Abstract
The intercellular adhesion molecule-1 (ICAM-1, CD54) serves as a counter-receptor for the β2-integrins, LFA-1 and Mac-1, which are expressed on leukocytes. Although expression of ICAM-1 on tumor cells has a role in tumor progression and development, information on ICAM-1 expression and its role in oral cancer has not been established. Normal human oral keratinocytes (NHOK), human papilloma virus (HPV)-immortalized human oral keratinocyte lines (HOK-16B, HOK-18A, and HOK-18C), and six human oral neoplastic cell lines (HOK-16B-BaP-T1, SCC-4, SCC-9, HEp-2, Tu-177 and 1483) were used to study ICAM-1 expression and its functional role in vitro. Our results demonstrated that NHOK express negligible levels of ICAM-1, whereas immortalized human oral keratinocytes and cancer cells express significantly higher levels of ICAM-1, except for HOK-16B-BaP-T1 and HEp-2. Altered mRNA half-lives did not fully account for the increased accumulation of ICAM-1 mRNA. Adhesion of peripheral blood mononuclear cells (PBMC) to epithelial cells correlated with cell surface ICAM-1 expression levels. This adhesion was inhibited by antibodies specific for either ICAM-1 or LFA-1/Mac-1, suggesting a role for these molecules in adhesion. In contrast, lymphokine-activated-killer (LAK) cell cytotoxic killing of epithelial cells did not correlate with ICAM-1 levels or with adhesion. Nonetheless, within each cell line, blocking of ICAM-1 or LFA-1/Mac-1 reduced LAK cells killing, suggesting that ICAM-1 is involved in mediating this killing.
Keywords: ICAM-1, Oral Epithelial Cells, Human Papilloma Virus, Peripheral Blood Mononuclear Cells, Adhesion, Lymphokine-Activated Killer Cells, Cytotoxicity, Oral cancer
Introduction
The intercellular adhesion molecule-1 (ICAM-1, CD54) is a member of the immunoglobulin gene superfamily. This cell surface glycoprotein serves as a ligand for the β2-integrins, LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18), which are expressed on leukocytes (1,2), and for leukosialin (CD43) expressed on T cells (3). The interactions between ICAM-1 and the β2-integrins play a pivotal role in the course of inflammatory and immune responses.
ICAM-1 is expressed by a wide variety of cells, including subpopulations of hemopoietic cells, vascular endothelium, fibroblasts and epithelial cells (4-7). In skin tissue, ICAM-1 is only expressed by epidermal keratinocytes at the site of T cell infiltration in inflammatory dermatitis (8, 9). Similarly, ICAM-1 is not usually detected in oral epithelium, except in the sulcular and junctional epithelium (10-12). ICAM-1 expression is induced in oral keratinocytes in recurrent oral ulceration (13).
A link between ICAM-1 expression and tumor progression was first demonstrated in melanoma cells in which ICAM-1 expression correlates with the risk of metastasis (14, 15). Similarly, in liver cancer patients, both tissue ICAM-1 and secreted ICAM-1 levels are higher in the group with metastasis than in the group without metastasis (16). Furthermore, malignant melanoma cells treated with TNF-α, which induces ICAM-1 expression, have a greater ability to metastasize when the cells are inoculated intravenously into nude mice, and that this effect can be reduced by the use of ICAM-1 antisense oligonucleotides (17).
Cancer cells expressing ICAM-1 may bind to leukocytes expressing LFA-1, thus using the leukocytes to bring these cancer cells to the endothelium. This enhances the potential for hematogenous metastasis (18). Alternately, ICAM-1 expression on tumor cells may be detrimental to tumor growth. ICAM-1 expression on tumor cells increases their interaction with the immunosurveillance system, thereby enhancing the response to adoptive immunotherapy and increasing susceptibility to tumor cell lysis (19-23). Immunohistochemical studies of ICAM-1 expression in invasive breast cancer have demonstrated that ICAM-1 expression has a negative correlation with tumor size and lymph node metastasis. Patients with ICAM-1-positive tumors had a better prognosis and overall survival rates (24). Over-expression of ICAM-1 on colorectal cancer cells might favor host anti-tumor defenses by trafficking of lymphocytes, which influences tumor progression (25).
Unfortunately, specific information regarding ICAM-1 expression in oral cancer is lacking. Therefore, this study was undertaken to investigate whether ICAM-1 expression in human oral epithelial cells is altered following immortalization and transformation using established cell lines, carcinoma cell lines, as well as the primary cultured cells. We present herein that immortalized and transformed oral epithelial cells constitutively express high levels of ICAM-1 both at the protein and mRNA level. The functional aspects of this increased expression were investigated using in vitro assay systems. The implication s of ICAM-1's role in vivo are discussed.
Materials and Methods
Cell cultures
Normal human oral keratinocytes (NHOK) were prepared from gingival tissue excised from healthy donors. The tissues were washed in calcium- and magnesium-free Hank's Balanced Saline Solution (HBSS, Life Technologies, Gaithersburg, MD), digested with dispase and collagenase to separate the epithelial layer, then digested with trypsin to separate individual cells. The dissociated epithelial cells were grown to confluency in keratinocyte growth medium (KGM) supplemented with 30 μg/ml bovine pituitary extract, 0.1 ng/ml human epidermal growth factor, 5 μg/ml bovine insulin, 0.5 μg/ml hydrocortisone, 50 μg/ml gentamicin and 50 ng/ml amphotericin-B (Clonetics Corp., San Diego, CA), at a density of 5 × 104 cells per 28 cm2 (26).
NHOK immortalized with HPV-16 DNA (HOK-16B) or HPV-18 DNA (HOK-18A and HOK-18C cells) (26, 27), were grown in supplemented KGM. The fully transformed cell line HOK-16B-BaP-T1, derived from exposing HOK-16B cells to chemical carcinogens, were grown in Dulbecco's Modified Eagle Medium (DMEM; Life Technologies) containing 4.5 g/L D-glucose and supplemented with 10% fetal bovine serum (FBS) and 0.4 μg/ml hydrocortisone (28). HEp-2 cells, a larynx carcinoma cell line (American Type Culture Collection (ATCC) CCL-23), were grown in Minimum Essential Medium (MEM) supplemented with 10% FBS. Oral carcinoma cell lines, SCC-4 (ATCC CRL-1624) and SCC-9 (ATCC CRL-1629), were grown in DMEM/F12 (1:1 mixture) with 10% FBS and 0.4 μg/ml hydrocortisone. Oral carcinoma cell lines, Tu-177 and 1483 (from Drs. G. L. Clayman and E. J. Shillitoe, Houston, Texas), were grown in DMEM/F12 (1:1 mixture) with 10% FBS. With the exception of SCC4, SCC-9, Tu-177 and NHOK, all other cell lines contain HPV DNA.
Northern blot analysis and mRNA half-life determination
Cellular RNA was isolated using RNA STAT-60 (Tel-Test “B”, Inc., Friendswood, TX). Fifteen μg of total RNA was size fractionated on a 1.5% formaldehyde-agarose gel, transferred to a nitrocellulose filter, and probed with a specific 32P-labeled cDNA fragment of human ICAM-1 targeting a 1,400 bp fragment in the coding region (XhoI restriction endonuclease digest of plasmid pICAM-1; kindly provided by Dr. B. Seed, Boston, MA). A 32P-labeled cDNA fragment of human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control probe.
Monolayers of oral epithelial cells were treated with actinomycin D (ActD; 10 μg/mL) to block transcription. At different time points following ActD treatment, RNA was isolated and subjected to Northern blot analysis. To enhance the detection of low level ICAM-1 message from NHOK, mRNA was isolated using the MessageMaker mRNA Isolation System (Life Technologies). In addition to ICAM-1, a short-lived message c-myc was also probed with a c-myc cDNA fragment (provided by Dr. R. Chiu, UCLA, Los Angeles, CA) to verify the activity of ActD. The signals were visualized by autoradiography. The x-ray images were scanned and specific bands were quantitated using an NIH image software program. The half-lives of the ICAM-1 transcripts were measured by comparison with the mRNA level at time 0 (100%).
ICAM-1 surface expression analysis
Cells were harvested by very brief exposure to 0.05% trypsin/EDTA solution in phosphate buffered saline (PBS), then incubated for 30 min on ice with a mouse monoclonal antibody specific for cell surface ICAM-1 (murine IgG1 anti-human CD54 (ICAM-1), AMAC Inc., Westbrook, ME). Cells were then washed with cold PBS and incubated with secondary antibody, goat anti-mouse IgG (H+L)-R-phycoerythrin (RPE) conjugate (Southern Biotechnology Assoc., Inc., Birmingham, AL), for 30 min on ice. Nonspecific background staining was determined using an isotype control antibody (mouse IgG, Sigma, St. Louis, MO) and the secondary antibody. Cells were then washed and fixed in 1% formaldehyde in PBS and subjected to flow cytometry (Becton-Dickinson FACScan).
Peripheral blood mononuclear cells (PBMC) adhesion assay
Epithelial cells were seeded into 48-well plates 2 d prior to the adhesion assays. Human PBMC were isolated from healthy donors using Ficoll-Paque density gradient centrifugation (Pharmacia Biotech, Uppsala, Sweden). The PBMC (1-5 × 107) were resuspended in 2 ml RPMI-1640 and labeled for 2 h at 37°C in 5% CO2 with 100 μCi of sodium [51Cr] chromate (ICN Pharmaceuticals, Costa Mesa, CA). Labeled PBMC were washed three times with PBS and resuspended in RPMI-1640 with 2% human serum (Sigma). Labeled PBMC were added to confluent monolayers of oral epithelial cells at a ratio of ∼8:1 PBMC per epithelial cell. The cultures were centrifuged at room temperature for 30 sec at 50 × g, then incubated for 30 min at 37°C in 5% CO2. The monolayers were gently washed three times with HBSS to remove nonadherent PBMC.
For blocking ICAM-1, the monolayers were pre-incubated for 30 min at room temperature with 20 μg/ml mouse anti-human CD54 (ICAM-1) (clone RR1, kindly provided by Dr. R. Rothlein) (29) or an isotype control antibody (mouse IgG, Sigma). For blocking neutrophil counter-receptors for ICAM-1, PBMC were pre-incubated with 10 μg/ml mouse anti-human CD18 (LFA-1β) (Biosource International, Camarillo, CA) for 30 min at room temperature. Antibodies were present throughout the subsequent incubation period. Adherent PBMC were lysed in 0.5% Triton X-100 in HBSS and the lysate was collected for γ-counts. Bound PBMC was measured as percent of the total PBMC added to the monolayers. Because the cell number at confluence was different among the various cell lines, a correction factor was used to normalize the percent of adherent PBMC, i.e., PBMC adhesion among different cell lines is presented as PBMC adhesion per same number of cells.
Lymphokine-activated-killer (LAK) cell cytotoxicity assay
51Cr labeling of target cells
Epithelial cells were seeded into 96-well plate ∼2 d prior to the killing assays. Confluent epithelial cells were labeled with 51Cr (50 μCi/ml) in RPMI-1640 containing 10% FBS and antibiotics (100 U/ml penicillin-G, 100 μg/ml streptomycin, 0.25 μg/ml Fungizone) for 8 h. Monolayers were then washed three times with RPMI before adding the LAK.
LAK cell preparation
Freshly isolated PBMC were resuspended in 10% human AB serum in RPMI-1640 containing antibiotics (as above), 10 mM HEPES, and 100 U/ml recombinant human IL-2 (rhIL-2; Amgen, Thousand Oaks, CA). Cells were incubated for 3 d and then washed three times with RPMI-1640 medium.
Cytotoxicity assay
LAK cells, resuspended in RPMI-1640 containing heat-inactivated 2% human serum and antibiotics, were added to epithelial cell monolayers at a ratio of ∼4:1 LAK cells per epithelial cell. Cultures were then incubated at 37°C in 5% CO2 for 8 h to allow LAK-mediated cytotoxic killing of the target monolayers. 51Cr labeling and incubation with LAK cells was performed on adherent epithelial cell monolayers to prevent potential damage of the ICAM-1 surface molecule from trypsin treatment for detaching epithelial cells.
For blocking ICAM-1, monolayers were pre-incubated for 30 min at room temperature with 20 μg/ml mouse anti-human ICAM-1 or an isotype control antibody as described above. For blocking the LAK cell ICAM-1 counter-receptors, LAK cells were pre-incubated with 10 μg/ml mouse anti-human CD18 (LFA-1β) for 30 min at room temperature. Antibodies were present throughout the subsequent incubation period. After incubation, supernatants were collected from the wells without LAK cells for analysis of spontaneous release or from wells with LAK cells for analysis of killing release. Total release was measured by adding 0.5% Triton X-100 in HBSS to lyse the cells. Supernatants (spontaneous, killing, or total release) were analyzed with a γ-counter. A correction factor was also applied to normalize the percentage of LAK killing.
Results
Altered cell surface ICAM-1 expression and ICAM-1 mRNA levels in transformed oral keratinocytes
Normal, HPV immortalized, and neoplastic cells were analyzed for surface ICAM-1 expression by FACScan. Figure 1A shows the relative levels of cell surface ICAM-1 expression on NHOK, and the HPV-16 DNA positive cell lines, HOK-16B and HOK-16B-BaP-T1. ICAM-1 expression on NHOK was low, whereas the level dramatically increased on the HPV-16 immortalized cell line HOK-16B. However, the ICAM-1 level was lower on the fully transformed HOK-16B-BaP-T1 cells, i.e., the level was similar to that of NHOK. Because different culture medium was used for the different cells, fresh medium or conditioned medium from each cell line was incubated with each of the other cell lines to ensure that the increased ICAM-1 on HOK-16B was not due to the effects of the culture medium. The results were no different than those using normal culture methods, i.e., HOK-16B produced a higher level of surface ICAM-1 than NHOK and the HOK-16B-BaP-T1 cell line regardless of which medium was used for culture. Northern blot analyses (Fig. 1B & C) showed that ICAM-1 mRNA levels correlated with the cell surface protein levels.
Fig. 1.
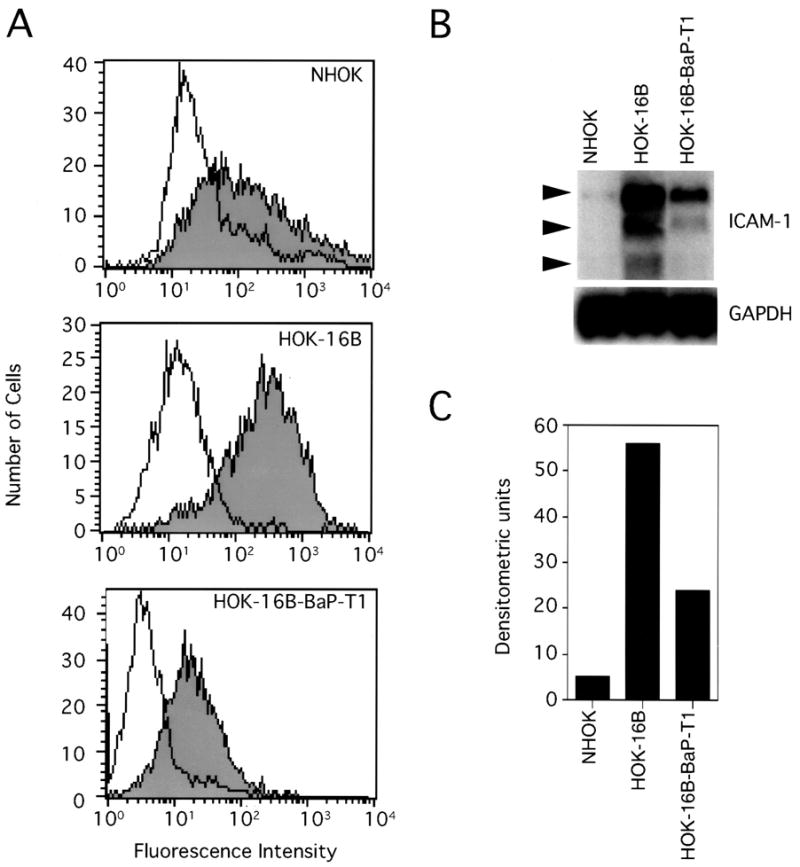
Expression of ICAM-1 by primary oral epithelial cells (NHOK) and two immortalized oral epithelial cell lines (HOK-16B and HOK-16B-BaP-T1) containing the HPV-16 genome. (A) Flow cytometric analysis of ICAM-1 expression on oral epithelial cells. Shaded areas represent constitutive ICAM-1 expression and non-shaded areas depict staining with an isotype control antibody. (B) Northern blot analysis of ICAM-1 mRNA expression in oral epithelial cells. Three major ICAM-1 mRNA forms were detected (3.4, 2.6 and 1.7 kb). (C) Relative signal densities of ICAM-1 mRNA among the cells measured by NIH imaging software. Data are from a representative experiment.
Comparison of ICAM-1 levels in the HPV-18 DNA positive cell lines (Fig. 2A) revealed that the HOK-18A and 1483 cells expressed high levels of ICAM-1 relative to NHOK, whereas HEp-2 had a lower level than NHOK. The three HPV DNA negative carcinoma cell lines, Tu-177, SCC-4 and SCC-9, expressed high levels of ICAM-1 (Fig. 2B). Northern blot analyses (Fig. 2C & D) demonstrated that the ICAM-1 mRNA levels in each of these cells correlated with their cell surface expression levels. Figure 3 summarizes the relative levels of ICAM-1 cell surface expression among all cells examined. Generally, their levels are correlated well with their mRNA levels (Figs. 1C, 2D).
Fig. 2.
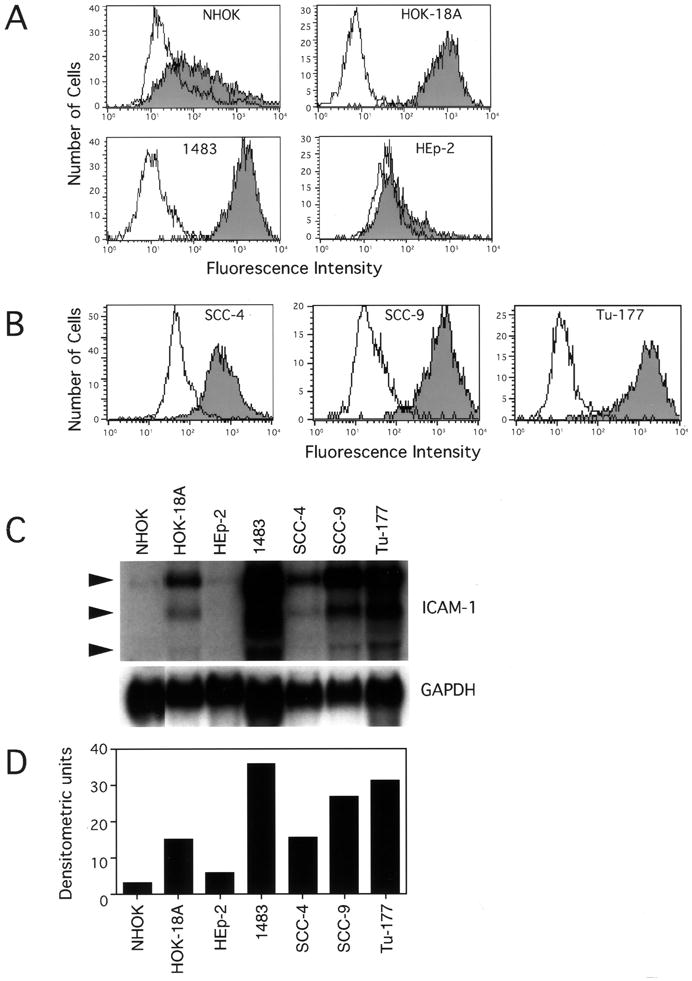
Expression of ICAM-1 by primary oral epithelial cells (NHOK) and six oral epithelial cell lines (HOK-18A, 1483, HEp-2, SCC-4, SCC-9, and Tu-177). Flow cytometric analysis of ICAM-1 expression on (A) NHOK and three oral cell lines containing HPV-18 and (B) three HPV-negative oral cancer cell lines. Shaded areas represent constitutive ICAM-1 expression and non-shaded areas depict staining with an isotype control antibody. (C) Northern blot analysis of ICAM-1 mRNA expression in oral epithelial cells. (D) Relative signal densities of ICAM-1 mRNA among the cells measured by NIH imaging software. Data are from a representative experiment.
Fig. 3.
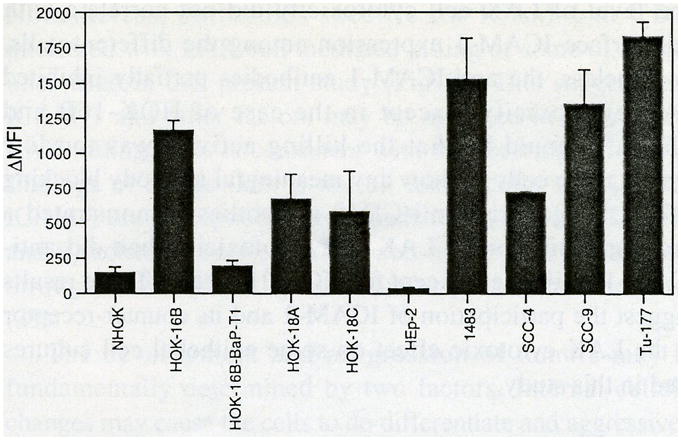
Relative cell surface ICAM-1 expression on NHOK and oral epithelial cell lines. Δ mean fluorescence intensity (MFI) = MFI with anti-ICAM-1 IgG minus MFI with isotype control. Data represent mean ± SEM of the results of at least two or more independent experiments.
Longer ICAM-1 mRNA half-life does not fully account for the increased mRNA expression
To determine whether increased ICAM-1 mRNA levels in certain cells was the result of an altered mRNA turnover rate, mRNA half-lives were measured using the transcription inhibitor ActD. Cells with low ICAM-1 mRNA levels (NHOK and HOK-16B-BaP-T1) and those with high ICAM-1 mRNA levels (HOK-16B and SCC-9) were used for these experiments (Fig. 4). The ICAM-1 mRNA turnover rate in HOK-16B was 2.4-fold higher than in NHOK. In the HOK-16B-BaP-T1 and SCC-9 cells, the turnover rate was approximately the same as in NHOK. In contrast, the mRNA level was 9- to 11-fold higher in both HOK-16B and SCC-9, and ∼5-fold higher in HOK-16B-BaP-T1 than in NHOK (Figs. 1C and 2D). Thus, altered ICAM-1 mRNA turnover rates in the HOK-16B and SCC-9 cells, compared with that of NHOK, did not fully account for differences in the ICAM-1 mRNA levels.
Fig. 4.
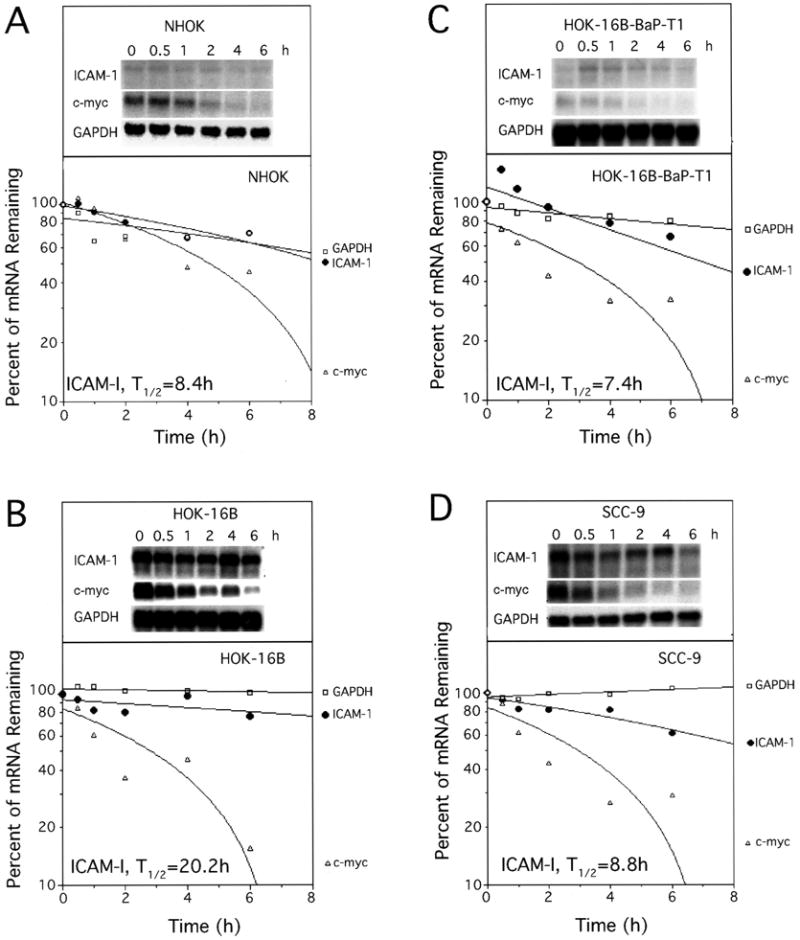
ICAM-1 mRNA half-lives in oral epithelial cells. At different time points (0, 0.5, 1, 2, 4 and 6 h) following ActD treatment, RNA were isolated and subjected to Northern blot analysis. Northern blot images and plots to determine the message half-lives are shown. The signal densities of ICAM-1 mRNA were measured by NIH imaging software. T1/2, mRNA half-life.
Increased cell surface ICAM-1 mediates adhesion of PBMC
The functional role of the increased ICAM-1 on these cell lines was assessed using the PBMC in vitro adhesion assay. Freshly isolated PBMC, which express the ICAM-1 receptors LFA-1/Mac-1, were used for the adhesion assay. The results demonstrated that the level of PBMC adhesion to each epithelial cell line approximately correlated to the level of surface ICAM-1 expression (Fig. 5; compare with Fig. 3). To verify whether this adhesion is mediated specifically by ICAM-1/LFA-1 or Mac-1 interaction, blocking antibodies reactive with either ICAM-1 or LFA-1/Mac (both receptors contain CD18) were preincubated with the epithelial cell monolayers or PBMC, respectively, prior to the adhesion assays. As shown in Fig. 5, 30-40% of PBMC binding was inhibited by the addition of anti-ICAM-1. Moreover, anti-CD18 inhibited up to 60-90% of adhesion. In contrast, isotype control antibodies did not inhibit adhesion. These data indicate that the higher levels of PBMC adhesion to HOK-16B, HOK-18A, SCC-4, and SCC-9 cells is mediated, at least in part, via ICAM/LFA-1 or Mac-1 interactions.
Fig. 5.
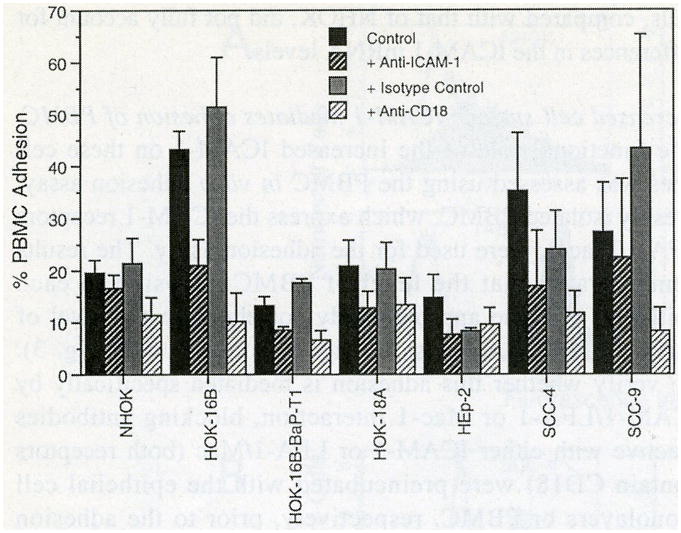
Relative levels of PBMC adherence to NHOK and oral epithelial cell lines. The amount of bound PBMC was measured as the percentage of the total PBMC added to the monolayers before removal of the unbound PBMC. Data are presented as mean ± SEM of the results of at least two or more independent experiments.
Increased cell surface ICAM-1 is involved in LAK cytotoxicity
To assess whether increased adhesion enhanced the cytotoxicity of LAK cells against these epithelial cells, killing assays were performed by preincubating PBMC with rhIL-2 to stimulate natural killer (NK) cells to become LAK cells. 51Cr-labelled epithelial cells were then used as target cells for the LAK cells. The results are summarized in Fig. 6. The level of LAK cell cytotoxicity did not correlate with cell surface ICAM-1 expression among the different cells. Nonetheless, the anti-ICAM-1 antibodies partially inhibited LAK cytotoxicity, except in the case of HOK-16B and HEp-2. It could be that the killing activity was too low against these cells to show any meaningful antibody blocking effects. In general, anti-CD18 antibodies demonstrated a stronger inhibition of LAK cell cytotoxicity than did anti-ICAM-1 antibodies, except for HOK-18A cells. These results suggest the participation of ICAM-1 and its counter-receptor in the LAK cytotoxic effect on some epithelial cell cultures used in this study.
Fig 6.
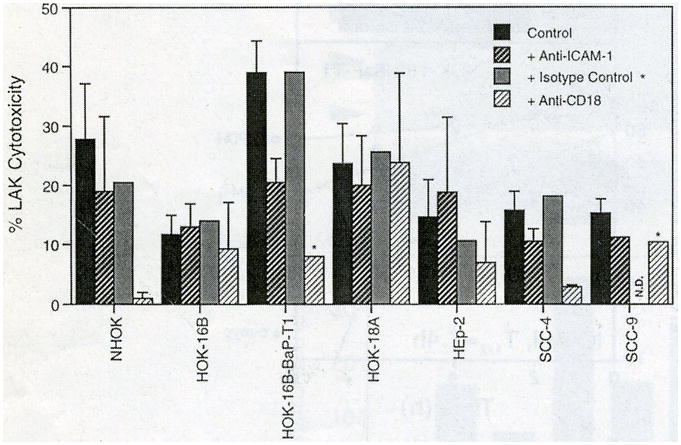
Relative levels of LAK cell cytotoxicity to oral epithelial cells. Data are presented as mean ± SEM of the results of at least two or more independent experiments except where indicated. N.D., not determined. *, single experiment.
Discussion
This study provides information on the alteration of ICAM-1 expression in transformed oral epithelial cells and on the potential implication of its altered expression in affecting tumor development in vivo. ICAM-1 expression was constitutively upregulated in most of the cell lines examined. The functional role of increased ICAM-1 expression was evidenced by the PBMC adhesion assay and LAK cell cytotoxicity analysis.
All three immortalized, nontumorigenic clones (HOK-16B, HOK-18A and HOK-18C) expressed significantly higher levels of ICAM-1 than did NHOK. It has been reported that infection with certain viruses or bacteria can upregulate ICAM-1 expression (30-33). Therefore, HPV integration into NHOK may concomitantly upregulate ICAM-1. Interestingly, HOK-16B-BaP-T1 cells expressed significantly lower ICAM-1 than HOK-16B (Fig. 1), suggesting that further transformation may inhibit the active production of ICAM-1. ICAM-1 expression by oral epithelial cells appears to be similar to that by cervical epithelial cells, i.e., ICAM-1 expression is negative on immortalized cervical keratinocytes not transformed by HPV-16, whereas HPV-16 positive, fully transformed cervical keratinocyte lines constitutively express ICAM-1 (34).
Two of the five human oral carcinoma cell lines examined, HEp-2 and 1483, contain HPV-18 (35). Interestingly, HEp-2 expressed very low levels of ICAM-1, whereas 1483 expressed the highest level. In addition, SCC-4, SCC-9, and Tu-177, all HPV DNA negative oral carcinoma cell lines, expressed relatively high levels of ICAM-1. This suggests that different control mechanisms have taken place in these cells during transformation resulting in differential ICAM-1 expression. Our data showed that an increased mRNA half-life in HOK-16B cells was not solely responsible for the increased ICAM-1mRNA level. The ICAM-1 mRNA half-life of SCC-9 is similar to that of NHOK, in contrast to the ICAM-1 mRNA level in SCC-9 which is ∼9-fold higher than that in NHOK. This suggests that regulation at the transcriptional level is more important. Studies have shown that potential ICAM-1 gene regulatory regions (enhancing or silencing elements) that interact with transcriptional factors differentially control the constitutive high or low expression of ICAM-1 (36).
Although the functional role of ICAM-1 in enhancing PBMC adhesion was clearly demonstrated (Fig. 5), the role that ICAM-1 plays in LAK cell cytotoxicity in this in vitro system was not clear. Nonetheless, antibodies specific for ICAM-1 or CD18 interfered with LAK cell mediated killing of some of the cell lines used in this present study (Fig 6). This suggests that ICAM-1 and other factors are involved in determining LAK killing. This is consistent with the seemingly disparate findings of others that: 1) not all tumor cells with induced ICAM-1 are susceptible to cell-mediated cytolysis (22, 37, 38), and 2) adhesion between killer and tumor cells is enhanced through ICAM-1, yet lysis of tumor cells is not increased (39).
The development and progression of tumors may be fundamentally determined by two factors. Internal cellular changes may cause the cells to de-differentiate and aggressively proliferate, or changes in the extracellular microenvironment may facilitate or prohibit tumor growth. To form a malignant tumor, genetic mutations in the cells that result in aberrant cell growth and escape from immuno-surveillance are important. It seems that expressing adhesion molecules, such as ICAM-1, that can interact with immune surveillant cells would not be beneficial to tumor survival. However, this does not appear to be the case for the tumor cells examined in this study. SCC-4, SCC-9, 1483 and Tu-177 are all ICAM-1 high-expressing, tumorigenic cells derived from human oral cancers. These ICAM-1 positive cancer cells were able to escape immune reaction with tumor killer cells. Therefore, ICAM-1 expression on tumor cells does not necessarily guarantee an advantage to the host. One possibility is that multiple factors are involved in effective immuno-surveillance and the absence of one or more of these important factors may be responsible for the failure of the immune cells to eliminate tumors (40). The lack of significant expression of adhesion molecules on peritumoral vascular endothelial cells has been considered as a possible mechanism that debilitates the recruitment of immune cells to tumor sites (41). Furthermore, ICAM-1 expressing tumors may metastasize more readily. Thus, a thorough understanding of the overall physiological role of ICAM-1 expression by a particular cancer cell type is required to control and predict the prognosis of the cancer.
Acknowledgments
This study was supported in part by UCLA Academic Senate Research Grant and NIDCR/NIH Grant DE12141-01.
References
- 1.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 2.Diamond MS, Staunton DE, De Fougerolles AR, Stacker SA, Garcia-Aguillar, Hibbs ML, Springer TA. ICAM (CD54)--a counter recepter for Mac-1 (CD11/CD18) J Cell Biol. 1990;111:3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenstein Y, Park JK, Hahn WC, Rosen FS, Bierer BE, Burakoff SJ. CD43, a molecule defective in Wiskott-Aldrich syndrome, binds ICAM-1. Nature. 1991;354:233–235. doi: 10.1038/354233a0. [DOI] [PubMed] [Google Scholar]
- 4.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL-1 and interferon-γ: tissue distribution, biochemistry and function of an adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 5.Kashihara-Sawami M, Norris D. The state of differentiation of cultured human keratinocytes determines the level of intercellular adhesion molecule-1 (ICAM-1) expression induced by γ-interferon. J Invest Dermatol. 1992;98:741–747. doi: 10.1111/1523-1747.ep12499938. [DOI] [PubMed] [Google Scholar]
- 6.Vignola AM, Chanez P, Campbell AM, Pinel AM, Bousquet J, Michel FB, Godard P. Quantification and localization of HLA-DR and intercellular adhesion molecule-1 (ICAM-1) molecules on bronchial epithelial cells of asthmatics using confocal microscopy. Clin Exp Immunol. 1994;96:104–109. doi: 10.1111/j.1365-2249.1994.tb06238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agace WW, Patarroyo M, Svensson M, Carlemalm E, Svanborg C. Escherichia coli induces transuroepithelial neutrophil migration by an intercellular adhesion molecule-1-dependent mechanism. Infect Immunol. 1995;63:4054–62. doi: 10.1128/iai.63.10.4054-4062.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths CE, Voorhees JJ, Nickoloff BJ. Characterization of intercellular-adhesion molecule 1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol. 1989;20:617–629. doi: 10.1016/s0190-9622(89)70073-6. [DOI] [PubMed] [Google Scholar]
- 9.Dustin ML, Singer KH, Tuck DT, Springer TA. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1) J Exp Med. 1988;167:1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford JA. Distribution of ICAM-1, LFA-3 and HLA-DR in healthy and diseased gingival tissues. J Periodont Res. 1992;27:291–298. doi: 10.1111/j.1600-0765.1992.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 11.Gemmell E, Walsh LJ, Savage NW, Seymour GJ. Adhesion molecule expression in chronic inflammatory periodontal disease tissue. J Periodont Res. 1994;29:46–53. doi: 10.1111/j.1600-0765.1994.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 12.Huang GTJ, Zhang X. Immunohistochemical analysis of interleukin-8 and intercellular adhesion molecule-1 in human gingival epithelium. Int J Oral Biol. 1999;24:7–16. [Google Scholar]
- 13.Healy CM, Thornhill MH. Induction of adhesion molecule expression on blood vessels and keratinocytes in recurrent oral ulceration. J Oral Pathol Med. 1999;28:5–11. doi: 10.1111/j.1600-0714.1999.tb01986.x. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JP, Stade BG, Holzmann B, Schwable W, Riethmuller G. De novo expression of intercellular-adhesion molecule 1 in melanoma correlates with increased risk of metastasis. Proc Natl Acad Sci USA. 1989;86:641–644. doi: 10.1073/pnas.86.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natali P, Nicotra MR, Cavaliere R, Bigotti A, Romano G, Temponi M, Ferrone S. Differential expression of intercellular adhesion molecule 1 in primary and metastatic melanoma lesions. Cancer Res. 1990;50:1271–1278. [PubMed] [Google Scholar]
- 16.Sun JJ, Zhou XD, Liu YK, Tang ZY, Feng JX, Zhou G, Xue Q, Chen J. Invasion and metastasis of liver cancer: expression of intercellular adhesion molecule 1. J Cancer Res Clin Oncol. 1999;125:28–34. doi: 10.1007/s004320050238. [DOI] [PubMed] [Google Scholar]
- 17.Miele ME, Bennett CF, Miller BE, Welch DR. Enhanced metastatic ability of TNF-α-treated malignant melanoma cells is reduced by intercellular adhesion molecule-1 (ICAM-1, CD54) antisense oligonucleotide. Exp Cell Res. 1994;214:231–241. doi: 10.1006/excr.1994.1253. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe K, Alexander JP, Steinbach F, Campbell S, Novick AC, Klein EA. Retroviral transduction of intercellular adhesion molecule-1 enhances endothelial attachment of bladder cancer. Urol Res. 1997;25:401–405. doi: 10.1007/BF01268855. [DOI] [PubMed] [Google Scholar]
- 19.Katsanis E, Bausero MA, Xu H, Orchard PJ, Xu Z, McIvor RS, Brian AA, Blazar BR. Transfection of the mouse ICAM-1 gene into murine neuroblastoma enhances susceptibility to lysis, reduces in vivo tumorigenicity and decreases ICAM-2-dependent killing. Cancer Immunol Immunother. 1994;38:135–141. doi: 10.1007/BF01526209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong AS, Boussy IA, Jiang XL, Lamas M, Graf LH., Jr CD54/ICAM-1 is a costimulator of NK cell-mediated cytotoxicity. Cell Immunol. 1994;157:92–105. doi: 10.1006/cimm.1994.1208. [DOI] [PubMed] [Google Scholar]
- 21.Burno DK, Kyprianou N, Sartor WM, Fabian DF, Turner J, Vu T, Patel A, Trimbach C, Lefor A. Transfection of a murine fibrosarcoma with intercellular adhesion molecule-1 enhances the response to adoptive immunotherapy. Surg. 1995;118:237–244. doi: 10.1016/s0039-6060(05)80329-0. [DOI] [PubMed] [Google Scholar]
- 22.Burno DK, Fabian DF, Lefor AT. ICAM-1 increases in vitro adhesion and cytotoxicity in a murine fibrosarcoma. J Surg Res. 1996;60:398–402. doi: 10.1006/jsre.1996.0065. [DOI] [PubMed] [Google Scholar]
- 23.Uzendoski K, Kantor JA, Abrams SI, Schlom J, Hodge JW. Construction and characterization of a recombinant vaccinia virus expressing murine intercellular adhesion molecule-1: induction and potentiation of antitumor responses. Human Gene Ther. 1997;8:851–860. doi: 10.1089/hum.1997.8.7-851. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa Y, Hirakawa K, Nakata B, Fujihara T, Sawada T, Kato Y, Yoshikawa K, Sowa M. Expression of intercellular adhesion molecule-1 in invasive breast cancer reflects low growth potential, negative lymph node involvement, and good prognosis. Clin Cancer Res. 1998;4:31–36. [PubMed] [Google Scholar]
- 25.Maurer CA, Friess H, Kretschmann B, Wildi S, Muller C, Graber H, Schilling M, Buchler MW. Over-expression of ICAM-1, VCAM-1 and ELAM-1 might influence tumor progression in colorectal cancer. Int J Cancer. 1998;79:76–81. doi: 10.1002/(sici)1097-0215(19980220)79:1<76::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Park NH, Min BM, Li SL, Huang MZ, Cherrick HM, Doniger J. Immortalization of normal human oral keratinocytes with type 16 human papillomavirus. Carcinogenesis. 1991;12:1627–1631. doi: 10.1093/carcin/12.9.1627. [DOI] [PubMed] [Google Scholar]
- 27.Shin KH, Min BM, Cherrick HM, Park N-H. Combined effects of human papillomavirus-18 and N-methyl-N′-nitro-N-nitrosoguanidine on the transformation of normal human oral keratinocytes. Mol Carcinogenesis. 1994;9:76–86. doi: 10.1002/mc.2940090205. [DOI] [PubMed] [Google Scholar]
- 28.Park NH, Gujuluva CN, Baek JH, Cherrick HM, Shin KH, Min BM. Combined oral carcinogenicity of HPV-16 and benzo(a)pyrene: An in vitro multistep carcinogenesis model. Oncogene. 1995;10:2145–2153. [PubMed] [Google Scholar]
- 29.Rothlein R, Dustin ML, Marlin SD, Springer TA. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- 30.Ito M, Watanabe M, Ihara T, Kamiya H, Sakurai M. Increased expression of adhesion molecules (CD54, CD29 and Cd44) on fibroblasts infected with cytomegalovirus. Microbiol Immunol. 1995;39:129–133. doi: 10.1111/j.1348-0421.1995.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 31.Patel JA, Kunimoto M, Sim TC, Garofalo R, Eliott T, Baron S, Ruuskanen O, Chonmaitree T, Ogra PL, Schmalstieg F. Interleukin-1 alpha mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol Biol. 1995;13:602–609. doi: 10.1165/ajrcmb.13.5.7576697. [DOI] [PubMed] [Google Scholar]
- 32.Huang GTJ, Eckmann L, Savidge TC, Kagnoff MF. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM-1) expression and neutrophil adhesion. J Clin Invest. 1996;98:572–583. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang GTJ, Kinder Haake S, Kim JW, Park NH. Differential expression of interleukin-8 and intercellular adhesion molecule-1 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans or Porphyromonas gingivalis infection. Oral Microbiol Immunol. 1998;13:301–309. doi: 10.1111/j.1399-302x.1998.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 34.Coleman N, Greenfield IM, Hare J, Kruger-Gray H, Chain BM, Stanley MA. Characterization and functional analysis of the expression of intercellular adhesion molecule-1 in human papillomavirus-related disease of cervical keratinocytes. Am J Path. 1993;143:355–367. [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MS, Li SL, Bertolami CN, Cherrick HM, Park NH. State of p53, Rb and DCC tumor suppressor genes in human oral cancer cell lines. Anticancer Res. 1993;13(5A):1405–1413. [PubMed] [Google Scholar]
- 36.Jahnke A, Van de Stolpe A, Caldenhoven E, Johnson JP. Constitutive expression of human intercellular adhesion molecule-1 (ICAM-1) is regulated by differentially active enhancing and silencing elements. Eur J Biochem. 1995;228:439–446. [PubMed] [Google Scholar]
- 37.Makgoba MW, Sanders ME, Ginther Luce GE, Gugel EA, Dustin ML, Springer TA, Shaw S. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytoxicity. Europ J Immun. 1988;18:637–40. doi: 10.1002/eji.1830180423. [DOI] [PubMed] [Google Scholar]
- 38.Akella R, Hall RE. Expression of the adhesion molecules ICAM-1 and ICAM-2 on tumor cell lines does not correlate with their susceptibility to natural killer cell-mediated cytolysis: evidence for additional ligands for effector cell β2 integrins. Eur J Immunol. 1992;22:1069–1074. doi: 10.1002/eji.1830220429. [DOI] [PubMed] [Google Scholar]
- 39.Scher RL, Carras A, Schwab D, Richtsmeier WJ, Koch WM. Interferon gamma enhances lymphokine-activated killer cell adhesion but not lysis of head and neck squamous cell carcinoma. Arch Otolaryngol--Head Neck Surg. 1995;121:1271–1275. doi: 10.1001/archotol.1995.01890110047009. [DOI] [PubMed] [Google Scholar]
- 40.Lang S, Whiteside TL, Lebeau A, Zeidler R, Mack B, Wollenberg B. Impairment of T-cell activation in head and neck cancer in situ and in vitro: strategies for an immune restoration. Arch Otolaryngol--Head Neck Surg. 1999;125:82–88. doi: 10.1001/archotol.125.1.82. [DOI] [PubMed] [Google Scholar]
- 41.Verhaegh M, Beljaards R, Veraart J, Hoekzema R, Neumann M. Adhesion molecule expression in basal cell carcinoma. Eur J Dermatol. 1998;8:252–255. [PubMed] [Google Scholar]


