated that Cl 13 targets APCs, and, in particular DCs (6). DCs are sentinels of the immune system; they efficiently capture viral antigens at the infection site and rapidly migrate to the lymph nodes where they initiate T cell responses (7). Thus, DCs are ideal targets for viral immunosuppression. Sevilla and colleagues demonstrate that Cl 13 adopts a surprising dual strategy for disabling DCs: inhibiting both their development and T cell stimulatory function (Figure 1). They clearly show that infection with Cl 13 impairs the expression of MHC and costimulatory molecules on both spleen myeloid (CD8α–) and lymphoid (CD8α+) DCs. As a result, DCs do not efficiently stimulate T cell proliferation ex vivo. DC function remains impaired as long as LCMV infection persists. Moreover, Cl 13 infects bone marrow precursors in vivo and in vitro, inhibiting development and differentiation of CD8α– and CD8α+ DCs.
Figure 1.
Immunosuppressive mechanisms of LCMV Cl 13. Cl 13 infects DC precursors and DCs, possibly using α-DG as the entry receptor. Cl 13 blocks development of CD8α+ and CD8α– DCs from DC precursors (preDCs) and prevents immature DCs (iDCs) from becoming mature DCs (mDCs), which express high levels of MHC, CD40, and B7, and initiate T cell responses. Blockade of CD8α+ DC development by Cl 13 requires type I IFN. The sites of action of other immunosuppressive viruses interfering with DC functions or DC–T cell interactions are indicated. EBV, Epstein-Barr virus; HSV, herpes simplex virus; VV, vaccinia virus; MCMV, murine cytomegalovirus; MV, measles virus.
Why does Cl 13 selectively affect DCs and their precursors? LCMV has been shown to bind α-dystroglycan (α-DG), a receptor for extracellular matrix proteins that is highly expressed on DCs and bone marrow precursors (8). Notably, Cl 13 has a higher affinity for α-DG than does wild-type ARM (8). Thus, it is possible that Cl 13 competes with extracellular matrix proteins for binding α-DG on DCs, thereby infecting them, whereas ARM does not.
The paradoxical role of type I IFN
The notion that DCs are major targets of immunosuppressive viruses is corroborated by several other types of infections (Figure 1). Measles virus (9, 10), herpes simplex virus (11), vaccinia virus (12), and murine cytomegalovirus (13) infect DCs and impair their capacity to stimulate T cells. Human immunodeficiency virus exploits DCs for transmission to T cells (14). Epstein-Barr virus inhibits the development of DCs by inducing apoptosis of their monocytic precursors without infecting them (15). Furthermore, DCs are eliminated by CTL-mediated responses elicited by some immunosuppressive LCMV variants (16). In comparison with these immunosuppressive mechanisms, the inhibition of DCs by Cl 13 reported by Sevilla and colleagues is remarkable in that the virus impairs both DC immunostimulatory function and the development of CD8α– and CD8α+ DCs. Moreover, Sevilla and colleagues demonstrate that type I IFN, i.e., IFN-α and/or IFN-β, is necessary for Cl 13–mediated blockade of CD8α+ DC development. Thus, type I IFN paradoxically contributes to immunosuppression rather than host defense. What is the mechanism? Previous studies have shown that Cl 13, in contrast to wild-type ARM, effectively triggers secretion of type I IFN by DCs (17). Thus, type I IFN may affect CD8α+ DC development through an autocrine loop. The paracrine action of type I IFN secreted by natural IFN-producing cells (IPCs) (18) in response to Cl 13 may also be involved. Another intriguing observation reported by Sevilla and colleagues is that Cl 13 inhibits expression of MHC in DCs cultured from bone marrow cells, although type I IFN would be expected to increase at least MHC class I expression. Whether Cl 13 directly inhibits MHC synthesis by mechanisms similar to those employed by herpes viruses (19) remains to be determined.
In conclusion, the Cl 13 infection model underscores the central role of DCs in mediating viral immunosuppression and describes novel methods by which a virus can impair DCs. It will be important to investigate the influence of viral burden on these mechanisms. Infection of bone marrow and suppression of DC development may require higher viral loads than infection of peripheral DCs. Another important question is whether Cl 13 immunosuppression involves other APCs that may participate in anti-LCMV immune responses, such as IPCs and macrophages. Certainly, Cl 13 infection will provide a valuable model to test whether increasing DC numbers, their maturation, and their T cell stimulatory capacity can improve the efficacy of vaccines in chronic infections associated with immunosuppression.
Footnotes
See the related article beginning on page 737.
Nonstandard abbreviations used: α-dystroglycan (α-DG); Armstrong 53b (ARM); clone 13 (Cl 13); natural IFN-producing cell (IPC); lymphocytic choriomeningitis virus (LCMV).
Conflict of interest: The author has declared that no conflict of interest exists.
References
- 1.Oldstone MB. How viruses escape from cytotoxic T lymphocytes: molecular parameters and players. Virology. 1997;234:179–185. doi: 10.1006/viro.1997.8674. [DOI] [PubMed] [Google Scholar]
- 2.Klenerman P, Zinkernagel RM. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 1998;394:482–485. doi: 10.1038/28860. [DOI] [PubMed] [Google Scholar]
- 3.Ciurea A, et al. Viral persistence in vivo through selection of neutralizing antibody-escape variants. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2749–2754. doi: 10.1073/pnas.040558797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MBA. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J. Clin. Invest. 2004;113:737–745. doi:10.1172/JCI200420243. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed R, Oldstone MB. Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 1988;167:1719–1724. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sevilla N, et al. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Sevilla N, Kunz S, McGavern D, Oldstone MB. Infection of dendritic cells by lymphocytic choriomeningitis virus. Curr. Top. Microbiol. Immunol. 2003;276:125–144. doi: 10.1007/978-3-662-06508-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Servet-Delprat C, Vidalain PO, Valentin H, Rabourdin-Combe C. Measles virus and dendritic cell functions: how specific response cohabits with immunosuppression. Curr. Top. Microbiol. Immunol. 2003;276:103–123. doi: 10.1007/978-3-662-06508-2_5. [DOI] [PubMed] [Google Scholar]
- 10.Karp CL, et al. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 11.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 1999;29:3245–3253. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Engelmayer J, et al. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 1999;163:6762–6768. [PubMed] [Google Scholar]
- 13.Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P, Degli-Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2001;2:1077–1084. doi: 10.1038/ni724. [DOI] [PubMed] [Google Scholar]
- 14.Steinman RM, et al. The interaction of immunodeficiency viruses with dendritic cells. Curr. Top. Microbiol. Immunol. 2003;276:1–30. doi: 10.1007/978-3-662-06508-2_1. [DOI] [PubMed] [Google Scholar]
- 15.Li L, et al. Epstein-Barr virus inhibits the development of dendritic cells by promoting apoptosis of their monocyte precursors in the presence of granulocyte macrophage-colony-stimulating factor and interleukin-4. Blood. 2002;99:3725–3734. doi: 10.1182/blood.v99.10.3725. [DOI] [PubMed] [Google Scholar]
- 16.Odermatt B, Eppler M, Leist TP, Hengartner H, Zinkernagel RM. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc. Natl. Acad. Sci. U. S. A. 1991;88:8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diebold SS, et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–328. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 18.Colonna M, Krug A, Cella M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 2002;14:373–379. doi: 10.1016/s0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 19.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu. Rev. Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]