Abstract
BACKGROUND
Despite dramatic positive effects, there is evidence that the androgen receptor (AR) may negatively influence prostate tumor progression. Understanding the AR repressor function and how it is subverted is of particular importance in anti-androgen and AR intervention strategies.
METHODS
AR, resident FGFR2IIIb and ectopic FGFR1 were expressed by transfection in the AR-negative epithelial cell line DTE that predominates in cell culture of AR-positive androgen-responsive model Dunning R3327 rat prostate tumors. Androgen-responsiveness at transcription was measured by a luciferase reporter. Cell population growth rates were assessed by cell counts, DNA synthesis and expression of cell cycle genes. AR variants (ARVs) were assessed by immunochemistry and nuclease protection of mRNA.
RESULTS
Expression of AR inhibited cell population growth of AR-negative DTE cells at the G1 to S phase of the cell cycle. Ectopic FGFR1, but not resident FGFR2IIIb abrogated the growth inhibitory effects of AR. Appearance of ARVs was coincident with co-expression of FGFR1 and AR and abrogation of the AR-dependent inhibition of cell growth.
CONCLUSIONS
DTE cells may represent nonmalignant AR-negative progenitors whose population is restricted by activation of AR in vivo. Ectopic expression of epithelial FGFR1, a common observation in tumors, overrides the inhibition of AR and thus may contribute to evolution of androgen and AR independent tumors. These results are consistent with the notion that some tumor cells are negatively restricted by AR and are unleased by androgen-deprivation or ectopic expression of FGFR1. ARV’s may play a role in the bypass of the negative restrictions of AR.
Keywords: castration-resistance, fibroblast growth factor signaling, stem cells, tumor suppression, tyrosine kinases
INTRODUCTION
A central dogma regarding development, progression and treatment of prostate cancer is that the androgen receptor (AR) is the major driver of both androgen-responsive and androgen-unresponsive tumors (1,2). Androgen deprivation therapy results in temporary regression of the majority of prostate tumors when first diagnosed, however, over time tumors become resistant to androgen deprivation therapy and progress with increasing malignancy and mortality. Tumors that are resistant to androgen deprivation referred to as castration-resistant tumors most often express AR and exhibit intact pathways associated with AR signaling (2,3). This underpins the idea that even though highly malignant tumors are resistant to anti-androgen therapy they may still be driven by androgen-independent AR signaling within tumor cells (1). The idea that AR and AR signaling drives progression to and supports castration-resistant cancers is complicated by observations that in some instances castration-resistant patients benefit from androgen replacement therapy (4,5). In a prevention study, long term depression of dihydotestosterone (DHT) with 5-(α)-reductase inhibitor finasteride reduced overall prostate tumor incidence but induced a higher grade of tumor (6). In castrated Nkx3.1;Pten mutant mice, restoration of low testosterone accelerated tumorigenesis relative to restoration of normal levels (7). Reduction of AR in mice and isolated prostate tumor cells in culture revealed that AR has positive and negative roles in prostate tumor development dependent on the prostate compartment or cell type in which the AR resides (3,8,9). The identification of the cell types in which AR signaling plays a repressor function in tumorigenesis and mechanisms of subversion of the repressor function is of particular importance in design of AR intervention strategies for all stages of prostate cancers.
Differentiated Dunning R3327 adenocarcinomas (DT) that originated in rat prostate constitute a unique model in which both epithelial and stromal compartments travel together in a transplantable tumor (10,11). The two compartments are dependent on each other for maintenance of the differentiated and nonmalignant state. The system has been instrumental in dissecting changes in fibroblast growth factor (FGF) isotypes and signaling between stroma and epithelium that occur during tumor progression (12–14). Epithelial cells from rat normal prostate and nonmalignant prostate (DT) tumors are characterized by the exclusive expression of the FGFR2 splice variant IIIb that responds to FGF7 and FGF10 from the stroma (10,15,16). In the reverse direction, epithelial cells express FGF9 that acts on stromal FGFR1 and FGFR3 (13,17). Generally similar to human prostate cancer, two-compartment DT tumors are well-differentiated morphologically, regress in response to androgen deprivation, but eventually progress to a highly malignant androgen-unresponsive state (18,19). AR gene expression without evidence of variants in AR mRNA is retained in some malignant sublines and lost in others. A loss of stromal FGF7 and FGF10-responsive FGFR2IIIb (20) concurrent with the ectopic appearance of normally mesenchyme-associated FGFR1 (11) occurs in epithelial cells during the progression. Forced expression of ectopic FGFR1 in non-malignant tumor epithelial cells by transfection accelerates the malignant phenotype (11,21) while restoration of FGFR2 to malignant cells retards their malignant properties (11,22). This profile has been recapitulated in human prostate and human prostate tumor cells (23–30) and in prostatic intraepithelial neoplasia (PIN) and prostate cancers induced in genetically altered mice (31–34).
Despite the dramatic changes in expression of FGFR isotypes associated with the progression to resistance against androgen deprivation therapy, few studies have directly addressed the relationship between FGFR and AR signaling in prostate tumor cells. In developing mouse prostate, FGFR2 influences prostate morphogenesis directed by androgen, but not prostate secretory function (35). Here we show that the predominant proliferative epithelial cell type (DTE) that emerges in culture from AR-expressing, nonmalignant two-compartment, androgen-responsive DT tumors (10,11,36) do not express AR. Expression of AR by transfection inhibited DTE cell proliferation. Ectopic expression of FGFR1, but not overexpression of resident FGFR2, overrode the negative influence of AR on the DTE cell proliferation. Surprisingly, specifically FGFR1 resulted in a variety of androgen receptor variants (ARVs) in the transgenic AR expression products concurrent with the override. The results support the notion that rare AR-negative epithelial cells with proliferative potential are hidden among the majority AR-positive and responsive prostate tumor cells. Activation of expression of AR inhibits expansion of that population. Ectopic FGFR1 may contribute to castration-resistant prostate tumors by overriding the negative influence of AR on proliferation of specific tumor epithelial cells subject to growth limitation by AR.
MATERIALS AND METHODS
Materials
Oligonucleotides were custom made from Integrated DNA Technology, Inc. (Coralville, IA), radiolabeled reagents were from Dupont NEN life Science Products (Boston, MA) and 5α-dihydrotestosterone (DHT) was from Sigma-Aldrich, Co. (St. Louis, MO).
Tissues and cell cultures
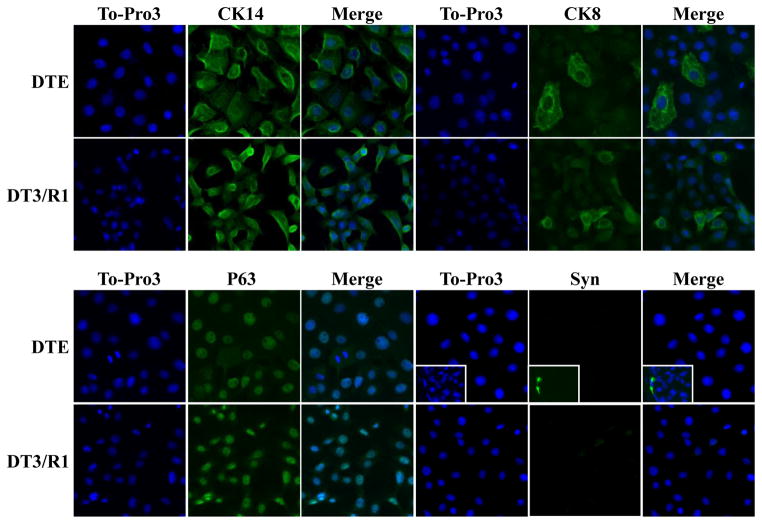
Normal prostate tissues, transplantable Dunning R3327PAP (DT) and AT3 tumors were prepared in Copenhagen rats and tumor derived cell lines DTE, DTE/R1, DTE/R2 were established, maintained and stable expression of FGFR monitored by binding and covalent affinity cross-linking of radiolabeled FGF1 or FGF2 as described previously (11,20,21,37). Cells grown on cover slides were characterized by immunohistochemistry using anti-epithelial cell cytokeratins CK14 and CK8, anti-basal cell marker p63 and anti-neuroendocrine cell marker synaptophysin. Antigens were retrieved by incubating in citrate buffer (10 mM) for 20 minutes at 100 C. The slides were incubated with 5 percent BSA in TBST buffer for 1 hour at room temperature to block non-specific binding sites, followed by incubating with primary antibodies for 1 hour at room temperature. The sources and concentrations of primary antibodies are: mouse anti-cytokeratin 8 (1:15 dilution) were from Fitzgerald (Concord, MA); mouse anti-cytokeratin 14 (1:100) and mouse anti-P63 (1:150 dilution) from Santa Cruz (Santa Cruz, CA); mouse anti-synaptophsin (1:100) from BD Biosciences (Rockville, MD); and Alexa Fluro 488 goat anti-mouse IgG (1:100). Specifically bound primary antibodies were then probed with respective secondary antibodies labeled with horseradish peroxidase (Bio-Rad Co., Hercules, CA). Immnuo-labeled proteins were detected by using TSATM Plus Fluorescence Systems from PerkinElmer (Boston, MA). To-Pro3 iodide (1:200) was used to stain nuclei (Invitrogen, La Jolla, CA).
Stable AR-expressing cell lines DTE/AR, DTE/R1AR, and DTE/R2AR were generated by transfection using CelluFectin (Invitrogen, Carlsbad CA) as suggested by the manufacturer. For cell growth assays, 5 × 104 cells per well were seeded in 24-well plates in RD media with 5 percent charcoal-stripped FBS. After the cells attached to the wells, DHT or ethanol (vehicle) was added to the medium at a final concentration of 10 nM or as specified in the text. Cells were harvested at 48, 96, and 144 hr after incubation and the cell numbers were counted with a Coulter counter (Hialeah, Florida). Cell growth rates were expressed as population doubling times. For DNA synthesis assays, 5 × 105 cells per well were seeded in 24-well plates in RD media with 5 percent charcoal-stripped serum FBS. After cells attached, the medium was replaced with serum-free RD medium. After culture for 48 hr, the medium was then replaced with 5 percent charcoal-stripped FBS and RD medium containing 0 to 100 nM DHT as indicated in the text. Six hr later, 0.5 μCi/ml of [3H]-thymidine (NEN, Boston, MA) was added. After 6 hr incubation, the cells were solubilized in 0.5 M NaOH after washing with PBS, 10 percent trichloroacetic acid, and then PBS. The radioactivity was quantitated by liquid scintillation counter. Experiments were replicated at least three times. Cell growth data were means ± SD from triplicate samples.
Gene Expression Analyses
cDNAs encoding rat AR mutants were generated with PCR from the AR cDNA template, cloned into pBlueScript SK vectors and sequences verified. PCR was carried out for 30 cycles at 94°C, 60°C, and 72°C for 1 min/cycle using PfuTurbo DNA Polymerase (Stratagene, La Jolla, CA). pBlueScript SK-β-actin was prepared as described (38). The ARR2PB composite promoter was a gift of Dr. R. Matusik (39), which was inserted into the pGL3-promoter vector (Promega, Madison, WI). The resultant reporter plasmid was designated as pGL3-ARR2PB-Luc.
mRNA was analyzed by the reverse transcription-polymerase chain reaction (RT-PCR) and RNase protection (RPA). Total RNA was extracted with the Ultraspec RNA isolation kit (Biotecx, Houston, TX) according to manufacturer’s instruction. For the RT-PCR, AR mRNA was analyzed using sense primer ARP5 (5′-GCCAGTGCGTGAGGAGAGG-3′) and antisense primer ARP3 (5′-TCTCTTGCAATAGGCTGCAC-3′), yielding products of 269 base pairs. Five μg RNA extracted from the tissues and cells were converted to cDNA and 10 percent of the RT products were used as the template in reactions. The PCR was carried out for 35 cycles at 94°C, 60°C, and 72°C for 1 min each using TaqDNA Polymerase (Promega). PCR products were analyzed on 1.5 percent agarose gels.
The RPA was carried out with the Hybspeed RPA kit (Ambion, Austin, TX) using the α-[32P]UTP radiolabeled probes indicated in the text transcribed with the Maxiscript kit (Ambion) as described (13). Rat β-actin mRNA was measured as a control. Unless otherwise indicated, each probe was hybridized with 25 μg of total RNA sample for 15 min and incubated in the presence of Ambion RNase A/T1 for 30 min. Protected fragments were separated on 5 percent polyacrylamide sequencing gels and subjected to autoradiography. Experiments were repeated at least three times.
Protein was measured by immunoblot from monolayer cells lysed with the lysis buffer (20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM dithiothreitol, 1 percent Nonidet P-40, 20 μg/ml leupeptin, 20 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride). Cell lysates were centrifuged at 10,000 × g for 30 min at 4°C. Supernatants were transferred to microcentrifuge tubes and protein determined by the BCA protein assay kit (Pierce, Rockford, IL). Lysates equivalent to 40 μg protein were separated on the SDS-PAGE and electrically blotted onto PVDF membranes, followed by detection with antibodies against AR (N-20 or C-19), cyclin A, cyclin D, p27, and p21 (Santa Cruz Biotech, Santa Cruz, CA), or βactin (Sigma) antibodies. The specifically bound antibodies were detected with the ECL-Plus chemoluminescent kit (Amersham Biosciences, Piscataway, NJ) or by the alkaline phosphatase method.
Luciferase reporter assays
DTE cells and transfected variant lines were transfected with pGL3-ARR2PB-Luc DNA and pcDNA3.1-Zeo-β-gal as a transfection efficiency standard, incubated at 37°C for 4 hr, and distributed into 24-well plates in RD medium with 5 percent charcoal-stripped FBS containing 10 μg/ml heparin at 37°C for 16 hr. The medium was then changed to serum-free RD medium containing 10 μg per ml heparin, 2 ng per ml FGF1 or FGF2, and 10 nM DHT. After incubation for 16 hr at 37 °C, the cells were lysed. The cell lysates were transferred to 96-well plates and mixed with 25 μl of luciferase luciferase assay kit (Promega), and the light intensity was measured with a microplate scintillation counter (Packard, Meriden, CT). β-gal activity was determined by the Gal-screen kit (Tropix, Bedford, MA) following manufacturer’s protocols. Luciferase activity was normalized to β-gal activity. Relative luciferase expression was defined as fold induction relative to solvent controls unless otherwise indicated. Data were means ± SD from triplicate samples.
RESULTS
AR suppresses cell population growth in AR-negative tumor epithelial cells
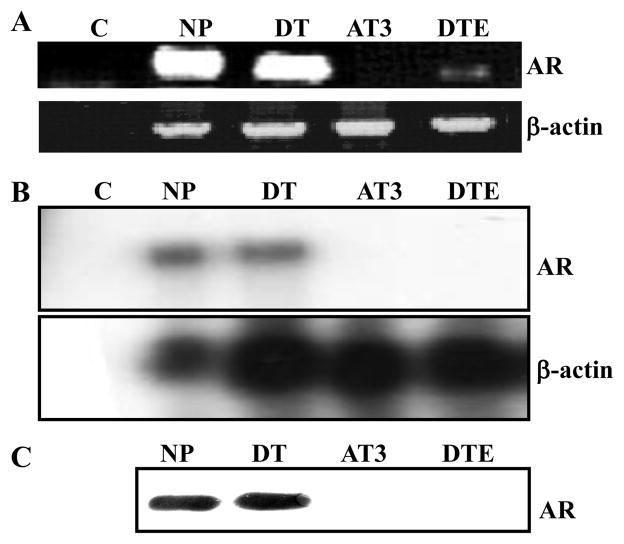
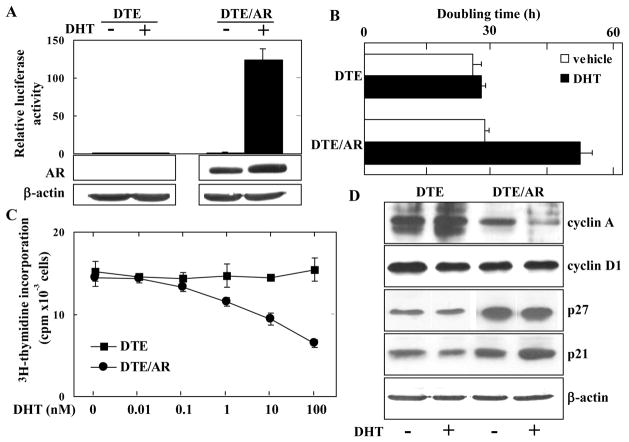
Nearly all epithelial cells capable of sustained proliferation in cultures (DTE) from the DT tumor displayed basal cell cytokeratin 14 coincident with basal cell marker p63 (Fig. 1). Notably 2 to 5 percent of cells displayed luminal cell cytokeratin CK8. No cells displaying neuroendrocine marker synaptophysin (Syn) were apparent. This indicates that, although DTE cultures consist of mostly basal-like cells, among them are transitional cells either displaying concurrently luminal markers or basal-like cells capable of transitioning into luminal-like cells. In contrast to normal prostate and slow-growing, non-malignant DT tumors, DTE cultures exhibited only trace AR mRNA levels that could be detected after amplification by RT-PCR (Fig. 2A). AR was undetectable by nuclease protection of mRNA (Fig. 2B) and immunoblot of AR protein with anti-AR antibody (Fig. 2C). To determine whether the AR-deficient cells retained ability to respond to activated AR, we transfected the DTE cells with cDNA encoding the light-emitting reporter luciferase driven by the androgen-dependent ARR2PB promoter. Consistent with the absence of AR, untransfected DTE cells exhibited no response to DHT whereas the transfected DTE/AR cells exhibited a robust response (Fig. 3A). This indicated that even though devoid of AR, the DTE cells are poised to respond to activated AR once it is expressed.
Fig. 1.
DTE cell cultures display basal and luminal epithelial cell markers, but no synaptophysin-positive neuroendocrine cells. DTE and DTE/R1 cells on cover slides were immunostained with the indicated antibodies. The nuclei were counterstained with To-Pro 3. Inserts are prostate tissue sections stained with synaptophysin for positive controls. Images were representative of the entire cultures collected with a Zeiss LSM 510 Confocal Microscope. CK, cytokeratin; Syn, synaptophysin.
Fig. 2.
AR expression in Dunning tumor tissue and cells. AR mRNA expression in the indicated tissues and cells were analyzed by RT-PCR (A) and nuclease protection of total RNA (40 ug) extracted from the indicated sources (B) as described in Materials and Methods. mRNA was protected with the 57-164 probe described in Fig. 4. C. Cell lysates were subjected to immunoblot analysis with anti-AR antibody. C, control; NP, normal rat prostate tissue; DT, Dunning R3327PAP tumor tissue; AT3, Dunning R3327AT3 tumor tissue; DTE, nonmalignant epithelial cells that emerge in culture from the Dunning R3327PAP tumor.
Fig. 3.
Expression of AR in DTE cells suppresses cell proliferation. A. Luciferase activity was assessed in control DTE and AR-expressing DTE/AR cells after transient transfection with pGL3-ARR2PB-LUC and pcDNA3.1-Zeo-β-gal in the presence or absence of DHT. Luciferase activity induced by DHT was normalized and expressed as the relative increase over DTE cells in absence of DHT. Expression of AR assessed by immunoblot is shown in the lower panels. B. DTE and DTE/AR cells were grown in 5 percent charcoal-stripped serum with or without 10 nM DHT. The population doubling times were calculated from time points described in Materials and Methods. C. Cells were grown in 5 percent charcoal-stripped serum with DHT added at the indicated concentrations and 3H-thymidine measured by the sequence described in Materials and Methods. D. Cells were treated with 10 nM DHT or vehicle for 24 hr and lysates then subjected to analyses by immunoblot with the indicated antibodies.
We compared the effect of DHT on DTE/AR cell population growth. No increase in the cell population was observed. Instead DHT caused a 1.8-fold extension in doubling time of the DTE/AR population (Fig. 3B) indicating that the activated AR restricts cell population growth. Analysis of 3H-thymidine incorporation confirmed the AR-dependence of inhibition of cell population growth rates and suggested the effect occurs through inhibition of DNA synthesis (Fig. 3C). Analysis of effect on cell cycle regulatory proteins further suggested that the activated AR likely inhibited cell growth at the G1 to S phase of the cell cycle (Fig. 3D). Cyclin A was reduced in the AR-expressing cells while cyclin-dependent kinase inhibitors p27kip1 and p21cip1 were increased. Cyclin D1 levels were unchanged.
FGFR1, but not FGFR2 abrogates AR-mediated growth inhibitory effects
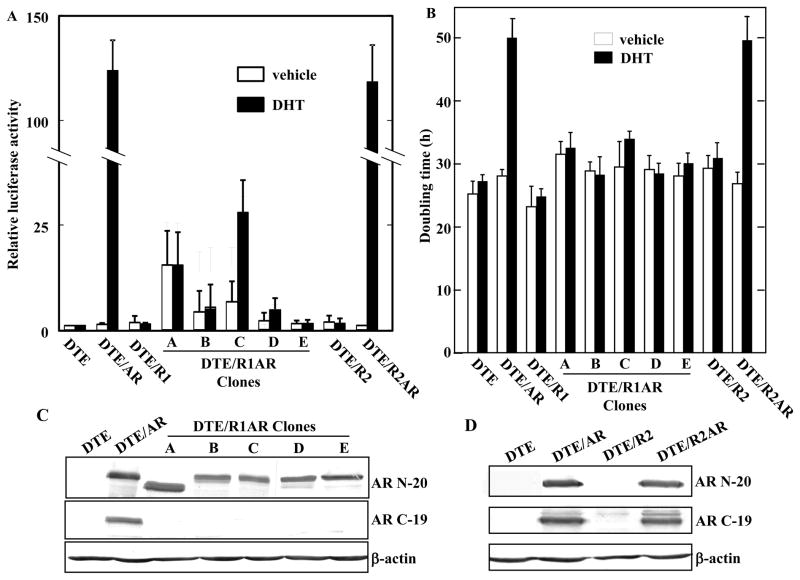
DTE cells are characterized by retention of expression of low levels of FGFR2IIIb that is a normal epithelial cell property and the complete absence of normally stromal-derived FGFR1 that often is expressed in and drives malignant tumors (40). After stable transfection with FGFR1, DTE cells exhibit accelerated rates of cell proliferation and progression to malignancy (11,21). This is in contrast to cells transfected with high levels of FGFR2 which have a biphasic effect on cell proliferation and net inhibitory effect on tumor progression (11,22). DTE cells transfected with FGFR1 (Fig. 1) and FGFR2 (not shown) exhibited relative proportions of cells expressing different cytoskeletal markers similar to parent DTE cultures. To determine whether the ectopic expression of FGFR1 could interfere with the negative effect of AR on proliferation of DTE cells, we examined the effect of expression of AR in DTE cells expressing FGFR1 (DTE/R1 cells). Five clonal lines (A–E) of DTE/R1 cells expressing AR based on mRNA (DTE/R1AR cells) were selected for analysis of response to DHT and effect on cell population doubling rates (Fig. 4). Although baseline AR transcriptional activity was increased in 3 of the 5 clones, a response to DHT was detectable in only one of the 5 lines (Fig. 4A). This was in marked contrast to DTE cells expressing FGFR2 (DTE/R2) instead of FGFR1 which exhibited a robust response to DHT similar to DTE/AR cells when transfected with AR. Similar to the mixed population of DTE/R1AR cells out of which the five lines were cloned (not shown), none of the FGFR1 and AR expressing clonal lines exhibited the extension of cell population doubling times induced by DHT that was observed in DTE/AR or DTE/R2AR cells (Fig. 4B). This suggested that the inhibition of cell proliferation rates by transfected AR was abrogated by the concurrent expression of specifically FGFR1. The abrogation of the AR inhibition was independent on baseline AR transcriptional activity and response to androgen.
Fig. 4.
Ectopic FGFR1 abrogates androgen response and AR-dependent inhibition of cell growth concurrent with C-terminal truncation of AR. A. The indicated permanently transfected variants of DTE cells were transiently transfected with pGL3-ARR2PB-LUC and pcDNA3.1-Zeo/β-gal and relative luciferase activity assess in presence or absence of DHT as described in Fig. 2A. A-E were five different clones of DTE/R1AR cells. B. Cell population doubling rates were assessed in the variant DTE cells in (A) as described in Fig. 2B. C, D. The indicated cell lysates were subjected to immunoblot analyses with anti-AR N-20 against the AR N terminus (upper panel) and anti-AR C-19 against the AR C-terminus (middle panel).
Co-expression of FGFR1 and AR causes AR variants
To determine whether co-expression with FGFR1 caused alteration of the AR transgene and its expression, we analyzed AR protein by immunoblot in the five DTE/R1AR lines using both N-terminal and C-terminal anti-AR antibodies. The N-terminal antibody revealed in all five DTE/R1AR clones strong bands slightly lower in molecular mass of the presumably full length intact AR in DTE/AR cells (Fig. 4C). Strikingly, no signal could be detected by the C-terminal antibody indicating a C-terminal truncation in the major AR translation products in all five lines expressing FGFR1. No alteration in AR was detected with either antibody in DTE/R2AR cells (Fig. 4D).
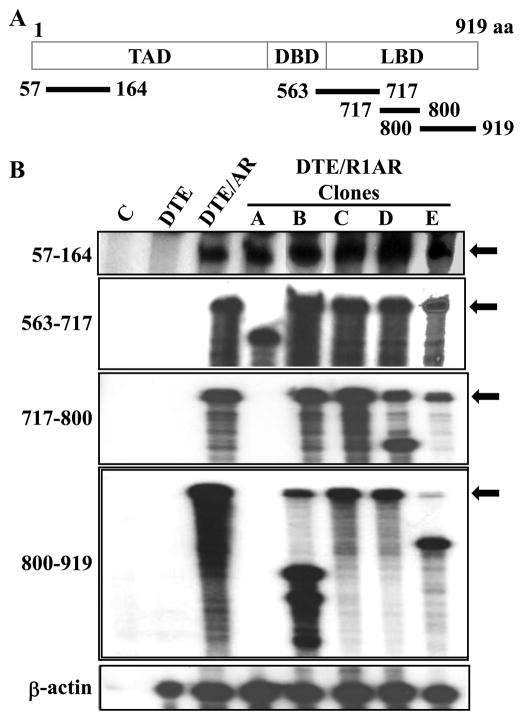
To test whether co-expression with FGFR1 caused AR mRNA variants in the co-expressing clones, we examined the mRNA expression pattern with nuclease protection probes, one complementary to sequence in the N-terminus and four sequences from the C-terminus (Fig. 5). All five DTE/R1AR clonal lines exhibited an intact AR coding sequence for N-terminal residues 55-164 (Fig. 5B). Clone C mRNA was fully protected by all four probes despite the fact that it did not react with the antibody against the C-terminal 19 amino acid residues (Fig. 4C) and the negative effect of AR on clone C proliferation was abrogated by FGFR1 (Fig. 4B). This may indicate a post-translational modification of the AR C-terminus. It is also noteworthy that clone C was the only one of the five DTE/R1AR clones that retained a transcriptional response to DHT (Fig. 4A). Clone A exhibited alterations in coding regions spanned by all four C-terminal probes, clone B only in probe 800-919, clone D in 717-800 and clone E in probe 800-919. These results indicate that in addition to the general AR C-terminal truncation, the AR mRNA coding sequences exhibit diverse variants induced by co-expression of the AR gene and specifically FGFR1. mRNA variants using the same probes were not observed in the DTE/R2AR cells (not shown).
Fig. 5.
Androgen receptor variants (ARVs) in cells expressing ectopic FGFR1 and AR. A. Schematic of AR mRNA protection probes. Probes are numbered in respect to amino acids (aa) coded by probes overlapping the N-terminal transactivating domain (TAD), the DNA binding domain (DBD) and the C-terminal ligand binding domain (LBD) (47). B. Total RNA from the indicated DTE variants and clonal lines cells (Fig. 3) was analyzed by RPA with the indicated probes. Arrows indicate the band corresponding to the full length probe.
DISCUSSION
Aberrant expression of FGFR1 and its activating FGF ligands and evolution of resistance to androgen-deprivation are coincident events during progression of prostate tumors (40). In addition in transplantable xenografts from tissue or cultured cells and in genetically engineered models of prostate cancer hyperactivity of FGFR1 has been demonstrated to drive tumor progression (11,33,34). Evolution of unresponsiveness to withdrawal of androgen (castration-resistance) and expression of FGFR1 has not been directly related. Similar to human prostate cancer, androgen-responsive transplantable Dunning prostate tumors evolve to tumors unresponsive to androgen after androgen-deprivation. The androgen-responsive tumors invariably give rise to androgen-unresponsive epithelial cells in culture (36) that in absence of differentiation-inducing stroma give rise to androgen-independent malignant tumors (20). Here we show that the androgen insensitive non-malignant epithelial cells (DTE) that emerge in cell culture from androgen-responsive Dunning prostate tumors are devoid of AR except for trace levels detected by RT-PCR amplification. Introduction of AR into the AR-negative cells inhibited cell proliferation rates. Cells expressing FGFR1 were resistant to the AR-dependent inhibition in contrast to cells expressing the normally epithelial cell resident FGFR2. Tumor cell-associated FGFR1 may drive tumors in addition to or independent of AR through its strong canonical signaling that enhances cell cycling at the G1-S transition and direct anti-apoptotic survival effects. However, our results also indicate that FGFR1 may play a role in evolution of androgen-independence by directly promoting bypass of the growth limiting influence of AR signaling in special cells, or selection of those cells that have bypassed the growth limiting AR effect by other mechanisms.
Understanding the cellular origin and evolution of castration-resistant prostate tumors that arise after failure of androgen-deprivation therapy and are eventually lethal to the host is a critical subject in design of castration-resistant therapy. The prevailing notion is that AR-expressing tumor cells that have bypassed a requirement for external androgen either by AR overexpression, activation of AR by internal factors or gain of function mutations in AR emerge by default after tumor cells dependent on external androgen die out (1,41,42). However, there is increasing evidence that androgen-deprivation therapy unleashes a population of tumor cells whose expansion is normally limited by androgen-activated AR (3,7,41). These are postulated to be intermediate or transient amplifying cell populations subject to normal expansion on demand somewhere between primitive stem cells that do not express AR (43) and fully differentiated and functional luminal secretory cells that are dependent on AR for survival and secretory function (3,41). The AR-negative DTE cells described here revealed by selection in cell culture, but hidden among the majority of AR-positive and responsive cells in the parent tumor in vivo (19) likely represent an AR-negative subpopulation within the greater intermediate/transitional cell population whose expansion is limited when AR is expressed.
Abnormal expression of FGFR1 is a frequent observation in prostate tumor epithelial cells and ectopic epithelial FGFR1 has been established as a driver of prostate tumors in animal models (40). This is in contrast to resident epithelial cell FGFR2IIIb that mediates both growth but also growth-limiting homeostasis-promoting instructions from stroma (10,11,15,16,44). Similar to other tumor cell associated genes, expression of FGFR1 may arise in FGFR1-negative epithelial cells by ectopic activation or by emergence of pre-existent rare and dormant FGFR1-expressing progenitors. FGFR1 is silent or very low in the epithelial cells of the mature prostate both in vivo and in vitro. However, FGFR1 has been widely implicated in expansion of stem/progenitor cells for lineage transitions (45,46) which could be a pre-existent source of FGFR1-expressing tumor cells. Similar to AR expression, FGFR1 remains absent from the emergent DTE cells described here after prolonged culture (10,11). DTE cells are remarkably resistant to the ectopic presence of FGFR1 and only become susceptible to the tumor-driving effects of FGFR1 after prolonged exposure and adaptation (21). This is in marked contrast to the observed dampening effects on cell population doubling rates due to overexpression of resident epithelial FGFR2 and expression of AR. Thus DTE cells appear to represent committed epithelial cells defined by exclusive expression of splice variant FGFR2IIIb that have shut down both FGFR1 expression and FGFR1 signaling reception mechanisms. Moreover, they likely represent the population of committed epithelial cells subject to recruitment for differentiation upon AR expression and whose population is limited by expression and activation of AR. Ectopic expression and acquisition of FGFR1 signaling during tumor progression subverts the AR-dependent limitation on expansion of the specialized cell population.
Here we also showed that cells that acquire the tumor-driving effects of ectopic FGFR1 and subvert the negative restrictions on cell growth rates induced by androgen-activated AR invariably exhibit AR variants (ARVs). The variants are diverse among clonal lines, but all have the common feature of truncation at the C-terminus. ARVs result from the presence of specifically FGFR1 since none have been observed in control DTE cells or DTE cells overexpressing resident FGFR2. Most commonly ARVs have been proposed as androgen-independent gain of function selectable drivers of castration-resistant tumors (1,42). However, recently ARVs have been shown to be much more diverse among different tumors and tumor models as well as function than previously expected. They can be induced by androgen deprivation, suppressed by androgen and dependent in some cases on intact full length AR (42). Thus generation and selection for cells expressing inactive or AR-inhibiting ARVs by tumor promoters as ectopic FGFR1 may also be a mechanism for bypass of the negative influence of AR on specific prostate tumor cell populations that are subject to growth inhibition by androgen and AR (3).
In summary, we showed here that the predominant proliferative FGFR2IIIb-positive, FGFR1-negative epithelial cell type (10,11,21) that emerges in culture from among the majority of AR-expressing cells of the classic androgen-responsive Dunning prostate tumor is AR-negative. Based on cytoskeletal markers, the cultures are characterized by predominantly basal type epithelial cells. The concurrent presence of cells positive for cytokeratin 8 associated with luminal cells indicates the presence of transitional cells within the cultures. The cell population is subject to restriction upon expression of AR. Ectopic expression of FGFR1, a common property observed in prostate tumors that drives malignancy in animal models, subverts the restrictions imposed on expansion of the cell population by AR. Our results support the notion that there is a minority population of tumor cells that are AR-negative and held in check by activation of expression of AR. This population is unleashed by androgen deprivation therapy and may contribute to castration-resistant malignancies. Ectopic FGFR1 may be a contributing factor in bypass of negative effects of both androgen-dependent and androgen-independent AR. Further work is needed to determine the role of FGFR1-induced ARVs on bypass of the negative restrictions imposed by AR.
Acknowledgments
We thank R. Matusik (Vanderbilt-Ingram Cancer Center) for the ARR2PB plasmid and Dr. M. Marcelli (Baylor College of Medicine) for plasmid pCMV3-AR.
Grant sponsor: Public Health Service grants R01CA96824 (FW) and P50CA140388 (FW/WLM), the Susan Komen Foundation (WLM) and aid from the John S. Dunn Research Foundation (WLM).
References
- 1.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 2.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24(18):1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu Y, Chang TM, Yeh S, Ma WL, Wang YZ, Chang C. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene. 2010;29(25):3593–3604. doi: 10.1038/onc.2010.121. [DOI] [PubMed] [Google Scholar]
- 4.Mathew P. Prolonged control of progressive castration-resistant metastatic prostate cancer with testosterone replacement therapy: the case for a prospective trial. Ann Oncol. 2008;19(2):395–396. doi: 10.1093/annonc/mdm568. [DOI] [PubMed] [Google Scholar]
- 5.Morris MJ, Huang D, Kelly WK, Slovin SF, Stephenson RD, Eicher C, Delacruz A, Curley T, Schwartz LH, Scher HI. Phase 1 trial of high-dose exogenous testosterone in patients with castration-resistant metastatic prostate cancer. Eur Urol. 2009;56(2):237–244. doi: 10.1016/j.eururo.2009.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA., Jr The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 7.Banach-Petrosky W, Jessen WJ, Ouyang X, Gao H, Rao J, Quinn J, Aronow BJ, Abate-Shen C. Prolonged exposure to reduced levels of androgen accelerates prostate cancer progression in Nkx3. 1; Pten mutant mice. Cancer Res. 2007;67(19):9089–9096. doi: 10.1158/0008-5472.CAN-07-2887. [DOI] [PubMed] [Google Scholar]
- 8.Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, Yao J, Yeh S, Chang C. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci U S A. 2008;105(34):12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niu Y, Altuwaijri S, Yeh S, Lai KP, Yu S, Chuang KH, Huang SP, Lardy H, Chang C. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc Natl Acad Sci U S A. 2008;105(34):12188–12193. doi: 10.1073/pnas.0804701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan G, McBride G, McKeehan WL. Exon skipping causes alteration of the COOH-terminus and deletion of the phospholipase C gamma 1 interaction site in the FGF receptor 2 kinase in normal prostate epithelial cells. Biochemical and biophysical research communications. 1993;194(1):512–518. doi: 10.1006/bbrc.1993.1849. [DOI] [PubMed] [Google Scholar]
- 11.Feng S, Wang F, Matsubara A, Kan M, McKeehan WL. Fibroblast growth factor receptor 2 limits and receptor 1 accelerates tumorigenicity of prostate epithelial cells. Cancer Res. 1997;57(23):5369–5378. [PubMed] [Google Scholar]
- 12.McKeehan WL, Wang F, Kan M. The heparan sulfate-fibroblast growth factor family: diversity of structure and function. Prog Nucleic Acid Res Mol Biol. 1998;59:135–176. doi: 10.1016/s0079-6603(08)61031-4. [DOI] [PubMed] [Google Scholar]
- 13.Jin C, Wang F, Wu X, Yu C, Luo Y, McKeehan WL. Directionally specific paracrine communication mediated by epithelial FGF9 to stromal FGFR3 in two-compartment premalignant prostate tumors. Cancer Res. 2004;64(13):4555–4562. doi: 10.1158/0008-5472.CAN-03-3752. [DOI] [PubMed] [Google Scholar]
- 14.McKeehan WL, Wang F, Luo Y. The fibroblast growth factor (FGF) signaling complex. In: Bradshaw R, Dennis E, editors. Handbook of Cell Signaling. 2. New York: Academic/Elsevier Press; 2009. [Google Scholar]
- 15.Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL. Heparin-binding keratinocyte growth factor is a candidate stromal-to- epithelial-cell andromedin. Molecular endocrinology (Baltimore, Md. 1992;6(12):2123–2128. doi: 10.1210/mend.6.12.1491693. [DOI] [PubMed] [Google Scholar]
- 16.Lu W, Luo Y, Kan M, McKeehan WL. Fibroblast growth factor-10. A second candidate stromal to epithelial cell andromedin in prostate. J Biol Chem. 1999;274(18):12827–12834. doi: 10.1074/jbc.274.18.12827. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Jin C, Wang F, Yu C, McKeehan WL. Stromal cell heterogeneity in fibroblast growth factor-mediated stromal-epithelial cell cross-talk in premalignant prostate tumors. Cancer Res. 2003;63(16):4936–4944. [PubMed] [Google Scholar]
- 18.Isaacs JT, Coffey DS. Adaptation versus selection as the mechanism responsible for the relapse of prostatic cancer to androgen ablation therapy as studied in the Dunning R-3327-H adenocarcinoma. Cancer Res. 1981;41(12 Pt 1):5070–5075. [PubMed] [Google Scholar]
- 19.Quarmby VE, Beckman WC, Jr, Cooke DB, Lubahn DB, Joseph DR, Wilson EM, French FS. Expression and localization of androgen receptor in the R-3327 Dunning rat prostatic adenocarcinoma. Cancer Res. 1990;50(3):735–739. [PubMed] [Google Scholar]
- 20.Yan G, Fukabori Y, McBride G, Nikolaropolous S, McKeehan WL. Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol Cell Biol. 1993;13(8):4513–4522. doi: 10.1128/mcb.13.8.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, McKeehan K, Yu C, McKeehan WL. Fibroblast growth factor receptor 1 phosphotyrosine 766: molecular target for prevention of progression of prostate tumors to malignancy. Cancer Res. 2002;62(6):1898–1903. [PubMed] [Google Scholar]
- 22.Matsubara A, Kan M, Feng S, McKeehan WL. Inhibition of growth of malignant rat prostate tumor cells by restoration of fibroblast growth factor receptor 2. Cancer Res. 1998;58(7):1509–1514. [PubMed] [Google Scholar]
- 23.Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res. 1999;5(5):1063–1071. [PubMed] [Google Scholar]
- 24.Ozen M, Giri D, Ropiquet F, Mansukhani A, Ittmann M. Role of fibroblast growth factor receptor signaling in prostate cancer cell survival. J Natl Cancer Inst. 2001;93(23):1783–1790. doi: 10.1093/jnci/93.23.1783. [DOI] [PubMed] [Google Scholar]
- 25.Naimi B, Latil A, Fournier G, Mangin P, Cussenot O, Berthon P. Down-regulation of (IIIb) and (IIIc) isoforms of fibroblast growth factor receptor 2 (FGFR2) is associated with malignant progression in human prostate. The Prostate. 2002;52(3):245–252. doi: 10.1002/pros.10104. [DOI] [PubMed] [Google Scholar]
- 26.Yasumoto H, Matsubara A, Mutaguchi K, Usui T, McKeehan WL. Restoration of fibroblast growth factor receptor2 suppresses growth and tumorigenicity of malignant human prostate carcinoma PC-3 cells. The Prostate. 2004;61(3):236–242. doi: 10.1002/pros.20093. [DOI] [PubMed] [Google Scholar]
- 27.Devilard E, Bladou F, Ramuz O, Karsenty G, Dales JP, Gravis G, Nguyen C, Bertucci F, Xerri L, Birnbaum D. FGFR1 and WT1 are markers of human prostate cancer progression. BMC Cancer. 2006;6:272. doi: 10.1186/1471-2407-6-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Expression of bFGF/FGFR-1 and vascular proliferation related to clinicopathologic features and tumor progress in localized prostate cancer. Virchows Arch. 2006;448(1):68–74. doi: 10.1007/s00428-005-0075-3. [DOI] [PubMed] [Google Scholar]
- 29.Sahadevan K, Darby S, Leung H, Mathers M, Robson C, Gnanapragasam V. Selective over-expression of fibroblast growth factor receptors 1 and 4 in clinical prostate cancer. The Journal of pathology. 2007 doi: 10.1002/path.2205. [DOI] [PubMed] [Google Scholar]
- 30.Matsubara A, Teishima J, Mirkhat S, Yasumoto H, Mochizuki H, Seki M, Mutaguchi K, McKeehan WL, Usui T. Restoration of FGF receptor type 2 enhances radiosensitivity of hormone-refractory human prostate carcinoma PC-3 cells. Anticancer Res. 2008;28(4B):2141–2146. [PubMed] [Google Scholar]
- 31.Foster BA, Kaplan PJ, Greenberg NM. Characterization of the FGF axis and identification of a novel FGFR1iiic isoform during prostate cancer progression in the TRAMP model. Prostate Cancer and Prostate Diseases. 1999;(2):76–82. doi: 10.1038/sj.pcan.4500297. [DOI] [PubMed] [Google Scholar]
- 32.Jin C, McKeehan K, Guo W, Jauma S, Ittmann MM, Foster B, Greenberg NM, McKeehan WL, Wang F. Cooperation between ectopic FGFR1 and depression of FGFR2 in induction of prostatic intraepithelial neoplasia in the mouse prostate. Cancer Res. 2003;63(24):8784–8790. [PubMed] [Google Scholar]
- 33.Wang F, McKeehan K, Yu C, Ittmann M, McKeehan WL. Chronic activity of ectopic type 1 fibroblast growth factor receptor tyrosine kinase in prostate epithelium results in hyperplasia accompanied by intraepithelial neoplasia. The Prostate. 2004;58(1):1–12. doi: 10.1002/pros.10311. [DOI] [PubMed] [Google Scholar]
- 34.Acevedo VD, Gangula RD, Freeman KW, Li R, Zhang Y, Wang F, Ayala GE, Peterson LE, Ittmann M, Spencer DM. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer cell. 2007;12(6):559–571. doi: 10.1016/j.ccr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y, Liu G, Zhang Y, Hu YP, Yu K, Lin C, McKeehan K, Xuan JW, Ornitz DM, Shen MM, Greenberg N, McKeehan WL, Wang F. Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development. 2007;134(4):723–734. doi: 10.1242/dev.02765. [DOI] [PubMed] [Google Scholar]
- 36.McKeehan WL, Adams PS, Fast D. Different hormonal requirements for androgen-independent growth of normal and tumor epithelial cells from rat prostate. In Vitro Cell Dev Biol. 1987;23(2):147–152. doi: 10.1007/BF02623596. [DOI] [PubMed] [Google Scholar]
- 37.Kan M, Shi EG, McKeehan WL. Identification and assay of fibroblast growth factor receptors. Methods in enzymology. 1991;198:158–171. doi: 10.1016/0076-6879(91)98017-z. [DOI] [PubMed] [Google Scholar]
- 38.Yu C, Wang F, Kan M, Jin C, Jones RB, Weinstein M, Deng CX, McKeehan WL. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J Biol Chem. 2000;275(20):15482–15489. doi: 10.1074/jbc.275.20.15482. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate- specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141(12):4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 40.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell cycle (Georgetown, Tex. 2009;8(4):580–588. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Litvinov IV, De Marzo AM, Isaacs JT. Is the Achilles’ heel for prostate cancer therapy a gain of function in androgen receptor signaling? J Clin Endocrinol Metab. 2003;88(7):2972–2982. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- 42.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107(39):16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawson DA, Witte ON. Stem cells in prostate cancer initiation and progression. The Journal of clinical investigation. 2007;117(8):2044–2050. doi: 10.1172/JCI32810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo Y, Yang C, Jin C, Xie R, Wang F, McKeehan WL. Novel phosphotyrosine targets of FGFR2IIIb signaling. Cellular signalling. 2009;21(9):1370–1378. doi: 10.1016/j.cellsig.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villegas SN, Canham M, Brickman JM. FGF signalling as a mediator of lineage transitions--evidence from embryonic stem cell differentiation. J Cell Biochem. 110(1):10–20. doi: 10.1002/jcb.22536. [DOI] [PubMed] [Google Scholar]
- 46.Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent cells. Development. 137(20):3351–3360. doi: 10.1242/dev.050146. [DOI] [PubMed] [Google Scholar]
- 47.Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl Recept Signal. 2008;6:e008. doi: 10.1621/nrs.06008. [DOI] [PMC free article] [PubMed] [Google Scholar]