Magic angle spinning solid-state NMR (MAS ssNMR) spectroscopy is a powerful method for atomic-resolution structure determination of biomacromolecules that are recalcitrant to crystallization (membrane proteins and fibrils).[1, 2, 2–4] Developments in pulse sequence design,[5–10] probes,[11] isotopic-labeling schemes,[12], sensitivity enhancement using paramagnetic effects [13], dynamic nuclear polarization (DNP)[14–16], and sample preparations have made it possible to advance resonance assignments and structure determination by ssNMR. However, as the biological systems under investigation increase in complexity, new methods are needed to improve spectral resolution and sensitivity as well as speed up NMR data acquisition.
Here we present a novel approach (which we refer as “dual acquisition MAS ssNMR or DUMAS ssNMR) for parallel acquisition of multidimensional ssNMR experiments without the need of additional hardware. DUMAS ssNMR is able to combine several of the above advancements to almost double the capability of the NMR spectrometers. Central to this technique are the following phenomena: 1) the long-living 15N polarization in biological solids; 2) Simultaneous Hartmann-Hahn (HH) cross polarization (CP) from 1H to 13C and 15N, and 3) 15N-13C dipolar decoupling under MAS conditions. DUMAS is not limited to specific pulse sequences, rather it represents a general approach to concatenate various multidimensional ssNMR experiments.
The classical ssNMR experiments start with HH-CP,[17, 18], which enhances the sensitivity of rare nuclei coupled to abundant 1H spin bath. However, during CP only part of the polarization from 1H spin bath is transferred to dilute spins (13C or 15N). Recently, Oschkinat and co-workers proposed to enhance 13C sensitivity with triple resonance (1H, 2H, 13C) CP using perdeuterated proteins.[19] Analogously, for DUMAS we utilize simultaneous CP (SIM-CP) to polarize 13C and 15N. We then exploit the polarization to acquire two 13C- and 15N-edited multidimensional experiments in parallel. Since the 15N nuclei have relatively long longitudinal spin relaxation times (T1),[20] the 15N z-magnetization can be stored for several milliseconds (while acquiring 13C coherences) and recovered for an additional multidimensional experiment.
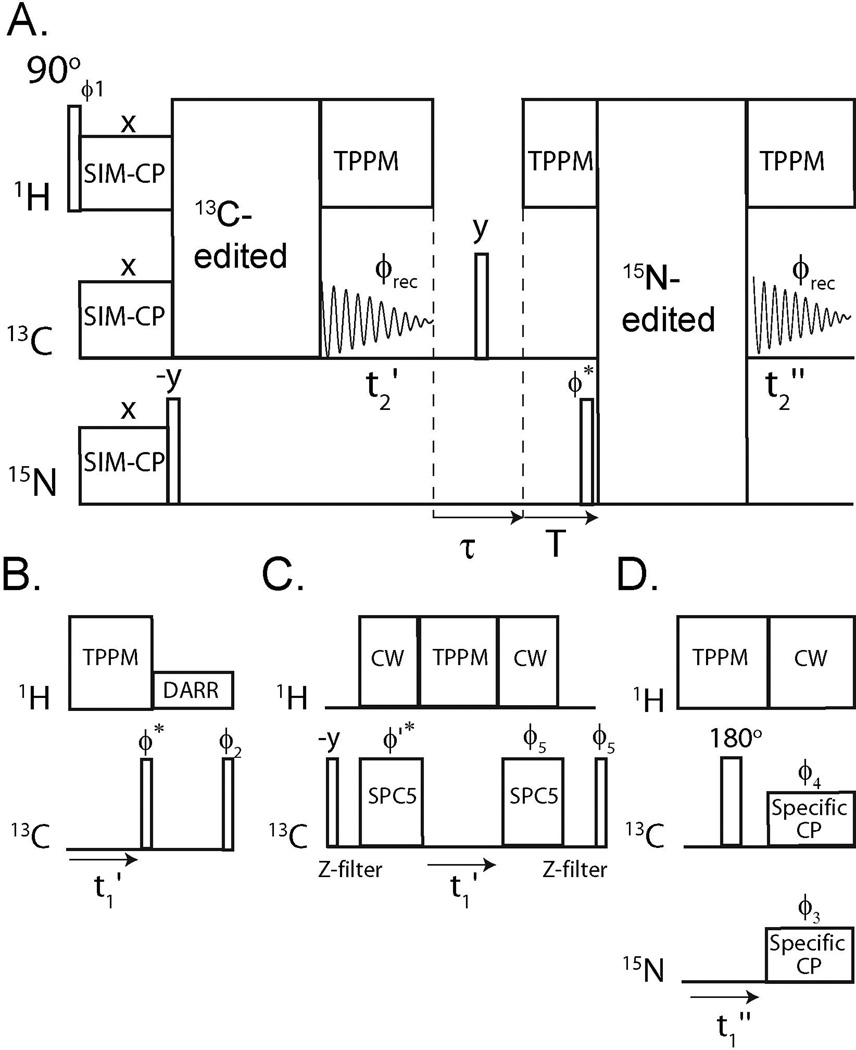
A schematic of DUMAS is reported in Figure 1A. A 90° pulse on 1H is followed by SIM-CP and creates the initial polarization of 13C and 15N nuclei. After SIM-CP, the 15N magnetization is tilted along the z-axis by a 90° pulse, whereas the 13C magnetization is used to record a 13C-edited spectrum with t1’ indirect evolution and t2’ acquisition under two-pulse phase modulated (TPPM) heteronuclear decoupling.[21]
Figure 1.
(A) General scheme for the DUMAS ssNMR. The 13C- and 15N-edited blocks are combined to give DUMAS-DARR-NCA (B and D blocks) or DUMAS-DQSQ-NCO (C and D blocks).
After the acquisition of the first experiment, a τ delay of 2 ms is used to dephase any residual magnetization. Namely, in the first τ/2 period, the transverse magnetization is dephased, while the longitudinal magnetization is dephased by a 90° pulse, followed by another τ/2 period. A delay T = [t1max’−dw(t1’)] is applied under heteronuclear decoupling, where t1max’ is the maximum t1’, and dw(t1’) is dwell time of the t1’ evolution period. This delay makes the total time of the 13C-edited acquisition identical for all of the t1’ increments, while the 15N magnetization is stored along the z-axis. A 90y° pulse recovers the 15N magnetization stored along the z-axis and places it along the x-axis. At this point, a 15N-edited sequence block is implemented followed by a second acquisition on 13C channel during t2”. Due to the different relaxation behaviors, the evolution times (t1) are usually longer for 15N than 13C. Our scheme uses separate evolution time periods (t1’ and t1”), which can be optimized for both sensitivity and resolution according to Table S1. Although it is possible to combine various multidimensional experiments, we demonstrate the DUMAS method concatenating DARR,[22], NCA,[23] DQSQ,[24], and NCO,[23] four of the most widely used pulse sequences for structure determination of proteins. Specifically, we incorporated the 13C- and 15N-edited blocks to create the DUMAS-DARR-NCA and DUMAS-DQSQ-NCO pulse sequences (Figure 1).
To test the performance of these experiments, we used uniformly (U)-13C, 15N ubiquitin in microcrystalline form, which is the benchmark for MAS ssNMR experiments on proteins.[25] U-13C, 15N labeled ubiquitin powder sample (10 mg) was dissolved in citrate buffer (20 mM, pH 4.1) to obtain a solution of 35 mg/mL. The protein was then crystallized by adding 2-methyl-2,4-pentanediol (60% final volume).[25, 26] Approximately, 5mg of microcrystalline ubiquitin was loaded into the MAS rotor. We also demonstrate that DUMAS pulse sequences can be applied to more challenging systems such as integral membrane protein phospholamban reconstituted in lipid membrane. Phospholamban (52 residues)[27–29] was expressed recombinantly in E. coli[30] and reconstituted in lipid membranes as previously reported[31].
For the DARR, DQSQ, NCA and NCO pulse sequences (Figures 1S, panels A–C), the initial 1H-13C or 1H-15N CP was optimized using the conventional CP experiment shown in Figure 1S. During SIM-CP, 1H-13C, or 1H-15N CP, the radio frequency (RF) field strengths of 1H, 13C and 15N were 45, 36.7, and 36.7 kHz, respectively, which corresponds to n=1 side band matching condition. The 1H RF amplitude was ramped by 10% with center of the slope equal to 45 kHz. For comparison, the spectra obtained with 1H/13C CP and 13C-detected 1H/13C/15N SIM-CP at various contact times are reported in Figure 2S. Notably, the sensitivity in the 13C spectral region between 0 and 80 ppm is nearly identical for both CP and SIM-CP at all contact times. The 13C sensitivity in the carbonyl region of 13C spectrum (Figure 3S) is 5–15 % higher for the spectrum acquired with CP. However, the carbonyl region of 13C-13C correlation spectra is often neglected due to inherent low sensitivity and resolution. The comparison of 1H/15N CP and 15N detected 1H/13C/15N SIM-CP spectra at various contact times (Figure 4S) shows a sensitivity loss of ~12–23 % for SIM-CP. The integrated peak intensities from the spectra (Figures 2S and 4S) as function of the contact time are plotted in Figure 5S. Note that we have also tested CP and SIM-CP experiments on U-13C,15N N-acetyl Valine-Leucine dipeptide powder. Indeed, we obtained relative sensitivities similar to those obtained for microcrystalline ubiquitin and phospholamban in lipids. Although it is difficult to derive a theoretical (quantitative) expression for SIM-CP, it should be noted that the rate of 13C and 15N polarization enhancement versus contact time is similar for both CP and SIM-CP experiments (Figures 5S). The latter indicates that the spin dynamics for CP and SIM-CP are qualitatively similar. Since only a moderate loss of sensitivity is observed for 15N spectra (10–23%), we deduce that more polarization from the 1H bath to the heteronuclei is transferred during SIM-CP compared to double resonance CP (1H-13C or 1H-15N). In fact Pines and co-workers [17] showed that more 1H polarization can be transferred using multiple contact periods of 1H-13C CP. For SIM-CP, the 13C and 15N spin lock periods do not have to be identical. For example longer 13C (or 15N) spin lock period can be used in SIM-CP to improve the sensitivity of dynamic regions of the biopolymers or for side chain detection. For the ubiquitin sample, we found that contact times of 400 and 600 µs give optimum sensitivity for 1H-13C and 1H-15N CP, respectively (Figures 2S and 4S); whereas 400 µs was optimal for SIM-CP. For PLN, we used CP contact times of 300 and 500 µs for 1H-13C and 1H-15N CP respectively, whereas 300 µs for SIM-CP. With the exception of the contact time, all of the other experimental parameters used for DUMAS were identical to the conventional pulse sequences. Moreover, we tested the decay of 15N magnetization stored along the z-axis after SIM-CP. Indeed, we found that the magnetization decay is negligible during the first 13C-edited acquisition (Figure 6S).
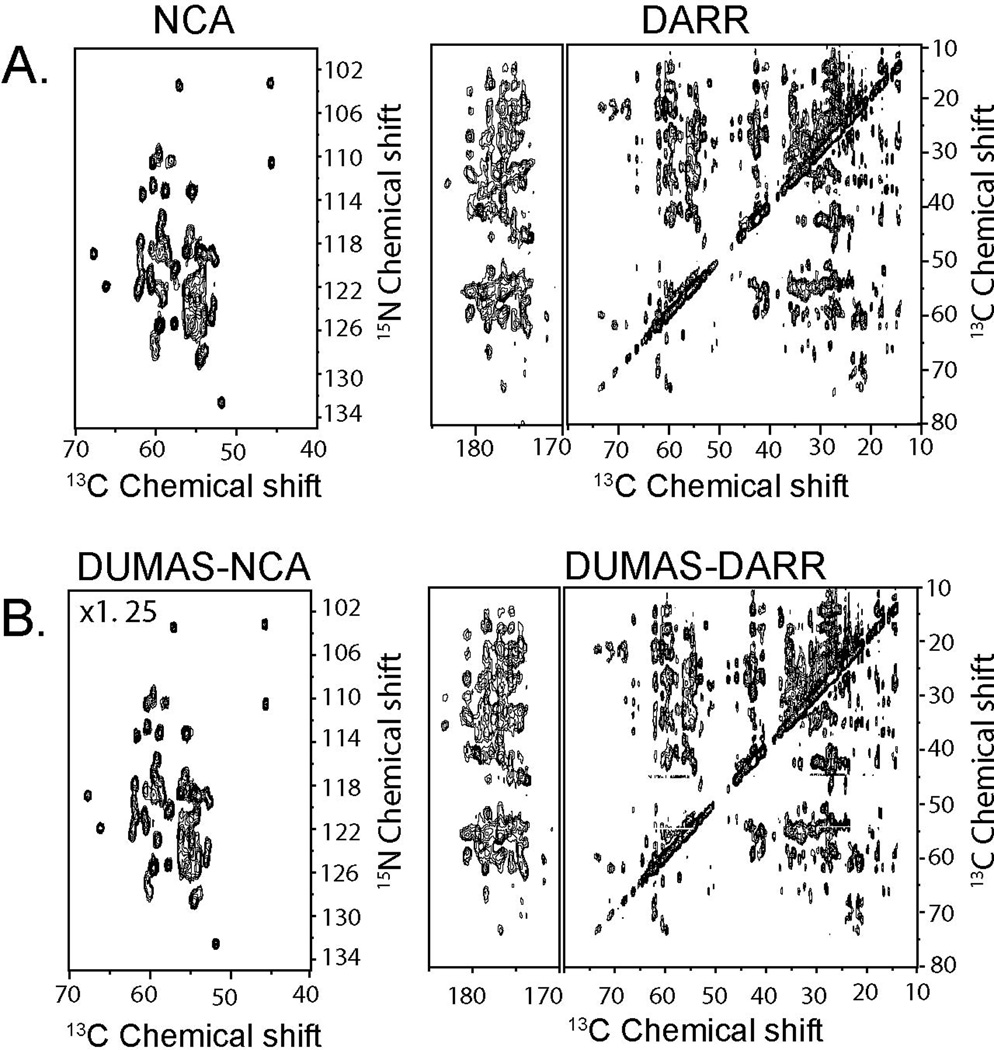
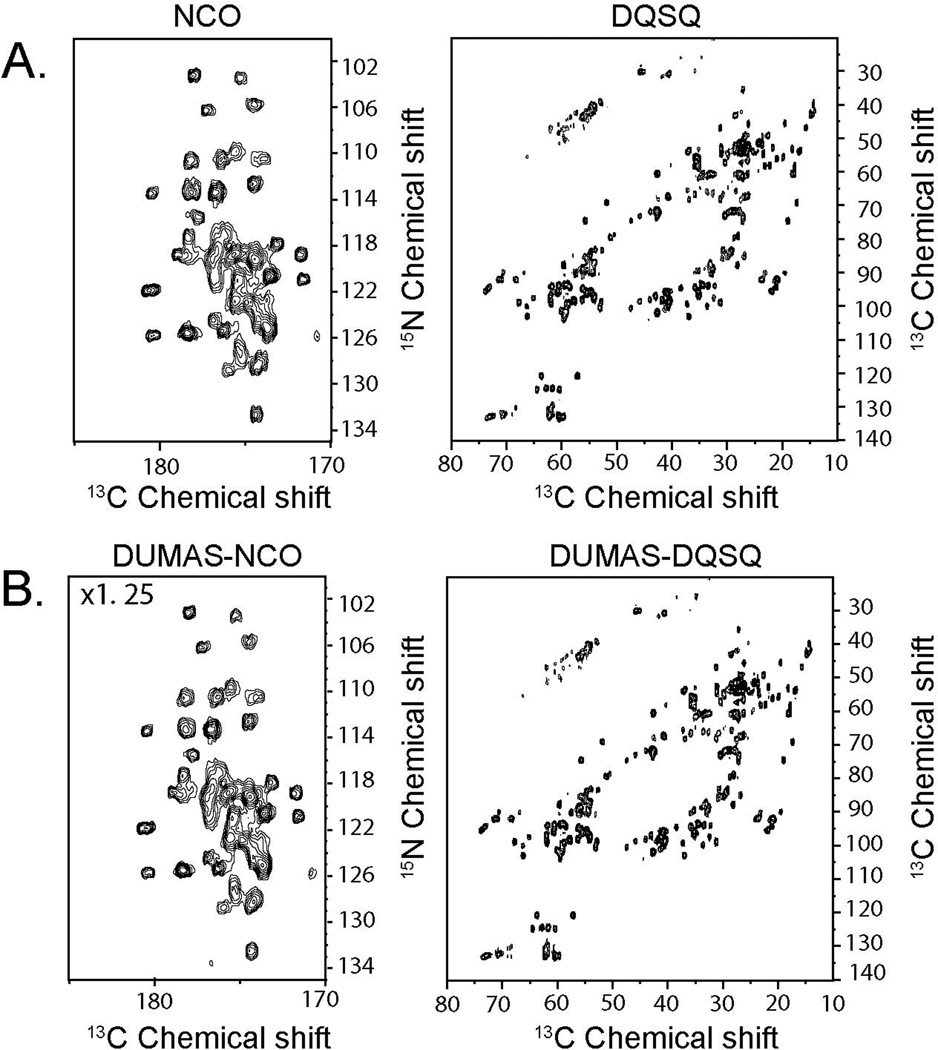
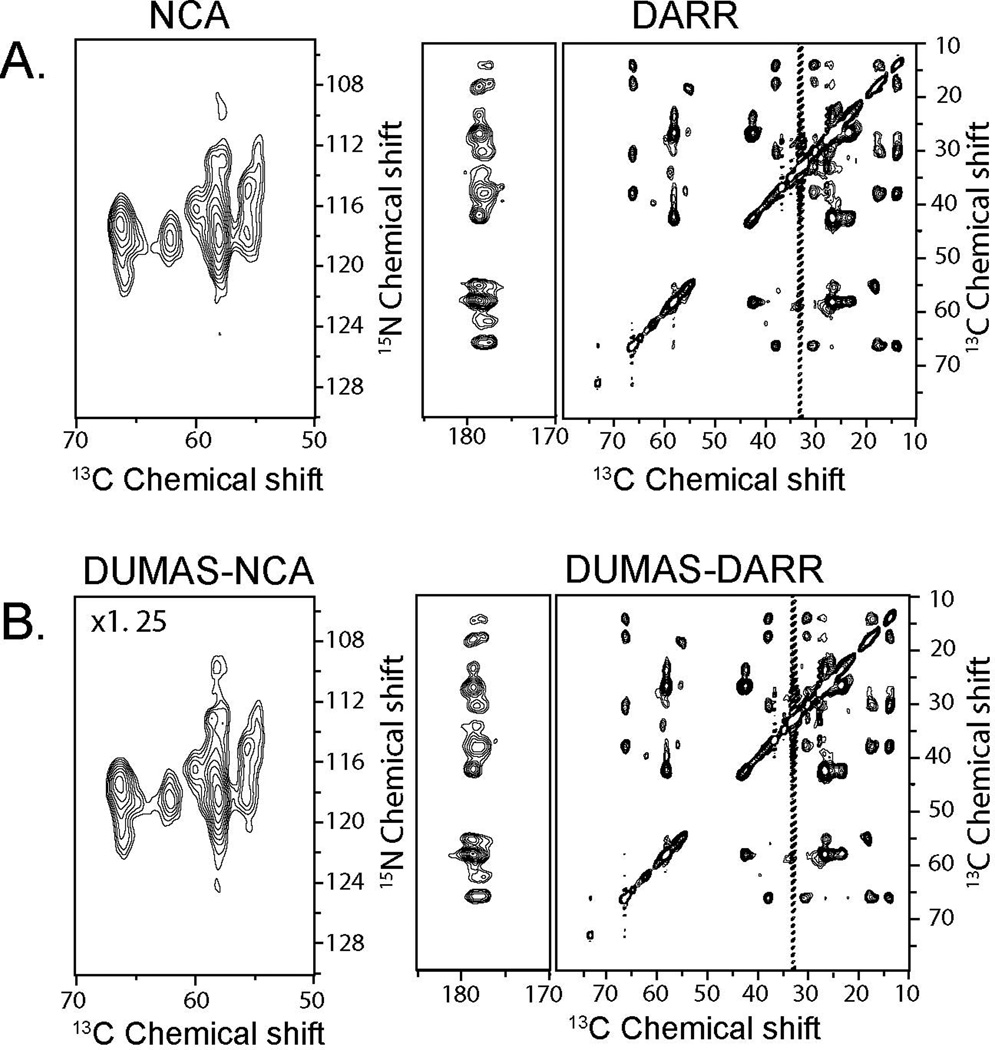
Figure 2A shows the NCA and DARR spectra recorded using conventional 2D pulse sequences (Figure 1S). The total experimental time for both spectra was 16.03 hours. Figure 2B shows the corresponding spectra recorded simultaneously using the DUMAS-DARR-NCA experiment. The total experimental time was 8.12 hours. As expected, the sensitivity of DUMAS-DARR is identical to the conventional DARR, whereas the sensitivity of DUMAS-NCA is about 80% of the NCA spectrum, which is due to the implementation of the SIM-CP. A similar situation is presented for the NCO and DQSQ spectra. Figure 3A shows the two spectra acquired with the conventional methods for a total acquisition time of 15.57 hours. Figure 3B shows the corresponding spectra acquired simultaneously with DUMAS-DQSQ-NCO with a total experimental time of 8.06 hours, which is roughly ½ the time spent with conventional methods. As for the NCA, the sensitivity loss for the DUMAS-NCO is approximately 20%, while the DUMAS-DQSQ spectrum is similar to the conventional DQSQ. Figure 4 shows the application of DUMAS on U-13C,15N labeled membrane protein phospholamban. Each of the conventional spectra DARR and NCA were recorded in ~16 hours, while DUMAS spectra were acquired simultaneously in ~16 hours. These results demonstrate that the DUMAS is extendable to integral membrane proteins as well.
Figure 2.
(A) 2D NCA and DARR spectra of U-13C,15N-labeled ubiquitin acquired using conventional methods (Figure 1S). (B) NCA and DARR spectra acquired simultaneously using DUMAS-DARR-NCA pulse scheme. DARR and DUMAS-DARR are plotted with the identical noise level. DUMAS-NCA is plotted at 1.25 times the signal intensity of NCA to account for 20% signal loss of 15N during SIM-CP.
Figure 3.
(A) 2D NCO and DQSQ spectra acquired using conventional methods (Figure 1S). (B) NCO and DQSQ spectra acquired simultaneously using DUMAS-DQSQ-NCO. DQSQ and DUMAS-DQSQ are drawn at the same noise level. To account for 20 % signal loss of 15N during SIM-CP, DUMAS-NCO is plotted with 1.25 times higher scale compared to NCO.
Figure 4.
(A) 2D NCA and DARR spectra of U-13C,15N-labeled phospholamban reconstituted in lipid membranes acquired using conventional methods (Figure 1S). (B) NCA and DARR spectra acquired simultaneously using DUMAS-DARR-NCA pulse scheme. As for the previous spectra, DARR and DUMAS-DARR are plotted with the identical noise level. DUMAS-NCA is plotted at 1.25 times the signal intensity of NCA to account for 20% signal loss of 15N during SIM-CP.
Note that for the DUMAS experiments, the parameters for the indirect acquisition of 13C and 15N are such that ni(t1’)∙ nt(t1’) = ni(t1”)∙nt(t1”) according to Table S1. In general, the optimum number of scans and increments (total number of 1D experiments) depend on the nature of 13C- and 15N-edited experiments. Since the 13C- and 15N- edited blocks are in different time periods, DUMAS gives the flexibility to acquire an insensitive 13C-edited experiment using a greater number of scans, and multiple (two or more) 15N-edited 2D experiments, or vice versa. In other words, a longer 13C-edited experiment can be combined with multiple 15N edited experiments, or vice versa.
In the past years, there has been a consistent effort to make the best out of polarization for both solution and solid-state NMR experiments[32–35]. An approach involves the recovery of discarded coherences to acquire an additional experiment[36–38] or obtain sensitivity enhancement of the NMR signals.[39–43] Another example is the simultaneous acquisition of 13C- and 15N-edited experiments in liquid-state NMR using single acquisition on the 1H channel[44]. In the latter experiment, the evolution of 13C and 15N nuclear spins is coupled to 1H spins through two-spin operators, limiting its performance. In ssNMR spectroscopy, 15N nuclei have long T1 relaxation values in the order of few hundred milliseconds. For the first time, we utilized this unique property in combination with a SIM-CP scheme to exploit an untapped source of polarization and generate multiple experiments simultaneously. More importantly, DUMAS can be applied in combination with several sensitivity and resolution enhancement methods. For instance, it can benefit from protein perdeuteration[45]. To this extent, it has been shown that the values of 15N T1 for perdeuterated protein samples are not significantly different from the protonated ones[46]. A recent paper by Oschkinat and co-workers[47] shows that triple CP 1H/2H/13C is substantially more sensitive than conventional 1H/13C CP. Based on this work, we can anticipate that quadruple 1H/2H/13C/15N CP using four channel probe with DUMAS acquisition will further enhance the sensitivity of these experiments.
DUMAS can also be used in concert with paramagnetic relaxation enhancements methods[13], obtaining faster data acquisition. In fact, it has been shown that for PRE samples the loss of 15N z-magnetization in the absence of 1H decoupling is less than ~10 % for 100 ms[48]. Therefore, DUMAS can also be successfully applied in combination with PRE enhancement [49, 50].
Another recent improvement for sensitivity enhancement of ssNMR is the implementation of DNP[14–16, 51]. This technique boosts the signal-to-noise ratio (S/N) of NMR spectra taking advantage of the polarization transfer of electronic spins to nuclear spins, doping the samples with nitroxide spin labels. If DNP is applicable to the sample under analysis, DUMAS can be coupled with methods to further speed up data acquisition and push the sensitivity limits of MAS ssNMR.
In conclusion, we introduce a novel approach for the simultaneous acquisition of 13C- and 15N-edited multidimensional MAS ssNMR experiments without additional hardware. Multidimensional DUMAS experiments are demonstrated on microcrystalline ubiquitin as well as PLN in lipid vesicles. The DUMAS scheme opens up new avenues for reducing the acquisition time for multi-dimensional experiments for biomolecular ssNMR by combining the sensitivity and resolution advancements obtained in the past few years. We anticipate that the combination of DUMAS with dynamic nuclear polarization[15, 16, 51], proton detection[8, 52], PRE and multiple receivers,[53] will further increase sensitivity, resolution as well as the number of experiments that can be acquired simultaneously.
Experimental Section
All of the NMR experiments were performed using a 700 MHz VNMRS spectrometer (Agilent Technologies). The spectrometer wasequipped with a 3.2 mm bioMAS scroll coil probe with reduced RF heating. [11] A spinning rate (ωr) of 8.333 kHz was used for all of the experiments. Temperature was held constant at 5 °C. Further details on data acquisition are provided in the Supporting Information.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM64742 and GM072701). We acknowledge M. Gustavsson, K. Mote, and F. Chao for preparing the samples and Dave Rice (Agilent) for helpful discussions.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org
References
- 1.McDermott A. Annu. Rev. Biophys. 2009;38:385–403. doi: 10.1146/annurev.biophys.050708.133719. [DOI] [PubMed] [Google Scholar]
- 2.Renault M, Cukkemane A, Baldus M. Angew. Chem. Int. Ed Engl. 2010;49:8346–8357. doi: 10.1002/anie.201002823. [DOI] [PubMed] [Google Scholar]
- 3.Renault M, Bos MP, Tommassen J, Baldus M. J. Am. Chem. Soc. 2011;133:4175–4177. doi: 10.1021/ja109469c. [DOI] [PubMed] [Google Scholar]
- 4.Tycko R. Annu. Rev. Phys. Chem. 2011;62:279–299. doi: 10.1146/annurev-physchem-032210-103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldus M. Progr NMR Spectr. 2002;41:1–47. [Google Scholar]
- 6.Griffin RG. Nat. Struct. Biol. 1998;5(Suppl):508–512. doi: 10.1038/749. [DOI] [PubMed] [Google Scholar]
- 7.Wylie BJ, Rienstra CM. J. Chem. Phys. 2008;128 doi: 10.1063/1.2834735. 052207. [DOI] [PubMed] [Google Scholar]
- 8.Linser R, Dasari M, Hiller M, Higman V, Fink U, Lopez Del Amo JM, Markovic S, Handel L, Kessler B, Schmieder P, Oesterhelt D, Oschkinat H, Reif B. Angew. Chem. Int. Ed Engl. 2011;50:4508–4512. doi: 10.1002/anie.201008244. [DOI] [PubMed] [Google Scholar]
- 9.Schuetz A, Wasmer C, Habenstein B, Verel R, Greenwald J, Riek R, Bockmann A, Meier BH. Chembiochem. 2010;11:1543–1551. doi: 10.1002/cbic.201000124. [DOI] [PubMed] [Google Scholar]
- 10.Franks WT, Kloepper KD, Wylie BJ, Rienstra CM. J. Biomol. NMR. 2007;39:107–131. doi: 10.1007/s10858-007-9179-1. [DOI] [PubMed] [Google Scholar]
- 11.Stringer JA, Bronnimann CE, Mullen CG, Zhou DH, Stellfox SA, Li Y, Williams EH, Rienstra CM. J. Magn. Reson. 2005;173:40–48. doi: 10.1016/j.jmr.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Lian L, Middleton DA. Progr NMR Spectr. 2001;39:171–190. [Google Scholar]
- 13.Wickramasinghe NP, Parthasarathy S, Jones CR, Bhardwaj C, Long F, Kotecha M, Mehboob S, Fung LW, Past J, Samoson A, Ishii Y. Nat. Methods. 2009;6:215–218. doi: 10.1038/nmeth.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maly T, Debelouchina GT, Bajaj VS, Hu KN, Joo CG, Mak-Jurkauskas ML, Sirigiri JR, van der Wel PC, Herzfeld J, Temkin RJ, Griffin RG. J. Chem. Phys. 2008;128 052211. [Google Scholar]
- 15.Matsuki Y, Maly T, Ouari O, Karoui H, Le Moigne F, Rizzato E, Lyubenova S, Herzfeld J, Prisner T, Tordo P, Griffin RG. Angew. Chem. Int. Ed Engl. 2009;48:4996–5000. doi: 10.1002/anie.200805940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbey U, Franks WT, Linden A, Lange S, Griffin RG, van Rossum BJ, Oschkinat H. Angew. Chem. Int. Ed Engl. 2010;49:7803–7806. doi: 10.1002/anie.201002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pines A, Gibby MG, Waugh JS. Journal of Chemical Physics. 1973;59:569–590. [Google Scholar]
- 18.Hartmann SR, Hahn EL. Phys. Rev. 1962;128:2042. [Google Scholar]
- 19.Akbey U, Camponeschi F, van Rossum BJ, Oschkinat H. Chemphyschem. 2011 doi: 10.1002/cphc.201100084. [DOI] [PubMed] [Google Scholar]
- 20.Giraud N, Bockmann A, Lesage A, Penin F, Blackledge M, Emsley L. J. Am. Chem. Soc. 2004;126:11422–11423. doi: 10.1021/ja046578g. [DOI] [PubMed] [Google Scholar]
- 21.Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. J Chem Phys. 1995;103:6951–6958. [Google Scholar]
- 22.Takegoshi K, Nakamura S, Terao T. Chem. Phys. Lett. 2001;344:631. [Google Scholar]
- 23.Baldus M, Petkova AT, Herzfeld JH, Griffin RG. Mol. Phys. 1998;95:1197–1207. [Google Scholar]
- 24.Hohwy M, Rienstra CM, Jaroniec CP, Griffin RG. J Chem Phys. 1999;110:7983–7992. [Google Scholar]
- 25.Zech SG, Wand AJ, McDermott AE. J. Am. Chem. Soc. 2005;127:8618–8626. doi: 10.1021/ja0503128. [DOI] [PubMed] [Google Scholar]
- 26.Igumenova TI, McDermott AE, Zilm KW, Martin RW, Paulson EK, Wand AJ. J. Am. Chem. Soc. 2004;126:6720–6727. doi: 10.1021/ja030547o. [DOI] [PubMed] [Google Scholar]
- 27.Traaseth NJ, Ha KN, Verardi R, Shi L, Buffy JJ, Masterson LR, Veglia G. Biochemistry. 2008;47:3–13. doi: 10.1021/bi701668v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traaseth NJ, Shi L, Verardi R, Mullen DG, Barany G, Veglia G. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10165–10170. doi: 10.1073/pnas.0904290106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verardi R, Shi L, Traaseth NJ, Walsh N, Veglia G. Proc Natl Acad Sci U S A. 2011;108:9101–9106. doi: 10.1073/pnas.1016535108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buck B, Zamoon J, Kirby TL, DeSilva TM, Karim C, Thomas D, Veglia G. Protein Expr Purif. 2003;30:253–261. doi: 10.1016/s1046-5928(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 31.Gustavsson M, Traaseth NT, Veglia G. BBA. 2011 [Google Scholar]
- 32.Freeman R, Kupce E. Top. Curr. Chem. 2011 doi: 10.1007/128_2010_103. [DOI] [PubMed] [Google Scholar]
- 33.Kupce R, Freeman E. J. Magn. Reson. 2003;163:56–63. doi: 10.1016/s1090-7807(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 34.Frydman L, Lupulescu A, Scherf T. J. Am. Chem. Soc. 2003;125:9204–9217. doi: 10.1021/ja030055b. [DOI] [PubMed] [Google Scholar]
- 35.Atreya HS, Szyperski T. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9642–9647. doi: 10.1073/pnas.0403529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuchi M, Takegoshi K. Solid State Nucl. Magn. Reson. 2008;34:151–153. doi: 10.1016/j.ssnmr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Kupce E, Kay LE, Freeman R. J. Am. Chem. Soc. 2010;132:18008–18011. doi: 10.1021/ja1080025. [DOI] [PubMed] [Google Scholar]
- 38.Gopinath T, Mote K, Veglia G. 2011 (Under Review). [Google Scholar]
- 39.Kay LE, Keifer E, Saarinen T. J. Am. Chem. Soc. 1992;114:10663–10665. [Google Scholar]
- 40.Tycko R. Chemphyschem. 2004;5:863–868. doi: 10.1002/cphc.200301208. [DOI] [PubMed] [Google Scholar]
- 41.Gopinath T, Veglia G. J. Am. Chem. Soc. 2009;131:5754–5756. doi: 10.1021/ja900096d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gopinath T, Verardi R, Traaseth NJ, Veglia G. J Phys Chem B. 2010;114:5089–5095. doi: 10.1021/jp909778a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gopinath T, Traaseth NJ, Mote K, Veglia G. J Am Chem Soc. 2010;132:5357–5763. doi: 10.1021/ja905991s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pascal SM, Muhandiram DR, Yamazaki T, Forman-Kay JD, Kay LE. J Magn Reson. 2004;103:197–201. [Google Scholar]
- 45.Crespi HL, Katz JJ. Methods Enzymol. 1972;26 PtC:627–637. doi: 10.1016/s0076-6879(72)26029-3. [DOI] [PubMed] [Google Scholar]
- 46.Chevelkov V, Diehl A, Reif B. J. Chem. Phys. 2008;128 doi: 10.1063/1.2819311. 052316. [DOI] [PubMed] [Google Scholar]
- 47.Akbey U, Camponeschi F, van Rossum BJ, Oschkinat H. Chemphyschem. 2011 doi: 10.1002/cphc.201100084. [DOI] [PubMed] [Google Scholar]
- 48.Nadaud PS, Helmus JJ, Sengupta I, Jaroniec CP. J. Am. Chem. Soc. 2010;132:9561–9563. doi: 10.1021/ja103545e. [DOI] [PubMed] [Google Scholar]
- 49.Nadaud PS, Helmus JJ, Hofer N, Jaroniec CP. J. Am. Chem. Soc. 2007;129:7502–7503. doi: 10.1021/ja072349t. [DOI] [PubMed] [Google Scholar]
- 50.Nadaud PS, Helmus JJ, Kall SL, Jaroniec CP. J. Am. Chem. Soc. 2009;131:8108–8120. doi: 10.1021/ja900224z. [DOI] [PubMed] [Google Scholar]
- 51.Matsuki Y, Eddy MT, Griffin RG, Herzfeld J. Angew. Chem. Int. Ed Engl. 2010;49:9215–9218. doi: 10.1002/anie.201003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huber M, Hiller S, Schanda P, Ernst M, Bockmann A, Verel R, Meier BH. Chemphyschem. 2011;12:915–918. doi: 10.1002/cphc.201100062. [DOI] [PubMed] [Google Scholar]
- 53.Kupce E, Freeman R, John BK. J. Am. Chem. Soc. 2006;128:9606–9607. doi: 10.1021/ja0634876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.