Abstract
Background
No prior studies have related a tobacco-specific carcinogen to risk of lung cancer in smokers. Of the over 60 known carcinogens in cigarette smoke, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is specific to tobacco and causes lung cancer in laboratory animals. Its metabolites, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides (total NNAL), have been studied as biomarkers of exposure to NNK. We studied the relation of prospectively measured NNK biomarkers to lung cancer risk.
Methods
In a case-control study nested in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, we randomly selected 100 lung cancer cases and 100 controls who smoked at baseline and analyzed their baseline serum for total NNAL, cotinine and r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene (PheT), a biomarker of polycyclic aromatic hydrocarbon exposure and metabolic activation. To examine the association of the biomarkers with all lung cancer and for histologic subtypes, we computed odds ratios (OR) for total NNAL, PheT and cotinine using logistic regression to adjust for potential confounders.
Findings
Individual associations of age, smoking duration, and total NNAL with lung cancer risk were statistically significant. After adjustment, total NNAL was the only biomarker significantly associated with risk (OR = 1.57 per unit standard deviation increase, 95% confidence interval: 1.08, 2.28). A similar statistically significant result was obtained for adenocarcinoma risk, but not for non-adenocarcinoma.
Conclusions
This first reporting of the effect of the prospectively measured tobacco-specific biomarker, total NNAL, on risk of lung cancer in smokers provides insight into the etiology of smoking-related lung cancer and reinforces targeting NNK for cancer prevention.
Introduction
Cigarette smoking causes 90% of lung cancer, the major cancer killer in the world, with 1.2 million deaths expected annually.(1) At least some of the over 60 established carcinogens in cigarette smoke undoubtedly are collectively responsible for lung cancer outcomes.(2) Which specific carcinogens are responsible for lung cancer and their contribution to risk is less clear. Tobacco carcinogen biomarkers, which are metabolites or macromolecular binding products related to specific carcinogens in cigarette smoke, have been widely used to measure exposure in smokers and to demonstrate the uptake of specific carcinogens under various conditions, including exposure to secondhand smoke.(3–5) However, no reports to date relate any specific tobacco carcinogen biomarker to lung cancer risk. Doing so could improve our understanding of lung cancer etiology in smokers and provide a basis for rational approaches to prevention and even therapy. Furthermore, such biomarkers could ultimately become part of a predictive algorithm for identifying those smokers most likely to get lung cancer. This algorithm, which has evaded researchers to date, could serve not only to identify long-term smokers needing more vigorous intervention or surveillance, but also perhaps to identify among smokers newly acquiring the habit those with demonstrably increased susceptibility, in hopes that the increased risk may help motivate them to give up smoking.
To begin to address this problem, we measured two tobacco carcinogen biomarkers: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides (together designated total NNAL) and r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene (PheT) in prospectively obtained blood samples in a large screening study. Total NNAL is a biomarker of exposure to the powerful tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) while PheT is a biomarker of uptake plus metabolic activation of phenanthrene, a representative of the polycyclic aromatic hydrocarbon (PAH) class of cigarette smoke carcinogens.(3, 6–8) We obtained serum samples from blood taken shortly after randomization at the first screening visit from a group of current smokers at baseline who participated in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO),(9) sponsored by the U.S. National Cancer Institute (NCI). We then analyzed those samples, all of which were taken before any diagnosis of lung cancer, for total NNAL and PheT. We also measured serum cotinine, a widely used biomarker of nicotine and tobacco exposure, but not a carcinogen biomarker.(10) By comparing serum levels in subjects with lung cancer to those in controls, we estimated the association of the biomarkers with the risk of lung cancer.
Methods
Parent Study
The PLCO is an NCI-funded multi-center randomized trial of screening for cancers of the prostate, lung, colorectum and ovaries that began in 1993 and is projected to end in 2011.(9) The group offered screening in the trial includes 77,468 men and women, of whom approximately 25,000 are current or former smokers. In addition to annually screening participants and carefully abstracting cancers from medical records, the PLCO has prospectively collected extensive information from study participants, including smoking history, family history of cancer, and demographic information collected at randomization, and it maintains a bio-repository of blood samples drawn over six annual screening visits starting in 1993. The PLCO trial made available its prospectively collected blood samples from the first screening visit and its extensive data, thus providing for the direct calculation of lung cancer risks in the groups with different baseline levels of biomarkers. In addition, the PLCO screening cohort ensured that all cases of lung cancer had been screened at least once and so the variability in diagnostic lead times and the potential confounding that variability can produce in unscreened cohorts was substantially reduced. At the time our study was approved, over 800 lung cancer cases had been diagnosed in the screening arm of the PLCO. We randomly selected cases and controls from subjects who reported currently smoking on the baseline questionnaire filled out at the time of randomization.
The PLCO was approved by the institutional review boards of each participating institution, and all subjects signed consents permitting the research represented here.
Case-control Study
We used a nested case-control approach wherein the source cohort consisted of PLCO participants who at randomization filled out a baseline questionnaire indicating they were free of cancer and currently smoking at least 10 cigarettes per day, and who contributed adequate blood samples to the biorepository. Cases were those diagnosed with lung cancer and controls were those with no diagnosis of lung cancer before the cut-off date (August 17, 2007). From this cohort, we randomly selected 100 incident lung cancer cases and 100 controls and obtained their demographic and other baseline data from the PLCO database, as well as serum samples adequate to measure cotinine, NNAL, and PheT. The intent was to determine whether or not biomarker levels in lung cancer subjects differ from those in non-lung cancer subjects. We hypothesized that the levels of tobacco carcinogen and their specific metabolites among long-term current smokers predispose them to higher risks of developing lung cancer. We did not match on any characteristics, choosing to control for age, sex, family history, and smoking exposure by post-adjustment; this avoided over-matching and allowed us to examine the risks associated with these factors in comparison to those for the biomarkers. (11) The sample size was determined to provide 80% power with 95% confidence for an OR of 1.5 for 1 standard-deviation difference in serum biomarker level.
Laboratory methods
The methods for assaying NNAL and PheT in blood samples have been previously published.(12) Both are stable compounds and have elimination half-lives of 40 – 45 days(13) and 2–3 days, respectively. Total cotinine (free cotinine plus cotinine N-glucuronide) concentration in serum was quantified by gas chromatography-mass spectrometry. The method was similar to that used previously to analyze urinary cotinine(13), with the addition of a solid phase extraction step carried out on an MCX column (Waters Corporation, Milford MA). The MCX column was prepared and the sample eluted as described previously.(14) All analyses were performed in sets with positive and negative controls for quality control, and without knowledge of sample identities. No duplicates were performed due to inadequate amounts of available serum.
Statistical Methods
Standard descriptive analyses were conducted on continuous and discrete variables. Bivariate associations between case/control status and each covariate were examined with t-test or chi-square statistics, as appropriate, and odds ratios were estimated with 95% confidence intervals. A simple causal diagram was constructed based on hypothesized relations between demographic and biologic variables, which modeled the relations between age, sex, socio-economic status (SES), enrollment and screening in PLCO, tobacco use, biomarkers, susceptibility, carcinogenesis, and lung cancer diagnosis. Based on the hypothesized causal diagram, we used logistic regression to adjust the biomarker effects for potential confounders(15) and for associated covariates to improve power. Specific covariates included in the regression were sex, age at randomization, family history of lung cancer, cotinine, total NNAL, PheT, and years of cigarette smoking. Untransformed biomarker measurements were used in the regression, as the distributions were reasonably symmetric. An advantage of this study design is that it allows for conditioning on PLCO enrollment; thus, potential confounding by factors that may affect diagnosis through screening, such as age, sex and SES, are controlled, at least in part. To improve power, age was included in the regression, as a continuous linear term (based on non-rejection of linearity). Adding sex and family history to the regression models addressed potential confounding through susceptibility. Family history of lung cancer was defined as having at least one first-degree relative who had been diagnosed with lung cancer. Reported years of smoking and cotinine level were used to control for duration and intensity of tobacco use, respectively. Both were continuous linear terms, based on non-rejection of linearity. Since all subjects were current smokers at baseline, no adjustment for time since quitting was necessary. Parameter estimates and corresponding odds ratios with 95% Wald confidence limits were estimated and intervals excluding 1 were considered statistically significant. All computations were done in SAS v. 9.1 (SAS Institute, Inc., Cary, NC, USA) for Windows XP OS (Microsoft, Inc., Redmond, WA, USA).
Results
Table 1 gives the distributions by case/control status and tests of significance for categorical variables, including age, sex, race/ethnicity, education, marital status, occupation, family history of lung cancer, and the usual number of cigarettes smoked per day. Table 2 gives descriptive statistics by case/control status and tests of significance for continuous variables, including duration of smoking and measured serum levels of cotinine, total NNAL and PheT. Only age (p = 0.0039), years of smoking (p < 0.0001), and serum level of total NNAL (p = 0.0084) were statistically significantly associated with lung cancer risk. Although not statistically significantly associated with risk, cotinine and PheT differed in the expected direction between cases and controls.
Table 1.
Comparing the age, sex, race/ethnicity, education, marital status, occupational status, family history of lung cancer, and cigarettes per day of cases and controls
| VARIABLES | Controls N=100 | Cases N=100 | P | ORa (95% CI)b |
|---|---|---|---|---|
| Age at Randomization | 0.0039 | |||
| ≤59 years | 51 (51%) | 28 (28%) | reference | |
| 60–64 years | 26 (26%) | 27 (27%) | 1.891 (0.931,3.843) | |
| 65–69 years | 18 (18%) | 36 (36%) | 3.642 (1.756,7.557) | |
| ≥70years | 5 (5%) | 9 (9%) | 3.278 (1.001,10.738) | |
| Sex | 0.2913 | |||
| Women | 36 (36%) | 29 (29%) | reference | |
| Men | 64 (64%) | 71 (71%) | 1.377 (0.760,2.495) | |
| Race/Ethnicity | 0.0958 | |||
| White, non-Hispanic | 93 (93%) | 84 (84%) | reference | |
| Black, non-Hispanic | 4 (4%) | 13 (13%) | 3.598 (1.129,11.465) | |
| Other | 3 (3%) | 3 (3%) | 1.107 (0.218,5.635) | |
| Education | 0.5518 | |||
| Less than 12 years | 10 (10%) | 15 (15%) | 1.345 (0.515,3.510) | |
| 12 yrs or completed high school | 26 (26%) | 29 (29%) | reference | |
| Post-high-school training other than college | 16 (16%) | 10 (10%) | 0.560 (0.216,1.450) | |
| Some college | 20 (20%) | 24 (24%) | 1.076 (0.486,2.383) | |
| College graduate | 19 (19%) | 13 (13%) | 0.613 (0.254,1.482) | |
| Post-graduate training | 9 (9%) | 9 (9%) | 0.897 (0.309,2.600) | |
| Marital Status | 0.8507 | |||
| Married or living as married | 75 (75%) | 69 (69%) | reference | |
| Widowed | 10 (10%) | 10 (10%) | 1.087 (0.427,2.770) | |
| Divorced | 11 (11%) | 16 (16%) | 1.581 (0.686,3.642) | |
| Separated | 2 (2%) | 2 (2%) | 1.087 (0.149,7.928) | |
| Never married | 2 (2%) | 3 (3%) | 1.630 (0.264,10.051) | |
| Occupation | 0.7811 | |||
| Homemaker | 6 (6%) | 5 (5%) | reference | |
| Working | 44 (44%) | 36 (36%) | 0.982 (0.277,3.482) | |
| Unemployed | 3 (3%) | 2 (2%) | 0.800 (0.093,6.848) | |
| Retired | 39 (39%) | 49 (49%) | 1.508 (0.428,5.311) | |
| Disabled | 4 (4%) | 5 (5%) | 1.500 (0.255,8.817) | |
| Other/not answered | 4 (4%) | 3 (3%) | 0.900 (0.133,6.080) | |
| Family History of Lung Cancer | 0.1456 | |||
| No | 94 (94%) | 88 (88%) | reference | |
| Yes | 6 (6%) | 12 (12%) | 2.136 (0.769,5.937) | |
| Cigarettes per Day | 0.0576 | |||
| 11–20 | 48 (48%) | 49 (49%) | reference | |
| 21–30 | 37 (37%) | 23 (23%) | 0.609 (0.316,1.173) | |
| 31–40 | 13 (13%) | 21 (21%) | 1.582 (0.712,3.515) | |
| 41 + | 2 (2%) | 7 (7%) | 3.429 (0.678,17.344) |
OR = odds ratio
(95% CI) = 95% confidence interval, given as (lower limit, upper limit)
Table 2.
Comparing cases and controls on serum levels of cotinine, NNAL, PheT, and years of smoking
| VARIABLES | Controls N=100 | Cases N=100 | Difference (controls-cases) | P |
|---|---|---|---|---|
| Mean ± SDa | Mean ± SD | Mean ± SEb | ||
| Years of Cigarette Smoking | 41.6 ± 7.2 | 45.4 ± 6.5 | −3.9 ± 1.0 | 0.0001 |
| Cotinine (ng/ml) | 217 ± 111 | 227 ± 93 | −10 ± 15 | 0.4681 |
| Total NNALc (fmol/ml) | 77.4 ± 39.3 | 92.4 ± 40.7 | −15.0 ± 5.7 | 0.0084 |
| PheTd (fmol/ml) | 76.3 ± 66.8 | 92.5 ± 107.6 | −16.1 ± 12.7 | 0.2039 |
SD = standard deviation
SE = standard error
NNAL = 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol; total includes its glucuronides.
PheT = r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene
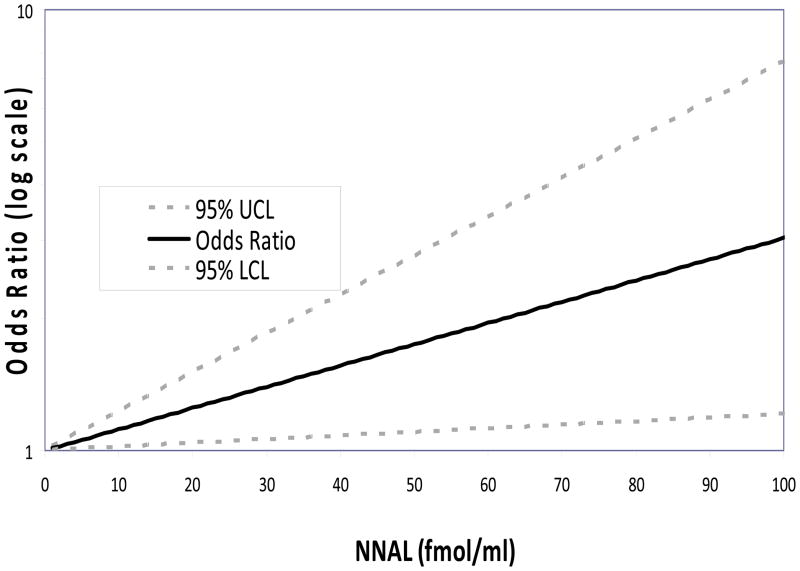
The results of the logistic regression to adjust the association of total NNAL, PheT, and cotinine to lung cancer risk for potential confounders and to improve the power of the test of the putative causal effect are given in Table 3. The effect of total NNAL was statistically significant, while those of PheT and cotinine were not. Note that neither PheT nor cotinine differed significantly between cases and controls (Table 2). The odds ratio for total NNAL suggests an approximate 57% increase in lung cancer risk (95% CI: 8%, 128%) for each standard deviation (sd; 40.0 fmol/ml) increase in total NNAL level,. Figure 1 shows the relation between total NNAL and the odds ratio for lung cancer risk based on the logistic model results.
Table 3.
Results of multiple logistic regression of lung cancer risk on sex, age at randomization, family history of lung cancer, cotinine, total NNALa, PheTb, and years of cigarette smoking
| VARIABLES | 95% Confidence Limits | P | ||
|---|---|---|---|---|
| ORc | Lower | Upper | ||
| Sex (men vs. women) | 1.38 | 0.68 | 2.79 | 0.3701 |
| Age at randomization | 1.10 | 1.02 | 1.18 | 0.0187 |
| Family history of lung cancer | 2.57 | 0.87 | 7.60 | 0.0891 |
| Cotinined | 0.85 | 0.59 | 1.23 | 0.3947 |
| Total NNALa,d | 1.57 | 1.08 | 2.28 | 0.0182 |
| PheTb,d | 1.23 | 0.88 | 1.72 | 0.2274 |
| Years of cigarette smokinge | 1.54 | 0.86 | 2.75 | 0.1454 |
NNAL = 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol; total includes its glucuronides.
PheT = r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene
OR = odds ratio
Represents OR associated with a unit standard deviation increase
Represents OR associated with a 10-year increase in smoking duration
Figure 1.
Logarithm of Odds Ratio by NNAL concentration estimated by multiple logistic regression controlling for sex, age at randomization, family history of lung cancer, serum cotinine level, serum PheT, and years of cigarette smoking.
The only other variable significant in the regression was age (p = 0.0187); sex, family history of lung cancer, and years of cigarette smoking were not significant.
Most of the lung cancers (59/100) were adenocarcinoma (including bronchioalveolar and adenosquamous carcinoma), with the second most frequent type being squamous cell carcinoma (30/100). The two other types were large-cell (7/100) and “non-small-cell, not otherwise specified” (4/100). For the purposes of further analysis, the non-adenocarcinoma lung cancers were grouped together, and separate regressions were run on the adenocarcinoma group and the non-adenocarcinoma group to see if the associations were consistent across histologic types. The estimated effect sizes were similar for adenocarcinoma and for the other cancers (1.3% vs. 0.8% increase per fmol/ml total NNAL, respectively); the effect for adenocarcinoma was statistically significant, whereas that for other cancers was not. Thus, a negligible effect for the other cancer types cannot be ruled out, nor can we conclude that the effects are the same.
Discussion
This study demonstrates that total NNAL in serum is significantly associated with lung cancer risk in samples from the PLCO study, even after controlling for intensity and duration of smoking. The analysis was controlled simultaneously for both duration (years of smoking) and intensity (cotinine levels) of smoking and so the estimated effect of NNAL is unlikely to be due to residual confounding from smoking patterns. Further, we simultaneously controlled for both the tobacco carcinogen biomarkers to avoid mutual confounding. Based on our regression, which assumes a linear relation between concentration and log odds, for a unit standard deviation increase in NNAL of 40 fmol per ml of serum, the odds for lung cancer increased 1.57 times (95% confidence interval:,1.08, 2.28). As suggested by Figure 1, the effect of other increases in NNAL can be predicted from the estimated relationship between total NNAL and risk. For example, the odds increase about 1.7 times (95% CI: 1.1, 2.8) from the 25th to 75th percentiles of total NNAL (57.3 to 108 fmol/ml), and about 4.2 times (95% CI: 1.3, 13.7) from the 5th to 95th percentiles (23.2 to 147.0 fmol/ml). This suggests that, among long-term heavy smokers, the total serum NNAL level is a major determinant of lung cancer risk conferred by cigarette smoking.
Age, the only other factor statistically significantly associated with increased lung cancer risk in the multiple logistic regression, was previously well established. Although the unadjusted two-way association of smoking duration with lung cancer risk was statistically significant, when controlled for the biomarkers and other factors it was not. This may be due to the relatively small sample size here and should not be interpreted to indicate that it is not an important risk factor. Note that the distributions of biomarkers observed in this study are similar to those measured in other studies.
To our knowledge, this is the first study to demonstrate a relation between a specific quantifiable tobacco-related lung carcinogen biomarker – total NNAL – and lung cancer in smokers. Several studies, including one prospective study, have examined the relation between lung cancer and “bulky DNA adducts,” “aromatic DNA adducts” or “PAH-DNA adducts,” in white blood cells of smokers using immunoassay or 32P-postlabelling; the results generally demonstrate increased risk with increased adduct levels.(16–21) However, these methods are non-specific to a particular carcinogen, and it is not known with certainty which cigarette-smoke carcinogens actually give rise to the measured adducts, although it is likely that PAHs are responsible, in part.
Our finding that total NNAL is related to lung cancer in smokers is biologically plausible. NNK has long been suspected to be a human lung carcinogen. NNAL and its glucuronides are metabolites of NNK in humans and do not come from any other sources; urinary total NNAL is related to NNK dose and correlates with total serum NNAL.(3, 5) NNK itself is extensively metabolized and cannot be detected in human urine. NNK causes lung cancer, predominantly adenocarcinoma, in all laboratory animal species tested, including rats, mice, hamsters, and ferrets, and it does so independently of the route of administration.(22, 23) It is particularly effective in rats, in which a total dose of only 6 mg/kg caused a significant incidence of lung tumors.(24) The carcinogenic activity of NNAL is similar to that of NNK.(22) Based on these data and associated mechanistic evidence from laboratory animals and humans, the International Agency for Research on Cancer has concluded that NNK and the related tobacco-specific nitrosamine N′-nitrosonornicotine are carcinogenic to humans,(25) and the present results certainly support that evaluation by directly estimating the effect in humans.
The measures of smoking duration (years of smoking) and intensity (serum cotinine) used are imperfect. One might hypothesize that NNAL is merely sweeping up residual effects of smoking duration and intensity through its correlation with other carcinogens, a hypothesis which an observational study cannot refute deductively. However, the fact that cotinine, for which NNAL may be a proxy, has too small a relationship with risk in this modestly sized study to be statistically significant, and inclusion of total NNAL reduces the otherwise statistically significant effect of duration, for which it is not a proxy, argues against this interpretation. Further, this hypothesis would require that NNAL, controlled for smoking duration and intensity, is highly associated with some other set of unmeasured carcinogens that do cause lung cancer, while the carcinogen with which it is most highly associated (NNK) does not cause lung cancer. This could happen if some strong physical or biological association between NNK and these putative carcinogens existed, for example, if they were products of the same biological process. But since total NNAL is most highly correlated with intake of NNK, a known lung carcinogen in many species of mammals, and much less highly correlated with other carcinogen intake (e.g., PAHs), we find this interpretation less plausible and find our results as highly confirmatory of the importance of NNK in lung cancer carcinogenesis among smokers.
The effect of total NNAL estimated in this study appears modest compared to the effect of smoking overall because it is the estimated difference in risk for individuals with a given smoking history, age, sex, and family history of lung cancer. Therefore, the reported effect is due only to differences in total NNAL levels. These differences could arise from differing concentrations of NNK in the cigarettes smoked or from biological variability in the absorption and metabolism of NNK. In any case, the NNAL effect cannot be compared to the effect of smoking vs. not smoking. Rather, this increased risk is a modifier of the known, large risk of smoking and thus, importantly, supports the hypothesis that NNK and NNAL are human lung carcinogens. Of further note, NNK and NNAL require metabolic activation to exert their carcinogenic effects.(22) Thus, the carcinogenic effects of NNK are related to dose plus the extent of metabolic activation. In this study we measured only dose; higher levels of metabolic activation (e.g., due to inherited differences or inducers/inhibitors) would potentially increase risk. We did not measure metabolites of NNK beyond total NNAL, but in future research inclusion of such biomarkers – or their ratios – may allow us to estimate the effects of activation and detoxification and to control for additional heterogeneity in risk among smokers. Such future studies may result in even stronger predictive relations between measured biomarker levels and lung cancer risk.
We did not observe associations of either PheT with lung cancer risk in this study. PheT has been proposed as a biomarker of PAH dose plus metabolic activation.(7, 8, 26) PheT levels correlate with those of 1-hydroxypyrene, a well established biomarker of PAH uptake. But PheT, the product of the PAH diol epoxide metabolic activation pathway, is also a measure of metabolic activation. The lack of an association of PheT with lung cancer in this study was somewhat surprising in view of the strong evidence linking PAH to lung cancer etiology in smokers.(4) However, unlike NNAL, PheT is not tobacco-specific, and diet is another more variable source of PAH exposure which may attenuate the observed effect of PAH from cigarette smoking. Also, note that the rather high variance of PheT measurements relative to mean levels makes the power for a given proportional change smaller than that for NNAL. This finding motivates further studies to evaluate PheT as a marker of risk for lung cancer. Markers of tobacco-specific PAH exposure in general may be intractable due to the many environmental sources for PAH; nonetheless, finding biomarkers of PAH metabolic activation would be valuable to understanding the role of tobacco smoke in lung cancer.
In this study, we observed no association between serum cotinine levels and lung cancer risk, although serum cotinine correlated with serum total NNAL and smoking intensity (data not shown), consistent with previous studies of urinary cotinine and total NNAL.(3) We included cotinine in the regression as a more accurate proxy for intensity of smoking exposure rather than using categories of reported number of cigarettes per day. Boffetta and co-workers found that serum cotinine was a predictor of lung cancer risk in a study of smokers which was far larger than ours;(27) however, they did not measure or control for total NNAL exposure. Our results suggest that total NNAL, metabolically formed from the carcinogen NNK, is a better risk marker than cotinine, a metabolite of the non-carcinogen nicotine.
Our study has certain limitations. Sample size was modest, but obviously adequate to detect the association for which it was designed. A larger study would, of course, have had more power to investigate the strength of the association of this and other biomarkers with lung cancer and within histologic subtypes. A larger study would also allow us to more closely examine the mathematical nature of the relation of NNAL to smoking patterns and to the other biomarkers and allow for more precise estimates of the relations of the biomarkers to risk. We hope these results will motivate such expanded research.
Each biological sample in this study represents only a single time point – the initial screening visit. Although no split-sample testing was done in this study due to the small amount of sample available, a previous publication estimated the within-day and between-day relative standard deviations for the NNAL laboratory assay to be 7.7% and 11.7%, respectively. However, we have shown, in a recently completed longitudinal study (unpublished), that the average coefficient of variation of serum NNAL levels over a one-year period in 50 smokers sampled every 2 months was about 30%. Thus, most of the variability within a subject comes from biological variability rather than assay reliability. This variability would serve to attenuate the estimated effect of the NNAL, suggesting that the true effect might likely be even larger than the estimated effect.
The results of this study have potentially profound implications for lung cancer etiology, because they support the hypothesis that NNK is a lung carcinogen in smokers. By focusing on NNK and the pathways related to its activation and detoxification, we may be able to develop preventive approaches. Previous studies using total NNAL to measure NNK uptake in smokers who used different products or reduced their smoking, and in non-smokers exposed to secondhand tobacco smoke demonstrate great variability in levels of exposure to NNK.(3, 5, 28–33) Now those data can also be seen to reflect potential variability in lung cancer risk and have implications for future studies that use total NNAL as a biomarker. Furthermore, lung cancer chemoprevention approaches based on NNK in rodent models now gain more salience to humans.(34–36) Ultimately, as further research refines its estimated effect and delineates how it interacts with other biological measures, total NNAL may become part of an algorithm that includes metabolic activation and DNA repair measures to predict lung cancer susceptibility in people who choose to smoke cigarettes. In long-term smokers, such an algorithm could be used in cessation programs or surveillance activities. In newer smokers, better risk information could better motivate early cessation efforts. The assay is limited in that it can only be done on current smokers, and so could not help those considering smoking initiation or those who have already given it up.
In summary, we report here for the first time that total NNAL, a biomarker of uptake of the tobacco-specific lung carcinogen NNK, is related to the risk of lung cancer in smokers. These results are consistent with previous carcinogenicity and mechanistic studies that point to NNK as an important cause of lung cancer in smokers.
Acknowledgments
Funding
U.S. National Cancer Institute, National Institutes of Health, Department of Health and Human Services [contract number N01-CN-25513]; National Institutes of Health [grant DA-13333].
References
- 1.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon, France: IARC; 2004. Tobacco Smoke and Involuntary Smoking, Tobacco Smoke, Section 5. Summary of Data Reported and Evaluation; pp. 1179–87. [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon, France: IARC; 2004. Tobacco Smoke and Involuntary Smoking, Tobacco Smoke, Section 1. Production, Composition, Use and Regulation; pp. 53–119. [PMC free article] [PubMed] [Google Scholar]
- 3.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 4.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 5.Hatsukami DK, Benowitz NL, Rennard SI, Oncken C, Hecht SS. Biomarkers to assess the utility of potential reduced exposure tobacco products. Nicotine Tob Res. 2006;8:600–22. doi: 10.1080/14622200600858166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecht SS, Carmella SG, Yoder A, et al. Comparison of polymorphisms in genes involved in polycyclic aromatic hydrocarbon metabolism with urinary phenanthrene metabolite ratios in smokers. Cancer Epidemiol Biomarkers Prev. 2006;15:1805–11. doi: 10.1158/1055-9965.EPI-06-0173. [DOI] [PubMed] [Google Scholar]
- 7.Hecht SS, Chen M, Yoder A, et al. Longitudinal study of urinary phenanthrene metabolite ratios: effect of smoking on the diol epoxide pathway. Cancer Epidemiol Biomarkers Prev. 2005;14:2969–74. doi: 10.1158/1055-9965.EPI-05-0396. [DOI] [PubMed] [Google Scholar]
- 8.Hecht SS, Chen M, Yagi H, Jerina DM, Carmella SG. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: a potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol Biomarkers Prev. 2003;12:1501–8. [PubMed] [Google Scholar]
- 9.Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: History, organization, and status. Control Clin Trials. 2000;21:251S–72S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL, Jacob P, 3rd, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268:296–303. [PubMed] [Google Scholar]
- 11.Rothman KJ, Greenland S, editors. Modern Epidemiology. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 12.Carmella SG, Yoder A, Hecht SS. Combined analysis of r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in smokers’ plasma. Cancer Epidemiol Biomarkers Prev. 2006;15:1490–4. doi: 10.1158/1055-9965.EPI-06-0199. [DOI] [PubMed] [Google Scholar]
- 13.Hecht SS, Carmella SG, Chen M, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–6. [PubMed] [Google Scholar]
- 14.Murphy SE, Villalta P, Ho SW, von Weymarn LB. Analysis of [3′,3′-d(2)]-nicotine and [3′,3′-d(2)]-cotinine by capillary liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:1–8. doi: 10.1016/j.jchromb.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 16.Kriek E, Rojas M, Alexandrov K, Bartsch H. Polycyclic aromatic hydrocarbon-DNA adducts in humans: relevance as biomarkers for exposure and cancer risk. Mutat Res. 1998;400:215–31. doi: 10.1016/s0027-5107(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 17.Tang D, Phillips DH, Stampfer M, et al. Association between carcinogen-DNA adducts in white blood cells and lung cancer risk in the physicians health study. Cancer Res. 2001;61:6708–12. [PubMed] [Google Scholar]
- 18.Wiencke JK. DNA adduct burden and tobacco carcinogenesis. Oncogene. 2002;21:7376–91. doi: 10.1038/sj.onc.1205799. [DOI] [PubMed] [Google Scholar]
- 19.Perera FP, Mooney LA, Stampfer M, et al. Associations between carcinogen-DNA damage, glutathione S-transferase genotypes, and risk of lung cancer in the prospective Physicians’ Health Cohort Study. Carcinogenesis. 2002;23:1641–6. doi: 10.1093/carcin/23.10.1641. [DOI] [PubMed] [Google Scholar]
- 20.Veglia F, Matullo G, Vineis P. Bulky DNA adducts and risk of cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003;12:157–60. [PubMed] [Google Scholar]
- 21.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon, France: IARC; 2004. Tobacco Smoke and Involuntary Smoking, Tobacco Smoke, Section 4. Other Data Relevant to an Evaluation of Carcinogenicity and its Mechanisms; pp. 1012–70. [PMC free article] [PubMed] [Google Scholar]
- 22.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Liu XS, Liu C, Smith DE, Russell RM, Wang XD. Induction of pulmonary neoplasia in the smoke-exposed ferret by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK): a model for human lung cancer. Cancer Lett. 2006;234:209–19. doi: 10.1016/j.canlet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 24.Belinsky SA, Foley JF, White CM, Anderson MW, Maronpot RR. Dose-response relationship between O6-methylguanine formation in Clara cells and induction of pulmonary neoplasia in the rat by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1990;50:3772–80. [PubMed] [Google Scholar]
- 25.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 89. Lyon, France: IARC; Smokeless Tobacco and Tobacco-specific Risks to Humans. in press. [Google Scholar]
- 26.Kim JY, Hecht SS, Mukherjee S, Carmella SG, Rodrigues EG, Christiani DC. A urinary metabolite of phenanthrene as a biomarker of polycyclic aromatic hydrocarbon metabolic activation in workers exposed to residual oil fly ash. Cancer Epidemiol Biomarkers Prev. 2005;14:687–92. doi: 10.1158/1055-9965.EPI-04-0428. [DOI] [PubMed] [Google Scholar]
- 27.Boffetta P, Clark S, Shen M, Gislefoss R, Peto R, Andersen A. Serum cotinine level as predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:1184–8. doi: 10.1158/1055-9965.EPI-06-0032. [DOI] [PubMed] [Google Scholar]
- 28.Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob Control. 2004;13(Suppl 1):i48–56. doi: 10.1136/tc.2002.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht SS, Carmella SG, Le KA, et al. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides in the urine of infants exposed to environmental tobacco smoke. Cancer Epidemiol Biomarkers Prev. 2006;15:988–92. doi: 10.1158/1055-9965.EPI-05-0596. [DOI] [PubMed] [Google Scholar]
- 30.Hatsukami D, Mooney M, Murphy S, LeSage M, Babb D, Hecht S. Effects of high dose transdermal nicotine replacement in cigarette smokers. Pharmacol Biochem Behav. 2007;86:132–9. doi: 10.1016/j.pbb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecht SS, Carmella SG, Murphy SE, Riley WT, Le C, Luo X, Mooney M, Hatsukami DK. Similar exposure to a tobacco-specific carcinogen in smokeless tobacco users and cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2007;16:1567–72. doi: 10.1158/1055-9965.EPI-07-0227. [DOI] [PubMed] [Google Scholar]
- 32.Hatsukami DK, Le CT, Zhang Y, et al. Toxicant exposure in cigarette reducers versus light smokers. Cancer Epidemiol Biomarkers Prev. 2006;15:2355–8. doi: 10.1158/1055-9965.EPI-06-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatsukami DK, Joseph AM, LeSage M, et al. Developing the science base for reducing tobacco harm. Nicotine Tob Res. 2007;9:S537–53. doi: 10.1080/14622200701679040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hecht SS, Trushin N, Rigotty J, et al. Complete inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced rat lung tumorigenesis and favorable modification of biomarkers by phenethyl isothiocyanate. Cancer Epidemiol Biomarkers Prev. 1996;5:645–52. [PubMed] [Google Scholar]
- 35.Conaway CC, Wang CX, Pittman B, et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–57. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 36.Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66:11494–501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]