Abstract
The renin-angiotensin system plays an important role in the control of blood pressure (BP) and renal function. To illuminate the importance of renin in the context of a disease background in vivo, we used zinc-finger nucleases (ZFNs) designed to target the renin gene and create a renin knockout in the SS/JrHsdMcwi (SS) rat. ZFN against renin caused a 10-bp deletion in exon 5, resulting in a frameshift mutation. Plasma renin activity was undetectable in the Ren−/− rat, and renin protein was absent from the juxtaglomerular cells in the kidney. Body weight was lower in the Ren−/− rats (than in the Ren+/− or wild-type littermates), and conscious BP on low-salt diet (0.4% NaCl) was 58 ± 2 mm Hg in the Ren−/− male rats versus 117 mm Hg in the Ren+/− littermates, a reduction of almost 50 mm Hg. Blood urea nitrogen (BUN) and plasma creatinine levels were elevated in the Ren−/− strain (BUN 112 ± 7 versus 23 ± 2 mg/dL and creatinine 0.53 ± 0.02 versus 0.26 ± 0.02 mg/dL), and kidney morphology was abnormal with a rudimentary inner renal medulla, cortical interstitial fibrosis, thickening of arterial walls, and abnormally shaped glomeruli. The development of the first rat knockout in the renin-angiotensin system demonstrates the efficacy of the ZFN technology for creating knockout rats for cardiovascular disease on any genetic background and emphasizes the role of renin in BP regulation and kidney function even in the low-renin SS rat.
Keywords: renin, zinc finger nucleases, hypertension, kidney, rat knockout
Renin is a limiting factor in the production of angiotensin II. While the kidney is the source of the bulk of circulating renin, nearly every tissue and organ express renin along with other components of the renin-angiotensin system (RAS) comprising the so-called “local” RASs.1 The RAS is involved in blood pressure regulation, kidney and vascular function, angiogenesis, thirst, and many other physiological and pathological conditions, and it has been widely studied in animals and humans, mostly through the use of angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors. Much of our understanding of the regulation of this system has come from a variety of animal models; however, renin function has not been fully characterized in laboratory rats, the preferred model for blood pressure regulation.
We recently produced the first site-directed gene knockout (KO) in the rat using a new technology called zinc-finger nucleases (ZFNs).2,3 ZFNs are engineered proteins that can be injected into the pronucleus of a single-cell embryo to induce deletion mutations in the target region, producing a gene KO and therefore enabling the direct correlation of gene function to phenotypes. Although the renin gene has been knocked out previously in the mouse,4 –10 some strains of mouse possess 2 renin genes as well as several other features that make the RAS different between mouse and human,11 opening the possibility to study renin function in another species.
In this study, we report the first physiological characterization of the renin KO rat. We have measured renin production and expression, kidney and heart histology, blood pressure, and renal function. Renin KO was validated by measuring renin expression and activity in plasma, kidney, and skeletal muscle.
Methods
All animal procedures and breeding were performed at the Medical College of Wisconsin under protocols approved by the Institutional Animal Care and Use Committee.
Generation of a ZFN-Mediated Renin Gene KO Rat
ZFN constructs specific for the rat renin gene were designed, assembled, and validated by Sigma-Aldrich, to target exon 5 (target sequence ACCCTTCATGCTGGCCAAGTTTGACGGGGTTCTGGGCATG) where 1 ZFN binds to each underlined sequence on opposite strands. The DNA recognition helices for the renin gene ZFNs are as follows: TSGHLSR RSDNLSV RNASRIT RSDNLSE DRSHLAR from the N to C terminus of the left-hand ZFN; RSDSLSV DRSHLAR RSDVLSE TSGSLTR RSDHLSR DRSNLTR from the N to C terminus of the right-hand ZFN. mRNA encoding the renin ZFNs was diluted in microinjection buffer (1 mmol/L Tris, 0.1 mmol/L EDTA, pH 7.4) at a concentration of 2 ng/μL and injected into 1-cell SS/McwiHsd (SS) rat embryos as described previously.3 Two hundred one embryos were injected and transferred to pseudopregnant Sprague Dawley females, of which 31 pups were born. At 10 days of age, pups were ear punched, and DNA was extracted and screened for ZFN-induced mutations as described previously.3 Briefly, DNA extracted from ear tissue was amplified using primers flanking the above target sequence Ren_F (5′-gtgaaagccagagcagatcc-3′) and Ren_R (5′-ggaccacctaatcaggagca-3′). PCR products were heat denatured, reannealed (95°C, 2 minutes; 95° to 85°C, −2°C/s; 85° to 25°C, −0.1°C/s; 4°C indefinitely), and subjected to cleavage by the Surveyor Nuclease (Cel-I; Trans-genomic) according to the manufacturer’s instructions. Ten microliters of each reaction were loaded on a 10% Tris/Borate/EDTA polyacrylamide gel (Bio-Rad Laboratories) and poststained with 1× GelStar nucleic acid gel stain (Cambrex Bio Science). Among the 31 pups born, 1 positive female was identified and sequenced to reveal a 10-bp frameshift deletion of gccaagtttg in exon 5 of the renin gene, resulting in truncation of the normal 402-amino acid renin protein after amino acid L185 and insertion of 28 additional nonsense amino acids before a stop codon is introduced. As previous reports2,12 have demonstrated that off-target effects of ZFNs are rare and can therefore be easily separated from the target locus by backcrossing, the founder female rat was backcrossed to an SS male. To further mitigate any possible effects of off-target mutations, multiple separate pairs of mutation-carrying progeny were then intercrossed to generate an F2 population that was used for phenotyping and breeding to homozygosity.
Measurement of Renin Activity, Plasma Ang I Levels, Renin Gene Expression, and Immunohistochemistry
Eight-week– old rats (Ren−/−, Ren+/−, and wild-type Ren+/+ littermates) were anesthetized with pentobarbital. One milliliter of blood was collected by direct cardiac puncture for plasma renin activity (PRA) and analyzed based on the method of Sealey et al.13,14 Kidneys from each animal were collected for expression and histological analysis. For histological analysis, 1 kidney from each animal was placed in a 10% formalin solution in phosphate buffer. The kidneys were paraffin embedded using an automatic tissue processor (Microm HMP 300), cut in 3 μm sections (Microm HM355S), and mounted on silanized/charged slides. Slides were stained with Gomori’s One-Step Trichrome or immunohistological detection of renin as described previously.15 Briefly, slides were deparaffinized and incubated in 0.3% hydrogen peroxide and 50% methanol for 30 minutes followed by the endogenous avidin and biotin blocking kit (Vector Laboratories). Slides were blocked in horse serum and incubated against antirenin antibody (1:100; Santa Cruz Biotechnology) for 1 hour. Sections were rinsed and incubated with a biotinylated anti-goat secondary antibody (Vector Laboratories) for 1 hour, followed by Vectastain Elite ABC reagent per the manufacturer’s instructions (Vector Laboratories). Staining was visualized with 3,3′-diaminobenzidine and peroxidase. Tissue sections were photographed using a Nikon E-400 fitted with a Spot Insight camera.
For measurement of renin mRNA expression, kidneys were quartered, and 1 kidney sample from each animal was homogenized in 1 mL of Trizol (Invitrogen) with a TissueLyser II including a 5-mm stainless steel bead, and 2 cycles of 30.0 Hz for 2 minutes. Total RNA was isolated by guanidinium isothiocyanatephenol-chloroform extraction per the manufacturer’s protocol. Briefly, RNA from homogenized samples was extracted with chloroform and precipitated with isopropanol. Precipitated RNA was washed 3 times with 70% (v/v) ethanol and resuspended in diethyl pyrocarbonate-treated water. Samples were treated with DNase I (Fermentas) per the manufacturer’s protocol, for 15 minutes at room temperature, to remove any potential genomic DNA contamination. RNA quality and concentration were assessed with a nano-drop spectrophotometer at 260 nm, and samples were immediately subjected real-time polymerase chain reaction (PCR) on a 7900HT real-time PCR machine (Applied Biosystems). Samples were run with the Taqman One-step kit (Applied Biosystems) per the manufacturer’s instructions and the following oligos: renin forward 5′-GGTGCCCTCCACCAAGTGT, renin reverse 5′-GCTAGAGGATTCCGAGGAGTC primers, renin probe 5′-[6FAM]TCCCCTCTACACTGCCTGTGAGATTCACA[TAMARA] (Sigma), or the Taqman Ribosomal control kit. Analysis was performed as described by Knoll et al.16
The method used for measurements of PRA and Ang I was performed as described previously.17 Briefly, arterial blood was collected in tubes containing K3EDTA and immediately centrifuged at 1500g and 4°C. The samples were thawed on ice, and neomycin sulfate (0.1%), phenylmethylsulfonyl fluoride (0.25%), and maleic anhydrate (0.2 mol/L) were added to 50 μL of sample to inhibit converting enzyme and protease activity. After generation, ANG concentration was determined by radioimmunoassay.17
Blood Pressure, Renal Function, and Serum Biochemistry
All rats (breeders and experimental animals) were fed 0.4% NaCl AIN-76 diet (Dyets). At 8 weeks of age, rats were placed in metabolic cages (40615; Laboratory Products) to acclimate for 24 hours, followed by a 24-hour urine collection. Urine was measured for total urine volume; Na, K, creatinine, protein, and microalbumin excretion; and urine osmolarity. At 9 weeks of age, rats were anesthetized with 2% isoflurane, and a blood pressure transmitter (PA-C40; DSI) was surgically implanted subcutaneously with the catheter tip secured in the abdominal aorta via the femoral artery. During the transmitter implant procedure, 1.2 mL of blood was collected from the femoral artery for serum analysis. After a 3-day recovery period, blood pressure was measured by radiotelemetry in conscious freely moving animals for 3 consecutive days, 3 hours/day, and averaged. At the end of the study, the 10-week– old rats were anesthetized with isoflurane, and retroperitoneal fat, kidneys, and heart were collected, weighed, fixed in formaldehyde, and stained for hematoxylineosin and trichrome for histological analysis. Serum collected during implant was sent to Marshfield Labs (a division of Marshfield Clinic) for analysis of serum glucose, aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase, total bilirubin, cholesterol, total protein, albumin, globulin, urea N, creatinine, phosphorous, Ca, Na, K, Cl, bicarbonate, anion gap, gamma-GT, albumin/globulin ratio (A/G ratio), lactate dehydrogenase (LDH), and creatine kinase (CK).
Results
The SS Ren−/− strain has a deletion of 10 bp in exon 5; this causes a truncation of the message from 1451 to 1441 bp leading to a frameshift and premature truncation of the normal open reading frame after codon 185.
Ang I Levels and Renin Expression, Activity, and Immunohistochemistry
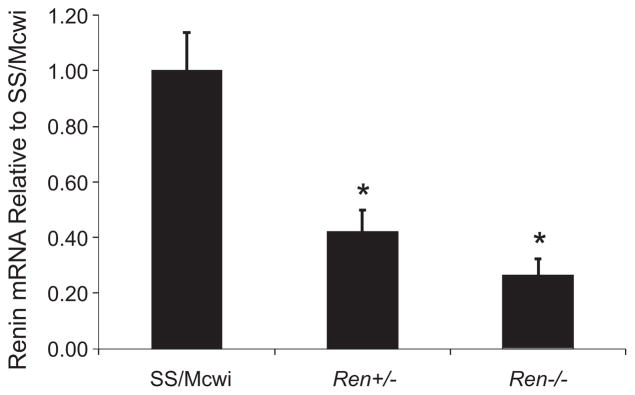
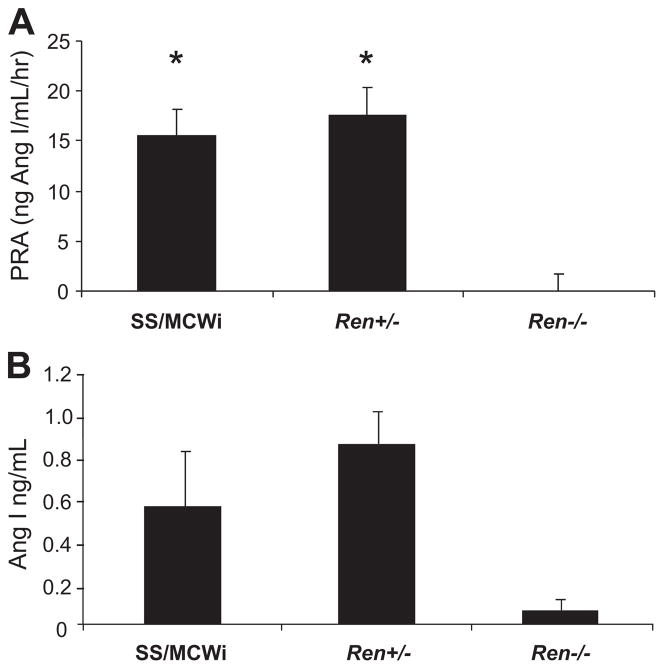
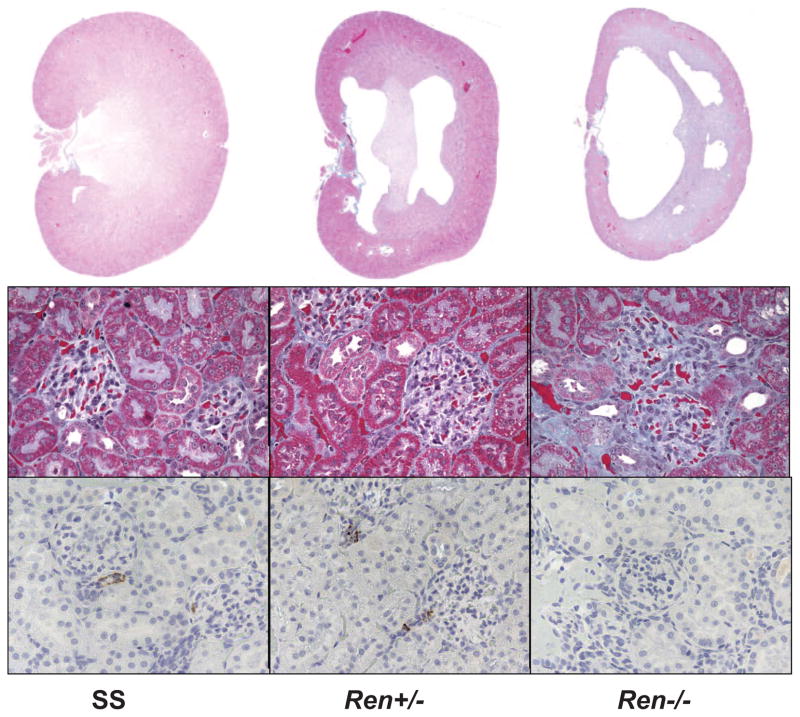
Figure 1 represents the gene expression of renin in the kidney of the Ren−/− compared to the wild-type rats and rats heterozygous for the mutation. Ren−/− rats had a 5-fold decrease in renin expression compared to the parental SS rat. The Ren+/− rat also showed a 60% decreased renin expression in the kidney compared to the parental rat. PRA measured in a deeply anesthetized state was 15.6 ± 2.9 ng Ang I/mL per hour in the SS rats, which was not different in the KO heterozygous rat (17.5 ± 1.6 ng Ang I/mL per hour (Figure 2). The PRA in the Ren−/− rats was undetectable (minimum detectable level of the assay was 0.025 ± 0.0 ng Ang I/mL per hour). Similarly, there was no difference in plasma Ang I levels between SS and heterozygous rats (0.55 ± 0.26 ng Ang I/mL in the SS rats versus 0.85 ± 0.16 ng Ang I/mL in the Ren+/− rat), while Ren−/− rats’ Ang I plasma levels were below background (0.06 ± 0.03 ng Ang I/mL), as shown in Figure 2. Also, there was no detectable renin in the juxtaglomerular cells of the Ren−/− rat compared to the SS and heterozygous rats (Figure 3).
Figure 1.
Renin kidney expression. Renal mRNA expression of Ren in wild-type (n = 5), Ren+/− (n = 4), and Ren−/− (n = 5) rats, as relative to control SS values. Data are expressed as mean ± SE. *P < 0.05, different from wild type by 1-way ANOVA.
Figure 2.
PRA and Ang I in anesthetized rats. A, PRA was compared in anesthetized wild-type (n = 4), Ren+/− (n = 13) and Ren−/− (n = 5) rats. Data are expressed as mean ± SE.*P < 0.005, different from Ren−/− by 1-way ANOVA. B, Plasma Ang I levels in the same animals.
Figure 3.
Histological data from SS renin KO rat. Top left, Magnified (×1) renal midline section from SS. Top middle, Magnified (×1) renal midline section from Ren+/−. Top left, Magnified (×1) renal midline section from Ren−/−. Middle row, Magnified (×20) view of renal cortical glomeruli from SS, Ren+/−, and Ren−/−. Bottom row, Staining for renin in SS, Ren+/−, and Ren−/−.
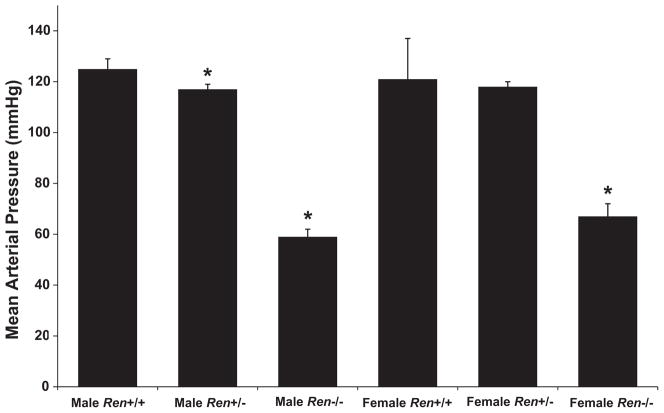
Morphometric and urine function measurements of Ren+/+, Ren+/−, and Ren−/− rats are shown in Table 1. Body weight was significantly lower in the Ren−/− strain, in both males and females. Heart weight per body weight was also reduced in the Ren−/−. As for body fat, retroperitoneal fat weight was reduced in both male and female Ren−/− rats. The KO of the renin gene did not affect the mortality rate within the first 6 months of life, which was approximately 10% in all strains. Blood pressure was reduced over 50 mm Hg in both male and female Ren−/− rats (Figure 4 and Table 2), and urine flow was significantly increased, but not sodium excretion.
Table 1.
Morphological and Biochemical Characterization of ZFN Renin KO and Control SS Rats
| Morphometric and Renal Function Parameters | Male
|
Female
|
||||
|---|---|---|---|---|---|---|
| Ren+/+ | Ren+/− | Ren−/− | Ren+/+ | Ren+/− | Ren−/− | |
| BW, g | 301.00 ± 18.06 | 321.00 ± 11.14 | 220.50 ± 10.70* | 204.50 ± 3.31 | 203.13 ± 3.02 | 148.75 ± 4.94* |
| %HW/BW | 0.39 ± 0.01 | 0.36 ± 0.01* | 0.31 ± 0.01* | 0.43 ± 0.02 | 0.43 ± 0.01 | 0.38 ± 0.01* |
| %RKW/BW | 0.49 ± 0.03 | 0.46 ± 0.02 | 0.46 ± 0.03 | 0.51 ± 0.02 | 0.51 ± 0.02 | 0.59 ± 0.03* |
| %FW/BW | 0.44 ± 0.07 | 0.49 ± 0.05 | 0.23 ± 0.04* | 0.32 ± 0.02 | 0.27 ± 0.03 | 0.17 ± 0.02* |
| UF, ml/day | 7.72 ± 1.55 | 7.04 ± 1.40 | 22.19 ± 1.98* | 5.09 ± 0.47 | 8.30 ± 2.15 | 17.38 ± 4.75* |
| Una, mEq/day | 0.65 ± 0.25 | 0.81 ± 0.08 | 0.46 ± 0.10 | 0.71 ± 0.08 | 0.55 ± 0.11 | 0.34 ± 0.14 |
| Uprot, mg/day | 93.08 ± 9.96 | 85.47 ± 10.93 | 7.83 ± 0.95* | 52.72 ± 10.67 | 55.45 ± 5.64 | 12.75 ± 8.08* |
| Ucreat, mg/day | 7.94 ± 0.82 | 8.41 ± 0.40 | 4.85 ± 0.28* | 6.05 ± 0.18 | 5.40 ± 0.18† | 3.88 ± 0.61* |
| Creat Cl/KW | 0.90 ± 0.1 | 0.83 ± 0.08 | 0.33 ± 0.02* | 0.87 ± 0.10 | 0.79 ± 0.07 | 0.30 ± 0.03* |
| BUN, mg/dL | 25.40 ± 1.96 | 23.12 ± 1.66 | 112.25 ± 7.52* | 21.88 ± 1.38 | 25.78 ± 1.15 | 108.00 ± 3.24* |
| PlCreat, mg/dL | 0.22 ± 0.03 | 0.26 ± 0.02 | 0.53 ± 0.02* | 0.27 ± 0.03 | 0.26 ± 0.02 | 0.43 ± 0.03* |
| ALT(GPT), U/L | 33.40 ± 1.93 | 35.78 ± 5.57 | 53.63 ± 5.74† | 27.63 ± 1.69 | 31.00 ± 2.36 | 47.50 ± 1.98* |
| PlCl, mmol/L | 97.8 ± 0.66 | 98.89 ± 0.72 | 104.00 ± 0.58* | 96.88 ± 0.56 | 99.00 ± 0.54 | 107.25 ± 0.87* |
| PlBicarb, mmol/L | 27.60 ± 0.58 | 27.67 ± 0.50 | 20.88 ± 0.72* | 24.00 ± 0.46 | 25.78 ± 0.79 | 16.75 ± 1.20* |
Values are mean ± SE. BW indicates body weight at 10 weeks of age; HW, heart weight; RKW, right kidney weight; FW, retroperitoneal fat weight; UF, urine flow; UNa, sodium excretion; Uprot, protein excretion; Ucreat, creatinine excretion; Creat Cl, creatinine clearance; BUN, blood urea nitrogen; PlCreat, plasma creatinine; PlCl, plasma chloride; PlBicarb, plasma bicarbonate.
P < 0.01 from wild type (+/+) within gender;
P < 0.05 from wild type (+/+) within gender.
Figure 4.
Mean arterial pressure in conscious renin KO rats. Blood pressure in the renin KO rats was significantly decreased in both male and female rats. Data are expressed as mean ± SE. *P < 0.05 from wild type (+/+).
Table 2.
Hemodynamic Characterization of ZFN Renin KO and Control SS Rats
| Hemodynamic Parameter | Male
|
Female
|
||||
|---|---|---|---|---|---|---|
| Ren+/+ | Ren+/− | Ren−/− | Ren+/+ | Ren+/− | Ren−/− | |
| SBP, mm Hg | 153.22 ± 1.42 | 144.19 ± 2.22* | 72.07 ± 2.42* | 138.76 ± 4.52 | 139.28 ± 2.25 | 76.38 ± 2.94* |
| MAP, mm Hg | 125.46 ± 2.07 | 116.63 ± 2.07* | 58.36 ± 2.38* | 112.80 ± 3.55 | 115.55 ± 1.50 | 61.59 ± 2.87* |
| DBP, mm Hg | 101.76 ± 2.81 | 93.33 ± 1.98† | 46.44 ± 2.34* | 89.42 ± 3.12 | 94.19 ± 1.82 | 49.10 ± 2.61* |
| HR, bpm | 366.02 ± 6.86 | 357.55 ± 50 | 402.94 ± 4.46* | 386.01 ± 5.71 | 390.95 ± 11.68 | 436.56 ± 7.76* |
Values are mean ± SE. SBP indicates systolic blood pressure; MAP, mean arterial pressure; DBP, diastolic blood pressure; HR, heart rate.
P < 0.05 from wild type (+/+) within gender.
P < 0.01 from wild type (+/+) within gender.
Despite having a reduced protein excretion, the Ren−/− rats had an elevated plasma urea and creatinine and a decreased creatinine excretion and clearance, indicating a reduced glomerular filtration. ALT levels were also elevated in the homozygous renin KO rats, which had also high plasma chloride and low bicarbonate, similar to what is observed in a nephrogenic diabetes insipidus.
Other parameters that were measured in serum but were not affected by renin KO were phosphorus, bilirubin, LDH, cholesterol and triglycerides, alkaline phosphatase, and globulin.
Histological Analysis of the Kidneys
Wild-type Ren+/+ kidneys showed a moderate degree of interstitial and perivascular fibrosis throughout the cortex and outer medulla (Figure 3). Moderate protein casts were visible in the cortical collecting ducts; however, the morphology of the inner and outer medulla was normal. Kidneys from both the homozygous and heterozygous renin KO rats were clearly atypical with the homozygous displaying an increasing degree of uncharacteristic features compared to the heterozygotes or the wild-type Ren+/+. Both Ren−/− and Ren+/− rats revealed significant architectural abnormalities including a displaced medulla, incomplete formation of the medullary rays, the presence of large central lesions, and cortical thinning, all of which were more pronounced in the homozygotes. Closer examination showed improper orientation of collecting ducts and distal tubules. Tubules were disorganized with no evidence of obstruction. The cortex displayed a “moth-eaten” appearance that continued into the medulla. Intracellular protein globules were evident in the tubular epithelium. Cortical glomeruli were scarce, and those that were present displayed attenuation of capillary loops. Arterioles were dramatically hypertrophied to the extent that many appeared to be completely closed. Both interstitial and perivascular fibrosis was apparent throughout the cortex. Among the most striking features of these kidneys was the extent of chronic inflammatory infiltrates forming fibroid follicles. Tubular lymphohistocytic infiltrate with abundant macrophage and lymphoblastic cell infiltration was most apparent in the homozygotes but was also observed, to a lesser extent, in the heterozygote KOs. Large numbers of apoptotic cells were evident throughout the kidney, but were most pronounced on the periphery of the central lesion and in the papillated villous-like stalks penetrating from the cortex into the pelvis. Hearts from the SS and KOs were morphologically normal. Myocytes appeared normal with no observable hypertrophy or disarray in any of the groups. There was no arteriolar hypertrophy in the hearts of the SS or the KOs.
Discussion
By the use of ZFNs, we have deleted 10 bp in exon 5 of the Ren gene in the rat, causing also a frameshift mutation. This caused inactivation of the renin gene, evidenced by the absence of renin granules in the juxtaglomerular cells of the renal afferent arteriole, and no measurable PRA in the Ren−/− rats. Although Ren expression was also reduced in the Ren+/− rats, this did not translate in reduced PRA or plasma Ang I levels, suggesting that the remaining, functional Ren allele is capable of maintaining normal PRA in adult rats. The reduced expression of the truncated message in the Ren−/− rats could mean an increased degradation of the “abnormal” message. The abnormal mRNA in the Ren−/− rats must be really very unstable (probably because of nonsense-mediated decay), since it can be expected that the lack of renin protein leads to a dramatic increase in renal renin transcription.
The disruption of the Ren gene caused profound changes in kidney morphology. This is similar to what has been observed in mouse after the ablation of renin cells.6 It is not possible to discern which of the observed changes are due specifically to the absence of renin as opposed to the lack of angiotensin II production, since it is known that Ang II inhibition causes changes in the number of glomeruli11,18 and mouse KO for other RAS components also show renal abnormalities.11
Both male and female Ren−/− rats show a greatly reduced blood pressure. This has been also observed in mice with different deletions of the RAS system.5,9,19 –22 This decrease in blood pressure is accompanied by a reduction in the protein excretion, characteristic of the SS rat.23 This observed reduction in protein excretion is most likely secondary to the lowering of blood pressure.24,25 There is also evidence of a decrease in renal function, reflected by the reduced creatinine excretion and creatinine clearance in the renin Ren−/− rats. This abnormal renal function could be secondary to a reduction in blood pressure below autoregulation limits or caused by a decrease in the number of glomeruli, and their abnormal morphology. The reduced blood pressure and kidney function did not translate in a higher mortality rate within the first 6 months of life in the renin Ren−/− rats, which could be surprising somehow. It is possible, though, that there is a higher mortality rate as rats age.
Other features observed in the Ren−/− rats are a decreased body weight and body fat, an increased urine flow with normal sodium excretion, and an increase in serum ALT.
It is interesting to note that the heterozygous Ren+/− rats captured some features of the homozygous Ren−/− rats, whereas other traits were not different from the parental SS rats. For example, both Ren+/− and Ren−/− animals displayed significant gross morphological and histochemical changes in the kidney that were qualitatively similar but of greater severity in Ren−/−. Despite that, renal function was preserved in the Ren+/−. In the heart, where the RAS has been reported to be an important contributor to the hypertrophy and fibrosis observed in the SS rat,26,27 there were no differences between the KO and the parental strain.
In the present study, we used ZFN technology to KO renin on the background of the inbred SS rat. This is significant because it allowed us to evaluate the role of renin in the context of a well-characterized disease model and revealed that in this low-renin hypertensive strain elimination of renin results in a profound reduction in blood pressure. Because ZFN technology allows the KO of genes on any genetic background with different susceptibility for specific diseases or traits, it is possible to evaluate the role of a gene such as renin in the pathogenesis of the disease compared to the normal state. This flexibility provides an exceptional tool to study gene-gene interaction and also eliminates the risk of introducing modifier loci that can confound the interpretation of KO experiments.
In conclusion, these studies demonstrate the efficacy of the ZFN technology for creating KO rats on a disease background and emphasize the role of renin in blood pressure regulation and kidney function even in the low-renin SS rat.
Perspectives
Current ZFN technology has enabled the development of gene-targeted KOs in rats in zygotes, without the need of homologous recombination in embryonic stem cells, a tool widely used to modify the mouse genome. Generation of conditional and tissue-specific mutants by homologous recombination in zygotes using ZFN is underway.28 This will provide an invaluable tool for the study of gene function and, in the case of renin, will allow us to tease out the different roles of the RAS in organ development and function.
Acknowledgments
We thank Carol Bobrowitz, Camille Torres, Jason Klotz, Rebecca Schilling, Jaime Foeckler, and Shawn Kalloway for technical assistance.
Sources of Funding
This work was supported by National Institutes of Health (NIH) grants HL-82798 and HL-101681, and NIH–National Heart, Lung, and Blood Institute contract N01-HV-28182.
Footnotes
Disclosures
None.
References
- 1.Dzau VJ. Circulating versus local renin-angiotensin system in cardiovascular homeostasis. Circulation. 1988;77:I4–I13. [PubMed] [Google Scholar]
- 2.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Menoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geurts AM, Cost GJ, Remy S, Cui X, Tesson L, Usal C, Menoret S, Jacob HJ, Anegon I, Buelow R. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol. 2010;597:211–225. doi: 10.1007/978-1-60327-389-3_15. [DOI] [PubMed] [Google Scholar]
- 4.Clark AF, Sharp MG, Morley SD, Fleming S, Peters J, Mullins JJ. Renin-1 is essential for normal renal juxtaglomerular cell granulation and macula densa morphology. J Biol Chem. 1997;272:18185–18190. doi: 10.1074/jbc.272.29.18185. [DOI] [PubMed] [Google Scholar]
- 5.Pentz ES, Lopez ML, Kim HS, Carretero O, Smithies O, Gomez RA. Ren1d and ren2 cooperate to preserve homeostasis: evidence from mice expressing gfp in place of ren1d. Physiol Genomics. 2001;6:45–55. doi: 10.1152/physiolgenomics.2001.6.1.45. [DOI] [PubMed] [Google Scholar]
- 6.Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol. 2004;286:R474–R483. doi: 10.1152/ajpregu.00426.2003. [DOI] [PubMed] [Google Scholar]
- 7.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi N, Lopez ML, Cowhig JE, Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- 9.Yanai K, Saito T, Kakinuma Y, Kon Y, Hirota K, Taniguchi-Yanai K, Nishijo N, Shigematsu Y, Horiguchi H, Kasuya Y, Sugiyama F, Yagami K, Murakami K, Fukamizu A. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem. 2000;275:5–8. doi: 10.1074/jbc.275.1.5. [DOI] [PubMed] [Google Scholar]
- 10.Matsusaka T, Kon V, Takaya J, Katori H, Chen X, Miyazaki J, Homma T, Fogo A, Ichikawa I. Dual renin gene targeting by cre-mediated inter-chromosomal recombination. Genomics. 2000;64:127–131. doi: 10.1006/geno.2000.6113. [DOI] [PubMed] [Google Scholar]
- 11.Fogo A, Ichikawa I. Renin angiotensin system in development of mice and men. Am J Pathol. 1996;149:1797–1801. [PMC free article] [PubMed] [Google Scholar]
- 12.Mashimo T, Takizawa A, Voigt B, Yoshimi K, Hiai H, Kuramoto T, Serikawa T. Generation of knockout rats with x-linked severe combined immunodeficiency (x-scid) using zinc-finger nucleases. PLoS One. 2010;5:e8870. doi: 10.1371/journal.pone.0008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amaral SL, Roman RJ, Greene AS. Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl s rats. Hypertension. 2001;37:386–390. doi: 10.1161/01.hyp.37.2.386. [DOI] [PubMed] [Google Scholar]
- 14.Sealey JE, Laragh JH. Radioimmunoassay of plasma renin activity. Semin Nucl Med. 1975;5:189–202. doi: 10.1016/s0001-2998(75)80033-x. [DOI] [PubMed] [Google Scholar]
- 15.Santos CF, Akashi AE, Dionisio TJ, Sipert CR, Didier DN, Greene AS, Oliveira SH, Pereira HJ, Becari C, Oliveira EB, Salgado MC. Characterization of a local renin-angiotensin system in rat gingival tissue. J Periodontol. 2009;80:130–139. doi: 10.1902/jop.2009.080264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoll KE, Pietrusz JL, Liang M. Tissue-specific transcriptome responses in rats with early streptozotocin-induced diabetes. Physiol Genomics. 2005;21:222–229. doi: 10.1152/physiolgenomics.00231.2004. [DOI] [PubMed] [Google Scholar]
- 17.Rieder MJ, Roman RJ, Greene AS. Reversal of microvascular rarefaction and reduced renal mass hypertension. Hypertension. 1997;30:120–127. doi: 10.1161/01.hyp.30.1.120. [DOI] [PubMed] [Google Scholar]
- 18.Saez F, Castells MT, Zuasti A, Salazar F, Reverte V, Loria A, Salazar FJ. Sex differences in the renal changes elicited by angiotensin ii blockade during the nephrogenic period. Hypertension. 2007;49:1429–1435. doi: 10.1161/HYPERTENSIONAHA.107.087957. [DOI] [PubMed] [Google Scholar]
- 19.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin ii type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 20.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (at2) angiotensin receptor-mediated blood pressure regulation in mice lacking both at1a and at1b receptors for angiotensin ii. Proc Natl Acad Sci U S A. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O’Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ace-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 22.Davisson RL, Kim HS, Krege JH, Lager DJ, Smithies O, Sigmund CD. Complementation of reduced survival, hypotension, and renal abnormalities in angiotensinogen-deficient mice by the human renin and human angiotensinogen genes. J Clin Invest. 1997;99:1258–1264. doi: 10.1172/JCI119283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension. 1985;7:340–349. [PubMed] [Google Scholar]
- 24.Moreno C, Kaldunski ML, Wang T, Roman RJ, Greene AS, Lazar J, Jacob HJ, Cowley AW., Jr Multiple blood pressure loci on rat chromosome 13 attenuate development of hypertension in the dahl s hypertensive rat. Physiol Genomics. 2007;31:228–235. doi: 10.1152/physiolgenomics.00280.2006. [DOI] [PubMed] [Google Scholar]
- 25.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW., Jr High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol. 2008;19:1472–1482. doi: 10.1681/ASN.2007121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakata Y, Masuyama T, Yamamoto K, Doi R, Mano T, Kuzuya T, Miwa T, Takeda H, Hori M. Renin angiotensin system-dependent hypertrophy as a contributor to heart failure in hypertensive rats: different characteristics from renin angiotensin system-independent hypertrophy. J Am Coll Cardiol. 2001;37:293–299. doi: 10.1016/s0735-1097(00)01064-0. [DOI] [PubMed] [Google Scholar]
- 27.Wake R, Kim-Mitsuyama S, Izumi Y, Yoshida K, Izumiya Y, Yukimura T, Shiota M, Yoshiyama M, Yoshikawa J, Iwao H. Beneficial effect of candesartan on rat diastolic heart failure. J Pharmacol Sci. 2005;98:372–379. doi: 10.1254/jphs.fp0050160. [DOI] [PubMed] [Google Scholar]
- 28.Meyer M, de Angelis MH, Wurst W, Kuhn R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proc Natl Acad Sci U S A. 2010;107:15022–15026. doi: 10.1073/pnas.1009424107. [DOI] [PMC free article] [PubMed] [Google Scholar]