Abstract
Cyanobacteria produce a diverse array of toxic or otherwise bioactive compounds that pose growing threats to human and environmental health. We utilized the zebrafish (Danio rerio) embryo, as a model of vertebrate development, to investigate the inhibition of development pathways (i.e. developmental toxicity) by the cyanobacterial toxin, cylindrospermopsin (CYN), as well as extracts from various isolates of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum. CYN was toxic only when injected directly into embryos, but not by direct immersion at doses up to 50 μg/ml. Despite the dose dependency of toxicity observed following injection of CYN, no consistent patterns of developmental defects were observed, suggesting that toxic effects of CYN may not target specific developmental pathways. In contrast, direct immersion of embryos in all of the extracts resulted in both increased mortality and reproducible, consistent, developmental dysfunctions. Interestingly, there was no correlation of developmental toxicity observed for these extracts with the presence of CYN or with previously reported toxicity for these strains. These results suggest that CYN is lethal to zebrafish embryos, but apparently inhibits no specific developmental pathways, whereas other apparent metabolites from C. raciborskii and A. ovalisporum seem to reproducibly inhibit development in the zebrafish model. Continued investigation of these apparent, unknown metabolites is needed.
Keywords: Cyanobacteria, Cylindrospermopsin, Cylindrospermopsis, Aphanizomenon, Zebrafish, Development toxicity
1. Introduction
Cyanobacteria (“blue-green algae”) produce a diverse array of toxic or otherwise bioactive metabolites. In freshwater environments, in particular, these compounds can pose serious threats to human and environmental health via contamination of drinking water, recreational exposure to waterborne toxins and possible accumulation of toxins in the food-web (e.g. Chorus et al., 2000; Paerl et al., 2001; Rao et al., 2002; Codd et al., 2005; Falconer and Humpage, 2006). Though toxicoses associated with exposure to cyanobacterial toxins are typically recognized from cases of acute poisoning, emerging studies support a likely role of these compounds in sub-acute health effects (e.g. Milutinovic et al., 2002; Dos et al., 2005; Falconer and Humpage, 2006; Sukenik et al., 2006). One such example is the growing evidence to indicate cyanobacterial (and other marine and freshwater algal) toxins may act as developmental toxins, inhibiting or impairing various pathways of vertebrate development (Oberemm et al., 1997; Papendorf et al., 1997; Pilotto et al., 1999; Jacquet et al., 2004; Wang et al., 2005; Bu et al., 2006; Sukenik et al., 2006; Berry et al., 2007; Palikova et al., 2007; Rogers et al., 2007; Wright et al., 2006; Lecoz et al., 2008).
The cyanobacterial toxin, cylindrospermposin (CYN; Fig. 1), is a hepatotoxic alkaloid first isolated from Cylindrospermopsis raciborskii, and subsequently characterized chemically, by Ohtani et al. (1992). Toxicity of CYN was first recognized when more than 100 children of Aboriginal families on Palm Island in Queensland, Australia were admitted to hospitals for various symptoms of gastroenteritis (Griffiths and Saker, 2003). The illness was eventually linked to contamination of the local water supply with a dense algal bloom, and specifically a strain of C. raciborskii. From this isolate, CYN was purified and characterized chemically as a highly water-soluble cyclic guanidinium alkaloid, containing a unique tricyclic hydroxymethyl uracil (Fig. 1; Ohtani et al.,1992). Subsequently, the toxin has been identified in at least five additional genera of cyanobacteria, including Anabaena, Aphanizomenon, Raphidiopsis, Lyngbya and Umezakia (Harada et al., 1994; Banker et al., 1997; Li et al., 2001a; Seifert et al., 2007), and CYN-producing strains of C. raciborskii and other cyanobacteria are being found to be increasingly prevalent in temperate and tropical freshwaters (Saker and Griffiths, 2001; Neilan et al., 2003; Saker et al., 2003).
Fig. 1.
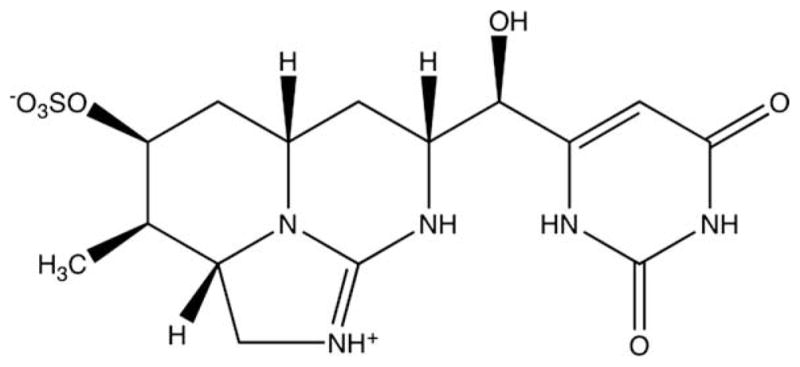
Chemical structure of cylindrospermopsin. Cylindrospermopsin is a water-soluble cyclic guanidinium alkaloid containing a tricyclic hydroxy-methyl uracil.
Despite considerable research, much remains to be clarified with respect to the toxicity of CYN. Initial evaluation of CYN indicated that the most likely mode of action of CYN is inhibition of protein synthesis, including observed detachment of ribosomes from membranes of the rough endoplasmic reticulum or possible interaction with soluble proteins involved in protein translation (Terao et al., 1994; Froscio et al., 2001; Runnegar et al., 2002; Froscio et al., 2008). Froscio et al. (2001), for example, using an in vitro assay system based on the rabbit reticulocyte lysate translation system, reported an IC50 of 120 nM and detection limit of 50 nM (equivalent to approximately 50 ng/mL and 21 ng/mL, respectively) for inhibition of protein synthesis by CYN. Subsequent studies (e.g. Runnegar et al., 2002; Froscio et al., 2008) using this same in vitro assay system have consistently confirmed inhibition at these concentrations. Likewise, evaluation of cellular inhibition of protein synthesis, specifically in mouse hepatocytes, similarly suggests complete inhibition at concentrations above 0.5 μM (Froscio et al., 2008). In addition, dose-dependent cytotoxicity of CYN was also observed in both rat and mouse hepatocytes at concentrations above 1 μM (Runnegar et al., 1994; Froscio et al., 2003). However, attenuation of this cytoxicity, and not protein synthesis, by cytochrome P450 inhibitors suggests that this acute toxicity to liver cells may be related to biotransformation products rather than inhibition of protein synthesis by CYN (Runnegar et al., 1995). Moreover, recent studies suggest CYN, with a potentially reactive guanidine, may also act through covalent binding and breakage of DNA in cells exposed at concentrations in the range of 1–10 μg/mL (Humpage et al., 2000; Shaw et al., 2000; Shen et al., 2002) leading to various cellular abnormalities. More recent studies though have suggested that cellular abnormalities may be unrelated to direct interaction with DNA (e.g. Fessard and Bernard, 2003). Clearly, given the increasing recognition of the apparently widespread distribution of CYN and CYN-producing cyanobacteria in freshwater systems, continued toxicological investigation will be essential to clarifying potential threats to human and environmental health.
Identification and characterization of developmental toxins from marine and freshwater algae has been facilitated by the use of several aquatic models, specifically including embryos of the zebrafish (Danio rerio) and other teleost fish species (reviewed by Berry et al., 2007). Owing to a number of practical advantages including small size, nearly transparent embryos, ease of husbandry, short embryogenesis and rapid sexual maturation, as well as growing knowledge of the species’ genome, the zebrafish has emerged as an especially important model system (Teraoka et al., 2003; Hill et al., 2005). This includes the use of zebrafish embryos to characterize developmental toxicity of a number of well-described toxins from marine algae, including saxitoxin (LeFebvre et al., 2004) and domoic acid (Tiedeken et al., 2005), and from freshwater cyanobacteria, particularly including microcystin-LR (Oberemm et al., 1997; Wang et al., 2005). In addition, the zebrafish embryo has been used to screen algal isolates for developmental toxins (Berry et al., 2007), and guide purification and characterization of novel or less well-known cyanobacterial metabolites with respect to their developmental toxicity, including the previously unknown mueg-gelone (Papendorf et al., 1997) from blooms of Aphanizomenon flosaquae, and hapalindole alkaloids from Fischerella (Berry et al., 2007). Here we employ the zebra-fish embryo as a model of vertebrate development to investigate the developmental toxicity of CYN, as well as other possible metabolites from C. raciborskii and other CYN-producing taxa of cyanobacteria.
2. Materials and methods
2.1. Chemicals
Solvents, including methanol (MeOH; Omnisolv®, 99.9% purity minimum) and HPLC-grade chloroform (CHCl3; 99.8% purity minimum), from EMD Chemicals, Inc. were purchased from Fisher Scientific. CYN, purified as described below, was obtained from the National Research Centre for Environmental Toxicology at the University of Queensland, Australia. All other chemicals and reagents were purchased from Sigma–Aldrich (St. Louis, MO, U.S.A.).
2.2. Isolation and culture of cyanobacteria
Seven strains of C. raciborskii (Table 1), and one strain of Aphanizomenon ovalisporum (Table 1), were isolated (by transfer of a single trichome), and cultured at 20 ± 1 °C with an incident light intensity of 10 μmol m−2 s−1 provided by cool white fluorescent tubes (14 h:10 h light/dark cycle), as previously reported by Saker and Eaglesham (1999) and Saker et al. (2003). Samples of biomass (130–300 mg dry weight) from cultured isolates were collected and lyophilized for extraction and fractionation of toxins (see below).
Table 1.
Strains of C. raciborskii and Aphanizomenon ovalisporum Extracted,a and tested for production of developmental toxins. Strains were isolated and cultured as reported previously by Saker and Eaglesham (1999) and Saker et al. (2003). Given are sources of isolate, and whether or not it was previously found to produce CYN (column labeled “CYN?”) or STX (column labeled “STX?”), or to be toxic in a mouse bioassay (column labeled “Mouse?”); “n.d.” indicates that the evaluation was not done to the author’s knowledge. References for the isolation, and testing of these isolates are given, along with alternative strain names used in these references and available GenBank accession codes for 16s rRNA gene sequences.
| Isolate | Species | Source | CYN? | STX? | Mouse? | Reference (alternative referenced isolate name) | GenBank |
|---|---|---|---|---|---|---|---|
| 4899 | C. raciborskii | Ardilla River, Portugal | No | n.d. | Toxic | Saker et al., 2003 (PT2); Neilan et al., 2003 (Marau2) | AF516740 |
| MARAU | C. raciborskii | Maranhao Reservoir, Portugal | No | n.d. | Toxic | Saker et al., 2003 (PT4); Neilan et al., 2003 (Marau1) | AF516739 |
| CAIA | C. raciborskii | Caia Reservoir, Portugal | No | n.d. | Toxic | Saker et al., 2003 (PT3); Neilan et al., 2003 | AF516742 |
| 4799 | C. raciborskii | Odivelas Reservoir, Portugal | No | n.d. | Toxic | Saker et al., 2003 (PT1); Neilan et al., 2003 | AF516741 |
| BRAZ | C. raciborskii | Brazil | n.d. | Yes | Toxic | Lagos et al., 1999 (T1); Neilan et al., 2003 (Braz2) | AF516734 |
| LJ | C. raciborskii | Lake Julius, Australia | No | n.d. | Not toxic | Saker and Griffiths, 2001; Saker and Neilan, 2001 (CR7); Neilan et al., 2003 | AF516725 |
| AQS | C. raciborskii | Townsville, Australia | Yes | n.d. | n.d. | Saker and Eaglesham, 1999; Saker and Neilan, 2001 (CR3) | N.A. |
| APH OVAL | A. ovalisporum | Israel | Yes | n.d. | n.d. | Banker et al., 1997; Pollingher et al., 1998 | N.A. |
Biomass from each strain was extracted sequentially in chloroform and 30% methanol (see Section 2).
2.3. Extraction and fractionation of toxins from cyanobacteria cultures
Samples of lyophilized biomass from cyanobacterial isolates (Table 1) were extracted sequentially with lipophilic (i.e. CHCl3) and polar (i.e. 30% MeOH in water) solvents. Specifically, biomass was first extracted in 10 mL of CHCl3 for 48 h with intermittent sonication (twice for 20 min each) with Biologics, Inc. Model 150 VT Ultrasonic Homogenizer, and subsequent freeze/thaw (twice). The extracts were filtered (Whatman 540 Hardened Ashless, > 8 μm), and the biomass rinsed with an additional 4 mL of CHCl3. The pooled extract and “rinse,” were taken to dryness by rotary evaporation. The remaining (dried) biomass was extracted a second time with 30% MeOH (in water) with sonication (once for 20 min) and freeze/thaw (once). The extracts were centrifuged, and the supernatant transferred to clean 50-mL tubes. The pellet was rinsed (by mixing) with an additional 4 mL of water and re-centrifuged. Subsequently, the “rinse supernatant” was pooled with the previous supernatant, frozen at −80 °C and lyophilized. For evaluation of developmental toxicity (see below), extracts were re-suspended in the appropriate extraction solvent (i.e. CHCl3 or 30% MeOH), and tested at final concentrations equivalent to approximately 14.3, 71.5 and 143 μg of lyophilized biomass per mL.
2.4. Pure CYN
CYN, obtained from the National Research Centre for Environmental Toxicology at the University of Queensland, Australia, was purified from spent culture medium by a method modified from Norris et al. (2001). Specifically, purification included solid-phase extraction (Alltech® Carbograph cartridges, 1000 mg; elution with 5% formic acid in MeOH) and sequential HPLC purification using a Prep Nova HR C18 radially compressed column (25 × 100 mm; 6 μm, 60A), followed by an Alltech, Apollo C18 (22 × 150 mm; 5 μm) column, using gradients of MeOH in water (W. Wickramasinghe, Pers. Comm.). The identity and purity (>95%) of CYN was confirmed by NMR and HPLC–MS/MS (Eaglesham et al., 1999).
2.5. Rearing and breeding of zebrafish
Zebrafish (Danio rerio) of the D31 and DGL lines (Gibbs and Schmale, 2000) were bred and maintained in the Rosenstiel School of Marine and Atmospheric Science at the University of Miami. The zebrafish were maintained in 30-L tanks at 28 °C with a 14 h:10 h light/dark cycle. To obtain embryos for evaluation of developmental toxicity, adults were bred as per the method described in Berry et al. (2007).
2.6. Zebrafish developmental toxicity assay
Developmental toxicity of CYN and extracts was initially evaluated, using zebrafish embryos, by a method slightly modified from Berry et al. (2007). Specifically, embryos (4-to 32-cell stage) were collected within 2 h post-fertilization (hpf), and exposed by direct immersion of embryos in wells of 24-well polypropylene plates (5 embryos per well) containing CYN or extracts/fractions in E3 medium (Brand et al., 2002). Assays were generally done in triplicate, and each test was typically duplicated to confirm observed results. Treated embryos were observed for 5 days post-fertilization (dpf) using a dissecting light microscope, and any morphological or other apparent developmental abnormalities were recorded and photographically documented. In later studies, primarily those investigating purified CYN, several alternative approaches were used to expose embryos to the toxin, specifically in order to circumvent the apparent inability of this water-soluble compound to permeate the chorion and/or cellular membranes of the developing embryo (discussed below). These approaches included addition of 2% dimethylsulfoxide (DMSO) to the rearing medium, partial dechorination of eggs and use of alternative media including distilled water (rather than E3 medium), as well as microinjection of the toxin directly into the fertilized egg (Table 2).
Table 2.
Zebrafish exposure methods used to test toxicity of purified CYN. Embryos were exposed by either immersion into medium containing CYN, or by micro-injection directly into embryos, as described in Section 2. Different media were used, and media were supplemented with 2% DMSO to facilitate passage of CYN across membranes. Additionally, mechanical dechorionation was used to circumvent possible impermeability of the chorion. CYN was dissolved in water for immersion exposure, or Hank’s balanced salt solution +0.04% phenol red for microinjection, and these were used for appropriate vehicle controls. Observed toxicity, specifically identified by mortality or deformity of zebrafish embryos, is indicated for each exposure method, and further discussed in the text.
| Exposure method | Medium used | 2% DMSO? | Dechorionation?a | Concentration rangeb | Toxic? |
|---|---|---|---|---|---|
| Immersion | E3c | No | No | Up to 50 μg/mL | No |
| Immersion | ERSd | No | No | Up to 50 μg/mL | No |
| Immersion | Distilled water | No | No | Up to 50 μg/mL | No |
| Immersion | E3 medium | Yes | No | Up to 50 μg/mL | No |
| Immersion | Distilled water | Yes | No | Up to 50 μg/mL | No |
| Immersion | E3 medium | Yes | Yes | Up to 50 μg/mL | No |
| Immersion | Distilled water | Yes | Yes | Up to 50 μg/mL | No |
| Microinjectione | E3 medium | No | No | 1.7–843 fmol/embryo (calculated dose injected) | Yesf |
Chorion removed mechanically with forceps.
For negative controls, vehicle added to the medium, except for microinjection exposures for which vehicle injected identically to treated embryos.
“Embryo rearing solution” described in Berry et al. (2007).
Injection into the yolk through the animal pole at 4–8 cell stage (see Section 2).
Toxic results shown in Fig. 2.
Microinjection of CYN was done by a method modified from Gibbs and Schmale (2000). The pure toxin was dissolved in Hank’s Balanced Salt Solution plus 0.04% phenol red to final concentrations of 50, 5, 1, 0.5, 0.2 and 0.1 μg/mL. Newly fertilized eggs of the D31 line were microinjected with a running syringe (Gilmont S-1100 with 5–10 μm needle tip). After trying several injection techniques, injection into the yolk between cells at the animal pole (of 4–8 cell embryos), followed by subsequent removal of non-dividing embryos (from both control- and CYN-injected embryos), was found to minimize leakage of injected solution, and increase survivorship of embryos (as determined by mortality in control-injected embryos), providing adequately quantitative data. Based on studies using microinjection of DNA (Gibbs and Schmale, 2000), it is known that the injection volume is equal to approximately 7 nL. “Intra-embryo concentrations” were accordingly calculated based on a 7-nL injection volume and average, approximate embryo volume of 0.2 μL (calculated based on an average measured diameter of the approximately spherical D31 embryo [without the chorion] of 725 μm). Dose of 50% lethality (LD50) was calculated by maximum likelihood regression using probit and logit analysis as per standard methods (Finney, 1971; EPA, 1991), specifically using ToxCalc v. 5.0.23 G (Tidepool Scientific Software, McKinleyville, CA).
3. Results
3.1. Toxicity of purfied CYN
No significant mortality or developmental effects were observed in embryos exposed (at 2 hpf for 5 dpf) by direct immersion in pure CYN at concentrations as high 50 μg/mL (Table 2). To investigate the possibility that the water-soluble CYN does not permeate either the chorion or cellular membranes, several procedural modifications were evaluated as means of delivering the toxin to embryos. Embryos were observed to develop normally in as much as 2% DMSO (data not shown) which has been well documented to enhance permeability and uptake in various cell biology techniques (see reviews by Yu and Quinn, 1998; Ingels and Augustijns, 2003). Thus, to facilitate passage of toxin into the egg, embryos were treated at the same range of CYN concentrations in the presence of 2% DMSO added to the medium. CYN was not toxic, as evidenced by no observed mortality (over 5 dpf), at toxin concentrations as high has 50 μg/mL in 2% DMSO (Table 2). Similarly no toxicity (up to 50 μg/mL for 5 dpf) was observed when embryos were exposed by direct immersion using distilled water or “embryo rearing solution” (ERS; Berry et al., 2007) rather than E3 medium (Table 2). The role of the chorion in this resistance to the toxin was further investigated using dechorionated embryos. No toxicity of CYN to embryos was observed (5 dpf) when dechorionated embryos were exposed by direct immersion in the toxin at concentrations as high as 50 μg/mL in either E3 medium or distilled water, with or without concomitant addition of 2% DMSO (Table 2).
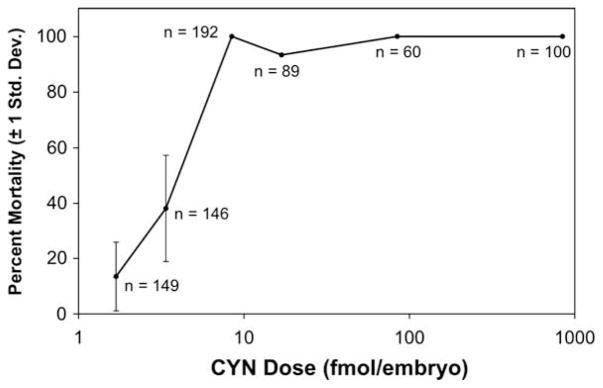
To further investigate the developmental toxicity of CYN, embryos were microinjected with the pure toxin. Embryos were microinjected with 50, 5, 1, 0.5, 0.2 and 0.1 μg/mL solutions of CYN, corresponding to approximately 843, 84, 17, 8.4, 3.4 and 1.7 fmol of CYN per embryo, or 4.2 × 103, 4.2 × 102, 84, 42, 17 and 8.4 nM calculated “intra-embryo” CYN concentrations (given a 7-nL injection into an approximately 0.2-μL volume; see Section 2), respectively. Dose-dependent mortality was observed at 1 dpf, (Fig. 2) and the percent mortalities for each treatment remained unchanged through 5 dpf (not shown). An average mortality rate of approximately 15.2% (±8.1%) was observed for control-injected embryos (receiving only 0.04% phenol red in HBSS). Injection at the highest concentrations of CYN (corresponding to 843, 84, 17 and 8.4 fmol/embryo) resulted in mortality or severe deformity of 100% of embryos at 1 dpf; specifically, all doses were scored as completely lethal, except 17 fmol/embryo for which 7% of the embryos (4 out of 60) survived to 5 dpf, but were all scored as severely deformed (Fig. 2). LD50 was calculated as 4.50 fmol CYN/embryo (95% confidence limit 3.35–6.18 fmol/embryo) and 4.19 fmol CYN/embryo (95% confidence limit 1.09–9.88 fmol/embryo) for 1 dpf and 5 dpf, respectively. This LD50 is equivalent to a calculated intra-embryo CYN concentration of 22.3 nM (95% confidence limit 16.8–31.0 nM) and 21.2 nM (95% confidence limit 5.5–49.5 nM) intra-embryo concentration of CYN, respectively, for 1 and 5 dpf embryos (based on calculated embryo volume; see Section 2).
Fig. 2.
Mortality of zebrafish embryos microinjected with cylindrospermopsin (CYN) at 1 day post-fertilization (dpf). Mortality was determined at calculated doses of 843, 84, 17, 8.4, 3.4 and 1.7 fmol of CYN/embryo (shown in logarithmic scale). The total number (n) of embryos tested at each dose is shown next to data points. Error bars, representing ±1 standard deviation, are given for the two lowest doses that resulted in less than 100% mortality or deformity. Error bars are not included for higher doses for which mortality was either 100%, and standard deviation is zero, or which were injected as a single group. As discussed in the text, mortality rate remained largely unchanged up to 5 dpf.
In addition to mortality, a non-dose-dependent increase in the number of deformed embryos was observed (at 5 dpf) for the lowest doses tested, compared to control-injected embryos (for which approximately 7% of the total embryos deformed). Specifically, approximately 20% and 16% of all embryos tested at 1.7 and 3.4 fmol/embryo, respectively, were deformed (corresponding to approximately 24% and 26% of the surviving embryos, respectively, at the two doses). The deformed embryos (not shown), in all cases, exhibited a wide range of abnormal morphologies with no consistent pattern of alterations in musculature, skeleton or soft tissues that would be consistent with disruption of a specific developmental pathway.
3.2. Developmental toxicity of extracts from C. raciborskii and A. ovalisporum
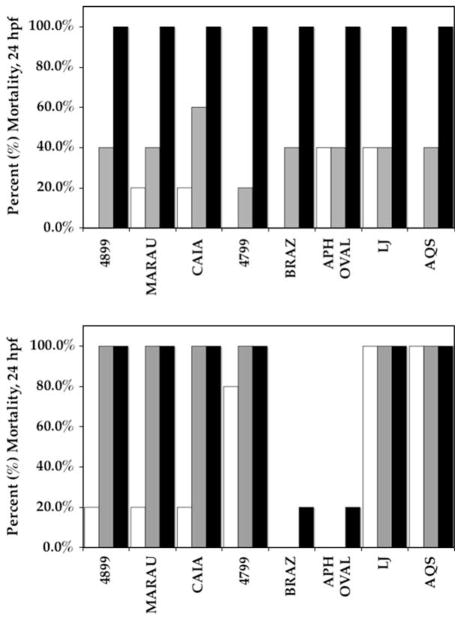
We additionally evaluated extracts from several isolates (Table 1) of C. raciborskii, and one isolate of A. ovalisporum, for toxicity in the zebrafish assay. All of the extracts were toxic to zebrafish embryos exposed by direct immersion at 2 hpf, causing mortality within 24 hpf at the highest concentration (i.e. 143 μg biomass/mL; Fig. 3). Mortality rates of 100% were observed with high and intermediate (143 and 71.5 μg biomass per mL, respectively) concentrations of all, but two, of the methanolic extracts (Fig. 3A), and with all of the high concentrations (143 μg biomass/mL) of the chloroform extracts (Fig 3B). At the lowest concentration of 30% MeOH extracts (14.3 μg biomass per mL), mortality rates were highly variable from 0% (2 extracts) to 100% (2 extracts), while CHCl3 extracts showed generally lower mortality rates (from 0 to 40%). By 2 dpf, 100% of embryos exposed to all extracts, except 30% MeOH extracts of C. raciborskii isolate BRAZ and A. ovalisporum isolate APH OVAL, at intermediate concentrations were dead or deformed (data not shown). Similarly, all embryos exposed to 30% MeOH extracts of C. raciborskii isolates CAIA and 4799 at even the lowest concentration tested (equivalent to 14.3 μg biomass per mL) were dead or deformed by 2 dpf (data not shown). No significant mortality or apparent dysfunction was observed for embryos exposed to solvent-only controls; specifically, mortality rates were less than the spontaneous mortality rate of approximately 5% that is typical for untreated embryos (see Berry et al., 2007).
Fig. 3.
Percent mortality (1 dpf) of 30% MeOH (A) and CHCl3 (B) extracts of cyanobacterial isolates (see Table 1, and Section 2) tested in the zebrafish embryo assay. Extracts were tested by direct immersion of embryos at concentrations equivalent to 14.3, 71.5 and 143 μg of lyophilized cyano-bacterial biomass per mL of E3 medium (white, gray and black bars, respectively).
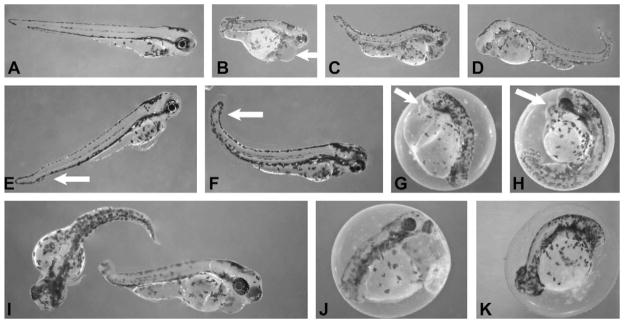
Moreover, various developmental effects were observed in embryos treated at sub-lethal concentrations of extracts. In particular, lipophilic (i.e. CHCl3) extracts of the isolates gave the most consistent patterns of abnormal development (Fig. 4), whereas more polar (i.e. 30% MeOH) extracts tended to lead to mortality with only sporadic incidence of deformity and little consistency in the observed phenotypes (not shown). A range of developmental defects was observed across extract types, however, certain patterns dominated (particularly for chloroform extracts). Specifically, bent or (less often) truncated body axes were frequently observed in combination with inhibition of organogenesis, and particularly impaired development of the eye, as well as edemas of the pericardium and other ventral cavities (Fig. 4B–K). In some cases, the treatment with extracts apparently delayed hatching, as evidenced by 4-dpf embryos frequently remaining in chorions, compared to control embryos that typically hatched within 3 dpf (data not shown).
Fig. 4.
Inhibition of zebrafish embryo development by chloroform extracts from various isolates of C. raciborskii and A. ovalisporum. All embryos shown are 4 dpf, and exposed at less than 2 hpf (typically 4- to 32-cell stage). A: control (untreated) embryo at 4 dpf. B: embryo exposed to extract of A. ovalisporum isolate (APH OVAL) at concentration equivalent to 14.3 μg biomass per mL (arrow indicates edema). C and D: embryos exposed to extracts of C. raciborskii isolates (4899 and MARAU) at concentrations equivalent to 71.5 μg biomass per mL. E–I: embryos exposed to extracts of C. raciborskii isolate (AQS) at concentrations 71.5 μg biomass per mL (G–I: arrow indicates edemas), and two-fold dilution (E–F: arrow indicates slightly bent tail). J–K: embryos exposed to extracts from C. raciborskii isolate (LJ) at concentration equivalent to 71.5 μg biomass per mL (K), and two-fold dilution (J).
The magnitude of these effects was notably dose-dependent. This is exemplified by chloroform extracts of C. raciborskii isolate AQS (Fig. 4E–I). Treatment of embryos with intermediate concentrations of these extracts (equivalent to 71.5 μg biomass per mL) produced deformities that ranged from undeveloped eyes, truncated body axis and severe edemas to curved, but otherwise elaborated, axes and relatively moderate edemas (Fig. 4G–I). Two-fold dilution of this extract, on the other hand, gave rather minimal developmental effects, characterized by only slightly bent tails and minor edemas (Fig. 4E–F) compared to control embryos (Fig. 4A), though it should be noted that this moderate phenotype was consistent for all surviving embryos treated at this lower concentration. Dose-dependence of the developmental toxicity is further supported here by observed effects of extracts from the non-CYN-producing C. raciborskii isolate LJ on embryos. Surviving embryos treated with intermediate concentrations of chloroform extracts from C. raciborskii isolate LJ (equivalent to 71.5 μg biomass per mL) were characterized by severely shortened, underdeveloped body axis and lack of eye development (Fig. 4K), whereas those treated with a two-fold dilution of the extract developed a notably less pronounced manifestation of the phenotype (Fig. 4J).
4. Discussion
Cyanobacterial toxins, particularly in freshwater systems, are of growing concern with respect to human and environmental health. CYN is being found to be increasingly prevalent in both temperate and tropical freshwater worldwide (e.g. Saker and Griffiths, 2001; Neilan et al., 2003; Saker et al., 2003). As reviewed previously (Griffiths and Saker, 2003; Rücker et al., 2007), environmental concentrations of CYN in freshwater habitats can vary considerably. Though no formal guideline exists for CYN levels, Humpage and Falconer (2003) have proposed a drinking water limit of 1 μg/L total CYN. Reported values have varied up to 800 μg/L of the toxin, as measured by Shaw et al. (2000) in farms dams in Australia, though Falconer and Humpage (2006) suggest a more typical range of 1–10 μg/L. Further complicating the issue, there is a considerable variability between relative proportions of extracellular/dissolved versus intracellular/particulate CYN. Saker and Eaglesham (1999), in their investigation of CYN in C. raciborskii-infested aquaculture ponds in Queensland, found that approximately 93% of the toxin was intracellular, whereas Rücker et al. (2007) reported that dissolved, extracellular CYN accounted for more than 80% of the toxin detected in temperate German lakes. Though the latter investigated lakes dominated by Aphanizomenon, other studies (e.g. Chiswell et al.,1999) have found similarly much higher proportions of CYN as the dissolved toxins in blooms of C. raciborskii. Likewise, cellular abundance of CYN-producing cyanobacteria (e.g. C. raciborskii, Aphanizomenon) can vary, and is not necessarily correlated with CYN concentrations. Densities as low as 15 × 103 cells/mL have been associated with levels as high as 20 μg/L (MacGregor and Fabbro, 2000), though blooms of 90 × 103 cells/mL have been reported with concentrations of toxin as low as 1 μg/L (Saker and Griffiths, 2001). Extreme blooms have been reported up to 32.5 × 106 cells/mL, with a corresponding toxin concentration of 589 μg/L (Saker and Eaglesham, 1999).
In addition, emerging evidence (Saker and Eaglesham, 1999; Saker et al., 2004; White et al., 2006) suggests that CYN may possibly accumulate in components of the freshwater food-webs. In particular, accumulation has been documented for several invertebrate species. For example, Saker et al. (2004) have shown that the freshwater mussel, Anodonta cygnea, exposed to CYN-producing cultures of C. raciborskii accumulate CYN concentrations up to 2.52 μg/g dry tissue weight. Suggesting accumulation of the toxin at environmentally relevant concentrations, Redclaw Crayfish (Cherax quadricarinatus) from an aquaculture pond contaminated with a bloom C. raciborskii (producing a measured total concentration of 589 μg/L CYN) accumulated the toxin to 4.3 μg/g and 0.9 μg/g in hepatopancreas and muscle tissue, respectively (Saker and Eaglesham, 1999). More recently, accumulation of CYN by the cane toad, Bufo marinus, as a vertebrate model, was found to accumulate the toxin at a maximum average concentration of 895 μg/kg following exposure of tadpoles to CYN-containing cells of C. raciborskii (White et al., 2007).
In addition to rather rapid and acute toxicity associated with CYN, growing evidence suggests possible a role of CYN in long-term effects of chronic and/or sub-acute exposure. Sukenik et al. (2006) reported that sub-acute levels of CYN in the range of 100–550 ng/mL/day in drinking water consumed by mice (equivalent to approximately 10–55 μg/kg dose per day) decreased red blood cell (RBC) counts accompanied by deformation of RBCs over the course of the 42-week period. Humpage and Falconer (2003) showed a range of effects on organs in exposed mice at ten-fold higher concentrations when exposed over 10 weeks. Preliminary evidence in mice (Falconer and Humpage, 2006) has also suggested that chronic exposure (30 weeks) to C. raciborskii extracts can lead to initiation of tumor formation. Moreover, recent evidence suggests that CYN may pose threat to vertebrate development; specifically studies in mice demonstrate induction of fetal toxicity following prenatal exposure (in the range 8–128 μg/kg) during late gestation (Rogers et al., 2007).
The widespread occurrence of CYN, taken together with the potent, acute toxicity as a protein synthesis inhibitor and possible genotoxin (Terao et al., 1994; Shaw et al., 2000; Shen et al., 2002; see discussion in Section 1), as well as possible chronic, sub-acute effects, clearly underscores the need for continued toxicological investigation of this compound. Here we investigated the developmental toxicity of CYN in the zebrafish embryo that has gained growing acceptance as a model for human toxicology (Shin and Fishman, 2002), and particularly of vertebrate development.
Though the zebrafish embryo has been used extensively as a model of vertebrate toxicology, including investigation of cyanobacterial toxins (e.g. Oberemm et al., 1997; Papendorf et al., 1997; LeFebvre et al., 2004; Tiedeken et al., 2005; Wang et al., 2005; Berry et al., 2007), our data interestingly suggest that CYN may not readily permeate cellular membranes in the zebrafish embryo. Indeed, this may point to a possibly general limitation of standard toxicological assays utilizing the zebrafish embryo model. Specifically, no toxicity was observed for embryos exposed by immersion to ambient levels of toxin as high 50 μg/mL that is several orders of magnitude above any reported environmental concentration of CYN, as well as the observed toxic concentrations observed for in vitro studies (as discussed above). Furthermore, none of the various procedural modifications, including dechorionation, addition of up to 2% DMSO or use of distilled water (rather than E3 medium), intended to increase passage of the toxin to the developing embryo, resulted in apparent inhibition of development.
Given the highly water-soluble nature of the zwitterionic alkaloid this is perhaps not surprising. A similar observation has been made, in fact, for the equally water-soluble cyanobacterial toxin, microcystin-LR (MC-LR), when investigated in the zebrafish embryo (Oberemm et al., 1997; Wang et al., 2005) and medaka (Oryzia latipes; Jacquet et al., 2004). In this case, initial studies of the developmental toxicity of MC-LR, specifically using direct immersion of embryos, suggest little or no effect of the otherwise potent phosphatase inhibitor on development of the zebrafish embryo (Oberemm et al., 1997). However, subsequent studies by Wang et al. (2005), using microinjection of MC-LR into zebrafish embryos, demonstrated potent developmental toxicity. A similar limitation of “ambient” exposure of embryos to MC-LR, compared to microinjection of the toxin, was also observed by Jacquet et al. (2004) when investigating developmental toxicity of the toxin in the medaka. Furthermore, a recent screening of extracts from freshwater cyanobacterial isolates for developmental toxicity in the zebrafish embryo model (Berry et al., 2007) indicates that a generally higher number of lipophilic extracts were active, compared to polar (i.e. aqueous/alcohol) extracts.
To circumvent any possible difficulties in the passage of CYN into embryos, we used a microinjection technique to expose developing embryos to the toxin. Specifically, injections were performed at the animal pole of 4- to 8-cell embryos (see Section 2). This technique differs somewhat from previous injection protocols (e.g. Gibbs and Schmale, 2000), but was found, after attempting other techniques, to specifically minimize, and largely prevent, leakage during injections (as visually evidenced by phenol red added to the injection medium), and consequently ensure quantitative exposure.
These microinjection studies show a dose-dependent toxicity of CYN, resulting in rapid mortality (≤1 dpf), at concentrations above approximately 1.7 fmol/embryo (Fig. 2), corresponding (given an approximate embryo volume of 0.2 μL) to 8.4 nM CYN concentration in the injected embryos. Likewise, the calculated LD50 was determined to be approximately 4.50 fmol CYN/embryo, or 22.3 nM CYN (intra-embryo concentration), after only 1 day post-fertilization. These findings correlate well with previously reported inhibition of protein synthesis in the nanomolar range (Terao et al., 1994; Froscio et al., 2001; Froscio et al., 2008). For example, Terao et al. (1994) reported that CYN inhibited protein synthesis in a cell-free rabbit reticulocyte lysate assay with an IC50 (50% inhibitory concentration) of 120 nm, and subsequent studies (Froscio et al., 2001; Froscio et al., 2008) have generally agreed with this range of activity. However, as might be expected for a general inhibition of protein synthesis, no apparent or reproducible inhibition of specific developmental pathways was evident, and CYN treatment generally resulted in mortality without obvious developmental toxicity, though some general deformity was observed at concentrations below 100% mortality (i.e. ≤8.4 fmol/embryo).
On the contrary, developmental toxicity (Fig. 4), as well as mortality (Fig. 3), was observed for lipophilic and polar extracts prepared from isolates of C. raciborskii and A. ovalisporum (Table 1). The authors have previously screened extracts from more than 200 isolates of freshwater cyanobacteria, and have generally observed developmental toxicity in about only 15–20% of extracts evaluated (Berry et al., 2007), suggesting that these observed effects on embryo development are related to specific metabolites these extracts, rather than a general toxicity of extracts, though the chemical nature of most of these compounds remains to be characterized. In particular, embryos exposed to extracts from isolates of C. raciborskii and A. ovalisporum showed developmental phenotypes largely, though not strictly, characterized by bent or twisted body axis, impairment of eye formation and edemas (Fig. 4; discussed above). Specifically, all isolates were toxic, by direct immersion of zebrafish embryos, at the highest concentrations of extracts (corresponding to ≥143 μg lyophilized biomass/mL). Using a conversion factor of 153 fg carbon per cell, as proposed by Hamasaki et al. (1999) for cyanobacteria, we can estimate equivalent densities of approximately 9.35 × 105 cells/mL corresponding to this ambient concentration of “biomass equivalents.” Though this estimation (falsely) assumes all of the lyophilized biomass is composed solely of carbon, any reduction in the contribution of carbon to the total biomass would effectively further lower the calculated cell density. Accordingly, it is proposed that these concentrations of cellular biomass evaluated here easily fall within the range of cell densities previously reported for blooms of C. raciborskii and Aphanizomenon in freshwater systems (as discussed above). Moreover, for several extracts, toxicity was observed at biomass concentration as much as ten-fold lower, corresponding to densities of 9.35 × 104 cell/mL that are typical of C. raciborskii blooms (see Griffiths and Saker, 2003).
Though all of the extracts were toxic to zebrafish, several lines of evidence suggest the toxic constituents are almost certainly not CYN. In addition to somewhat reproducible developmental effects, not seen in CYN-treated embryos, no apparent correlation was found between the observed mortality or developmental effects of extracts and the previously reported (Saker and Eaglesham, 1999; Saker et al., 2003) presence of CYN, or toxicity in the mouse bioassay, for these isolates. For example, though extracts from both A. ovalisporum isolate APH OVAL and C. raciborskii isolate AQS (Fig. 4B and E–I, respectively) that are known to produce CYN were toxic, isolates including C. raciborskii isolates 4899 and MARAU (Fig. 4C and D, respectively) which do not produce CYN, and even C. raciborskii isolate LJ (Fig. 4J–K) which was found to neither produce CYN, nor to be toxic in the mouse bioassay, showed equally profound inhibition of embryo development at comparable concentrations.
Furthermore, it was shown in studies with the pure toxin that CYN is not readily taken up by the embryo without microinjection (discussed above), yet toxicity of all extracts was observed by direct immersion of embryos in the diluted extracts. Specifically, it was shown that direct immersion of embryos in concentrations of the pure toxin as high as 50 μg/mL produced no significant mortality or apparent inhibition of development. Yet, in the case of 30% MeOH extracts from C. raciborskii isolate AQS, for example, though this isolate has been shown to produce CYN, 100% mortality was observed at concentrations equivalent to 14.3 μg of biomass (or less) per mL, such that exceeding 50 μg/mL of CYN (or more than three times this dry weight of biomass) would be impossible.
Further supporting the presence of non-CYN toxins in the isolates, all of the chloroform extracts were toxic to the zebrafish embryos, causing mortality and various developmental defects (Fig. 3B and 4). It is not expected that a lipophilic solvent, such as CHCl3, would extract the highly water-soluble CYN. Though it is conceivable that CYN might be partially extracted by lipophilic solvent, given the previous reasoning regarding calculated levels of CYN in the biomass, and the observation that all of the chloroform extracts produced 100% mortality at the highest concentration (i.e. 143 μg biomass per mL), it would be necessary for CYN in chloroform extracts to account for greater than 35% of the total dry weight of cyanobacterial biomass in all of the isolates to support the role of CYN in the observed toxicity of the chloroform extracts. Though CYN has been detected in both C. raciborskii isolate AQS and A. ovalisporum isolate APH OVAL, for example, even in these isolates this high concentration is extremely unlikely. Indeed, re-extraction and re-testing of chloroform extracts of C. raciborskii isolate AQS, for example, with 30% MeOH confirmed (data not shown) that the toxic component is not soluble or extractable in the latter, more polar solvent as would be expected for CYN.
In addition to CYN, C. raciborskii has also been shown (Lagos et al., 1999; Norris et al., 2001) to produce deoxy-cylindrospermopsin (deoxyCYN) and saxitoxin (STX) that might be proposed to contribute to developmental toxicity of these extracts. DeoxyCYN lacks a hydroxyl group (replaced by –H) at the “uracil bridge,” and it is conceivable that the decreased polarity and water-solubility (Norris et al., 2001) might explain some of the developmental toxicity of the more lipophilic fractions. Indeed, deoxyCYN has been shown to be equally inhibitory to protein synthesis and other indicators of toxicity (Neumann et al., 2007). Furthermore, though previous studies typically report deoxyCYN at approximately 10% of the concentrations of CYN found (e.g. Li et al., 2001b), Seifert et al. (2007) identified deoxyCYN in Lyngbya wollei at concentrations as high 300 times that of CYN. That said, it is not likely that the structural difference between deoxyCYN and CYN would make the former significantly lipophilic, and the apparent decrease in polarity is, in fact, rather slight (Norris et al., 2001). Likewise, though the inhibition of protein synthesis may be comparable between the two congeners, it would still not be expected that this general mechanism would contribute to the inhibition of specific developmental pathways. In fact, though protein synthesis inhibition seems to be unaffected by dehydroxylation of deoxyCYN (e.g. Neumann et al., 2007), Norris et al. (2001) found that intraperitoneal injection of mice with deoxyCYN was not toxic at doses four-fold higher than the lethal dose of CYN.
On the other hand, unlike CYN, LeFebvre et al. (2004) recently demonstrated developmental toxicity of the water-soluble toxin, saxitoxin (STX), in the zebrafish embryo exposed to the toxin by direct immersion. As mentioned, STX has been previously isolated from C. raciborskii (Lagos et al., 1999), including one of the strains tested here (C. raciborskii isolate BRAZ), as well as other various cyanobacterial taxa, including Aphanizomenon (e.g. Negri and Jones, 1995; Mahmood and Carmichael, 1986). Given the water-solubility of STX and its related gonyau-toxins it would be perhaps surprising to find these compounds in lipophilic extracts, however, it has not yet been ruled-out here. Furthermore, though developmental toxicity in the zebrafish has been reported for STX, the observed effects are not identical or even particularly related to those observed for extracts evaluated here (Fig. 4). Continuing studies are underway to assess the presence of deoxyCYN and STX in these extracts, as well as to possibly purify and characterize otherwise unknown constituents from C. raciborskii and A. ovalisporum.
5. Conclusions
The zebrafish embryo is emerging as an important model of vertebrate development, including evaluation of known and otherwise uncharacterized developmental toxins. This system has been previously applied to characterize the developmental toxicity of a number of known algal metabolites, and also used to identify and characterize novel or less well-known developmental toxins from marine and freshwater microalgae (reviewed by Berry et al., 2007).
It is clear from results presented here that the widespread cyanobacterial toxin, CYN, is toxic to the zebrafish embryo resulting in considerable mortality of toxin-injected embryos, though embryos seem to be impermeable to the water-soluble toxin. However, the presumed inhibition of protein synthesis by CYN does not result in any consistent pattern of developmental defects, suggesting that specific developmental pathways are probably not affected. In contrast, direct immersion of zebrafish embryos in 30% MeOH and CHCl3 extracts from several isolates of C. raciborskii and A. ovalisporum resulted in high levels toxicity and reproducible patterns of developmental defects. These results suggest that these extracts contain toxic metabolites other than CYN. Isolation and characterization of these compounds, utilizing the zebrafish embryo as a model system, is currently being pursued.
Acknowledgments
Support for this research was provided by a pilot project from an NIH-National Institute of Environmental Health Sciences (NIEHS) ARCH grant (ES11181), and an NIH-NIEHS R21 Exploratory/Developmental Research Grant (ES014 037). The authors would like to thank Dr. Wasa Wickra-masinghe of the National Research Centre for Environmental Toxicology (Queensland, Australia) for kindly providing purified CYN, and Dr. Bob Gerdes of UM RSMAS Division of Marine Biology and Fisheries for assistance with statistical analyses.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Banker R, Carmeli S, Hadas O, Teltsch B, Porat R, Sukenik A. Identification of cylindrospermopsin in Aphanizomenon ovalisporum isolated from Lake Kinneret, Israel. J Phycol. 1997;33 (4):613–616. [Google Scholar]
- Berry JP, Gantar M, Gibbs PD, Schmale MC. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145 (1):61–72. doi: 10.1016/j.cbpc.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Granato M, Nüsslein-Volhard C. Keeping and Raising Zebrafish. In: Nüsslein-Volhard C, Dahm R, editors. Zebrafish. Oxford University Press; United Kingdom: 2002. pp. 7–37. [Google Scholar]
- Bu YZ, Li XY, Zhang BJ, Chung IK, Lee JA. Microcystins cause embryonic toxicity in mice. Toxicon. 2006;48 (8):966–972. doi: 10.1016/j.toxicon.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Chiswell RK, Shaw GR, Eaglesham GK, Smith MJ, Norris RL, Seawright AA, Moore MR. Stability of cylindrospermopsin, the toxin from the cyanobacterium Cylindrospermopsis raciborskii: effect of pH, temperature, and sunlight on decomposition. Environ Toxicol. 1999;14 (1):155–165. [Google Scholar]
- Chorus I, Falconer IR, Salas HJ, Bartram J. Health risks caused by freshwater cyanobacteria in recreational waters. J Toxicol Environ Health B Crit Rev. 2000;3 (4):323–347. doi: 10.1080/109374000436364. [DOI] [PubMed] [Google Scholar]
- Codd GA, Morrison LF, Metcalf JS. Cyanobacterial toxins: risk management for health protection. Toxicol Appl Pharmacol. 2005;203 (3):264–272. doi: 10.1016/j.taap.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Dos JM, Vieira S, de P Azevedo MT, de Oliveira Azevedo SM, Honday RY, Correa B. Toxic cyanobacteria and microcystin concentrations in a public water supply reservoir in the Brazilian Amazonia region. Toxicon. 2005;45 (7):901–909. doi: 10.1016/j.toxicon.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Eaglesham GK, Norris RL, Shaw GR, Smith MJ, Chiswell RK, Davis BC, Neville GR, Seawright AA, Moore MR. Use of HPLC-MS/MS to monitor cylindrospermopsin, a blue-green algal toxin, for public health purposes. Environ Toxicol. 1999;14:151–154. [Google Scholar]
- Falconer IR, Humpage AR. Cyanobacterial (blue-green algal) toxins in water supplies: cylindrospermopsins. Environ Toxicol. 2006;21 (4):299–304. doi: 10.1002/tox.20194. [DOI] [PubMed] [Google Scholar]
- Fessard V, Bernard C. Cell alterations but no DNA strand breaks induced in vitro by cylindrospermopsin in CHO K1 cells. Environ Toxicol. 2003;18 (5):353–359. doi: 10.1002/tox.10136. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit analysis. Cambridge University Press; Cambridge: 1971. p. 333. [Google Scholar]
- Froscio SM, Humpage AR, Burcham PC, Falconer IR. Cell-free protein synthesis inhibition assay for the cyanobacterial toxin cylindrospermopsin. Environ Toxicol. 2001;16 (5):408–412. doi: 10.1002/tox.1050. [DOI] [PubMed] [Google Scholar]
- Froscio SM, Humpage AR, Burcham PC, Falconer IR. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocyte. Environ Toxicol. 2003;18 (4):243–251. doi: 10.1002/tox.10121. [DOI] [PubMed] [Google Scholar]
- Froscio SM, Humpage AR, Wickramasinghe W, Shaw G, Falconer IR. Interaction of the cyanobacterial toxin cylindrospermopsin with the eukaryotic protein synthesis system. Toxicon. 2008;51 (2):191–198. doi: 10.1016/j.toxicon.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Gibbs PD, Schmale MC. GFP as a genetic marker scorable throughout the life cycle of transgenic zebrafish. J Mar Biotechnol. 2000;2 (2):107–125. doi: 10.1007/s101269900014. [DOI] [PubMed] [Google Scholar]
- Griffiths DJ, Saker ML. The Palm Island Mystery Disease 20 years on: a review of research on the cyanotoxin cylindrospermopsin. Environ Toxicol. 2003;18 (2):78–93. doi: 10.1002/tox.10103. [DOI] [PubMed] [Google Scholar]
- Hamasaki K, Satoh F, Kikuchi T, Toda T, Satoru T. Biomass and production of cyanobacteria in a coastal water of Sagami Bay, Japan. J Plankton Res. 1999;21 (8):1583–1591. [Google Scholar]
- Harada KI, Ohtani I, Iwamoto K, Suzuki M, Watanabe MF, Watanabe M, Terao K. Isolation of cylindrospermopsin from a cyanobacterium Umezekia natans and its screening method. Toxicon. 1994;32 (1):73–84. doi: 10.1016/0041-0101(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86 (1):6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Humpage AR, Fenech M, Thomas P, Falconer IR. Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutat Res. 2000;472 (1–2):155–161. doi: 10.1016/s1383-5718(00)00144-3. [DOI] [PubMed] [Google Scholar]
- Humpage AR, Falconer IR. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: determination of no observed adverse effect level for deriving a drinking water guideline value. Environ Toxicol. 2003;18 (2):94–103. doi: 10.1002/tox.10104. [DOI] [PubMed] [Google Scholar]
- Ingels FM, Augustijns PF. Biological, pharmaceutical and analytical considerations with respect to the transport media used in the absorption screening system, Caco-2. J Pharm Sci. 2003;92 (8):1545–1558. doi: 10.1002/jps.10408. [DOI] [PubMed] [Google Scholar]
- Jacquet C, Thermes V, de Luze A, Puiseux-Dao S, Bernard C, Joly JS, Bourrat F, Edery M. Effects of microcystin-LR on development of medaka fish embryos (Oryzias latipes) Toxicon. 2004;43 (2):141–147. doi: 10.1016/j.toxicon.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Lagos N, Onodera H, Zagatto PA, Andrinolo D, Azevedo SM, Oshima Y. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsin raciborskii, isolated from Brazil. Toxicon. 1999;37 (10):1359–1373. doi: 10.1016/s0041-0101(99)00080-x. [DOI] [PubMed] [Google Scholar]
- Lecoz N, Malécot M, Quiblier C, Puiseux-Dao S, Bernard C, Crespeau F, Edery M. Effects of crude cyanobacterial crude extracts from Planktothrix agardhii on embryo-larval development of medaka fish, Oryzias latipes. Toxicon. 2008;51 (2):262–269. doi: 10.1016/j.toxicon.2007.09.011. [DOI] [PubMed] [Google Scholar]
- LeFebvre KA, Trainer VL, Scholz NL. Morphological abnormalities and sensorimotor deficits in larval fish exposed to dissolved saxitoxin. Aquat Toxicol. 2004;66 (2):159–170. doi: 10.1016/j.aquatox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Li R, Carmichael WW, Brittain S, Eaglesham GK, Shaw GR, Liu Y, Watanabe MM. First report of the cyanotoxins cylindrospermopsin and deoxycylindrospermopsin from Raphidiopsis curvata. J Phycol. 2001a;37 (6):1121–1126. [Google Scholar]
- Li R, Carmichael WW, Brittain S, Eaglesham GK, Shaw GR, Mahakhant A, Noparatnaraporn N, Yongmanitchai W, Kaya K, Watanabe MM. Isolation and identification of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermposin from a Thailand strain of Cylindrospermopsis raciborskii. Toxicon. 2001b;39 (7):973–980. doi: 10.1016/s0041-0101(00)00236-1. [DOI] [PubMed] [Google Scholar]
- MacGregor GB, Fabbro LD. Dominance of Cylindrospermopsis raciborskii in Queensland tropical and subtropical reservoirs: implications for monitoring and management. Lakes Reserv Res Manage. 2000;5:195–205. [Google Scholar]
- Mahmood NA, Carmichael WW. Paralytic shellfish poisons produced by the freshwater cyanobacterium Aphanizomenon flosaquae NH-5. Toxicon. 1986;24 (2):175–186. doi: 10.1016/0041-0101(86)90120-0. [DOI] [PubMed] [Google Scholar]
- Milutinovic A, Sedmak B, Horvat-Znidarsic I, Suput D. Renal injuries induced by chronic intoxication with microcystins. Cell Mol Biol Lett. 2002;7 (1):139–141. [PubMed] [Google Scholar]
- Negri AP, Jones GJ. Bioaccumulation of paralytic shellfish poisoning (PSP) toxins from the cyanobacterium Anabaena circinalis by the freshwater mussel Alathyria condola. Toxicon. 1995;33 (5):667–678. doi: 10.1016/0041-0101(94)00180-g. [DOI] [PubMed] [Google Scholar]
- Neilan BA, Saker ML, Fastner J, Törökne A, Burns BP. Phylo-geography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Mol Ecol. 2003;12 (1):133–140. doi: 10.1046/j.1365-294x.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- Neumann C, Bain P, Shaw G. Studies on the comparative in vitro toxicology of the cyanobacterial metabolite deoxycylindrospermopsin. J Toxicol Environ Health A. 2007;70 (19):1679–1686. doi: 10.1080/15287390701434869. [DOI] [PubMed] [Google Scholar]
- Norris RL, Eaglesham GK, Shaw GR, Senogles P, Chiswell RK, Smith MJ, Davis BC, Seawright AA, Moore MR. Extraction and purification of the zwitterions cylindrospermopsin and deoxy-cylindrospermopsin from Cylindrospermopsis raciborskii. Environ Toxicol. 2001;16 (5):391–396. doi: 10.1002/tox.1048. [DOI] [PubMed] [Google Scholar]
- Oberemm A, Fastner J, Steinberg CEW. Effects of microcystin-LR and cyanobacterial crude extracts on embryo-larval development of zebrafish (Danio rerio) Water Res. 1997;31 (11):2918–2921. [Google Scholar]
- Ohtani I, Moore RE, Runnegar MTC. Cylindrospermopsin: a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J Am Chem Soc. 1992;114 (20):7942–7944. [Google Scholar]
- Palikova M, Krejci R, Hilscherova K, Babica P, Navratil S, Kopp R, Blaha L. Effect of different cyanobacterial biomasses and their fractions with variable microcystin content on embryonal development of carp (Cyprinus carpio) Aquat Toxicol. 2007;81 (3):312–318. doi: 10.1016/j.aquatox.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Fulton RS, Moisander PH, Dyble J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Scientific WorldJournal. 2001;4:76–113. doi: 10.1100/tsw.2001.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papendorf O, König GM, Wright AD, Chorus I, Oberemm A. Mueggelone, a novel inhibitor of fish development from the freshwater cyanobacterium Aphanizomenon flosaquae. J Nat Prod. 1997;60 (12):1298–1300. doi: 10.1021/np970231s. [DOI] [PubMed] [Google Scholar]
- Pilotto LS, Kliewer EV, Davies RD, Burch MD, Attewell RG. Cyanobacterial (blue-green algal) contamination in drinking water and perinatal outcomes. Aust NZJ Public Health. 1999;23 (2):154–158. doi: 10.1111/j.1467-842x.1999.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Pollingher U, Hada O, Yacobi YZ, Zohary T, Berman T. Aphanizomenon ovalisporum in Lake Kinneret, Israel. J Plankton Res. 1998;20 (7):1321–1339. [Google Scholar]
- Rao PV, Gupta N, Bhaskar AS, Jayaraj R. Toxins and bioactive compounds from cyanobacteria and their implications on human health. J Environ Biol. 2002;23 (3):215–224. [PubMed] [Google Scholar]
- Rogers EH, Zehr RD, Gage MI, Humpage AR, Falconer IR, Marr M, Chernoff N. The cyanobacterial toxin, cylindrospermopsin, induces fetal toxicity in the mouse after exposure late in gestation. Toxicon. 2007;49 (6):855–864. doi: 10.1016/j.toxicon.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Rücker J, Stüken A, Nixdorf B, Fastner J, Chorus I, Wiedner C. Concentrations of particular and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon. 2007;50 (6):800–809. doi: 10.1016/j.toxicon.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Runnegar MT, Kong SM, Zhong YZ, Ge JL, Lu SC. The role of glutathione in the toxicity of a novel cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem Biophys Res Commun. 1994;201 (1):235–241. doi: 10.1006/bbrc.1994.1694. [DOI] [PubMed] [Google Scholar]
- Runnegar MT, Kong SM, Zhong YZ, Lu SC. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocyes. Biochem Pharmacol. 1995;49 (2):219–225. doi: 10.1016/s0006-2952(94)00466-8. [DOI] [PubMed] [Google Scholar]
- Runnegar MT, Chaoyu X, Snider BB, Wallace GA, Weinreb SM, Kuhlenkamp J. In vitro hepatotoxicity of the cyanobacterial alkaloid cylindrospermopsin and related synthetic analogues. Toxicol Sci. 2002;67 (1):81–87. doi: 10.1093/toxsci/67.1.81. [DOI] [PubMed] [Google Scholar]
- Saker ML, Eaglesham GK. The accumulation of cylindrospermopsin from the cyanobacterium Cylindrospermopsis raciborskii in tissues of the Redclaw crayfish Cherax quadricarinatus. Toxicon. 1999;37 (7):1065–1077. doi: 10.1016/s0041-0101(98)00240-2. [DOI] [PubMed] [Google Scholar]
- Saker ML, Griffiths DL. Occurrence of blooms of the cyanobacterium Cylindrospermopsis raciborskii in a north Queensland domestic water supply. Mar Freshw Res. 2001;52:907–915. [Google Scholar]
- Saker ML, Neilan BA. Varied diazotrophies, morphologies, and toxicity of genetically similar isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from Northern Australia. Appl Environ Microbiol. 2001;67 (4):1839–1845. doi: 10.1128/AEM.67.4.1839-1845.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saker ML, Nogueira ICG, Vaconcelos VM, Neilan BA, Eaglesham GK, Pereira P. First report and toxicological assessment of the cyanobacterium Cylindrospermopsis raciborskii from Portuguese freshwaters. Ecotoxicol Envriron Saf. 2003;55 (2):243–250. doi: 10.1016/s0147-6513(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Saker ML, Metcalf JS, Codd GA, Vasconcelos VM. Accumulation and depuration of the cyanobacterial toxin cylindrospermopsin in the freshwater mussel Anodonta cygnea. Toxicon. 2004;43 (2):185–194. doi: 10.1016/j.toxicon.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Seifert M, McGregor G, Eaglesham G, Wickramasinghe W, Shaw G. First evidence for the production of cylindrospermopsin and deoxy-cylindrospermopsin by the freshwater benthic cyanobacterium, Lyngyba wollei. Harmful Algae. 2007;6 (1):73–80. [Google Scholar]
- Shaw RG, Seawright AA, Moore MR, Lam PK. Cylindrospermopsin, a cyanobacterial alkaloid: evaluation of its toxicological activity. Ther Drug Monit. 2000;22 (1):89–92. doi: 10.1097/00007691-200002000-00019. [DOI] [PubMed] [Google Scholar]
- Shen X, Lam PK, Shaw GR, Wickramasinghe W. Genotoxicity investigation of a cyanobacterial toxin, cylindrospermopsin. Toxicon. 2002;40 (10):499–501. doi: 10.1016/s0041-0101(02)00151-4. [DOI] [PubMed] [Google Scholar]
- Shin JT, Fishman MC. From zebrafish to human: modular medical models. Annu Rev Genomics Hum Genet. 2002;3:311–340. doi: 10.1146/annurev.genom.3.031402.131506. [DOI] [PubMed] [Google Scholar]
- Sukenik A, Reisner M, Carmeli S, Werman M. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in mice: long-term exposure to low doses. Environ Toxicol. 2006;21 (6):575–582. doi: 10.1002/tox.20220. [DOI] [PubMed] [Google Scholar]
- Terao K, Ohmori S, Igarishi K, Ohtani I, Watanabe MF, Harada KI, Ito E, Watanabe M. Electron microscope studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green alga Umezekia natans. Toxicon. 1994;32 (7):833–843. doi: 10.1016/0041-0101(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Tiedeken JA, Ramsdell JS, Ramsdell AF. Developmental toxicity of domoic acid in zebrafish (Danio rerio) Neurotoxicol Teratol. 2005;27 (5):711–717. doi: 10.1016/j.ntt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Hiraga T. Zebrafish as a novel experimental model for developmental toxicology. Congenit Anom. 2003;43 (2):123–132. doi: 10.1111/j.1741-4520.2003.tb01036.x. [DOI] [PubMed] [Google Scholar]
- US EPA. Multifactor probit analysis. Washington, D.C: 1991. EPA 600/X-91-101. [Google Scholar]
- Wang PJ, Chien MS, Wu FJ, Chou HN, Lee SJ. Inhibition of embryonic development by microcystin-LR in zebrafish, Danio rerio. Toxicon. 2005;45 (3):303–308. doi: 10.1016/j.toxicon.2004.10.016. [DOI] [PubMed] [Google Scholar]
- White SH, Duivenvoorden LJ, Fabbro LD, Eaglesham GK. Influence of intracellular toxin concentrations on cylindrospermopsin bioaccumulation in a freshwater gastropod (Melanoides tuberculata) Toxicon. 2006;47 (5):497–509. doi: 10.1016/j.toxicon.2005.12.011. [DOI] [PubMed] [Google Scholar]
- White SH, Dulvenvoorden LJ, Fabbro LD, Eaglesham GK. Mortality and toxin bioaccumulation inBufo marinus following exposure to Cylindrospermopsis raciborskii cell extracts and live cultures. Environ Pollut. 2007;147 (1):158–167. doi: 10.1016/j.envpol.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Wright AD, Papendorf O, König GM, Oberemm A. Effects of cyanobacterium Fischerella ambigua isolates and cell-free culture media on zebrafish (Danio rerio) embryo development. Chemosphere. 2006;65 (4):604–608. doi: 10.1016/j.chemosphere.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Yu ZW, Quinn PJ. The modulation of membrane structure and stability by dimethyl sulphoxide. Mol Membr Biol. 1998;15 (2):59–68. doi: 10.3109/09687689809027519. [DOI] [PubMed] [Google Scholar]