Abstract
Objectives
There is still considerable debate and controversy about whether EEG can be used as a robust clinical tool for assessment of mild traumatic brain injury (MTBI). Nonhomogeneous subject populations, inaccurate assessment of severity of brain injury, time since injury when EEG testing was performed, the lack of EEG research conducted serially and in conjunction with other behavioral measures as injury evolves over time may contribute to the existing controversies. In this study, we implemented a concussion assessment protocol combining a series of EEG and balance measures throughout one year post-injury to document the efficacy of EEG and balance measures as relate to differential recovery of patients suffering from MTBI.
Methods
Three hundred and eighty subjects at risk for MTBI were initially recruited for baseline testing. Forty nine from this initial subjects pool subsequently suffered a single episode of concussive blow and were tested on day 7, 15, 30 days, 6 months and 12 months post-injury. EEGs were recorded while sitting, standing on the force plate and then on a foam base of support with eyes open/closed conditions. EEG alpha power (8–12 Hz) and its percent suppression from sitting to standing postures were computed. The center of pressure (COP) measures were obtained from the force platform and analyzed for eyes open and eyes closed conditions.
Results
Percent alpha power suppression from sitting to standing postural conditions significantly increased in MTBI subjects shortly after the injury (p < 0.01). Percent alpha power suppression significantly correlated with increased area of COP during standing posture with eye closed (r2 = 0.53, p < 0.01). The magnitude of alpha power suppression predicted the rate of recovery of this measure in sub-acute and chronic phases of injury (r2 = 0.609, p < 0.01). Finally, 85% of MTBI subjects who showed more than 20% of alpha power suppression in the acute phase of injury did not return to pre-injury status up to 12 months post-injury.
Conclusions
The efficacy of serially implemented EEG measures in conjunction with balance assessment over the course of MTBI evolution to document residual cerebral dysfunction was demonstrated. Specifically, alteration of EEG alpha power dynamics in conjunction with balance data in the acute phase of injury with respect to baseline measures may predict the rate of recovery from a single concussive blow.
Significance
Neurophysiological measures are excellent tools to assess the status and prognosis of patients with MTBI.
Keywords: EEG, Mild traumatic brain injury, Balance, Center of pressure (COP) area
1. Introduction
Mild traumatic brain injury (MTBI), most commonly known as concussion, with an annual reported incidence of 1.4 million cases in the United States alone (Bazarian et al., 2005; Langlois et al., 2006) accounts for 80% of all traumatic brain injuries (Risdall and Menon, 2011; Ruff, 2011). There are a number of immediate physical, cognitive, and emotional symptoms that arise from MTBI that include headache, dizziness, unsteady gait, nausea, slurred speech, poor concentration, and short-term memory loss (McCrory et al., 2009). For the most part sports-related typical MTBI recovery is rapid, with spontaneous symptom resolution within 10 days post-injury (Webbe and Barth, 2003; Makdissi et al., 2010); yet upwards to 15–38% of individuals suffering from MTBI have symptoms that persist beyond 3 months post-injury (Broglio et al., 2007; Witt et al., 2010; Sedney et al., 2011). Prolonged recovery time is referred to as atypical MTBI, that may be classified as “complicated mild TBI” with post-traumatic amnesia (PTA), EEG abnormalities, pathological reflexes and fractures (Hessen, 2010).
One of the challenges confronting TBI researchers is defining exactly what is meant by ‘mild’ injury. Inconsistencies and scientific disagreements have resulted in varying definitions over the years. Multiple signs and symptoms are commonly used alone or in combination to define such injury, including headache, dizziness, vomiting, feeling dazed, disorientation, and focal neurological deficits (Iverson et al., 2006; Lovell et al., 2006; Lau et al., 2011). Many of these signs and acute characteristics are non-specific and may have multiple causes aside from brain injury that obscures the diagnostic process. There is a suggestion for diagnostic criteria of MTBI to include a spectrum of severity from “uncomplicated” injuries with no signs of neurological damage to more complicated injuries with post-traumatic amnesia (PTA), loss of consciousness (LOC), EEG abnormalities, pathological reflexes and fractures (Hessen, 2010). Accumulated evidence from advanced brain imaging studies (Slobounov et al., 2011; Johnson et al., 2012; Zhang et al., 2010) suggests that most structural and functional abnormalities are present far beyond 10 days post-injury, meaning that majority of MTBI cases might well be classified as complicated and/or atypical injuries. Therefore, it is critically important to link behavioral and clinical symptoms to their underlying sources (neural underpinnings) as injury evolves over time, an approach implemented in this study.
Historically, electroencephalography (EEG) was the first brain imaging assessment tool to demonstrate the alteration of brain functions in subjects suffering from traumatic brain injury (Glaser and Sjaardema, 1940; Jasper et al., 1940; Williams, 1941). Since then, considerable empirical evidence was accumulated indicating both (a) clinical value of EEG in terms of the accuracy of assessment of MTBI and (b) conceptual significance of EEG in enabling the examination of neural substrates underlying neurological, behavioral and neuropsychological alterations in MTBI subjects (see Arciniegas, 2011 for review). In our work, significant reduction of the cortical potential amplitude and concomitant alteration of gamma activity (40 Hz) were observed in MTBI subjects performing force production tasks 3 years post-injury (Slobounov et al., 2002). More recently, we showed a significant reduction of EEG power within theta and delta frequency bands during standing postures in subjects with single and multiple concussions up to 3 years post-injury (Thompson et al., 2005).
Similar to typical course of clinical recovery after MTBI, abnormalities on conventional EEG recording tend to resolve during the first several months post-injury (see Nuwer et al., 2005 for review). EEG assessment could contribute to the development and refinement of differential diagnostic information among subjects with atypical clinical recovery following MTBI. That said, it is important to note that the differences in EEG profiles in subjects showing the typical and atypical functional recovery after MTBI may serve as a starting point from which to begin more fully investigating the neural substrates for differential recovery after traumatic brain injury (Arciniegas, 2011). In our previous pilot research, we reported that excessive alpha power suppression as a function of postural task may be used as a sensitive tool for sub-acute diagnosis of MTBI (Slobounov et al., 2005; Thompson et al., 2005). Accordingly, in this longitudinal study, we combined EEG (alpha power difference during sittting versus standing upright postures) and balance measures (standing still with eyes open and closed) in subjects prior to injury (baseline testing) and serially over one year post-injury to further examine the neural substrates of cerebral brain dysfunctions in MTBI subjects as injury evolves over time.
2. Methods
2.1. Subjects
A total of 380 subjects were initially recruited (baseline testing) for this sports-related concussion study. All subjects were Pennsylvania State University athletes at high risk for traumatic brain injury (collegiate rugby, football, ice hockey and soccer players), aged between 18 and 25 years, male (n = 270, mean age 21.8 years) and female (n = 110, mean age 20.1 years). None of these subjects had reported a concussion history at the time of baseline testing. In this study we included data from 49 subjects (male, n = 31; female, n = 18) from the initial subject pool, who suffered from a single episode of concussion within 6 months after baseline testing. All concussed subjects suffered from grade 1 MTBI (Cantu Data Driven Revised Concussion Grading Guideline, 2006).
Computerized balance and EEG data available for all MTBI subjects, including baseline testing and five consecutive follow-ups within one year post-injury were included in the final analysis. All subjects were clinically asymptomatic on day 7–10 after MTBI and were cleared for sport participation based upon neurological assessments (Co-operative Ataxia Rating Scale, World Federation of Neurology, Trouillas et al., 1997) as well as clinical symptom resolution. According to subjects’ reports, they did not take any medications that may influence neuropsychological, balance and EEG data.
2.2. Neuropsychological (NP) assessments
The neuropsychological tests were administered at baseline testing, on day 7 and day 14 post-injury as standard paper and pencil tests. The subject was instructed to complete the tests as quickly and accurately as possible. The NP testing battery consisted of three segments: Subjective Symptom Rating scale (e.g., Penn State University Standard Concussion Rating Scale) to assess MTBI symptom severity; Symbol Digit Substitution test to assess information processing speed and working memory; trails “B” test to assess information processing speed and scanning ability (Randolph, 2001). The neuropsychological testing component lasted approximately 15 min.
2.3. EEG recording
Subjects were seated and standing upright on the force platform with eyes open and eyes closed in an electrically shielded and dimly lit environment. The continuous EEG was recorded using Ag/AgCl electrodes mounted in a 19-channel spandex Electro-cap (Electro-cap International Inc., Eaton, OH). Conducting gel (Electro-gelTM, Electro-Cap International Inc., Eaton, OH) was injected into each electrode to connect the recording surface of the electrode with the scalp. The electrical activity from the scalp was recorded at 19-sites: FP1, FP2, FZ, F3, F4, F7, F8, CZ, C3, C4, T3, T4, T5, T6, PZ, P3, P4, O1, O2, according to the International 10–20 system (Jasper, 1958). The ground electrode was located 10% anterior to FZ, linked earlobes served as reference and electrode impedances were below 5 kΩ. It should be noted that there is no “gold standard” for reference electrode/sites in EEG studies. It is possible that the type of referencing (i.e., common reference, linked ears, linked mastoid, forehead, virtual reference free montage, etc. commonly reported in EEG literature) may influence topographical distribution of EEG signal. EEG signals were recorded using a programmable DC coupled broadband SynAmps amplifier (NeuroScan, Inc., El Paso, TX.). The EEG signals were amplified (gain 2500, accuracy 0.033/bit) with a recording range set for ±55 mV in the DC to 70-Hz frequency range. The EEG signals were digitized at 1000 Hz using 16-bit analog-to-digital converters.
2.4. EEG data processing and analysis
The EEG data were processed offline using EEGLAB 5.03 software (Delorme and Makeig, 2004) using Matlab open sources toolbox (Mathworks, Natick, MA). Imported data were down sampled to 250 Hz to reduce computing time and epoching from 0 to approximately 4 s and at least 2 min of artifact free EEG signals were subjected to further analysis for each subject. This procedure allows the removal of epochs containing signal values exceeding 3 SD and controls for artifacts such as eye blinks, eye movements, heartbeats etc. In order to reduce the number of independent variables (e.g., the number of possible pairings) and to avoid the loss of statistical power, we collapsed 19 EEG channels into five topographical regions of interest (ROIs): frontal (Fp1, Fp2, Fz, F4, F3, F8, F7), temporal (T3, T4, T5, T6), central (C4, C3, Cz), parietal (Pz, P4, P3) and occipital (O1, O2), similar to Oken and Chiappa (1986).
To compute power spectra during both sitting and standing postures, Fast Fourier Transform (FFT) power calculations were performed within each epoch and then averaged across them for each frequency band (delta, theta, alpha, and beta). To ensure homogeneous data processing, relative power for each frequency band was used instead of absolute power. In this paper we focused on alpha power difference (suppression) as a function of postural task, as the most sensitive EEG rhythm towards manipulation of whole body postural configurations (Slobounov et al., 2005; Thompson et al., 2005) in subjects suffering from MTBI. Also, we focused on this frequency band because alpha power coincides with increased cortico–cortical and cortico–spinal excitation of sensory-motor cortex (Rau et al., 2003) with a spatial topography that is specific to the somatotopic representation of cortex relevant to the movement task (Pfurtscheller et al., 2000).
The percent alpha power suppression from sitting to standing postures was calculated as:
| (1) |
2.5. Postural data acquisition
An Advanced Mechanical Technology, Inc. (AMTI) force platform was used to collect and process the center of pressure (COP) data. Three force components (Fx, Fy and Fz) along with three respective moment components (Mx, My and Mz) were simultaneously measured from the force platform. The signals were amplified through a six-channel AMTI model SGA6–4 amplifier. A maximum gain of 4000 was used, with a low-pass filter of 10.5 Hz. The bridge excitation was set to 10 V. All six channels were factory calibrated. The data were collected with a sample frequency of 90 Hz.
2.6. Postural data analysis
The traditional assessment of postural stability has included the center of pressure motion (COP) along the X and Y axes, COP area, standard deviation (SD), COP velocity and acceleration time series. The center of pressure at each instantaneous time point defined by the sample rate reflecting the degree of postural motion was calculated using the customized software as:
| (2) |
| (3) |
The % change of the area of the center of pressure (COP) two-dimensional area from standing eyes open to standing eyes closed was calculated as:
| (4) |
3. Results
3.1. Neuropsychological testing
None of the subjects reported the symptoms of concussion (i.e., headache, light sensitivity, dizziness, memory and concentration problems, disorientation etc.) on day 7 after the MTBI. As noted before, all MTBI subjects were cleared for sport participation on average 10 days post-injury based upon clinical and neurological symptom resolution.
3.2. EEG data
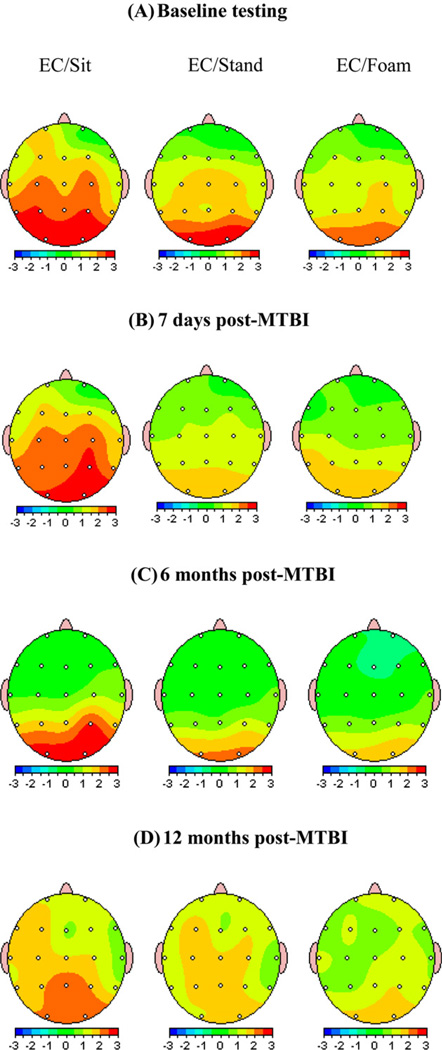
The results from EEG analysis are shown in Fig. 1. At baseline testing, the alpha power suppression at occipital ROI from sitting to standing posture was on average 9.7 ± 3% with slight increase, not exceeding 3–5%, while standing on the foam. On day 7th post-MTBI alpha power suppression from sitting to standing postures increased on average to 33.3 ± 10% with no further change while standing on the foam. T-test revealed significant differences in alpha power suppression on day 7 post-injury in occipital [F = 11.77 (1,48), p < 0.01] and parietal, [F = 8.2 (1,48), p = 0.038] ROIs. There were no significance differences in alpha power while standing on force plate versus on foam neither pre or post-injury (p > 0.05).
Fig. 1.
Two-dimensional plots of grand-average of alpha power at baseline (A) and pre-post injury [7 days (B), 6 months (C) and 12 months (D) post-MTBI] during sitting, standing on the force plate and standing on the foam conditions. Note a significant suppression of alpha power at occipital and parietal ROI during standing posture both on force plate and on the foam after MTBI on day 7 post-injury. Also note (C and D) MTBI subjects who showed more than 20% of alpha power suppression in the acute phase of injury did not return to pre-injury status up to 12 months post-injury. Visually, no significant differences in alpha power were noticed after MTBI while standing on the force plate or on the foam on day 7, 6 months and 12 months post-injury.
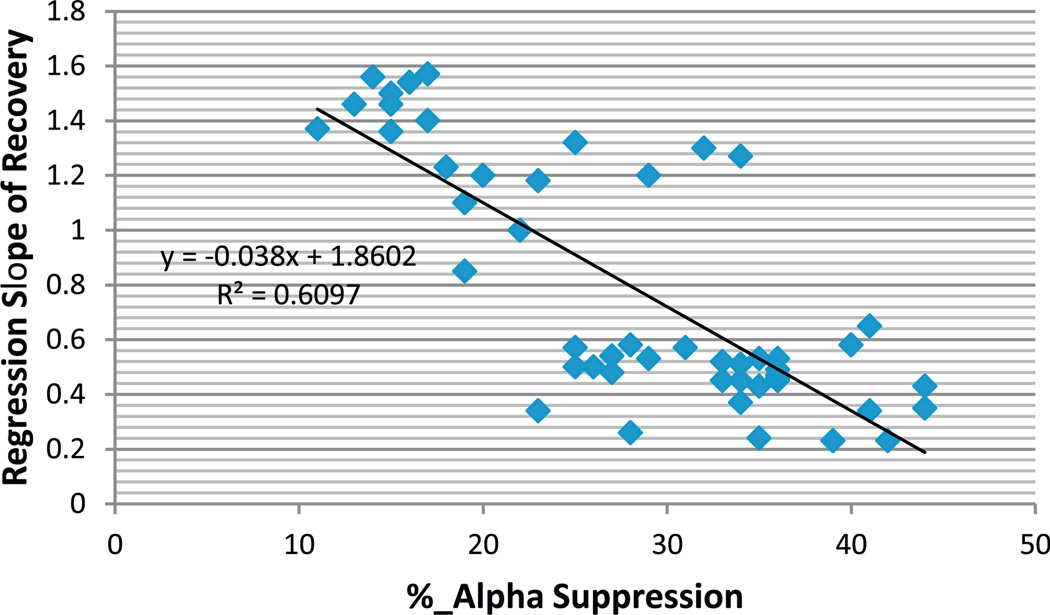
As noted before (see Section 2), the changes of alpha power suppression for all MTBI subjects under study were monitored as injury evolved over the year time frame. The relationship between percent alpha power suppression and rate of “recovery” of this index over the course of injury is shown in Fig. 2. As can be seen from this figure, the % of alpha power suppression at the occipital ROI highly correlated with the rate of recovery (r2 = 0.609, p < 0.01). Further analysis revealed that MTBI subjects, who experienced 20% and higher alpha suppression from sitting to standing postures in the acute phase of injury (on day 7th post-MTBI) did not return to pre-injury status on this EEG measure during the year of observation. It should be noted that no differences were observed as a function of testing day (p > 0.05) in NV group, n = 15) within 6 months post-baseline testing.
Fig. 2.
The relationship between % alpha power suppression in occipital ROI from sitting to standing posture with eyes closed at day 7th post-concussion and rate of recovery (regression slope, data from testing on day 7, 15, 30th, 6 months and 12 months post-injury) of this index within 12 months post-injury. Note, the larger % alpha power suppression in acute phase of injury the longer it takes to recover.
3.3. Balance data
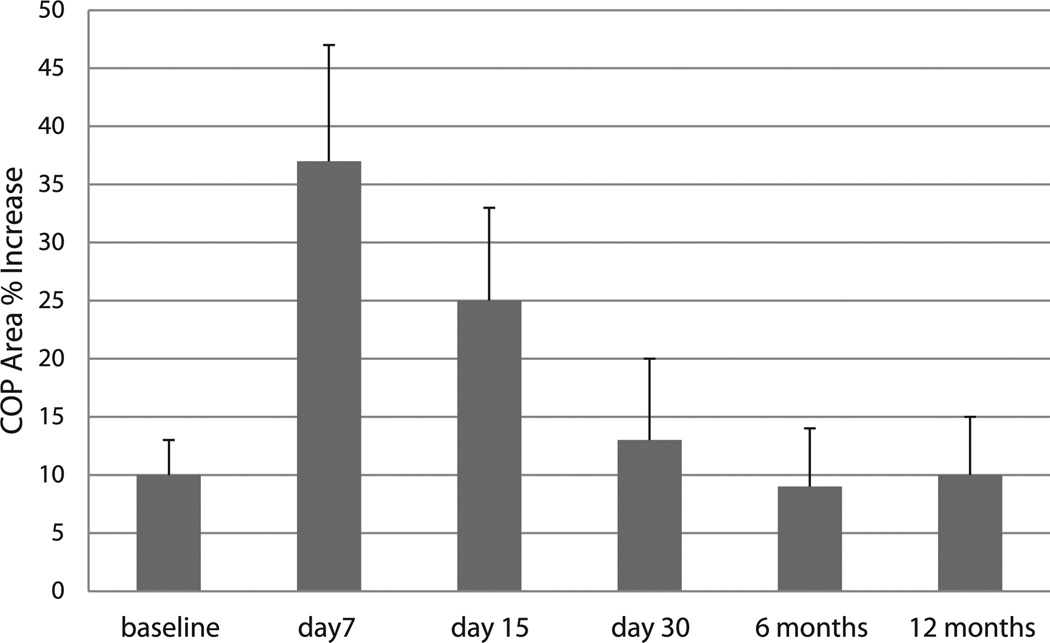
Percent increase of the center of pressure (COP) area from standing with eyes open to standing with eyes closed postures as a function of testing session is shown in Fig. 3. Clearly, there was non-significant (approximately 10%) increase of the COP area during standing with eyes closed in all subjects at baseline testing (p > 0.05). However, this % increase was highly significant at day 7th and 15th post-injury [F = 13.62 (1,48), p < 0.01]. It should be noted that this balance index reversed to baseline level at day 30th post-injury testing (p > 0.05), regardless of initial increment at day 7th post-injury.
Fig. 3.
The center of pressure (COP) area % increase from standing with eyes open to eyes closed postures as injury evolves. * Indicates significant differences with respect to baseline values (p < 0.01).
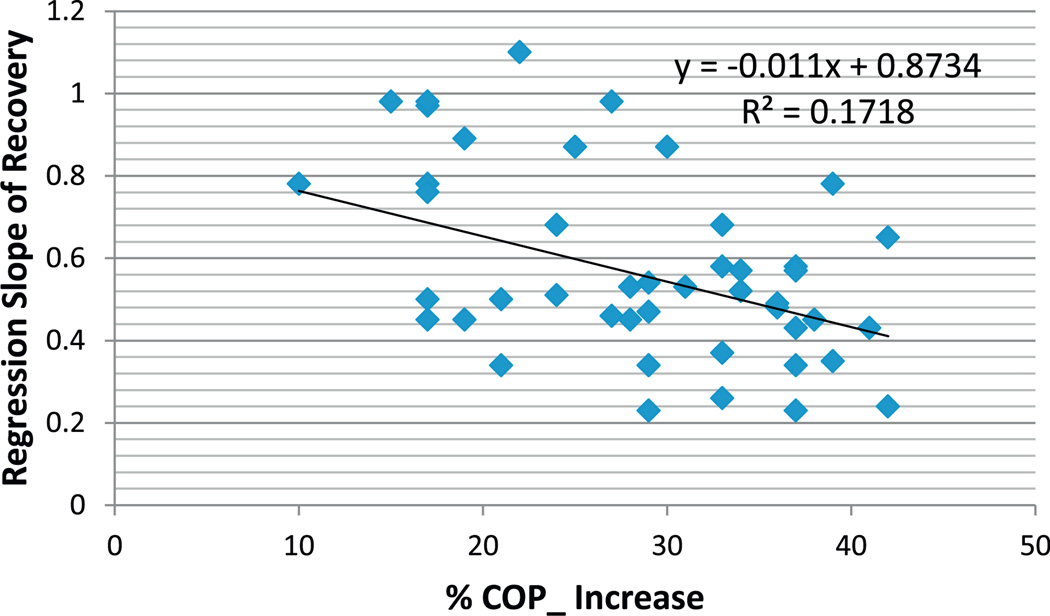
Unlike EEG data, the magnitude of increase of the COP area did not correlate with the rate of recovery of this index over one year time frame, most likely due to the fact that no significant differences with respect to baseline values were observed already at day 30th post-injury (see Fig. 4).
Fig. 4.
The relationship between % increase of the center of pressure area from standing with eyes open to eyes closed postures at day 7th post-concussion and rate of recovery (regression slope, data from testing on day 7, 15, 30th, 6 months and 12 months post-injury) of this index within 12 months post-injury. Note, the larger % increase of COP area in acute phase of injury did not predict the recovery rate.
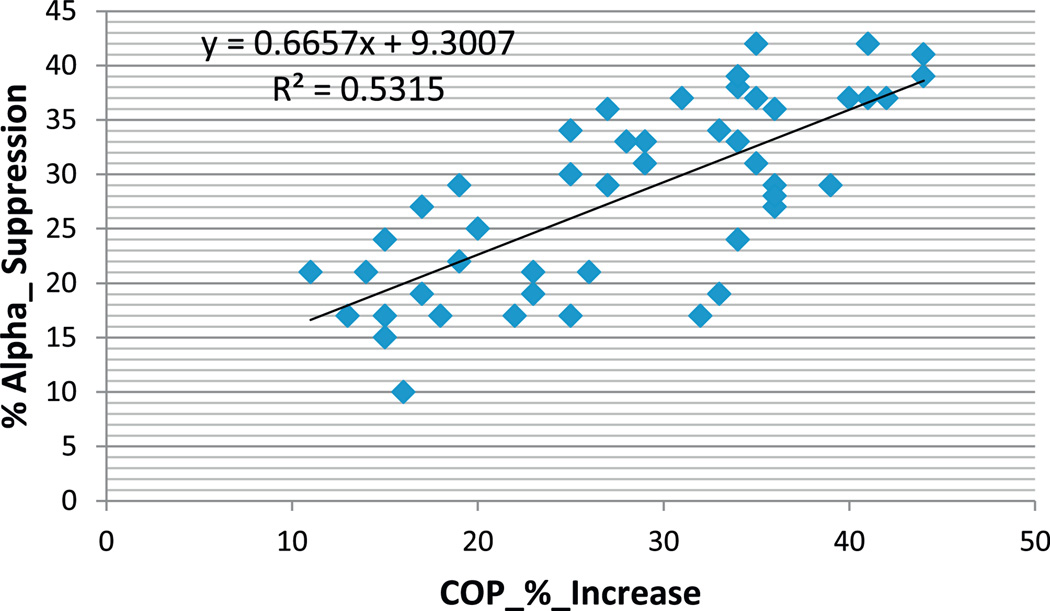
Additionally, there was a significant correlation (r2 = 0.53, p < 0.01) between % increase of the center of pressure area and % EEG alpha power suppression at day 7th post-injury. In other words, the larger % alpha suppression from sitting to standing postures, the less stable posture was prominent in acute phase of injury (see Fig. 5). This effect was not present at day 15th and 30th post-injury (r2 = 0.12, p > 0.01).
Fig. 5.
The relationship between EEG alpha power % suppression from sitting to standing postures with closed eyes and the center of pressure (COP) area % increase from eyes open to eyes closed postures at day 7th post-MTBI.
4. Discussion
Conventional wisdom holds that typical recovery following a sports-related mild traumatic brain injury (MTBI) is rapid, with most acute clinical symptoms resolving within hours, and with a person symptom-free by around 10 days post-injury. However, there is growing evidence of an atypical evolution of MTBI whereby physical, neurocognitive, and emotional symptoms persist months or even years post-injury. There are numerous factors that could influence the differential evolution of MTBI, including inaccurate diagnosis of the severity of brain injury leading to improper prognosis and management, i.e., premature return-to-play. Therefore, discovering the differential dynamics of cerebral dysfunctions and their underlying neural mechanisms in well-controlled multi-modal experimental settings may have dramatic effects on the field of concussion research. These results should also contribute to the understanding of numerous puzzles, such as why “there is no concussion alike in terms of its… symptoms and unpredictable symptom resolution” (Cantu, 2006).
Recently, Arciniegas (2011) outlined the major limiting factors contributing the limited capacity of EEG measures in clinical assessment of MTBI, including: (a) the lack of control for subjects’ homogeneity, (b) lack of research when EEG assessment was performed immediately after and serially over the first year of injury, (c) poor experimental designs when EEG data are collected independently from performance-based assessment of patients’ functional status and (d) different time frame since injury when EEG measures were obtained. To address at least some of these limiting factors, in this study we serially examined dynamics of balance abnormalities (i.e., a common symptom of MTBI at least in acute phase of injury, Guskiewicz, 2003) in MTBI subjects with a history of a single episode of concussive blow and associated changes in the brain electrical activity (i.e., EEG in the frequency domain during sitting and standing postures) with respect to pre-injury status and as MTBI evolved over the first year post-injury.
There are two major findings of interest. First, the magnitude of alpha power suppression in occipital ROI during standing versus sitting postures with respect to baseline measures may predict the rate of return to pre-injury status. The larger percent of alpha suppression was recorded shortly after the injury, the longer it takes to recover over one year time frame. It should be noted that this EEG measure obtained in acute phase of injury was highly correlated with abnormal balance measures. Specifically, we consistently observed an increased area of the center of pressure (COP), especially during the eyes closed condition that has been traditionally considered as an index of postural instability (Barin, 1992; Goldie et al., 1989; Slobounov and Newell, 1994).
Converging evidence indicates that the amplitude of alpha-band oscillations is negatively correlated with cortical excitability and task performance in sensory cortices (see Palva and Palva, 2011 for review). Also, the alpha amplitude decrease is often taken to reflect a state of increased neuronal excitability/disinhibition (Pfurtscheller, 2003), or task-relevant gating when active processing of information is facilitated (Klimesch et al., 2007). Along the line of these views, an increased cerebral effort and/or attentional demand (Gevins et al., 1997), due to increased postural task difficulty, might be required for MTBI patients to retain stable posture with eyes closed, and this may be responsible for the increased percent of alpha suppression (e.g., decrease of relative alpha power). Similarly, an inverse relationship between alpha power and task difficulty has been reported, and interpreted as alpha sensitivity to task difficulty, cortical idling or disengagement (Buzsaki, 2006). Standing on foam should be more difficult than on a firm surface, but we did not find this additional test productive, perhaps because the flat surface itself already produces a strong increase in effort.
Thus, the proposed EEG measure (e.g., % alpha power suppression in standing versus sitting postures) appeared to be a sensitive tool for detecting residual cerebral dysfunction in MTBI. Previously, reduced alpha power in posterior cortical regions, which was attributed to mechanical head injury, has been reported by Thatcher et al. (1989). That said, it should be noted that a comprehensive report concluded that no clear EEG features are unique to mild TBI, especially later after the injury (Nuwer et al., 2005). Overall, an approach combining EEG and balance measures as injury evolves may potentially be used as a clinical tool for accurate assessment of cerebral dysfunctions induced by concussive blow.
Second, impaired postural stability as reflected in increased area of the center of pressure was documented at least within 15 days post-injury. This finding is consistent with previous reports indicating postural stability deficits in concussed individuals in acute phase of injury (Guskiewicz et al., 1997; Guskiewicz, 2001, 2003; Rieman and Guskiewicz, 2002; Valovich et al., 2003; Peterson et al., 2003). However, unlike EEG data, the magnitude of increase of the COP area from eyes open to eyes closed conditions did not correlate with the rate of recovery of this measure within one year post-injury. This postural measure, similar to other traditional measures for assessment of postural stability, may not be sensitive enough to detect residual balance problems in MTBI subjects beyond 15 days post-injury. In fact, more recent studies by Cavanaugh et al. (2005a,b, 2006) have shown that advanced methods from nonlinear dynamics (i.e., Approximate Entropy, ApEn) may detect changes in postural control in athletes with “normal” postural stability measures based upon conventional balance testing longer than 3–4 days after cerebral concussion. Our own study has also shown alteration of virtual-time-to-contact (VTC) measures in the absence of traditional measures of postural instability in concussed individuals (Slobounov et al., 2008). In addition, we documented residual postural abnormalities at day 30th post-injury in MTBI subjects that can be detected via Virtual Reality tools (Slobounov et al., 2006a,b). Overall, these findings suggest that residual balance abnormalities in concussed individuals may be undetected using isolated unimodal and/or traditional research methods. Although, these measures may be complementary to an accurate assessment of cerebral dysfunction in MTBI subjects at least within 30 days post-injury if used in conjunction with EEG measures. That said, consistent and repetitive subconcussive blows that contact sport athletes experience on a daily basis upon return-to-play may be a confounding factor in this study. This important clinical question requires further research which is beyond the scope of this paper.
In conclusion, MTBI indeed produces cerebral dysfunction, as evidenced by EEG and balance measures that are not yet restored to pre-injury levels even when there has been resolution of the initial clinical symptoms and a return to baseline on neuropsychological testing. Similar findings have been recently reported in the brain imaging literature (Vagnozzi et al., 2010; Henry et al., 2011). Specifically, there is growing evidence through advanced neuroimaging techniques that despite a return to premorbid status (based upon current clinical measures), there are still residual deficits within brain structural and functional networks (Zhang et al., 2010; Johnson et al., 2012; Mayer et al., 2010; Slobounov et al., 2011) in the sub-acute phase of MTBI. This should be a major clinical concern when clearing athletes for sport participation, since subtle alteration of brain networks due to prior history of MTBI may put an individual at a higher risk from subsequent brain injuries.
HIGHLIGHTS.
Alpha power depression predicts recovery rate from mild traumatic brain injury (MTBI).
Postural instability correlates with EEG measures in MTBI subjects.
EEG and balance measures are excellent tools to assess prognosis of MTBI.
Acknowledgment
This work was supported by National Institutes of Health Grant RO1 NS056227-01A2 “Identification of Athletes at Risk for Traumatic Brain Injury”.
References
- Arciniegas D. Clinical electrophysiologic assessments and mild traumatic brain injury: state-of-the-science and implications for clinical practice. Int J Psychophysiol. 2011;82:41–52. doi: 10.1016/j.ijpsycho.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Barin K. Dynamic posturagraphy analysis of error in force plate measurement of postural sway. IEEE Eng Med Biol. 1992;11:52–56. [Google Scholar]
- Bazarian JJ, McClung J, Shan MN, Cheng YT, Flesher, Schneider S. Emergency department management of mild traumatic brain injury. Acad Emerg Med. 2005;1:199–214. doi: 10.1136/emj.2004.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Macciocchi SN, Ferrara MS. Neurocognitive performance of concussed athletes when symptom free. J Athl Train. 2007;42:504–508. [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain, Cycle 12″ coupling of systems by oscillations. Oxford University Press; 2006. p. 341. [Google Scholar]
- Cantu R. Concussion classification: ongoing controversy. In: Semyon Slobounov, Wayne Sebastianelli., editors. Foundations of sport-related brain injuries. NY: Springer; 2006. pp. 87–111. [Google Scholar]
- Cavanaugh J, Guskiewicz K, Stergiou N. A nonlinear dynamic approach for evaluating postural control: new directions for the management of sport-related cerebral concussion. Sports Med. 2005a;35:935–950. doi: 10.2165/00007256-200535110-00002. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Guskiewicz K, Giuliani C, Marshall S, Mercer V, Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005b;39:805–811. doi: 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Merser VS, Stergion N. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athl Train. 2006;41:305–313. [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Glaser MA, Sjaardema H. The value of the electroencephalograph in cranio-cerebral injuries. West Surg. 1940;48:6989–6996. [Google Scholar]
- Goldie PA, Bach TM, Evans OM. Force platform measures for evaluating postural control: reliability and validity. Arch Phys Med Rehabil. 1989;70:510–517. [PubMed] [Google Scholar]
- Guskiewicz KM, Riemann BL, Perrin DH, Nashner LM. Alternative approaches to the assessment of mild head Injury in athletes. Med Sci Sports Exerc. 1997;29:213–221. doi: 10.1097/00005768-199707001-00003. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001;11:82–189. doi: 10.1097/00042752-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K. Assessment of postural stability following sport-related concussion. Curr Sport Med Rep. 2003;2:24–30. doi: 10.1249/00149619-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Kershman J, Elvidge AR. Electroencephalographic study in clinical cases of injury of the head. Arch Neurol Psychiatry. 1940:328–348. [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephal Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Henry LC, Tremblay J, Tremblay S, Lepore N, Theoret H, Ellemberg D, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28:2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- Hessen E. Very long-term neuropsychological and behavioral consequences of mild and complicated mild MTI: increased impact of pediatric versus adult TBI. In: Alderson V, Yeates K, editors. Pediatric traumatic brain injury: new frontiers and translational research. Cambridge University Press; 2010. pp. 118–144. [Google Scholar]
- Iverson GL, Brooks BL, Collins MW, Lovell MR. Tracking neuropsychological recovery following concussion in sport. Brain Inj. 2006;20:245–252. doi: 10.1080/02699050500487910. [DOI] [PubMed] [Google Scholar]
- Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, et al. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage. 2012;59:511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology was impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Lovell MR, Iverson GL, Collins MW, Podell K, Johnston KM, Pardini D, et al. Measurement of symptoms following sport-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13:166–174. doi: 10.1207/s15324826an1303_4. [DOI] [PubMed] [Google Scholar]
- Lau BC, Collins MW, Lovell MR. Sensitivity and specificity of subacute computerized neuropsychological testing and symptom evaluation in predicting outcomes after sports-related concussion. Am J Sports Med. 2011;39:1209–1216. doi: 10.1177/0363546510392016. [DOI] [PubMed] [Google Scholar]
- Makdissi M, Darby D, Maruff P, Ugoni A, Brukner P, McCrory PR. Natural history of concussion in sport: markers of severity and implication for management. Am J Sports Med. 2010;38:464–471. doi: 10.1177/0363546509349491. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on concussion in sport - The 3rd International Conference on concussion in sport, held in Zurich, November 2008. J Clin Neurosci. 2009;16:755–763. doi: 10.1016/j.jocn.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Nuwer MR, Hovda DA, Schrader LM, Vespa PM. Routine and quantitative EEG in mild traumatic brain injury. Clin Neurophysiol. 2005;116:2001–2025. doi: 10.1016/j.clinph.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Oken BS, Chiappa KH. Statistical issues concerning computerized analysis of brainwave topography. Ann Neurol. 1986;19:493–497. doi: 10.1002/ana.410190511. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Krausz G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin Neurophysiol. 2000;111:1873–1879. doi: 10.1016/s1388-2457(00)00428-4. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Induced oscillations in the alpha band: functional meaning. Epilepsia. 2003;44(Suppl 12):2–8. doi: 10.1111/j.0013-9580.2003.12001.x. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva M. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front Psychol. 2011;2:204–208. doi: 10.3389/fpsyg.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C, Ferrara M, Mrazik M, Piland S, Elliott R. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sport. Clin J Sport Med. 2003;13:230–237. doi: 10.1097/00042752-200307000-00006. [DOI] [PubMed] [Google Scholar]
- Randolph C. Implementation of neuropsychological testing models for the high school, collegiate and professional sport setting. J Athl Train. 2001;36:288–296. [PMC free article] [PubMed] [Google Scholar]
- Rau C, Plewnia C, Hummel F, Gerloff C. Event-related desynchronization and excitability of the ipsilateral motor cortex during simple self-paced finger movement. Clin Neurophysiol. 2003;114:1819–1826. doi: 10.1016/s1388-2457(03)00174-3. [DOI] [PubMed] [Google Scholar]
- Rieman B, Guskiewicz K. Effect of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2002;35:19–25. [PMC free article] [PubMed] [Google Scholar]
- Risdall JE, Menon DK. Traumatic brain injury. Philos Trans R Soc Lond B Biol Sci. 2011;366:241–250. doi: 10.1098/rstb.2010.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RM. Mild traumatic brain injury and neural recovery: rethinking the debate. NeuroRehabilitation. 2011;28:167–180. doi: 10.3233/NRE-2011-0646. [DOI] [PubMed] [Google Scholar]
- Sedney CL, Orphanos J, Bailes JE. When to consider retiring an athlete after sports-related concussion. Clin Sports Med. 2011;30:189–200. doi: 10.1016/j.csm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Slobounov SM, Newell KM. Postural dynamic as a function of skill level and task constraints. Gait Posture. 1994;2:85–93. [Google Scholar]
- Slobounov S, Sebastianelli W, Simon R. Neurophysiological and behavioral concomitants of mild brain injury in collegiate athletes. Clin Neurophysiol. 2002;113:185–193. doi: 10.1016/s1388-2457(01)00737-4. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Sebastianelli W, Moss R. Alteration of posture-related cortical potentials in mild traumatic brain injury. Neurosci Lett. 2005;383:251–255. doi: 10.1016/j.neulet.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Slobounov E, Newell K. Application of virtual reality graphics in assessment of concussion. Cyberpsychol Behav. 2006a;9:188–191. doi: 10.1089/cpb.2006.9.188. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Tutwiler R, Sebastianelli W, Slobounov E. Alteration of postural responses to visual field motion in mild traumatic brain injury. Neurosurgery. 2006b;59:134–139. doi: 10.1227/01.NEU.0000219197.33182.3F. [DOI] [PubMed] [Google Scholar]
- Slobounov SM, Cao C, Sebastianelli W, Slobounov E, Newell K. Residual deficits from concussion as revealed by virtual time-to-contact measures of postural stability. Clin Neurophysiol. 2008;119:281–289. doi: 10.1016/j.clinph.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Slobounov SM, Gay M, Zhang K, Johnson B, Pennell D, Sebastianelli W, et al. Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. Neuroimage. 2011;55:1716–1727. doi: 10.1016/j.neuroimage.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Gerson I, Geisler FH. EEG discriminant analyses of mild head injury. EEG Clin Neurophysiol. 1989;73:94–106. doi: 10.1016/0013-4694(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Thompson J, Sebastianelli W, Slobounov S. EEG and postural correlates of mild traumatic brain injury in athletes. Neurosci Lett. 2005;377:158–163. doi: 10.1016/j.neulet.2004.11.090. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier D, Subramony S, Wessel K, et al. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. J Neurol Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Valovich T, Periin D, Gansneder B. Repeat administration elicits a practice effect with the balance error scoring system but not with the standardized assessment of concussion in high school athletes. J Athl Train. 2003;38:51–56. [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgro E, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- Webbe FM, Barth JT. Short-term and long-term outcome of athletic closed head injuries. Clin Sports Med. 2003;22:577–592. doi: 10.1016/s0278-5919(02)00103-5. [DOI] [PubMed] [Google Scholar]
- Williams D. The electro-encephalogram in acute head injuries. J Neurol Psychiatry. 1941;4:107–130. doi: 10.1136/jnnp.4.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ST, Lovejoy DW, Pearlson GD, Stevens MC. Decreased prefrontal cortex activity in mild traumatic brain injury during performance of an auditory oddball task. Brain Imaging Behav. 2010;4:232–247. doi: 10.1007/s11682-010-9102-3. [DOI] [PubMed] [Google Scholar]
- Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI and DTI study. Exp Brain Res. 2010;204:57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]