Abstract
Numerous studies have examined the effects of anthropogenic endocrine disrupting compounds; however, very little is known about the effects of naturally occurring plant-produced estrogenic compounds (i.e., phytoestrogens) on vertebrates. To examine the seasonal pattern of phytoestrogen consumption and its relationship to hormone levels (407 fecal samples analyzed for estradiol and cortisol) and social behavior (aggression, mating, and grooming) in a primate, we conducted an 11-month field study of red colobus (Procolobus rufomitratus) in Kibale National Park, Uganda. The percent of diet from estrogenic plants averaged 10.7% (n = 45 weeks; range: 0.7 – 32.4%). Red colobus fed more heavily on estrogenic Millettia dura young leaves during weeks of higher rainfall, and the consumption of this estrogenic item was positively correlated to both their fecal estradiol and cortisol levels. Social behaviors were related to estradiol and cortisol levels, as well as the consumption of estrogenic plants and rainfall. The more the red colobus consumed estrogenic plants the higher their rates of aggression and copulation and the lower their time spent grooming. Our results suggest that the consumption of estrogenic plants has important implications for primate health and fitness through interactions with the endocrine system and changes in hormone levels and social behaviors.
Keywords: estradiol, cortisol, environmental endocrinology, herbivory, mating, phytoestrogen, aggression, Kibale National Park, grooming, legume
INTRODUCTION
Endocrine disruption is of major concern because many synthetic chemicals pose a threat to humans and wildlife through interference with the vertebrate endocrine system (Hayes, 2005; Propper, 2005). Much research has focused on the physiological and behavioral effects of anthropogenic endocrine disrupting compounds, particularly those with estrogenic activity (Colborn et al., 1993; Guillette, 2000; Hayes et al., 2002; Milnes et al., 2006). Effects include modifying the developing reproductive system (e.g., genital deformation) and altering steroid hormone profiles, gamete production, and sex-typical behaviors in exposed adults (Hayes et al., 2002; Milnes et al., 2006). Much less is known about the effects of consuming natural plant-produced estrogenic compounds (i.e., phytoestrogens; Wynne-Edwards, 2001). Most primates depend heavily on a plant-based diet, thus the consumption of estrogenic plants may have important implications for their behavior, ecology, and evolution. Further, as half of all primate species are at risk of extinction (Chapman and Peres, 2001), a better understanding of the relationship between primates and naturally occurring phytoestrogens present in their foods is needed because such compounds have the potential to alter fertility and mating behavior (Cederroth et al., 2010a; Simon et al., 2004; Whitten and Patisaul, 2001; Wynne-Edwards, 2001).
Phytoestrogen consumption has been documented to disrupt fertility and affects behavior in laboratory and domesticated species, including rodents, monkeys, sheep, and cattle, as well as humans (Adams, 1990, 1995; Cederroth et al., 2010b; Whitten and Patisaul, 2001). Due to the conservative nature of the endocrine system across vertebrates, such effects are expected in wild primates feeding on similar estrogenic plant compounds (Hayes, 2005; Thornton, 2001). Previously we confirmed the presence of estrogenic plant foods in the diet of red colobus monkeys (Procolobus rufomitratus) of Kibale National Park, Uganda, using transient transfection assays (Wasserman et al., 2012). Three staple estrogenic foods with activity at estrogen receptor beta (ERβ) made up 10% of the diet (Millettia dura [Fabaceae] young leaves, Ficus natalensis [Moraceae] young leaves, and bark from non-native Eucalyptus grandis [Myrtaceae]), while non-staple estrogenic foods only made up 0.6% of the diet. Here, our objective was to determine if the consumption of these estrogenic plant foods by red colobus monkeys altered their physiology and behavior via interaction with their endocrine system.
Two hormonal axes are important for understanding the potential for phytoestrogens to alter physiology and behavior. The first, the hypothalamo-pituitary gonadal axis (HPG), plays a central role in regulating the development and maintenance of the mammalian reproductive system through the production of sex steroids (i.e., estrogens and androgens) and their downstream effects on reproductive physiology and behavior (Hadley, 1999; Wingfield and Sapolsky, 2003). Interference with the HPG axis can result in altered fertility in males, due to the role of testosterone and gonadotropins in sperm production, and females, due to the role of estrogens and gonadotropins in their estrous cycle (Hadley, 1999). The second hormonal axis, the hypothalamo-pituitary adrenal axis (HPA), plays a central homeostatic role through the production of glucocorticoids (Sapolsky, 2005; Wingfield and Sapolsky, 2003). There is strong evidence that these two hormone axes interact as the HPA axis can suppress the HPG axis so that stress can suppress reproduction (Wingfield and Sapolsky, 2003) and estrogens can suppress the negative feedback loop of the HPA axis, thus increasing production of glucocorticoids (Weiser and Handa, 2009).
We examined patterns of phytoestrogen consumption, fecal hormone levels, and social behavior of red colobus (P. rufomitratus) in Kibale over 11 months. Specifically, we determined how weekly variation in the time spent feeding on estrogenic plants related to fecal estradiol and cortisol levels in adult males. As both consumption of estrogenic plants and red colobus hormone levels may vary seasonally, their relationships to rainfall and temperature were examined. Climate can mediate the relationship between phytoestrogen consumption and primate hormone levels through three main mechanisms that are not mutually exclusive: (1) its effects on food availability (i.e., if plant phenology is linked to climatic variables) (Chapman et al., 1999; Struhsaker, 1997), (2) its effects on phytoestrogen levels (i.e., plants contain more or less of these compounds during certain times of the year) (Leopold et al., 1976; Mazur and Adlercreutz, 1998; Morrison et al., 2010), and (3) its direct effects on hormone levels (i.e., primates may suffer climate related stress) (Gogarten et al., In Press; Wingfield, 2005; Wingfield et al., 1983). To determine if any hormonal changes related to phytoestrogen consumption translated into altered social behavior, we examined the influence of climate, phytoestrogen consumption, and hormonal status on rates of aggression and mating and time spent grooming.
MATERIAL AND METHODS
Study site and species
Kibale National Park (795 km2) is a moist evergreen forest in western Uganda (0 13′ – 0 41′ N and 30 19′ – 30 32′ E) (Chapman et al., 2010). This forest receives an average of 1696 mm annually (1990–2011; Chapman and Chapman, unpublished data), with most falling during two rainy seasons (Chapman et al., 2010). The Ugandan red colobus (Procolobus rufomitratus tephrosceles) is considered endangered by the IUCN (Struhsaker, 2008), with the only viable population remaining in Kibale (Struhsaker, 2005). The Kibale population consists of numerous multimale-multifemale groups with an average group size of 65 individuals (Snaith et al., 2008). As a forestomach-fermenting obligate folivore dependent upon symbiotic gut bacteria for securing nutrients from its leaf based diet (Bauchop and Martucci, 1968; Chapman et al., 2002; Lambert, 1998; Milton, 1980), the red colobus is an ideal subject for examining the effects of phytoestrogens. With their specialized forestomach system, colobines may be particularly susceptible to the estrogenic activity of plants, as has been documented for foregut-fermenting livestock (e.g., “clover disease” of sheep) (Adams, 1990, 1995; Bennetts, 1946). Data show that phytoestrogens are often more active after bacterial metabolism (Gultekin and Yildiz, 2006; Setchell and Clerici, 2010). For example, a number of phytoestrogens (e.g., formononetin, daidzein) are converted to the more bioactive compound, equol, via bacterial metabolism in a number of species, including foregut fermenters (Setchell and Clerici, 2010).
Assessment of red colobus diet and social behavior
We determined the red colobus diet, rates of mating and aggression, and time spent grooming by collecting behavioral data from one group (~ 70 individuals) in Kibale from August 13, 2007 to June 27, 2008 (258 days of sampling), for a total of 1327 observation hrs. Data were collected six days per week from 0800 to 1300 h using scan samples of five individuals every 30 minutes. When feeding, plant species and the part being consumed were identified. We calculated the percent of diet for each item at the weekly scale by summing the number of observations of feeding on each plant item, and dividing this by the total number of feeding observations that week. Using these values, we calculated the percentage of the diet coming from each of the staple estrogenic foods, as well as a sum of all rare estrogenic plant foods, for each week (n = 45). A staple food was defined as a plant item that was fed on > 1% of the total time, while a rare food was any plant item that was fed on < 1% of the total time. Estrogenic activity of plant foods was tested using transient transfection assays (Wasserman et al., 2012). We also calculated the weekly percent of diet for each of the 13 most fed on dietary items as listed in Wasserman et al. (2012). Behavioral data on feeding came from 37 known individuals in the group, including 22 adult females, 13 adult males (including all ten males examined in the hormonal analyses), and two subadult males. Differences in feeding behavior between males and females were not apparent, especially for the three estrogenic staple foods (e.g., Millettia dura young leaf consumption was 5.2 ± 1.4% for adult males and 5.4 ± 1.6% for adult females), so we used our entire dataset to determine diet for the ten adult males as this likely provided the most robust and appropriate statistical sample estimate of their weekly diet.
Three types of social behavior were examined: mating, aggression, and grooming. Grooming data were collected during the scan samples and calculated as percent of time spent grooming (i.e., providing) or being groomed (i.e., receiving). Copulation rates (#/hr) were determined using ad libitum observations. Aggression rates (#/hr) (i.e., sum of number of chases and fights) were also collected through ad libitum observations. All social behavior data used came from only the 13 adult males.
Assessment of climate and plant phenology
Rainfall and temperature data were collected from an area within the home range of the group. Trees were monitored monthly for ripe fruits, unripe fruits, mature leaves, young leaves, and flowers (abundance ranked from 0 to 4). We calculated the mean monthly phenological score for the staple estrogenic plant foods to provide an index of their availability. Young leaves of Ficus natalensis (n = 2 trees) and Millettia dura (n = 11) were monitored, but Eucalyptus grandis bark was not because it does not vary in seasonal availability.
Assessment of fecal hormone levels
Although evidence of effects of phytoestrogen consumption similar to those of laboratory studies are difficult to obtain in field studies, indirect measures can provide evidence of changes in either the HPG or HPA axes, indicating disruption of reproductive capabilities. These hormonal effects can be examined noninvasively using the measurement of excreted estradiol and cortisol metabolites in the feces (Heistermann et al., 1993; Touma and Palme, 2005; Wasser et al., 1988; Whitten et al., 1998; Ziegler and Wittwer, 2005). Thus, fecal samples were collected immediately upon defecation from ten known adult males (out of 13 in the group). Adult males were selected because female reproductive state can greatly influence steroid hormone levels (Weingrill et al., 2004). Fecal samples were collected between 0830 and 1230 hrs to reduce the contribution of diurnal variation seen in the excretion patterns of fecal steroids (Sousa and Ziegler, 1998; Wasserman unpublished data for this species).
We collected samples approximately once a week for each male (n = 407). Fecal samples were immediately placed in sterile vials and stored in a cooler with ice packs until placed in a −20°C freezer later that day. On the day of extraction, samples were taken from the −20°C freezer and thawed. Each sample was homogenized using a spatula and 0.5g was weighed into a test tube. We added 5 ml of 5.0 pH citrate buffer and 5 ml 95% ethanol to each fecal sample, and this fecal material solution was mixed on a homogenizer for 24 hours. Steroid hormones were then separated from the fecal pellet using a centrifuge, and 2ml of supernatant were passed through a preconditioned solid phase extraction cartridge at a flow rate of 4ml/min. The steroid hormones were then stored in these cartridges with both ends capped. Capped samples were stored out of direct light until analysis via radioimmunoassay (RIA) for estradiol content and enzyme immunoassay (EIA) for cortisol content by MW at the Wisconsin National Primate Research Center (WNPRC).
At WNPRC, the cartridges were washed with 1ml of 5% methanol and the steroid hormones were collected using 2ml of 100% methanol passed through the cartridge at a 1ml/min flow rate. The methanol was then evaporated off and steroids hormones were reconstituted in 1 ml of 100% ethanol and stored in a 4°C refrigerator until analyses. For the estradiol RIA, 25 μL of sample was used and recovery was 107.44% 2.53%. Parallelism was demonstrated with no significant difference between slopes of the serial dilution of the sample pool and standard curve (p > 0.05). Inter-assay variation for the high pool was 14.62% and for the low pool was 9.6%, while intra-assay variation was 4.53% for the high pool and 7.51% for the low pool. The estradiol antibody used was antiserum from Holly Hill Biologicals, Inc., Hillsboro, Oregon, diluted so that ~ 50% of the tritiated estradiol was bound in the absence of unlabeled steroid (French et al., 1983). Cross-reactivity was 0.2% for estriol, 3.12% for estrone, and less than 0.01% for all other steroids tested (i.e., testosterone, DHT, androstenedione, progesterone, cortisone, cortisol, epiandrosterone, DHEA, and androstenediol). Because the cross-reactivities with other endogenous estrogens were low, it is unlikely that our antibody that is specific to estradiol would have enough cross-reactivity with phytoestrogens in the red colobus fecal samples to influence our results. For the cortisol EIA, 50 μL of sample was used and recovery was 125.27% 3.18%. Parallelism was demonstrated using serial dilution curves, with no significant difference between the sample pool and standards (p > 0.05). Inter-assay variation for the high pool was 18.83% and for the low pool was 16.62%, while intra-assay variation was 6.24% for the high pool and 6.26% for the low pool. The cortisol antibody used was R4866 (anti-cortisol-BSA) developed by Stabenfeldt & Munro at the University of California, Davis. It was diluted to 1:22,000 with 50 mM bicarbonate buffer (pH 9.6), as per Ziegler et al. (1995). The final hormone values are given in ng of steroid hormone per g of dry feces. The dry matter content of all fecal samples was calculated in the field by drying 0.5g of each sample to a constant weight. Biological validation of our cortisol EIA previously showed this to be an effective index of stress for the red colobus (Wasserman et al., In review).
Statistical analyses
To determine if the consumption of estrogenic plant foods interfered with the endocrine system and if climate played an important mediating role in this relationship, we tested two hypotheses: (1) temporal changes in red colobus hormonal levels were related to changes in phytoestrogen consumption and (2) climatic factors influenced hormone levels directly and/or indirectly though their effects on the timing of estrogenic plant availability and/or consumption. We used fecal estradiol level as an index of reproductive physiology and fecal cortisol level as an index of stress physiology. To determine the relative importance of phytoestrogen consumption and climatic seasonality to red colobus hormonal state, we used stepwise regression including six predictor variables: (1) % of diet from rare estrogenic foods, (2–4) % of diet from each of the three staple estrogenic foods (Millettia dura young leaves, Ficus natalensis young leaves, and Eucalyptus grandis bark), (5) weekly total rainfall, and (6) mean maximum temperature, and two outcome variables: (1) fecal estradiol and (2) fecal cortisol. We used the median of weekly estradiol and cortisol levels in our analyses since both hormone data sets were not normally distributed. We also used stepwise regression with the red colobus’ 13 most fed on dietary items (11 non-estrogenic and 2 estrogenic, accounting for 66% of total diet; Wasserman et al. 2012) and rainfall as predictor variables and fecal estradiol as the outcome variable to test if seasonal changes in diet, other than consumption of phytoestrogens, influenced male reproductive state and thus hormone levels.
To examine seasonality in diet, relationships between rainfall and the red colobus’ 13 most fed on dietary items, including the three estrogenic staple foods, were examined using Pearson correlations (n = 45 weeks). To further clarify if seasonality influenced the consumption of estrogenic plants, we examined the relationship between rainfall and phenology of Millettia dura young leaves and Ficus natalensis young leaves, as well as the relationship between their availability and the time the red colobus fed on each at the monthly level using Spearman rank correlations (n = 11 months).
To examine the influence of hormonal state, climate, and phytoestrogen consumption on behavior, we employed a regression tree analysis. Regression trees are ideally suited for the analysis of such complex data (De’ath and Fabricius, 2000). Data often display nonlinear relationships and complex higher-level interactions between variables (Elith et al., 2008). Regression trees explain variation in a single response variable using one or more explanatory variables and exhibit a number of advantages over many commonly used statistics: they are non-parametric, which is often useful when considering behavioral data, well-suited for examining complex interactions in explanatory variables, and easy to interpret (De’ath, 2007; De’ath and Fabricius, 2000). Regression trees use a recursive partitioning approach to split the variable space into smaller regions that minimize a measure of variation within the partitions. We used an algorithm that split the data so as to minimize the sum of squares, where splitting continues until some stopping criterion is achieved; in this case that any additional splits do not improve the cost-complexity criterion of the tree (i.e., that increasing complexity does not significantly improve the fit of the tree) (De’ath and Fabricius, 2000).
We built regression trees to predict: (1) % of time grooming, (2) copulation rate, and (3) rate of aggression using ‘rpart’ implemented in R version 2.12.2 (R-Development-Core-Team, 2012; Therneau et al., 2012). We predicted red colobus behaviors with median fecal cortisol and estradiol levels, rainfall, maximum temperature, and the percentage of the diet consisting of estrogenic foods (% diet from all estrogenic plants, % diet from staple estrogenic plants, % diet from rare estrogenic plants, and % diet from each of the estrogenic staples). Partitions near the top of the regression tree reflect strong relationships between predictor and response variables, with the length of the branch corresponding to the proportion of the sum of squares explained by each split in the tree (De’ath and Fabricius, 2000). Regression trees are read from the base of the tree (i.e., the root) down the branches to the terminal nodes (i.e., leaves), following a number of splits at each node. The number displayed at the tip is the mean value of the dependent variable in that subset of the data and n represents the number of samples in the data that are categorized into that leaf.
RESULTS
Estrogenic plants in red colobus diet
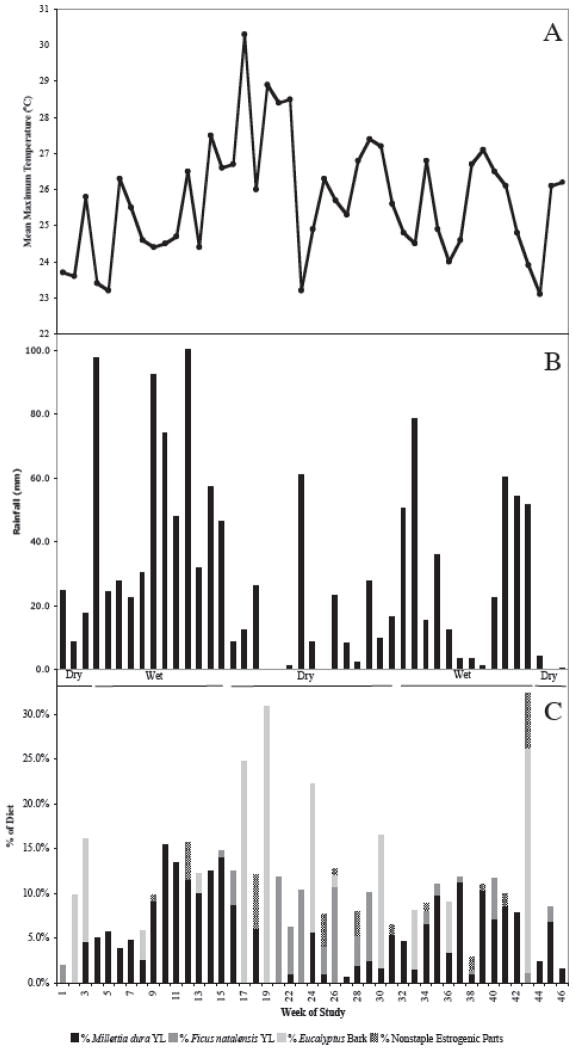
On average 10.7% of the diet was from estrogenic plants (range: 0.7% – 32.4% across weeks; Fig. 1C). This was mainly due to the consumption of three staple estrogenic plants and alone their contribution to the diet averaged 10.1% (range: 0.7% – 31.0%). The red colobus fed on at least one of the three staple estrogenic plants during each week, but never fed on all three during the same week. Of these three estrogenic staples, Millettia dura young leaves were fed on most, with an average of 5.1% of the diet and a range from none during 8 weeks to 15.5%. Eucalyptus grandis bark was fed on second most with an average of 3.4% of the diet and a range from none during 33 weeks to 31.0%. Ficus natalensis young leaves were fed on least of these staple estrogenic plants with an average of 1.5% of the diet and a range from none fed on during 28 weeks to 11.8%. As for the rare (non-staple) estrogenic plant foods (Erythrina abyssinica young leaves and flowers, Ficus sansibarica unripe fruit and young leaves, Ficus natalensis unripe fruit), their contribution to the diet averaged 0.6%, with a range from none fed on during 33 weeks to 6.1%.
Figure 1.
Weekly (n = 45) A) mean maximum temperature (°C) and B) amount of rainfall (mm) at Makerere University Biological Field Station, Kibale National Park, Uganda, with wet and dry seasons indicated. C) Percent of weekly diet from each of the estrogenic staple plant foods, as well as a sum of the percent of diet from all non-staple estrogenic plant foods, of the Ugandan red colobus monkey.
Climatic variation and plant phenology
Rainfall was highly variable temporally, both between weeks during the dry seasons and wet seasons and across weeks within a particular season (Fig. 1B). Total rainfall during the 45 weeks was 1304.5 mm, with an average of 28.99 mm falling per week (range = 0 – 100.33 mm). Weekly mean maximum temperature averaged 25.7 °C (range = 23.1 – 30.3 °C) (Fig. 1A).
Young leaves of both M. dura and F. natalensis were available throughout the study. The availability of M. dura young leaves was related to rainfall with a one-month time lag (i.e., rainfall in month 1 resulted in an increase in availability of M. dura young leaves in month 2; rs = 0.778, p = 0.008). The availability of F. natalensis young leaves was unrelated to rainfall (rs = −0.075, p = 0.836). Even though some young leaves of F. natalensis were available year-round, there were months when the red colobus did not feed on them, while M. dura young leaves were fed on in every month. However, there was no relationship between proportion of their feeding time spent eating M. dura young leaves and its availability (rs = 0.232, p = 0.492), nor did they feed on F. natalensis young leaves more when they were more available (rs = −0.041, p = 0.905).
There was a positive relationship between rainfall and the time spent feeding on M. dura young leaves (r = 0.414, p = 0.005), while there were no significant relationships between the time spent feeding on any of the 11 most fed on non-estrogenic plant items, or the other two estrogenic foods, and rainfall (all p > 0.107).
Adult male red colobus fecal estradiol and cortisol levels
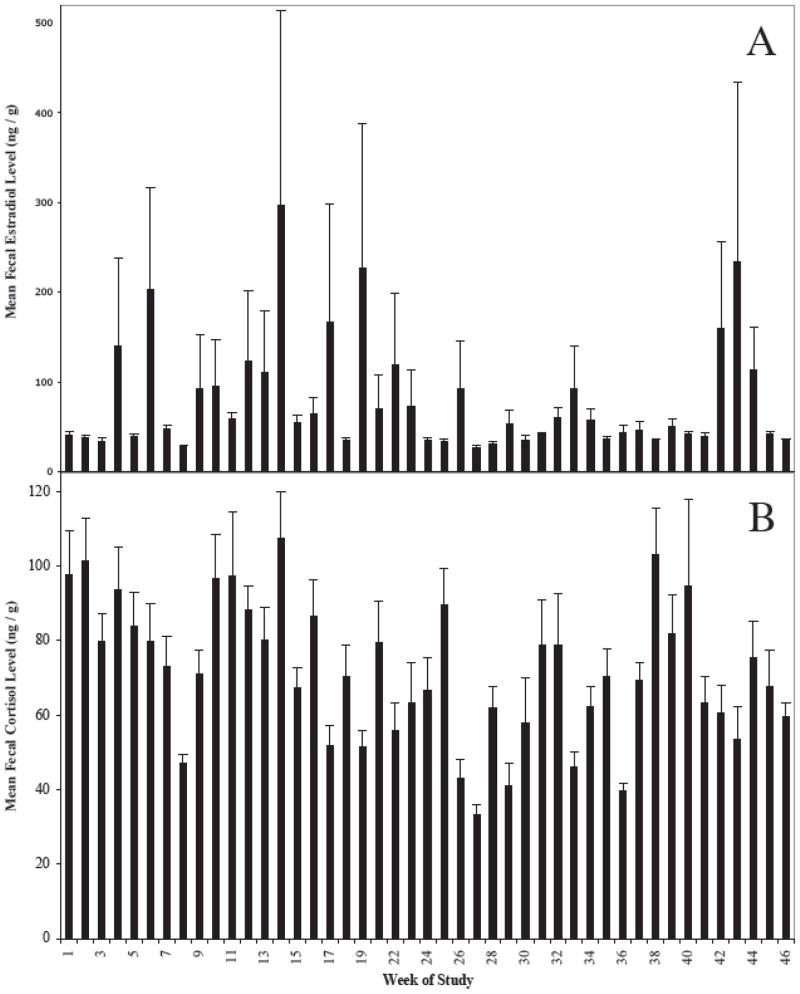
Fecal estradiol levels averaged 80.03 ng/g, with a range from 27.32 to 297.69 ng/g (n = 45; Fig. 2A). However, individual samples had much greater variation in estradiol levels, with a range from 19.62 to 2237.81 ng/g (n = 407). Due to one or two individuals having extremely high levels during a few weeks (e.g., 2237.81 ng/g), certain weeks had very high levels of variation, with a range in the standard error of the mean (SEM) from 1.07 to 216.95 ng/g and an average of 38.66 ng/g. Five of the weeks had one sample > 1000 ng/g, while 14 weeks had one or two samples > 500 ng/g. These one or two extremely high samples were responsible for the high SEM during those weeks. There was no apparent reason to suspect that these high values were a result of methodological error, as the cortisol values for these same samples were not outliers. If the samples had been contaminated or incorrectly processed, it is expected that their cortisol levels would have also reflected this error.
Figure 2.
Weekly mean (+/− SEM) fecal A) estradiol level and B) cortisol level (ng/g dry feces) from ten adult male red colobus living in one group at Kibale National Park, Uganda.
Mean fecal cortisol levels averaged 71.58 ng/g dry feces, with a range from 33.17 to 107.59 ng/g (n = 45; Fig. 2B). Individual samples did not show as much variation for cortisol levels as they did for estradiol levels, with a range from 18.63 to 200.12 ng/g (n = 407). The average SEM was 8.67 ng/g, with a range from 1.86 to 23.12 ng/g.
Influence of phytoestrogen consumption, non-estrogenic dietary items, and climate on fecal hormone levels
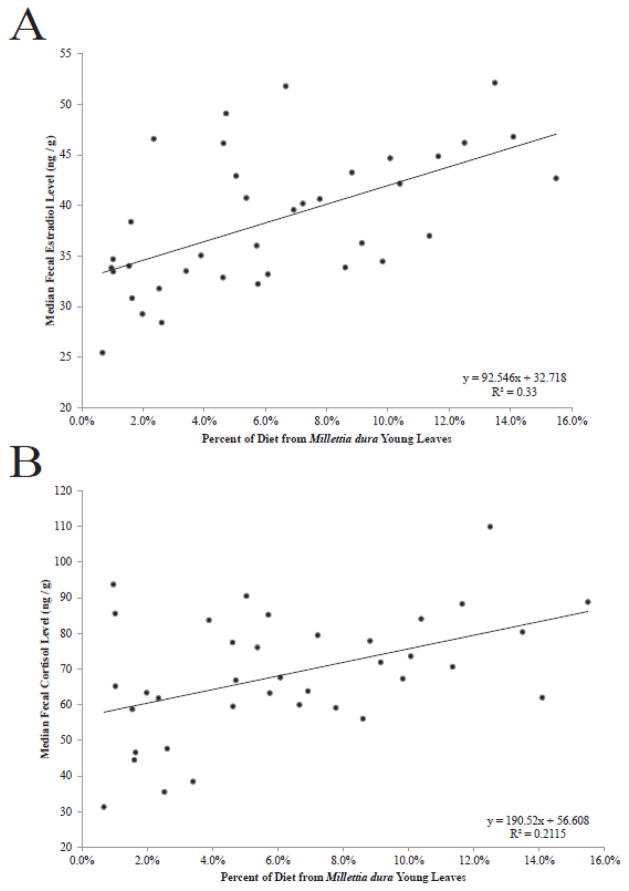
In the stepwise regressions predicting the median fecal estradiol levels with estrogenic dietary items and climatic variables, the best predictor was simply the percent of diet from M. dura young leaves (R2 = 0.362, p < 0.001). The relationship between fecal estradiol and M. dura consumption was also significant when only examining weeks in which it was fed on (r = 0.574, p < 0.001, n = 37; Fig. 3A). The median fecal cortisol levels were also best predicted by percent of diet from M. dura young leaves (R2 = 0.128, p = 0.016) in the stepwise regressions. The relationship between fecal cortisol and M. dura consumption was also significant when only examining weeks in which it was fed on (r = 0.461, p = 0.004, n = 37; Fig. 3B).
Figure 3.
Relationships between percent of weekly diet from Millettia dura young leaves and A) median fecal estradiol level and B) median fecal cortisol level for weeks when the red colobus fed on that particular estrogenic plant item (n = 37). Hormone levels are weekly calculations from ten adult male red colobus living in the same group at Kibale National Park, Uganda. Correlations are significant (p < 0.05).
In the stepwise regression predicting median fecal estradiol level that included the red colobus’ 13 most fed on dietary items (11 non-estrogenic and 2 estrogenic) and rainfall, two significant models were produced: 1) just M. dura young leaves (R2 = 0.355, p < 0.001) and 2) M. dura young leaves and Prunus africana young leaves (R2 = 0.424, p < 0.001). Although P. africana consumption was not related to rainfall (r = −0.028, p = 0.855), it was negatively related to median estradiol levels (r = −0.395, p = 0.007). It is important to note that it also showed a marginally significant negative relationship with M. dura consumption (r = −0.267, p = 0.076).
Influence of phytoestrogen consumption, hormonal state, and climate on social behavior
In the regression tree predicting aggression rate, we found median fecal estradiol to be the primary explanatory factor, followed by the percentage of diet from estrogenic staple foods (Fig. 4A; R2 = 0.437), with the highest rates of aggression associated with intermediate levels of fecal estradiol and lowest rates when estradiol was low. Estrogenic plant consumption was related to aggression rate when estradiol was greater than 34.35 ng/g, with more consumption relating to higher rates of aggression.
Figure 4.
Regression trees explaining variance in A) number of aggressive interactions per hour, B) number of copulations per hour, and C) % of time spent grooming for red colobus males using median fecal cortisol and estradiol, phytoestrogen consumption, rainfall, and temperature as predictor variables. Values in the ovals represent the mean value of the dependent variable at that node and the number of samples falling along that branch. Values in the rectangles represent the leaves of the regression tree and correspond to the mean value of the dependent variable and the number of samples that fall along that particular branch. For example, in figure 4C; when median cortisol was lower than 79.91 and the consumption of estrogenic plants was greater than 12.05% of the diet, the mean percent of time spent grooming was 4.609% and 11 of our samples fell into this category. For a detailed description of the mechanics and interpretation of regression trees see De’ath and Fabricius (2000); in brief the tree is created by repeatedly splitting the data on a single explanatory variable such that the variance in the dependent variable is minimized. The splitting is continued trying all explanatory variables, until the added variance explained is outweighted by the complexity of adding more splits.
With copulation rate the primary split was based on median fecal cortisol levels, with more copulations when cortisol was lower, and secondary splits based on rainfall and M. dura young leaf consumption (Fig. 4B; R2 = 0.388). Copulations were highest when median fecal cortisol was low and consumption of M. dura young leaves was high, while they were lowest when cortisol and rainfall were high (Fig. 4B).
With grooming time, we found median fecal cortisol levels to be the primary explanatory factor followed by the percent of all estrogenic foods in the diet and then median estradiol (Fig. 4C, R2 = 0.352). Red colobus males groomed most when cortisol was low, less estrogenic foods were consumed, and estradiol was high, while they groomed the least when cortisol was high (Fig. 4C).
DISCUSSION
Our results suggest that plant chemistry could act as a selective pressure on primates, and other herbivores, in ways that have yet to be fully appreciated because we found that adult male red colobus fecal estradiol and cortisol levels were related to the consumption of estrogenic plant foods, while social behaviors were related to both endogenous hormonal state and consumption of phytoestrogens. By altering steroid hormone levels that are important to reproductive physiology and the tendency to engage in aggressive, grooming, and mating behaviors, phytoestrogens could directly alter the fitness of the individuals ingesting them. The strongest relationship we documented was the positive association between median estradiol level and the consumption of M. dura young leaves. Millettia dura is a legume of the Papilionoideae subfamily, which is known to contain estrogenic isoflavonoids (Reynaud et al., 2005). Studies demonstrate that the ingestion of papilionoids by livestock can dramatically impair reproduction (Adams, 1990, 1995; Bennetts and Underwood, 1951), and similar detrimental effects on fertility may occur in humans (Cederroth et al., 2010a). However, it is also suggested that phytoestrogens may provide health benefits such as cancer prevention or alleviation of menopausal disorders (Dixon, 2004; Leitman et al., 2010; Ososki and Kennelly, 2003; Setchell and Cassidy, 1999). Based on these studies and the relationships found here, it is possible that M. dura exerted an important influence on the red colobus endocrine system. However, as red colobus have had a long evolutionary relationship with this native species, it is possible that they have evolved adaptations to protect against any potential endocrine disruption caused by ingesting leaves of M. dura (Wynne-Edwards, 2001).
Feeding on M. dura young leaves also had a positive relationship with fecal cortisol, and it is likely that the phytoestrogens interacted not only with the HPG axis, but also with the HPA axis. Studies on amphibians have shown that anthropogenic endocrine disruptors in pesticides can lead to an increase in stress hormone production (Hayes et al., 2006). Further, estrogens are able to alter the negative feedback loop of the HPA axis, thus changing production of glucocorticoids (Weiser and Handa, 2009). Therefore, it is possible that the phytoestrogens of M. dura young leaves influenced cortisol levels in red colobus. This supports the conclusion that the consumption of estrogenic plant foods affected red colobus physiology. The relationship with fecal estradiol may simply have been due to the RIA antibody binding to metabolites of the phytoestrogens passing through the monkeys, but this is unlikely due to our antibody’s cross-reactivities. However, the additional relationship with cortisol provided strong evidence that the phytoestrogens were absorbed and affected the endocrine system through the HPG and HPA axes, since metabolites of phytoestrogens would not bind to the cortisol antibody. Increased cortisol production from phytoestrogen consumption may result in a synergistic threat to endangered primates which live in environments with unusually high proportions of estrogenic plants, both through the suppression of their immune and reproductive systems by elevated cortisol (Sapolsky, 2005) and by altered fertility through phytoestrogen interaction with estrogen receptors (Cederroth et al., 2010a).
The relationships found here between the consumption of estrogenic plants and red colobus hormone levels were correlative; therefore, as with any non-experimental approach, there is the possibility that these relationships were due to spurious correlations. However, by including rainfall and temperature in the multiple regressions, we have attempted to control for other factors related to seasonality that could influence hormone levels. In the tropics, rainfall is the most important factor influencing seasonality. Because the consumption of M. dura young leaves was the only significant variable for predicting both median estradiol and cortisol in the multiple regressions, we think this strongly supports that this estrogenic plant influenced red colobus hormone levels and that this relationship was not driven by another seasonal process. Furthermore, red colobus do not have a breeding season, as births occur in all months of the year, and Kibale is known for being relatively aseasonal in terms of plant phenology and availability of primate plant foods (Chapman et al., 2005; Struhsaker, 1997). Supporting the aseasonality of Kibale, we found none of the other 13 most fed on dietary items correlated with rainfall, besides Millettia dura young leaves. Thus, large changes in red colobus male reproductive state related to dietary quality or climatic variables were not expected. Variation in the consumption of estrogenic plants remains as the most plausible explanation for the changes in hormonal status we found and these changes could translate into variation in fertility.
Additionally, if components of the diet other than phytoestrogens were responsible for the relationships found here it would be expected that consumption of other staple dietary items would relate to rainfall (none were) and non-estrogenic items would explain variation in estradiol levels. In the multiple regression model incorporating non-estrogenic dietary items, estrogenic M. dura young leaves explained most of the variation in red colobus estradiol levels. Of the other 12 most fed on items, only non-estrogenic Prunus africana young leaves slightly improved the model. However, their consumption was negatively correlated with consumption of M. dura and negatively correlated with estradiol. Thus, this relationship was likely due to the red colobus feeding less on M. dura in weeks they fed more on P. africana. Alternatively, there is published evidence that P. africana interacts with the endocrine system as it is used to prevent and treat prostate disorders and its extract displaces both estrogens and androgens (Shenouda et al., 2007). Although our previously reported transfection assay did not find this species to have estrogenic activity at either estrogen receptor (Wasserman et al, 2012), steroidal activity through other mechanisms may be responsible for the negative relationship between its consumption and estradiol levels found here. Therefore, it does not appear that the relationships between consumption of estrogenic plants and red colobus hormone levels found here were due to seasonal changes in non-estrogenic components of diet. This was further supported by the fact that P. africana did not show seasonality in consumption, while M. dura and estradiol did.
Our results showing behavioral relationships with the consumption of estrogenic plants provided further support that the correlations found here between phytoestrogens and red colobus estradiol and cortisol levels had biological significance. Although estradiol levels were most important to predicting rates of aggression and cortisol levels were most important to predicting rates of copulations and percent of time spent grooming, the consumption of estrogenic plants was secondarily important to predicting these social behaviors. We found that the greater the consumption of estrogenic plants, the more the red colobus males displayed aggressive behaviors and copulated and the less they groomed. These results are supported by captive studies that documented changes in behavior due to phytoestrogens. In captive adult male cynomolgus monkeys (Macaca fascicularis), a 15-month soy-based high-isoflavone diet resulted in increased aggressive behaviors (i.e., 67% more frequent compared to individuals fed an isoflavone-free diet) and a decrease in affiliative behaviors (i.e., 68% less time in body contact and 30% more time alone) (Simon et al., 2004). It was postulated that these effects were likely due to the weaker action of isoflavones on ER than estradiol, thus reducing the inhibition of the aggression–promoting action of ER (Simon et al., 2004). Male rats fed a high-isoflavone diet spent less time in social interactions and had higher corticosterone response to stress than rats fed an isoflavone-free diet (Hartley et al., 2003). Similarly, the ER agonist equol increased aggression and anxiety in male rats that were exposed neonatally (Patisaul and Bateman, 2008).
In contrast, our finding that the more the red colobus fed on M. dura young leaves the more they copulated does not agree with other studies that document fewer copulations with consumption of phytoestrogens (Jaroenporn et al., 2006; Wisniewski et al., 2003). It may be that the nutritional benefits obtained from this plant item outweigh any reproductive costs through increased cortisol and estradiol or the red colobus may use this plant as a chemical cue for reproductive behavior, where males are confused by the altered physiology of females due to feeding on the same plant (Berger et al., 1981; Higham et al., 2007; Leopold et al., 1976). In future studies we will examine tradeoffs between nutrient content and phytoestrogen presence in the foods of the red colobus, as well as the physiological and behavioral effects of these estrogenic plants on female red colobus.
Finally, although red colobus did not feed more heavily on M. dura young leaves when they were more available, they did feed more heavily on them during weeks with more rain. This might mean that variation in the phytoestrogen content of these leaves in response to increased rainfall is more important than availability in determining when and how much red colobus will feed on them. This is further supported by the fact that consumption of the other 13 most fed on dietary items was not related to rainfall. Increased phytoestrogen content may either be attractive as a form of self-medication (Forbey et al., 2009; Glander, 1980; Huffman, 1997; Strier, 1993) or act as a feeding deterrent if phytoestrogen consumption reduces fertility (Harborne, 1993; Hughes, 1988; Wynne-Edwards, 2001). Increases in isoflavone concentration (i.e., phytoestrogens) with increased precipitation have been documented in soybeans (Glycine max), which are members of the same subfamily (Papilionoideae) of legumes as M. dura (Morrison et al., 2010). Variation in the nutritional content of plant foods is important to primate diet selection (Milton, 1979; Rothman et al., 2011; Rothman et al., 2006) and can affect reproduction and steroid production (Lu et al., 2011), so tradeoffs between phytoestrogen consumption and nutrient intake may be critical to a primate’s decision to feed on a specific plant and warrant future study.
CONCLUSIONS
Our results show that the ingestion of estrogenic plants related to adult male red colobus fecal estradiol and cortisol levels, as well as aggressive, mating, and grooming behaviors. Further, rainfall was important for understanding seasonality in the consumption of certain estrogenic plant foods, but not to variation in estradiol and cortisol levels or social behaviors. Climate can affect red colobus phytoestrogen consumption and hormone levels through three main mechanisms: (1) effects on food availability/nutritional content, (2) effects on phytoestrogen levels, and (3) effects on hormone levels. Although these mechanisms are not mutually exclusive, our results suggest that the effect of rainfall on phytoestrogen levels of M. dura young leaves is the most important factor affecting the timing and magnitude of red colobus feeding on estrogenic plants. Subsequently, the amount of M. dura young leaves in the diet related to changes in red colobus estradiol and cortisol levels, which influenced social behaviors. We suggest that the consumption of estrogenic plant foods has important implications for their health and fitness through changes in physiology and behavior. In addition, this study raises the possibility of additive effects of natural plant-based endocrine disruptors and climate change on primates, which might threaten their long-term survival. Three findings warrant this: (1) climate change in the form of increased rainfall (c. 300 mm more per year now as compared to the early 1900’s) has been documented in Kibale (Chapman et al., 2005), (2) red colobus were found to feed more heavily on M. dura young leaves during wetter weeks, and (3) agricultural studies have documented increased levels of phytoestrogens with increased rainfall in soy (Morrison et al., 2010). Further study of interactions between estrogenic plants and primates in a natural setting should provide many exciting new discoveries relevant to a broad range of fields, including medicine, conservation, ecology, and evolution.
HIGHLIGHTS.
Phytoestrogen consumption influenced red colobus hormone levels and social behavior
Estrogenic Millettia dura young leaf consumption related positively to rainfall
Consumption of M. dura young leaves was related to higher estradiol and cortisol
Estradiol and cortisol levels were related to aggression, grooming, and mating
Phytoestrogen consumption resulted in more aggression and mating and less grooming
Acknowledgments
We would like to thank the Uganda Wildlife Authority and Uganda National Council for Science and Technology for providing permission to conduct this research. MW received funding from the National Science Foundation (DDIG #0823651 and Graduate Research Fellowship Program), the International Primatological Society, the University of California, Berkeley (UCB) Department of Environmental Science, Policy, and Management, UCB Center for African Studies, the UCB Chang-Lin Tien Scholars Program (funded by the Philomathia Foundation), and McGill University’s Tomlinson Postdoctoral Fellowship to support this research. JFG was supported by the National Science Foundation Graduate Research Fellowship under Grant No. (NSF 11-582). This publication was made possible in part by Grant Number P51 RR000167 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), to the Wisconsin National Primate Research Center, University of Wisconsin-Madison. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. These funding sources had no involvement with our research other than providing financial support. Research conducted complied with all regulations regarding the study of field animals and with Ugandan and U.S. laws. We would like to thank Musunguzi Hillary, Baguma Charles, Katusabe Swaibu, Mutegeki Richard, Patrick Omeja, Dennis Twinomugisha, Kaganzi Clovis, Lauren Chapman, Jessica Rothman, and Julie Wasserman for providing assistance in the field at Kibale. Tyrone Hayes, Isao Kubo, Paul Falso, Alexandra Taylor-Gutt, Mariah Hopkins, Tarek Milleron, Karen Strier, and Andrew Ritchie provided useful comments throughout the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael D. Wasserman, Email: michael.wasserman@mail.mcgill.ca.
Colin A. Chapman, Email: colin.chapman@mcgill.ca.
Katharine Milton, Email: kmilton@berkeley.edu.
Jan F. Gogarten, Email: jan.gogarten@gmail.com.
Dan J. Wittwer, Email: wittwer@primate.wisc.edu.
Toni E. Ziegler, Email: ziegler@primate.wisc.edu.
References
- Adams NR. Permanent infertility in ewes exposed to plant oestrogens. Aust Vet J. 1990;67:197–201. doi: 10.1111/j.1751-0813.1990.tb07758.x. [DOI] [PubMed] [Google Scholar]
- Adams NR. Detection of the effects of phytoestrogens on sheep and cattle. J Anim Sci. 1995;73:1509–1515. doi: 10.2527/1995.7351509x. [DOI] [PubMed] [Google Scholar]
- Bauchop T, Martucci RW. Ruminant-like digestion of the langur monkey. Science. 1968;161:698–700. doi: 10.1126/science.161.3842.698. [DOI] [PubMed] [Google Scholar]
- Bennetts HW. Metaplasia in the sex organs of castrated male sheep maintained on early subterranean clover pastures. Aust Vet J. 1946;22:70–78. doi: 10.1111/j.1751-0813.1946.tb06451.x. [DOI] [PubMed] [Google Scholar]
- Bennetts HW, Underwood EJ. The oestrogenic effects of subterranean clover (Trifolium subterraneum); uterine maintenance in the ovariectomised ewe on clover grazing. Aust J Exp Biol Med Sci. 1951;29:249–253. doi: 10.1038/icb.1951.29. [DOI] [PubMed] [Google Scholar]
- Berger PJ, Negus NC, Sanders EH, Gardner PD. Chemical triggering of reproduction in Microtus montanus. Science. 1981;214:69–70. doi: 10.1126/science.7025210. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Auger J, Zimmermann C, Eustache F, Nef S. Soy, phyto-oestrogens and male reproductive function: a review. Int J Androl. 2010a;33:304–316. doi: 10.1111/j.1365-2605.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Zimmermann C, Beny JL, Schaad O, Combepine C, Descombes P, Doerge DR, Pralong FP, Vassalli JD, Nef S. Potential detrimental effects of a phytoestrogen-rich diet on male fertility in mice. Mol Cell Endocrinol. 2010b;321:152–160. doi: 10.1016/j.mce.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Chapman LJ, Gillespie TR. Scale issues in the study of primate foraging: Red colobus of Kibale National Park. Am J Phys Anthropol. 2002;117:349–363. doi: 10.1002/ajpa.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CA, Chapman LJ, Jacob AL, Rothman JM, Omeja P, Reyna-Hurtado R, Hartter J, Lawes MJ. Tropical tree community shifts: Implications for wildlife conservation. Biol Conserv. 2010;143:366–374. [Google Scholar]
- Chapman CA, Chapman LJ, Struhsaker TT, Zanne AE, Clark CJ, Poulsen JR. A long-term evaluation of fruiting phenology: importance of climate change. J Trop Ecol. 2005;21:31–45. [Google Scholar]
- Chapman CA, Peres CA. Primate conservation in the new millennium: The role of scientists. Evol Anthropol. 2001;10:16–33. [Google Scholar]
- Chapman CA, Wrangham RW, Chapman LJ, Kennard DK, Zanne AE. Fruit and flower phenology at two sites in Kibale National Park, Uganda. J Trop Ecol. 1999;15:189–211. [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De’ath G. Boosted trees for ecological modeling and prediction. Ecology. 2007;88:243–251. doi: 10.1890/0012-9658(2007)88[243:btfema]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- De’ath G, Fabricius KE. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–3192. [Google Scholar]
- Dixon RA. Phytoestrogens. Annu Rev Plant Biol. 2004;55:225–261. doi: 10.1146/annurev.arplant.55.031903.141729. [DOI] [PubMed] [Google Scholar]
- Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- Forbey JS, Harvey AL, Huffman MA, Provenza FD, Sullivan R, Tasdemir D. Exploitation of secondary metabolites by animals: A response to homeostatic challenges. Integr Comp Biol. 2009;49:314–328. doi: 10.1093/icb/icp046. [DOI] [PubMed] [Google Scholar]
- French JA, Abbott DH, Scheffler G, Robinson JA, Goy RW. Cyclic excretion of urinary estrogens in female tamarins (Saguinus oedipus) J Reprod Fertil. 1983;68:177–184. doi: 10.1530/jrf.0.0680177. [DOI] [PubMed] [Google Scholar]
- Glander KE. Reproduction and population growth in free-ranging mantled howling monkeys. Am J Phys Anthropol. 1980;53:25–36. doi: 10.1002/ajpa.1330530106. [DOI] [PubMed] [Google Scholar]
- Gogarten JF, Brown LM, Chapman CA, Cords M, Doran-Sheehy D, Fedigan LM, Grine FE, Perry S, Pusey AE, Sterck EHM, Wich SA, Wright PC. Seasonal mortality patterns in non-human primates: Implications for variation in selection pressures across environments. Evolution. doi: 10.1111/j.1558-5646.2012.01668.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LJ., Jr Contaminant-induced endocrine disruption in wildlife. Growth Horm IGF Res. 2000;10(Suppl B):S45–50. doi: 10.1016/s1096-6374(00)80009-x. [DOI] [PubMed] [Google Scholar]
- Gultekin E, Yildiz F. Introduction to phytoestrogens. In: Yildiz F, editor. Phytoestrogens in Functional Foods. CRC Press; Boca Raton, Florida: 2006. pp. 3–18. [Google Scholar]
- Hadley M. Endocrinology. 5. Prentice Hall; Upper Saddle River, New Jersey: 1999. [Google Scholar]
- Harborne J. Introduction to Ecological Biochemistry. 4. Elsevier Academic Press; San Francisco, California: 1993. [Google Scholar]
- Hartley DE, Edwards JE, Spiller CE, Alom N, Tucci S, Seth P, Forsling ML, File SE. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology. 2003;167:46–53. doi: 10.1007/s00213-002-1369-7. [DOI] [PubMed] [Google Scholar]
- Hayes TB. Welcome to the revolution: integrative biology and assessing the impact of endocrine disruptors on environmental and public health. Integr Comp Biol. 2005;45:321–329. doi: 10.1093/icb/45.2.321. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Case P, Chui S, Chung D, Haeffele C, Haston K, Lee M, Mai VP, Marjuoa Y, Parker J, Tsui M. Pesticide mixtures, endocrine disruption, and amphibian declines: Are we underestimating the impact? Environ Health Perspect. 2006;114:40–50. doi: 10.1289/ehp.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci U S A. 2002;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistermann M, Tari S, Hodges JK. Measurement of faecal steroids for monitoring ovarian function in New World primates, Callitrichidae. J Reprod Fertil. 1993;99:243–251. doi: 10.1530/jrf.0.0990243. [DOI] [PubMed] [Google Scholar]
- Higham JP, Ross C, Warren Y, Heistermann M, MacLarnon AM. Reduced reproductive function in wild baboons (Papio hamadryas anubis) related to natural consumption of the African black plum (Vitex doniana) Horm Behav. 2007;52:384–390. doi: 10.1016/j.yhbeh.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Huffman MA. Current evidence for self-medication in primates: A multidisciplinary perspective. Yearb Phys Anthropol. 1997;40:171–200. [Google Scholar]
- Hughes CL., Jr Phytochemical mimicry of reproductive hormones and modulation of herbivore fertility by phytoestrogens. Environ Health Perspect. 1988;78:171–174. doi: 10.1289/ehp.8878171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroenporn S, Malaivijitnond S, Wattanasirmkit K, Trisomboon H, Watanabe G, Taya K, Cherdshewasart W. Effects of Pueraria mirifica, an herb containing phytoestrogens, on reproductive organs and fertility of adult male mice. Endocrine. 2006;30:93–101. doi: 10.1385/ENDO:30:1:93. [DOI] [PubMed] [Google Scholar]
- Lambert JE. Primate digestion: Interactions among anatomy, physiology, and feeding ecology. Evol Anthropol. 1998;7:8–20. [Google Scholar]
- Leitman DC, Paruthiyil S, Vivar OI, Saunier EF, Herber CB, Cohen I, Tagliaferri M, Speed TP. Regulation of specific target genes and biological responses by estrogen receptor subtype agonists. Curr Opin Pharmacol. 2010;10:629–636. doi: 10.1016/j.coph.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold AS, Erwin M, Oh J, Browning B. Phytoestrogens: adverse effects on reproduction in California quail. Science. 1976;191:98–100. doi: 10.1126/science.1246602. [DOI] [PubMed] [Google Scholar]
- Lu A, Beehner JC, Czekala NM, Koenig A, Larney E, Borries C. Phytochemicals and reproductive function in wild female Phayre’s leaf monkeys (Trachypithecus phayrei crepusculus) Horm Behav. 2011;59:28–36. doi: 10.1016/j.yhbeh.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Mazur W, Adlercreutz H. Naturally occurring oestrogens in food. Pure Appl Chem. 1998;70:1759–1776. [Google Scholar]
- Milnes MR, Bermudez DS, Bryan TA, Edwards TM, Gunderson MP, Larkin IL, Moore BC, Guillette LJ., Jr Contaminant-induced feminization and demasculinization of nonmammalian vertebrate males in aquatic environments. Environ Res. 2006;100:3–17. doi: 10.1016/j.envres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Milton K. Factors influencing leaf choice by howler monkeys: a test of some hypotheses of food selection by generalist herbivores. Am Nat. 1979;114:362–378. [Google Scholar]
- Milton K. The Foraging Strategy of Howler Monkeys: A Study in Primate Economics. Columbia University Press; New York: 1980. [Google Scholar]
- Morrison MJ, Cober ER, Saleem MF, McLaughlin NB, Fregeau-Reid J, Ma BL, Woodrow L. Seasonal changes in temperature and precipitation influence isoflavone concentration in short-season soybean. Field Crop Res. 2010;117:113–121. [Google Scholar]
- Ososki AL, Kennelly EJ. Phytoestrogens: a review of the present state of research. Phytother Res. 2003;17:845–869. doi: 10.1002/ptr.1364. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav. 2008;53:580–588. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Propper CR. The study of endocrine-disrupting compounds: past approaches and new directions. Integr Comp Biol. 2005;45:194–200. doi: 10.1093/icb/45.1.194. [DOI] [PubMed] [Google Scholar]
- R-Development-Core-Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- Reynaud J, Guilet D, Terreux R, Lussignol M, Walchshofer N. Isoflavonoids in non-leguminous families: an update. Nat Prod Rep. 2005;22:504–515. doi: 10.1039/b416248j. [DOI] [PubMed] [Google Scholar]
- Rothman JM, Raubenheimer D, Chapman CA. Nutritional geometry: gorillas prioritize non-protein energy while consuming surplus protein. Biol Lett. 2011;7:847–849. doi: 10.1098/rsbl.2011.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JM, Van Soest PJ, Pell AN. Decaying wood is a sodium source for mountain gorillas. Biol Lett. 2006;2:321–324. doi: 10.1098/rsbl.2006.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140:1355S–1362S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenouda NS, Sakla MS, Newton LG, Besch-Williford C, Greenberg NM, MacDonald RS, Lubahn DB. Phytosterol Pygeum africanum regulates prostate cancer in vitro and in vivo. Endocrine. 2007;31:72–81. doi: 10.1007/s12020-007-0014-y. [DOI] [PubMed] [Google Scholar]
- Simon NG, Kaplan JR, Hu S, Register TC, Adams MR. Increased aggressive behavior and decreased affiliative behavior in adult male monkeys after long-term consumption of diets rich in soy protein and isoflavones. Horm Behav. 2004;45:278–284. doi: 10.1016/j.yhbeh.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Snaith TV, Chapman CA, Rothman JM, Wasserman MD. Bigger groups have fewer parasites and similar cortisol levels: a multi-group analysis in red colobus monkeys. Am J Primatol. 2008;70:1072–1080. doi: 10.1002/ajp.20601. [DOI] [PubMed] [Google Scholar]
- Sousa MB, Ziegler TE. Diurnal variation on the excretion patterns of fecal steroids in common marmoset (Callithrix jacchus) females. Am J Primatol. 1998;46:105–117. doi: 10.1002/(SICI)1098-2345(1998)46:2<105::AID-AJP1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Strier KB. Menu for a monkey. Nat Hist. 1993;102:34–43. [Google Scholar]
- Struhsaker T. Ecology of an African Rain Forest: Logging in Kibale and the Conflict between Conservation and Exploitation. University Press of Florida; Gainesville, Florida: 1997. [Google Scholar]
- Struhsaker TT. Conservation of red colobus and their habitats. Int J Primatol. 2005;26:525–538. [Google Scholar]
- Struhsaker TT. IUCN Red List of Threatened Species, Version 2011.2. IUCN; 2008. Procolobus rufomitratus ssp. tephrosceles. 2011. [Google Scholar]
- Therneau TM, Atkinson B, Ripley MB. rpart: Recursive Partitioning, R package version 3.1–51. 2012 http://mayoresearch.mayo.edu/mayo/research/biostat/splusfunctions.cfm.
- Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci U S A. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C, Palme R. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann N Y Acad Sci. 2005;1046:54–74. doi: 10.1196/annals.1343.006. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Risler L, Steiner RA. Excreted steroids in primate feces over the menstrual cycle and pregnancy. Biol Reprod. 1988;39:862–872. doi: 10.1095/biolreprod39.4.862. [DOI] [PubMed] [Google Scholar]
- Wasserman M, Chapman C, Milton K, Goldberg T, Ziegler T. Physiological and behavioral effects of capture darting on red colobus monkeys (Procolobus rufomitratus) with a comparison to chimpanzee predation. Int J Primatol In review. [Google Scholar]
- Wasserman MD, Taylor-Gutt A, Rothman JM, Chapman CA, Milton K, Leitman DC. Estrogenic plant foods of red colobus monkeys and mountain gorillas in uganda. Am J Phys Anthropol. 2012;148:88–97. doi: 10.1002/ajpa.22045. [DOI] [PubMed] [Google Scholar]
- Weingrill T, Gray DA, Barrett L, Henzi SP. Fecal cortisol levels in free-ranging female chacma baboons: relationship to dominance, reproductive state and environmental factors. Horm Behav. 2004;45:259–269. doi: 10.1016/j.yhbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten PL, Brockman DK, Stavisky RC. Recent advances in noninvasive techniques to monitor hormone-behavior interactions. Am J Phys Anthropol Suppl. 1998;27:1–23. doi: 10.1002/(sici)1096-8644(1998)107:27+<1::aid-ajpa2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Whitten PL, Patisaul HB. Cross-species and interassay comparisons of phytoestrogen action. Environ Health Perspect. 2001;109(Suppl 1):5–20. doi: 10.1289/ehp.01109s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield JC. The concept of allostasis: Coping with a capricious environment. J Mammal. 2005;86:248–254. [Google Scholar]
- Wingfield JC, Moore MC, Farner DS. Endocrine responses to inclement weather in naturally breeding populations of white-crowned sparrows (Zonotrichia leucophrys pugetensis) Auk. 1983;100:56–62. [Google Scholar]
- Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: When and how. J Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Klein SL, Lakshmanan Y, Gearhart JP. Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats. J Urol. 2003;169:1582–1586. doi: 10.1097/01.ju.0000046780.23389.e0. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE. Evolutionary biology of plant defenses against herbivory and their predictive implications for endocrine disruptor susceptibility in vertebrates. Environ Health Perspect. 2001;109:443–448. doi: 10.1289/ehp.01109443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm Behav. 1995;29:407–424. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Wittwer DJ. Fecal steroid research in the field and laboratory: improved methods for storage, transport, processing, and analysis. Am J Primatol. 2005;67:159–174. doi: 10.1002/ajp.20175. [DOI] [PubMed] [Google Scholar]