Abstract
Objectives
To determine whether quality of life (QOL) ratings are reduced in mild cognitive impairment (MCI) and analyze correlations between QOL ratings and cognitive, neuropsychiatric and functional indices in MCI.
Design
Cross-sectional.
Setting
Easton Center for Alzheimer’s Disease Research at UCLA.
Participants
205 individuals who met criteria for normal cognition (NC; n=97) or MCI (n=108). The MCI group included amnestic (AMN; n=72) and non-amnestic (NON; n=36) MCI.
Measurements
QOL was assessed using subject and informant ratings on the Quality of Life-Alzheimer’s Disease (QOL-AD) scale. Cognitive performance was assessed with the National Alzheimer’s Disease Coordinating Center Uniform Data Set neuropsychological battery. Neuropsychiatric symptoms were assessed with the Geriatric Depression Scale (GDS) and the Neuropsychiatric Inventory (NPI). Functional abilities were assessed with the Functional Activities Questionnaire (FAQ).
Results
The NC group had significantly higher QOL-AD scores than the MCI group on both subject and informant assessments. Individual item analyses indicated that the largest group differences were seen on the mood and memory items. Similar QOL-AD scores were seen in the AMN and NON MCI subgroups. Multiple regression analyses within the MCI group indicated that QOL-AD ratings were not correlated with neuropsychological performance. Subject QOL-AD ratings were inversely correlated with GDS scores and informant QOL-AD ratings were inversely correlated with GDS, NPI, and FAQ scores.
Conclusions
Significant declines in QOL are seen in MCI and are associated with neuropsychiatric symptoms and functional decline. Interventions targeting mood symptoms and/or instrumental activities of daily living may improve QOL in MCI.
Keywords: quality of life, mild cognitive impairment, cognitive, neuropsychiatric, functional
INTRODUCTION
Mild cognitive impairment (MCI) often represents a transitional period between normal cognitive aging and early dementia. Individuals meeting criteria for MCI have subjective cognitive complaints and objective cognitive impairment, but essentially intact activities of daily living (1). They progress to dementia, primarily Alzheimer’s disease (AD), at higher rates than cognitively normal elderly (2). Although diagnostic criteria for MCI focus upon cognitive deficits, MCI is also characterized by increased neuropsychiatric symptoms (3) and mild declines in functional abilities (4–8) that also lie on the continuum between normal aging and early dementia.
The cognitive, behavioral, and functional symptoms seen in dementia can significantly impact patients’ general well-being or quality of life (QOL). Although there are different approaches to conceptualizing QOL associated with dementia (9), multiple studies using various rating scales demonstrate decreased QOL in demented subjects relative to cognitively normal elderly (10–13). Since symptoms consistent with incipient dementia are present in MCI, mild reductions in QOL might also be expected in this population. Investigators have examined different QOL indices in MCI (10, 11, 13–15) and found mixed results, with only a single study reporting decreased QOL in MCI (15). The explanation for these divergent results remains uncertain, but may be related to relatively small study sample sizes and/or the use of general QOL scales that may be less sensitive to potential QOL changes associated with MCI.
In the current study, we sought to address the potential limitations of the previous reports by including a larger cohort of MCI subjects and measuring QOL with a widely used assessment tool explicitly designed for older adults with cognitive impairment, the Quality of Life-Alzheimer’s Disease (QOL-AD) scale (16). The QOL-AD was originally designed and validated with AD patients and their caregivers (16, 17) and effectively distinguishes demented subjects from cognitively normal age-matched elderly (12). A recent study investigating the use of the QOL-AD in MCI reported excellent reliability and validity (18). We hypothesized that this strategy would allow us to identify subtle QOL deficits in subjects meeting criteria for MCI and examine QOL in amnestic and non-amnestic MCI. Since MCI often represents incipient dementia, similar underlying factors may influence QOL in both MCI and dementia. We explored this possibility by using multiple regression analyses to identify associations between QOL-AD scores and demographic, cognitive, neuropsychiatric, and functional indices.
METHODS
Research participants
Subjects were drawn from an ongoing research study coordinated through the Easton Center for Alzheimer’s Disease Research at UCLA. Participants meeting criteria for MCI (n=108) were recruited from patients assessed in the Memory Disorders Clinics at the UCLA Medical Center, Olive View-UCLA Medical Center, and Marina Campus of the Centinela-Freeman Medical Center. The normal comparison group (NC; n=97) included individuals initially seen in the Memory Disorders Clinics or recruited from the community who performed in the normal range on all neuropsychological assessments, irrespective of subjective cognitive complaints. Written consent, approved by the Institutional Review Boards at UCLA and participating medical centers, was obtained from each subject and informant.
Inclusion criteria included a multi-disciplinary evaluation resulting in a diagnosis of MCI or normal cognition. Exclusion criteria included: 1) age < 50, 2) diagnosis of dementia by Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria (19) or diagnosis of AD by National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) criteria (20), and 3) MRI or CT of the brain demonstrating any major focal lesions (mild to moderate microvascular ischemic changes or isolated lacunes noted on clinical neuroradiology reports were permitted).
Evaluations included neuropsychological testing, physician interview, and neurological examination. MCI was a consensus diagnosis based on modified Petersen criteria: 1) subjective cognitive complaint, 2) essentially intact activities of daily living, 3) objective cognitive impairment, and 4) not demented (1). Cognitive performance was assessed for diagnostic purposes in the domains of memory, attention, language, visuospatial, and executive function using a battery of neuropsychological tests as previously described (21). Subjects were considered cognitively impaired if performance on at least one test in any domain was ≥ 1.5 SD below published age and education adjusted normative means. MCI was further sub-classified as amnestic or non-amnestic based on the presence or absence of memory impairment. Global cognitive functioning was assessed using the Mini-Mental Status Examination [MMSE (22)]. Interviews included questions regarding performance of basic and instrumental activities of daily living (ADLs). The presence of essentially intact ADLs was determined through consensus clinician judgment during a subsequent multi-disciplinary conference.
Assessment tools
QOL was assessed using the QOL-AD (16), which is separately administered to subjects and informants and asks them to rate subjects’ QOL in 13 domains [physical health, energy level, mood, living situation, memory, relationships with family, marriage (i.e. relationship with spouse), relationships with friends, self as a whole, ability to do chores around the house, ability to do things for fun, money (i.e. financial situation), and life as a whole] on a 4-point scale (1: poor; 2: fair; 3: good; 4: excellent). QOL ratings were analyzed using 1) total QOL-AD scores (only included subjects with valid responses for all items) and 2) average QOL-AD item scores (included all subjects). Higher scores on these indices indicate better QOL.
Additional cognitive, neuropsychiatric, and functional assessments were performed to determine which clinical factors were associated with subject and informant QOL ratings. Cognitive functioning was examined using measures included in the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS) neuropsychological battery, which consists of Wechsler Memory Scale–Revised (WMS-R) Logical Memory IA and IIA, WMS-R forward and reverse digit span, verbal category fluency for animals and vegetables, Trail-Making Test Parts A and B, Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol, and the 30 odd-numbered items of the Boston Naming Test (23). Of note, the third editions of the WMS and WAIS tests were administered and scores were prorated to match the revised versions of the same tests included in the NACC. Namely, the WMS-3 version of the Digit Span subtest contains different digits and the WAIS-3 version of the Digit Symbol subtest was timed for 120 seconds instead of 90 seconds; therefore, the score was divided by 120 and then multiplied by 90 to derive a WAIS-R equivalent score. For each subject, z scores on each test were calculated using the large set of normative data from cognitively normal participants included in the NACC UDS and averaged to generate composite z scores for each of the four cognitive domains (executive/processing speed,memory, language, and attention) and for overall cognitive performance as previously described (8).
Informant ratings of neuropsychiatric symptoms were obtained with the Neuropsychiatric Inventory [NPI (24)], which measures the presence, frequency, and severity of 12 categories of behavioral disturbances. Composite scores for each category were calculated by multiplying frequency and severity scores. The NPI full scale score represents the sum total of all category-specific composite scores, with higher scores indicative of more frequent/severe neuropsychiatric symptoms. Subjects’ ratings of their own depressive symptoms were obtained with the Geriatric Depression Scale [GDS (25)], a 30-item questionnaire. Higher scores on the GDS suggest more depressive symptomatology.
Instrumental activities of daily living (IADLs) were assessed with the Functional Activities Questionnaire [FAQ (26)], which incorporates informant ratings of subjects’ performance on 10 categories of IADLs, with higher scores denoting increasing impairment. Activities that could not be rated because the subject never performed them prior to developing cognitive difficulties or because the informant had insufficient information to provide a valid response were not scored. Since not all subjects had data for all items, overall FAQ performance was evaluated using the average score across FAQ items with valid responses (mean FAQ item score) as previously described (8).
Data analyses
Statistical analyses were performed using SPSS 16.0 for Mac (SPSS Inc., Chicago IL). Demographic, QOL, cognitive, neuropsychiatric, and FAQ data were compared between groups using unpaired t-tests (for continuous variables) or chi-squared tests (for categorical variables) with Bonferroni correction for multiple comparisons where appropriate. Effect sizes were calculated using Cohen’s d statistic. Pearson’s correlation coefficients were calculated to determine concordance between subject and informant QOL ratings. Multiple regression models were used to determine the relative contributions of demographic, cognitive, neuropsychiatric, and functional variables to QOL indices.
RESULTS
Demographic, cognitive, neuropsychiatric, and functional comparisons between the NC and MCI groups are shown in Table 1. The two groups were similar in age, level of education, and gender and ethnic distribution. Analyses of cognitive data indicated that the MCI group performed more poorly on the MMSE and in each of the domains that were assessed. The MCI group exhibited greater neuropsychiatric symptomatology (as measured by the NPI), but did not report higher levels of depression (as measured by the GDS). The MCI group also exhibited poorer performance of IADLs (as measured by the FAQ).
Table 1.
Demographic, neuropsychological, neuropsychiatric, and functional data for the normal control (NC) and mild cognitive impairment (MCI) cohorts. Standard deviations are shown in parentheses. Scattered data points were missing for some variables, resulting in different degrees of freedom (df) for different comparisons.
| NC | MCI | t/χ2 | p | |
|---|---|---|---|---|
| N | 97 | 108 | -- | -- |
|
| ||||
| Demographics | ||||
|
| ||||
| Age | 70.1 (8.6) | 72.0 (9.5) | −1.53a | 0.13 |
| Years of education | 16.5 (2.9) | 15.9 (3.1) | 1.56a | 0.12 |
| MMSE | 28.8 (1.3) | 27.1 (2.6) | 5.73a | <0.001 |
| % Male | 53.6% | 43.5% | 2.08a | 0.15 |
| % Caucasian | 77.3% | 72.2% | 0.70a | 0.40 |
|
| ||||
| Cognitive Performance | ||||
|
| ||||
| Executive/Speed z | 0.05 (0.70) | −0.90 (1.37) | 6.03b | <0.001 |
| Memory z | −0.02 (0.97) | −1.32 (1.20) | 8.36c | <0.001 |
| Language z | 0.09 (0.65) | −0.48 (0.86) | 5.26d | <0.001 |
| Attention z | 0.01 (0.83) | −0.45 (0.90) | 3.79e | <0.001 |
| Composite Cognitive z | 0.04 (0.51) | −0.78 (0.81) | 8.33f | <0.001 |
|
| ||||
| Neuropsychiatric Symptoms | ||||
|
| ||||
| NPI full scale score | 1.59 (4.17) | 3.89 (8.32) | −2.43c | 0.016 |
| GDS | 1.85 (3.16) | 2.44 (3.16) | −1.34e | 0.18 |
|
| ||||
| Functional Abilities | ||||
|
| ||||
| Avg FAQ item | 0.06 (0.24) | 0.16 (0.24) | −3.17e | 0.002 |
df=203;
df=197;
df=199;
df=196;
df=202;
df=190
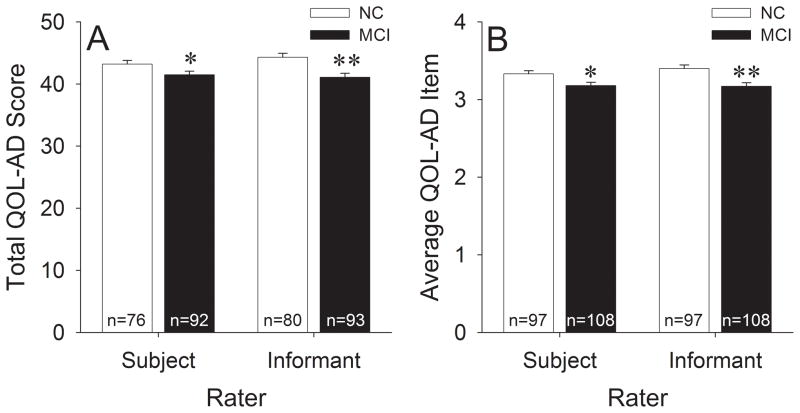
Global QOL-AD indices for the NC and MCI groups are shown in Figure 1. Analyses of total QOL-AD scores (Figure 1A; includes only subjects with valid data for all items) indicated that overall QOL was significantly impaired in the MCI group when using either subject [t(166)=2.07, p=0.04; d=0.32] or informant [t(171)=3.63, p<0.001; d=0.55] ratings. Analyses of average QOL-AD item scores (Figure 1B; includes all subjects) also demonstrated impaired overall QOL in the MCI group with either subject [t(203)=2.46, p=0.015; d=0.35] or informant [t(203)=3.68, p<0.001; d=0.52] ratings.
Figure 1.
A) Total QOL-AD scores and B) average QOL-AD item scores in the NC and MCI groups. Error bars represent standard error of the mean. The number of subjects used for each analysis is indicated at the base of each bar. **p<0.05 vs. NC; **p<0.001 vs. NC.
In the overall study sample, subject and informant ratings were moderately well correlated [total QOL-AD: r(165)=0.57; average QOL-AD item: r(205)=0.58; both p’s<0.001]. When the NC and MCI groups were separately analyzed, correlations between subject and informant ratings were numerically higher in the MCI group [total QOL-AD: r(90)=0.64; average QOL-AD item: r(108)=0.65; both p’s<0.001] than in the NC group [total QOL-AD: r(75)=0.44; average QOL-AD item: r(97)=0.46; both p’s<0.001]. However, differences between the correlation coefficients seen in the two groups did not reach statistical significance [total QOL-AD: z(163)=1.73, p=0.083; average QOL-AD item: z(203)=1.92, p=0.055]. Furthermore, paired t-test comparisons of subject and informant ratings also failed to show significant differences on global QOL-AD indices in the overall study sample or in the individual groups (all p’s>0.10).
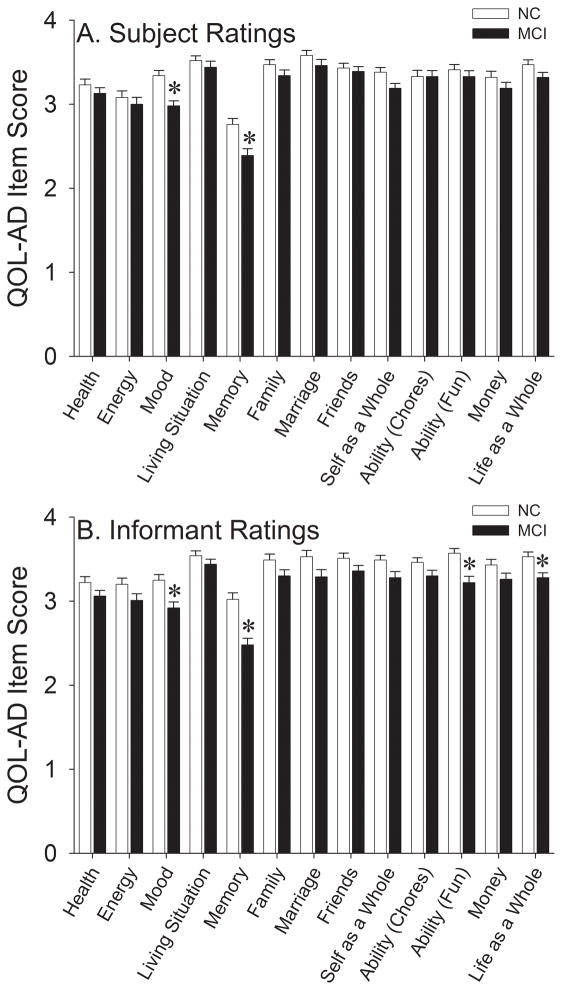
We subsequently sought to determine which individual QOL-AD items were primarily responsible for lower global QOL-AD indices in the MCI group. Subject ratings of individual QOL-AD items are shown in Figure 2A. After Bonferroni correction (13 comparisons; critical p=0.004), the MCI group was found to have poorer subject-rated QOL for the mood [t(203)=4.14, p<0.001; d=0.58] and memory [t(203)=3.49, p=0.001; d=0.49] items. A small number of subject ratings were absent for the family (1 MCI subject) and marriage (21 NC and 15 MCI subjects) items. Individual item analyses were also performed for informant QOL-AD ratings (Figure 2B), which revealed significantly impaired QOL in the MCI group for the mood [t(202)=3.43, p=0.001; d=0.48], memory [t(203)=4.91, p<0.001; d=0.69], ability to do things for fun [t(203)=3.65, p<0.001; d=0.52], and life as a whole [t(203)=3.16, p=0.002; d=0.45] items. A small number of informant ratings were absent for the mood (1 MCI subject), family (3 MCI subjects), and marriage (16 NC and 12 MCI subjects) items.
Figure 2.
Individual QOL-AD item scores in the NC and MCI groups using A) subject and B) informant ratings. Error bars represent standard error of the mean. *p<0.004 vs. NC.
Since the MCI group had lower QOL-AD scores for the memory item and included both amnestic (AMN) and non-amnestic (NON) subjects, we performed additional analyses to determine whether QOL differed between these subgroups. Demographic, cognitive, neuropsychiatric, and functional data for the AMN and NON subgroups are shown in Table 2. The AMN and NON subgroups were similar across all variables except for the expected poorer memory performance in the AMN group (which also resulted in lower overall composite cognitive z-scores). However, analyses of global QOL-AD indices did not reveal significant differences between the MCI subgroups using subject total QOL-AD scores [mean AMN=40.97 (SD=5.61); NON=42.47 (5.22); t(90)=−1.25, p=0.21], informant total QOL-AD scores [AMN=40.66 (6.02); NON=41.94 (6.03); t(91)=−0.98, p=0.33], subject average QOL-AD item scores [AMN=3.14 (0.46); NON=3.26 (0.39); t(106)=−1.38, p=0.17], or informant average QOL-AD item scores [AMN=3.13 (0.47); NON=3.24 (0.46); t(106)=−1.07, p=0.29]. Comparisons of individual QOL-AD items also did not reveal significant differences between the AMN and NON subgroups. Although there was a trend towards lower scores on the memory item in the AMN subgroup using either subject [AMN=2.26 (0.81); NON=2.64 (0.83); t(106)=−2.26, p=0.026] or informant [AMN=2.33 (0.73); NON=2.78 (0.87); t(106)=−2.80, p=0.006] ratings, neither of these findings survived Bonferroni correction (13 comparisons; critical p=0.004).
Table 2.
Demographic, neuropsychological, neuropsychiatric, and functional data for the amnestic (AMN) and non-amnestic (NON) MCI subgroups. Standard deviations are shown in parentheses. Scattered data points were missing for some variables, resulting in different degrees of freedom (df) for different comparisons.
| AMN | NON | t/χ2 | p | |
|---|---|---|---|---|
| N | 72 | 36 | -- | -- |
|
| ||||
| Demographics | ||||
|
| ||||
| Age | 72.2 (9.4) | 71.7 (9.7) | 0.24a | 0.81 |
| Years of education | 15.8 (2.9) | 16.0 (3.4) | −0.27a | 0.79 |
| MMSE | 26.8 (2.8) | 27.8 (2.1) | −1.75b | 0.08 |
| % Male | 41.7% | 47.2% | 0.30a | 0.58 |
| % Caucasian | 76.4% | 63.9% | 1.87a | 0.17 |
|
| ||||
| Cognitive Performance | ||||
|
| ||||
| Executive/Speed z | −0.97 (1.45) | −0.76 (1.18) | −0.73c | 0.46 |
| Memory z | −1.66 (1.18) | −0.64 (0.91) | −4.55d | <0.001 |
| Language z | −0.57 (0.92) | −0.32 (0.71) | −1.38e | 0.17 |
| Attention z | −0.47 (0.91) | −0.41 (0.87) | −0.33b | 0.74 |
| Composite Cognitive z | −0.90 (0.85) | −0.55 (0.68) | −2.04f | 0.044 |
|
| ||||
| Neuropsychiatric Symptoms | ||||
|
| ||||
| NPI full scale score | 4.46 (9.39) | 2.71 (5.52) | 1.02b | 0.31 |
| GDS | 2.63 (3.38) | 2.06 (2.67) | 0.89b | 0.37 |
|
| ||||
| Functional Abilities | ||||
|
| ||||
| Avg FAQ item | 0.17 (0.22) | 0.16 (0.28) | 0.24b | 0.81 |
df=106;
df=105;
df=102;
df=104;
df=100;
df=97
The MCI group had lower cognitive z-scores, more neuropsychiatric symptoms (i.e. higher NPI and GDS scores), and poorer IADL performance (i.e. higher FAQ scores) than the NC group (Table 1). We wanted to determine which of these deficits were most closely associated with decreased QOL in the MCI group. Therefore, we performed multiple regression analyses on subject- and informant-rated average QOL-AD item scores in the MCI group and included the following variables: age, education, executive/processing speed z-score, memory z-score, language z-score, attention z-score, GDS score, NPI full scale score, and average FAQ item score. The results of these analyses are shown in Table 3. The only significant predictor of subject-rated average QOL-AD item scores was the GDS score, which was inversely correlated with overall QOL. Significant predictors of informant-rated average QOL-AD item scores included the average FAQ item score, the NPI full scale score, and the GDS score, each of which was also inversely correlated with overall QOL. Notably, none of the cognitive testing z-scores were significant predictors of overall QOL. In the MCI group, the z-scores for separate cognitive domains were all significantly correlated (all r’s>0.25, all p’s≤0.005), raising the possibility that multicollinearity might obscure the effects of cognitive performance on QOL. Therefore, we conducted additional multiple regression analyses substituting either the MMSE or the composite cognitive z-score for domain-specific z-scores. The inclusion of these global cognitive variables did not significantly alter our findings (data not shown).
Table 3.
Multiple linear regression analyses of average QOL-AD item scores for the MCI group as rated by subject or informant.
| Subject Ratings | Informant Ratings | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| β | t(86) | p | β | t(86) | p | |
|
| ||||||
| Age | 0.059 | 0.621 | 0.537 | 0.077 | 0.852 | 0.396 |
| Education | −0.092 | −0.938 | 0.351 | −0.022 | −0.235 | 0.815 |
| Executive/Processing Speed z | −0.015 | −0.122 | 0.903 | −0.229 | −1.909 | 0.060 |
| Memory z | 0.114 | 1.047 | 0.298 | 0.073 | 0.707 | 0.482 |
| Language z | −0.040 | −0.339 | 0.736 | 0.066 | 0.587 | 0.559 |
| Attention z | 0.098 | 0.890 | 0.376 | 0.179 | 1.712 | 0.091 |
| NPI full scale score | 0.050 | 0.529 | 0.598 | −0.244 | −2.739 | 0.007 |
| GDS | −0.493 | −4.818 | <0.001 | −0.231 | −2.364 | 0.020 |
| Avg FAQ item | −0.155 | −1.561 | 0.122 | −0.381 | −4.025 | <0.001 |
|
| ||||||
| Overall Model | r=0.564 | r=0.618 | ||||
We also investigated the contribution of cognitive, neuropsychiatric, and functional variables to QOL in the NC group using the same multiple regression analyses. None of the included variables were significantly associated with subject QOL-AD ratings (all p’s>0.05), and only the executive functioning z-score (β=0.293, t(81)=2.61, p=0.011) was significantly associated with informant QOL-AD ratings.
DISCUSSION
Our results indicate that individuals meeting criteria for MCI have modest decreases in QOL relative to age-matched cognitively normal elderly. Reductions in global QOL-AD indices in our MCI cohort were driven by decreased ratings for the mood and memory items on both subject and informant questionnaires. These findings are consistent with a prior study that showed impaired psychological (but not physical, social relationship, or environmental) QOL in MCI subjects using the short version of the World Health Organization Quality of Life (WHOQOL-BREF) assessment tool (15). QOL deficits in MCI may not have been apparent in other earlier reports because of their relatively small (n<40) MCI cohorts (10, 11,13) and/or their use of generic QOL instruments, such as the Linear Analogue Self Assessment scale (13) or the COOP/Wonca charts (14), that may be less sensitive to QOL related to cognitive impairment. Therefore, the current study examined a larger MCI cohort (n=108) with the QOL-AD, which targets QOL deficits associated with cognitive impairment in the elderly population.
Total QOL-AD scores in the overall cohort were quite high (Figure 1). Our NC group had slightly higher scores than a previous report that included QOL-AD scores for cognitively normal elderly [mean subject rating 41.1, mean informant rating 38.9 (12)]. Similarly, QOL-AD scores in our MCI group were higher than those reported for AD patients, which range from 29.1 to 40.7 for subject ratings and 24.7 to 35.9 for informant ratings (27, 28), and higher than those reported in a recent study of MCI subjects [mean subject rating 29.8, mean informant rating 29.1; (18)]. QOL-AD studies have been performed several countries, and the wide range of reported results may be related to cultural influences on QOL ratings. The average magnitudes of measured declines in total QOL-AD scores in MCI were relatively modest: −1.71 with subject ratings and −3.21 with informant ratings (scale ranges from 13–52). Calculated effect sizes were in the small to medium range, which parallel the relatively modest cognitive, behavioral and functional impairments reported in other studies of MCI.
Reduced QOL-AD ratings in the MCI group relative to the NC group were seen with both subject and informant responses, which were moderately well correlated. However, group differences were more robust with informant ratings. Analyses of global QOL-AD indices did not reveal an effect of rater or a group × rater interaction, although there was a trend towards stronger correlations between subject and informant ratings in the MCI group relative to the NC group. Caregivers of patients with dementia frequently report lower patient QOL ratings than patients themselves (29). This discrepancy has been noted in previous studies utilizing the QOL-AD scale (28, 30–32), increases with dementia severity (16), and correlates with anosognosia (30), which may explain the better subject/informant agreement in our MCI cohort, which would be expected to have milder cognitive deficits and greater insight than cohorts of persons with dementia.
Previous studies have not compared QOL between amnestic and non-amnestic MCI. Given the higher rates of progression to dementia in amnestic MCI relative to non-amnestic MCI (33–36), we expected to find impaired QOL in the AMN group relative to the NON group. Although global QOL-AD indices were numerically lower in the AMN group than the NON group, these differences failed to reach statistical significance. One interpretation of these results, supported by the absence of significant correlations between cognitive performance and QOL-AD ratings, is that memory performance, which represents the key difference between the AMN and NON groups (Table 2), plays a relatively minor role in global QOL. Alternatively, it also possible that the QOL-AD scale, though likely more sensitive than generic instruments for identifying reductions in QOL in MCI, may still be insufficiently sensitive for the detection of smaller QOL differences expected between amnestic and non-amnestic MCI, particularly because our AMN vs. NON comparisons were relatively underpowered relative to our MCI vs. NC comparisons. The similarity in average FAQ item scores between our AMN and NON subjects may have also affected our ability to detect a difference in QOL-AD ratings between these subgroups. A previous study conducted with a much larger MCI sample found significantly higher FAQ scores (i.e. impaired IADL performance) in amnestic MCI than in non-amnestic MCI (8). Since average FAQ item scores were inversely associated with informant-rated average QOL item scores in the current study, the relatively preserved functional performance of our AMN group may have further limited the sensitivity of our analyses.
Multiple linear regression analyses in the MCI group indicated that both subject and informant QOL-AD ratings correlated with depressive symptoms, while informant QOL-AD ratings additionally correlated with a broader spectrum of behavioral symptoms and IADL performance. Conversely, QOL-AD scores were not significantly associated with cognitive performance. These results parallel QOL-AD findings from cohorts of patients with dementia, most of which have also suggested that QOL correlates better with mood, behavioral, and/or functional disturbances rather than severity of cognitive impairment (12, 28, 30–32, 37–39). In contrast, when the same multiple regression analyses were applied to our NC group, a distinctly different pattern emerged. One possible conclusion from these findings is that non-cognitive symptoms may be primarily responsible for decreased QOL in MCI. Alternatively, our results could indicate that the QOL-AD, NPI, GDS, and FAQ simply represent different overlapping approaches for measuring the same underlying phenomena.
A number of factors may limit the interpretation of our findings. The QOL-AD was designed and validated in cohorts comprised of patients with dementia (16, 17). Therefore, QOL-AD scores may be less applicable to MCI, although a recent study suggests this instrument may have sufficient validity and reliability in MCI (18). Effect sizes for global QOL deficits in our MCI cohort were modest in magnitude, and when subject ratings were analyzed, only the mood and memory items differed significantly between the NC and MCI groups. It is possible that these results reflect MCI subjects’ retained insight into their symptoms rather than actual QOL deficits. This distinction remains difficult to determine, particularly given the different approaches to conceptualizing and measuring QOL in cognitively impaired individuals (29). However, the numerically lower subject ratings on other QOL-AD items in MCI group relative to the NC group, the reasonable concordance between subject and informant ratings, and the different patterns of predictors for global QOL-AD indices between the NC and MCI groups all appear to support our conclusion of reduced in QOL in MCI. The NACC neuropsychological battery is relatively limited in scope (8) and in particular, does not assess visuospatial function (23). Therefore, our multiple linear regression analyses may have been less sensitive to the contributions of cognitive impairment to perceived QOL. Nevertheless, when a variable assessing visuospatial abilities (Rey Complex Figure copy score) was added to our regression models (data not shown), it was not significantly associated with average QOL-AD item scores and did not affect the overall results of the models.
Taken together, our data suggest modest declines in QOL are present in MCI and most closely associated with neuropsychiatric and functional changes. These findings, which require confirmation in larger MCI samples, are concordant with previous studies of QOL in dementia and consistent with the hypothesis that MCI lies on the continuum between normal cognitive aging and dementia. QOL indices may be more sensitive than standard cognitive assessments to clinically relevant outcomes after experimental interventions (40). Our results suggest that interventions targeting behavioral or functional symptoms in MCI may be most effective in improving QOL for these individuals.
Acknowledgments
This research was supported by grants from the National Institute on Aging (P50 AG 16570, K23 AG 028727 [to PL], and K08 AG 34628 [to ET; jointly sponsored by NIA, AFAR, the John A. Hartford Foundation, the Atlantic Philanthropies, the Starr Foundation and an anonymous donor]), the Alzheimer’s Disease Research Centers of California, and the Sidell-Kagan Foundation.
Footnotes
Disclosures: No disclosures to report.
References
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr. 2004;16:129–140. doi: 10.1017/s1041610204000092. [DOI] [PubMed] [Google Scholar]
- 3.Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25:115–126. doi: 10.1159/000112509. [DOI] [PubMed] [Google Scholar]
- 4.Albert SM, Michaels K, Padilla M, et al. Functional significance of mild cognitive impairment in elderly patients without a dementia diagnosis. Am J Geriatr Psychiatry. 1999;7:213–220. doi: 10.1097/00019442-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 6.Perneczky R, Pohl C, Sorg C, et al. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. Int J Geriatr Psychiatry. 2006;21:158–162. doi: 10.1002/gps.1444. [DOI] [PubMed] [Google Scholar]
- 7.Jefferson AL, Byerly LK, Vanderhill S, et al. Characterization of activities of daily living in individuals with mild cognitive impairment. Am J Geriatr Psychiatry. 2008;16:375–383. doi: 10.1097/JGP.0b013e318162f197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng E, Becker BW, Woo E, et al. Subtle deficits in instrumental activities of daily living in subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;30:189–197. doi: 10.1159/000313540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ettema TP, Droes RM, de Lange J, et al. The concept of quality of life in dementia in the different stages of the disease. Int Psychogeriatr. 2005;17:353–370. doi: 10.1017/s1041610205002073. [DOI] [PubMed] [Google Scholar]
- 10.Ready RE, Ott BR, Grace J. Patient versus informant perspectives of Quality of Life in Mild Cognitive Impairment and Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19:256–265. doi: 10.1002/gps.1075. [DOI] [PubMed] [Google Scholar]
- 11.Missotten P, Squelard G, Ylieff M, et al. Quality of life in older Belgian people: comparison between people with dementia, mild cognitive impairment, and controls. Int J Geriatr Psychiatry. 2008;23:1103–1109. doi: 10.1002/gps.1981. [DOI] [PubMed] [Google Scholar]
- 12.Rosas-Carrasco O, del Torres-Arreola LP, de Guerra-Silla MG, et al. Validation of the Quality of Life in Alzheimer’s Disease (QOL-AD) scale in Mexican patients with Alzheimer, vascular and mixed-type dementia. Rev Neurol. 2010;51:72–80. [PubMed] [Google Scholar]
- 13.Lapid MI, Rummans TA, Boeve BF, et al. What is the quality of life in the oldest old? Int Psychogeriatr. 2011;23:1003–1010. doi: 10.1017/S1041610210002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurz X, Scuvee-Moreau J, Vernooij-Dassen M, et al. Cognitive impairment, dementia and quality of life in patients and caregivers. Acta Neurol Belg. 2003;103:24–34. [PubMed] [Google Scholar]
- 15.Muangpaisan W, Assantachai P, Intalapaporn S, et al. Quality of life of the community-based patients with mild cognitive impairment. Geriatr Gerontol Int. 2008;8:80–85. doi: 10.1111/j.1447-0594.2008.00452.x. [DOI] [PubMed] [Google Scholar]
- 16.Logsdon RG, Gibbons LE, McCurry SM, et al. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64:510–519. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Thorgrimsen L, Selwood A, Spector A, et al. Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis Assoc Disord. 2003;17:201–208. doi: 10.1097/00002093-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Tatsumi H, Yamamoto M, Nakaaki S, et al. Utility of the Quality of Life-Alzheimer’s Disease Scale for mild cognitive impairment. Psychiatry Clin Neurosci. 2011;65:533. doi: 10.1111/j.1440-1819.2011.02245.x. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington: APA; 1994. [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:253–259. doi: 10.1159/000107100. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 25.Yesavage JA. Geriatric Depression Scale. Psychopharmacology Bulletin. 1988;24:709–711. [PubMed] [Google Scholar]
- 26.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 27.Matsui T, Nakaaki S, Murata Y, et al. Determinants of the quality of life in Alzheimer’s disease patients as assessed by the Japanese version of the Quality of Life-Alzheimer’s disease scale. Dement Geriatr Cogn Disord. 2006;21:182–191. doi: 10.1159/000090744. [DOI] [PubMed] [Google Scholar]
- 28.Shin IS, Carter M, Masterman D, et al. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:469–474. doi: 10.1176/appi.ajgp.13.6.469. [DOI] [PubMed] [Google Scholar]
- 29.Ettema TP, Droes RM, de Lange J, et al. A review of quality of life instruments used in dementia. Qual Life Res. 2005;14:675–686. doi: 10.1007/s11136-004-1258-0. [DOI] [PubMed] [Google Scholar]
- 30.Vogel A, Mortensen EL, Hasselbalch SG, et al. Patient versus informant reported quality of life in the earliest phases of Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:1132–1138. doi: 10.1002/gps.1619. [DOI] [PubMed] [Google Scholar]
- 31.Hoe J, Katona C, Orrell M, et al. Quality of life in dementia: care recipient and caregiver perceptions of quality of life in dementia: the LASER-AD study. Int J Geriatr Psychiatry. 2007;22:1031–1036. doi: 10.1002/gps.1786. [DOI] [PubMed] [Google Scholar]
- 32.Karttunen K, Karppi P, Hiltunen A, et al. Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer’s disease. Int J Geriatr Psychiatry. 2011;26:473–482. doi: 10.1002/gps.2550. [DOI] [PubMed] [Google Scholar]
- 33.Rasquin SM, Lodder J, Visser PJ, et al. Predictive accuracy of MCI subtypes for Alzheimer’s disease and vascular dementia in subjects with mild cognitive impairment: a 2-year follow-up study. Dement Geriatr Cogn Disord. 2005;19:113–119. doi: 10.1159/000082662. [DOI] [PubMed] [Google Scholar]
- 34.Alexopoulos P, Grimmer T, Perneczky R, et al. Progression to Dementia in Clinical Subtypes of Mild Cognitive Impairment. Dement Geriatr Cogn Disord. 2006;22:27–34. doi: 10.1159/000093101. [DOI] [PubMed] [Google Scholar]
- 35.Busse A, Hensel A, Guhne U, et al. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 36.Manly JJ, Tang MX, Schupf N, et al. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naglie G, Hogan DB, Krahn M, et al. Predictors of family caregiver ratings of patient quality of life in Alzheimer disease: cross-sectional results from the Canadian Alzheimer’s disease quality of life study. Am J Geriatr Psychiatry. 2011;19:891–901. doi: 10.1097/JGP.0b013e3182006a7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naglie G, Hogan DB, Krahn M, et al. Predictors of patient self-ratings of quality of life in Alzheimer disease: cross-sectional results from the Canadian Alzheimer’s disease quality of life study. Am J Geriatr Psychiatry. 2011;19:881–890. doi: 10.1097/JGP.0b013e3182006a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuh JL, Wang SJ. Assessing quality of life in Taiwanese patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:103–107. doi: 10.1002/gps.1425. [DOI] [PubMed] [Google Scholar]
- 40.Scholzel-Dorenbos CJ, van der Steen MJ, Engels LK, et al. Assessment of quality of life as outcome in dementia and MCI intervention trials: a systematic review. Alzheimer Dis Assoc Disord. 2007;21:172–178. doi: 10.1097/WAD.0b013e318047df4c. [DOI] [PubMed] [Google Scholar]