Summary
Amplified fragment length polymorphism (AFLP) is a PCR-based technique that uses selective amplification of a subset of digested DNA fragments to generate and compare unique fingerprints for genomes of interest. The power of this method relies mainly in that it does not require prior information regarding the targeted genome, as well as in its high reproducibility and sensitivity for detecting polymorphism at the level of DNA sequence. Widely used for plant and microbial studies, AFLP is employed for a variety of applications, such as: to assess genetic diversity within species or among closely related species, to infer population-level phylogenies and biogeographic patterns, to generate genetic maps and to determine relatedness among cultivars. Variations of standard AFLP methodology have been also developed for targeting additional levels of diversity, like transcriptomic variation and DNA methylation polymorphism.
Keywords: AFLP, cDNA, DNA methylation, Epigenetics, Genetic diversity, Isoschizomers, Ligation, MSAP, Restriction enzymes, Transcriptomics
1. Introduction
Amplified fragment length polymorphism (AFLP) is a PCR-based fingerprinting technique that was first described by Vos et al. (1). Since then several modified protocols have been reported, but all typically include five main steps: (a) restriction of genomic DNA (see Note 1) and ligation of adaptors (most often performed together) to restricted fragments; (b) preselective PCR amplification of a subset of the restricted fragments; (c) selective PCR amplification, reducing further fragment number; (d) electrophoretic separation of amplified DNA fragments; (e) scoring and interpretation of the data. We detail below one of the protocols that uses a RedTaq polymerase.
The power of AFLP analysis derives from its ability to quickly generate large numbers of marker fragments for any organism, without prior knowledge of genomic sequence. In addition, AFLP requires only small amounts of starting template and, in comparison with other fingerprinting techniques such as RAPD (random amplified polymorphic DNA) and ISSR (inter-simple sequence repeats) it exhibits much higher reproducibility (but see Note 2). Despite the fact that AFLP is a relatively labour-intensive method, it can be easily multiplexed and frequently is used to amplify in the same batch hundreds of genomic fragments from hundreds of individuals.
When setting up an AFLP assay, an optimization step may be necessary to identify those primer combinations that will generate sufficient polymorphic marker fragments for a study (see Note 3). The researchers will usually choose the best three primer combinations from a dozen that have been tested across few individuals, enabling them to identify the optimal pairs for a given organism without having to design, synthesize, or perform quality control tests of their primers. The success of an AFLP assay depends on four factors: optimized reagents (see Materials section), standardized reaction conditions (see Note 4), a robust and reliable electrophoresis platform (see Note 5), and accurate sizing software.
While the standard AFLP protocol normally starts from genomic DNA to target genome-wide genetic loci, modifications of it have been developed to target other levels of information, such as the transcriptome and DNA methylation variation. Although expressed DNA segments (coding genes together with promoter regions and other regulatory elements) make up only a limited fraction of the genome, they are exactly the elements producing differences between phenotypes. A comprehensive transcript-profiling method that has been used to compare gene expression between individuals and groups of phenotypes is the cDNA-AFLP (2-6). For this purpose, the standard AFLP procedure (1) is performed on cDNA (the complement of mRNA) generated from a given tissue (see Note 6). Compared with other transcriptomic techniques, cDNA-AFLP is fast, requires only a small quantity of mRNA, can be applied in the absence of prior sequence data, is reproducible and sensitive and exhibits a good correlation with Northern analysis or RT-PCR (2, 4).
Another AFLP-derived protocol is methylation sensitive amplified polymorphism (MSAP) that targets the variation in the distribution of DNA methylation in given tissues/cell lines (7-10). DNA methylation signals are chemical modifications of the DNA bases (especially cytosines) that are relatively stable over cell generations but can differ dramatically between individuals or even between cell types within one individual. They are essential for regulating development through their influence on gene transcription; they are also involved in a number of specific biological processes, such as gene silencing and mobile element control (6). In the MSAP protocol (see Note 6), the standard AFLP technique is applied to genomic DNA by making use of two isoschizomers (methylation-sensitive/insensitive restriction enzymes that recognize the same DNA sequence e.g., MspI and HpaII) as frequent cutters in parallel restriction reactions. A difference in banding patterns indicates methylation variation. Most of the polymorphic MSAP fragments are probably made up of non-coding regions; nevertheless a fraction of MSAP variation among individuals is expected to correspond to epigenetic gene silencing/activation.
2. Materials
Most chemicals to be used in an AFLP assay can be bought ready made. As much as possible the same chemicals (i.e., vial or batch) and in the same concentration should be used for all individuals to be analysed in the AFLP experiment. Use always ultrapure water (hereafter ddH2O), prepared by purifying deionised water to attain a sensitivity of 18 MΩ at 25 °C or buy e.g., Purified Water (Oxoid Ltd, U.K.). Store all reagents at -20 °C (unless indicated otherwise).
2.1. DNA template preparation
1x TE buffer: dissolve 10 mM TrisHCl (or use 10 mM of Tris, and adjust to pH 8 with HCl) and 1 mM EDTA (Ethylenediaminetetraacetic acid) in 1 L ddH2O, and adjust to pH 8. Store at room temperature.
2.2. Restriction-ligation (RL)
MseI restriction endonuclease (the “frequent cutter” – recognizes a four bases motif, i.e., 5′-TTAA). Per individual to be analysed 1 U MseI will be needed. Restriction enzymes are thermo-sensitive and should be taken as briefly as possible out of the freezer. Usually delivered in a solution containing Glycerol, therefore it will not freeze at −20 °C.
EcoRI restriction endonuclease (the “rare cutter” – recognizes a six bases motif, i.e., 5′-GAATTC). Per individual to be analysed 5 U MseI will be needed. Restriction enzymes are thermo-sensitive and should be taken as briefly as possible out of the freezer. Usually delivered in a solution containing Glycerol, therefore it will not freeze at −20 °C.
MseI-adaptor pair: 5′-GACGATGAGTCCTGAG and 5′-TACTCAGGACTCAT. Store each adaptor primer individually at −20 °C as stock with concentration of 100 μM. Immediately prior to adding to the RL reaction mix in proportion 1:1 (to obtain a concentration of 50 μM for each), then denature (i.e., heat up at 95 °C for 5 min) the required amount of combined MseI adaptors and allow slow renature (let them cool slowly at room temperature for ca 10 min) to form double stranded adaptor. Spin briefly.
EcoRI-adaptor pair: 5′-CTCGTAGACTGCGTACC and 5′-AATTGGTACGCAGTCTAC. Store each adaptor primer individually at −20 °C as stock with concentration of 100 μM. Immediately prior to adding to the RL reaction mix in proportion 1:1:18 with ddH2O (to obtain a concentration of 5 μM for each adaptor), then denature the required amount of combined EcoRI adaptors and allow slow renature. Spin briefly.
T4 DNA ligase. Per individual to be analysed 0.6 U T4 DNA ligase will be needed. Restriction enzymes are thermo-sensitive and should be taken as briefly as possible out of the freezer. Minimise the time and intensity of any spin or vortex of the ligase vial. Usually delivered in a solution containing Glycerol, therefore it will not freeze at −20 °C.
T4 DNA ligase buffer. Usually delivered with the DNA ligase. Contains: 50 mM Tris-HCl, 10 mM MgCl2, 1 mM ATP and 10 mM Dithiothreitol in a solution of 7.5 pH at room temperature. Can precipitate with time; before use it should be placed at 37 °C and be vortexed thoroughly from time to time until fully homogenized. It is sensitive.
BSA (Bovine Serum Albumin). Store as a stock solution of 10 mg/ml. Dilute prior to use to a working concentration of 1 mg/ml.
0.5 M NaCl.
1x TE 0.1 M buffer: dissolve 20 mM TrisHCl (or use 20 mM of Tris and adjust to pH 8 with HCl) and 0.1 mM EDTA in 1 L ddH2O, and adjust to pH 8. Store at room temperature.
10x TBE buffer (stock solution): dissolve 108 g Tris base, 55 g boric acid and 8.1 g Na2EDTA in 1L ddH2O. Correct acidity to pH 8.2-8.3. Store at room temperature. The concentration of the working solution is 1x TBE buffer.
Size ladder with fragments in the range smaller than 1,500 bp.
Electrophoresis loading buffer: buy a ready-made buffer or prepare yourself one, e.g., Orange G Loading buffer. Disolve 1.5 g Ficoll 400 and 0.015 g Orange G in 1 ml 0.5M EDTA, and make volume up to 10 ml with ddH2O.
2.3. Preselective PCR amplification
Taq DNA polymerase e.g., RedTaq (Sigma-Aldrich) or AmpliTaq (Life Technologies). DNA polymerases are termo-sensitive and should be taken as briefly as possible out of the freezer. Minimise the time and intensity of any spin or vortex of the Taq vial. The protocol given at Methods uses RedTaq that contains an inert red dye. The dye enables visual recognition of reactions to which polymerase has been added, confirmation of complete mixing, as well as direct loading of PCR products onto an agarose gel without the addition of electrophoresis loading buffer.
Taq DNA polymerase buffer. Use the recommended buffer for the chosen Taq polymerase. The protocol given at Methods uses 10x RedTaq PCR Reaction buffer (Sigma-Aldrich Co.). Some amounts of buffer are usually delivered with the DNA polymerase, but additional quantities may have to be ordered separately.
Deoxynucleotide Mix (dNTPs) in concentration 10 mM each dATP, dCTP, dGTP, dTTP. We recommend the use of a ready-made mix (e.g., GeneAmp dNTP Blend, 10mM from Life Technologies). If the dNTPs are purchased individually, a mixture has to be performed to achieve the required concentration. Aliquots of the dNTPs mixture can be stored at −20 °C for several months.
EcoRI primer: 5′-GACTGCGTACCAATTCA (see Note 8). Store as stock solution of 100 μM.
MseI primer: 5′-GATGAGTCCTGAGTAAC (see Note 8). Store as stock solution of 100 μM.
1x TE 0.1 M buffer (prepared as above).
Size ladder with fragments in the range smaller than 1,000 bp.
2.4. Selective PCR amplification
RedTaq (see 2.3).
RedTaq buffer (see 2.3).
dNTPs (see 2.3.)
EcoRI primers: 5- GACTGCGTACCAATTCXXX (see Note 8) where X stands for selective nucleotides (they vary after case). The EcoRI primers are usually fluorescently labelled. The working concentration of the EcoRI selective primer is 1 μM. As fluorescently labelled primers, the EcoRI selective primers are light-sensitive. Store as stock solution (100 μM) for several years and as working solution (1 μM) for several months.
MseI primers: 5- GATGAGTCCTGAGTAAXXX (see Note 8) where X stands for selective nucleotides (they vary after case). The working concentration of the MseI selective primer is 5 μM. Store as stock solution (100 μM) for several years and as working solution (5 μM) for several months.
2.5. Separation and visualization of fragments
Sephadex G-50 Fine or Superfine (Sigma Aldrich). Weigh 10 g of powder and mix with 120 ml ddH2O and 100 μl 100x TE buffer. Let it stand for a couple of hours. Store at room temperature and use within one week. The solution of Sephadex settles out, you must resuspend it before using.
Multi Screen HV plates (Millipore). Store at room temperature.
GeneScan ROX (Life Technologies) or another fluorescently-labelled, internal ladder suitable for sequencers. Store at 4 °C.
Hi-Di formamide (Life Technologies).
Polymer and buffers, specific for the type of sequencer used. Usually stored at 4 °C.
3. Methods
Carry out all steps on ice, unless otherwise specified. Allow frozen chemicals (except enzymes) to fully thaw and vortex the vials briefly before using.
3.1. DNA template preparation
The AFLP procedure (1) requires c. 500 ng of genomic DNA (see Note 9) in 5.5 μl volume as starting material. Therefore, proceed to quantify DNA. To obtain the desired concentration the DNA extract can be concentrated in a vacuum oven at 60 °C or diluted using 1x TE buffer. This entire step can be performed at room temperature.
3.2. RL
Calculate the appropriate amount of each chemical for the required number of reactions according to Table 1. For the three enzymes calculate the required amount according to their concentration (see Note 10). Do not forget to account also for replicates, blanks (see Note 2) and two more samples as a tolerance for potential pipeting inaccuracies.
Heat the required amount of MseI and EcoRI adaptor pairs at 95 °C for 5 min, each pair in a separate vial. Allow them to cool gradually to room temperature for c. 10 min. Spin briefly in a microcentrifuge for 10 s.
Prepare a master mix for all samples that you plan to analyse in one batch, starting with ddH2O, T4 ligase buffer, NaCl, BSA, both adaptor pairs and finishing with the three enzymes (see Note 11). Spin briefly.
Aliquot 5.5 μl of the master mix in individual tubes.
For each sample, add 5.5 μl DNA (containing 500 ng – see 3.1) in one tube. The final reaction volume will be 11 μl. Vortex and centrifuge briefly.
Incubate the reactions at 37 °C for at least 3 h in a thermal cycler with heated cover. Continue to incubate at 17 °C overnight, or at least for 3 h (17 °C is the optimum temperature for ligation activity). In case of PCR machines without heated lid the reaction mix has to be covered with a drop of mineral oil; evaporation leads to EcoRI Star Activity (non site-specific cutting; see Note 10).
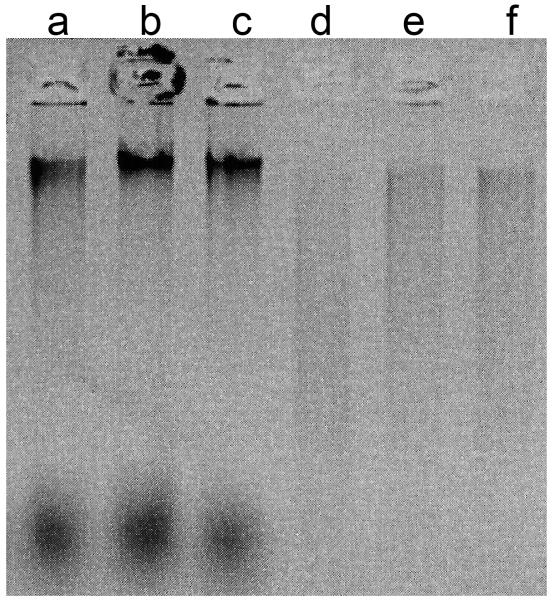
You can test the efficiency of the restriction reaction (see Notes 12-13) by running 5 μl of several of the reactions (mixed with loading buffer) on a 1.5% agarose gel in 1x TBE buffer (see Note 14) for 20 min at 90 V. When preparing the agarose gel add ethidium bromide (attention! carcinogenic) or GelRed to view the RL product under UV lamp (Fig. 1d-f).
Stop the reaction by diluting it 20-fold with 1x TE 0.1 M buffer. Mix thoroughly. For the samples for which an aliquot of the PCR product has been run on agarose gel (in step 7), do not forget to reduce the dilution volume.
Store the diluted RL reactions in the fridge for one day. The RL reactions can be stored for months at −20 °C.
Table 1.
Chemicals necessary for RL.
| Chemicals | μl per sample | Comments |
|---|---|---|
| 10x T4Ligase Buff. | 1.1 | vortex before use |
| 0.5 M NaCl | 1.1 | |
| 1 mg/ml BSA | 0.55 | freshly diluted |
| MseI adaptor pair (50 μM) | 1 | already annealed |
| EcoRI adaptor pair (5 μM) | 1 | already annealed |
| MseI restriction endonuclease | … (1 unit) | |
| EcoRI restriction endonuclease | … (5 units) | |
| T4 DNA ligase | … (0.6 unit) | |
| ddH2O (to make the total volume of 5.5 μl per sample) |
… … |
Figure 1.
Examples of agarose gel images for DNA extractions (a-c) and successful RL (d-f). See also Notes 12-13.
3.3. Preselective PCR amplification
Calculate the appropriate amount of each chemical for the required number of reactions according to Table 2. Carry over all replicates and blanks. Do not forget to account also for two more samples as a tolerance for potential pipeting inaccuracies.
Starting from the stock solutions (100 μM), dilute and mix preselective primers in proportion of 1:1:18 with ddH2O to result in a working concentration of 5 μM each primer.
Prepare a master mix for all samples that you plan to analyse in one batch, starting with ddH2O, 10x Taq-Buffer, dNTPs, primers and finishing with the Taq (see Note 11). Spin briefly.
Aliquot 8 μl of the master mix in individual tubes.
Add 2 μl of the diluted RL product to each tube. The final reaction volume will be 10 μl. Vortex and centrifuge (2,000 rpm) briefly.
Use a thermal cycler with heated cover and run the following program: one hold of 72 °C for 2 min, 20 cycles of (94 °C for 1 sec, 56 °C for 30 sec and 72 °C for 2 min), and finish with a hold of 60 °C for 30 min. Program the cycler to keep the reactions at 4 °C until you remove them.
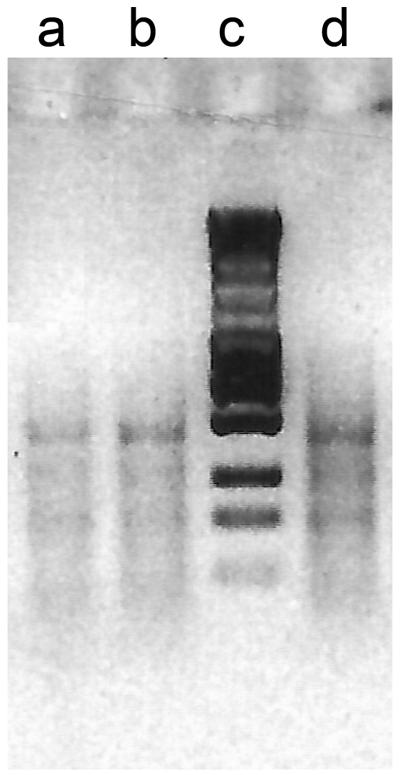
You can test the efficiency of the preselective amplification by running 5 μl of several of the reactions on a 1.5% agarose gel in 1x TBE buffer (see Note 14) for 20 min at 90 V. If using the Red Taq polymerase no loading buffer has to be used. When preparing the agarose gel add ethidium bromide (attention! carcinogenic) to view the RL product under UV lamp (Fig. 2). A smear product with few brighter bands in the 100-1500 base pair range should be visible.
Diluting the preselective reactions 20-fold with 1x TE 0.1 M buffer. Mix thoroughly. For the samples for which an aliquot of the PCR product has been run on agarose gel (in step 7), do not forget to reduce the dilution volume.
Store the diluted preselective reactions in the fridge for one day and at −20 °C for months.
Table 2.
Chemicals necessary for the preselective and selective amplification.
| Chemicals | μl per preselective sample | μl per selective sample |
|---|---|---|
| ddH2O | 5.86 | 5.50 |
| 10x Red Taq Buffer | 1.14 | 1 |
| Primers | 0.58 of the mixed primer pair | 0.54 MseI primer (5 μM) 0.54 EcoRI primer (1 μM) |
| dNTPs (10 mM) | 0.22 | 0.22 |
| Red Taq (1U/ μl) | 0.2 | 0.2 |
Figure 2.
Examples of successful preselective reactions (a-b, d). (c) ladder.
3.4. Selective PCR amplification
-
1.
Calculate the appropriate amount of each chemical for the required number of reactions according to Table 2. Carry over all replicates and blanks. Do not forget to account also for two more samples as a tolerance for potential pipeting inaccuracies.
-
3.
Prepare a master mix for all samples that you plan to analyse in one batch, starting with ddH2O, 10x Taq-Buffer, dNTPs, primers and finishing with the Taq (see Note 11). Spin briefly. If you are doing several primer combinations, the master mix for each combination must be prepared in separate vials.
4. Aliquot 8 μl of the master mix in individual tubes. If you are doing several primer combinations, aliquot each combination in separate tubes.
-
5.
Add 2 μl of the diluted preselective product to each tube. The final reaction volume will be 10 μl. Vortex and centrifuge (2,000 rpm) briefly.
-
6.
Use a thermal cycler with heated cover and run the following program (90% ramp time): one hold of 94 °C for 2 min, 9 cycles of (94 °C for 1 sec, 65 °C – 1 °C every cycle for 30 sec and 72 °C for 2 min), followed by 23 cycles of (94 °C for 1 sec, 56 °C for 30 sec and 72 °C for 2 min) and finish with a hold of 60 °C for 30 min. Program the cycler to keep the reactions at 4 °C until you remove them.
-
7.
We recommend freezing the selective reactions as soon as possible. They can, however, be kept for one day in the fridge. If necessary, selective reactions can be stored for several months at −20 °C, but we recommend running them as soon as possible in the sequencer.
3.5. Separation and visualization of fragments
Apply 200 μl of mixed Sephadex solution to each well of a Multi Screen (MS) plate. Place the MS plate on top of a microtiter plate to collect water. Pack the Sephadex by spinning at 600 g for 1 min. Discard water that has been collected in the microtiter plate.
Repeat step 1.
Repeat once again step 1, but this time pack the Sephadex by spinning at 600 g for 5 min.
Place MS plate with the Sephadex filter on top of a clean microtiter plate to collect the filtered selective product (see Note 15).
Mix together the selective reactions of up to three primer combinations corresponding to one individual sample, by applying 5 μl each selective PCR-product (e.g., labelled green, yellow and blue). Spin the MS plate (on top of the clean microtiter plate) at 600 g for 5 min (see Note 16).
Discard the Sephadex filter. Wash the HV plate with ddH2O and let it dry at room temperature. The HV plate can be re-used for up to 10 times.
Make up the loading mixture for the number of samples to be loaded on the sequencer using 9.8 μl Hi-Di formamide and 0.2 μl of GeneScan ROX per sample. Do not forget to account also for two more samples as a tolerance for potential pipeting inaccuracies.
Aliquot 10 μl of loading mixture to each well of a clean microtiter plate.
Add 1.2 μl of the filtered, combined selective products to each well. Vortex and centrifuge briefly.
Cover the microtiter plate containing loading mixture and sample; heat it up at 95 °C for 5 min. Cool the plate on ice immediately to denature the AFLP fragments.
Load the plate containing the denatured samples onto the sequencer.
Acknowledgement
O.P. was financed by an Austrian Science Fund (FWF) project (P222260-B16).
Footnotes
The AFLP technique requires good quality DNA; degraded DNA will produce non-comparable profiles between individuals. The best is to use silica gel dried material.
In standardized conditions, AFLP profiles usually display high levels of reproducibility. However, reproducibility has to be always tested for each batch of reactions by using replicates. It has been recommended to replicate c. 20% of the samples (11). They will then be used to calculate the error rate as the number of errors divided by the number of phenotypic comparisons within replicated samples. Acceptable error rates for AFLP are lower than 5% (11). In addition to replicates, always use negative controls to test for possible systematic contamination. This is very important as any DNA present in the reaction will produce fragments, which cannot be distinguished from the targeted products. In most cases negative controls will produce some peaks in the low base pair range due to the amplification of excess preselective primers in the selective amplification (1).
We recommend choosing six individuals that may be representative for the samples to be analyzed (e.g., select individuals from different regions, or different taxa). Look for primer combinations that produce an appropriate number of fragments (c. 100), that are evenly distributed across the range to be analyzed (50-500 bp). By combining different restriction enzymes (e.g., by using frequently cutting enzymes only) and using primers with two to four selective nucleotides, the number of AFLP fragments per profile can be optimized. See also Note 8.
Take measures to standardize each of the AFLP steps. Run all samples in a minimum amount of time. Use as much as possible the same PCR machine (or at least the same model) for all samples investigated for each primer pair. This will contribute towards keeping the error rate to a minimum (see Note 1).
Always use the same internal size standard for each sample run on the sequencer. Make sure to perform the sizing of the AFLP fragments based on the same fragments from the internal size standard.
Extract total RNA (e.g., with SV Total RNA Isolation System, Promega) from tissue material fixed in RNAlater (Sigma) and stored at −20 °C. Then, synthesize cDNA from mRNA (e.g., with SuperScriptTM Double-Stranded cDNA Synthesis Kit, Invitrogen), using an oligo (dT)12-18 primer. Apply the standard AFLP protocol further to obtain cDNA-AFLP fingerprints. As cDNA comprises much less DNA than the entire genome, use selective primers with only two selective nucleotides (or one primer with two selective nucleotides and one with three).
The MSAP technique uses two methylation-sensitive isoschizomers (e.g., MspI and HpaII) as frequent cutters, each in combination with the same rare cutter (e.g., EcoRI) in parallel reactions (7). The two isoschizomers recognize the same sequence (5′-CCGG) but differ in their sensitivity to DNA methylation. Comparison of the two profiles for each individual allows assessment of the methylation state of the restriction sites. MeCpG sites are recognized by MspI only, whereas plant-specific hemiMeCpCpG sites are recognized by HpaII only (9-10). Sites that are hypermethylated (i.e., both at the internal and external Cs), and sites that are fully methylated at the external Cs (i.e., on both strands) are not cut by either enzyme, whereas sites that are free from methylation are recognized by both. Normally the MSAP protocol uses an excess of restriction enzymes (10 U per sample of each restriction enzyme – use highly concentrated enzymes) in the RL, which uses also longer times (overnight).
According to the genome size and the abundance of restriction sites in the genomes to be analyzed you may need to increase or decrease the number of selective nucleotides of the primers. For a small genome use shorter selective primers, with only two selective nucleotides. For a large genome use longer preselective (with two selective nucleotides) and selective (with four selective nucleotides) primers. The tuning of the number of AFLP fragments can be also obtained by using a different combination of restriction enzymes (e.g., two “rare” cutters or two “frequent” cutters).
Genomic DNA should be extracted from similar amounts of tissue (c. 10 mg) to ensure a comparable concentration of DNA extractions across all samples.
Non-standard conditions that can induce Star Activity of EcoRI include high glycerol concentration, an excessive amount of enzyme used, low salt concentration, high pH (pH > 8) and contamination with organic solvents (see http://www.neb.com/nebecomm/products/faqproductR0101.asp#1). To prevent Star Activity avoid evaporation, use highly concentrated enzymes (to reduce the amount of glycerol introduced in the reaction) and use as little amounts of enzyme as possible to ensure complete digestion.
Always start preparing Master Mixes with the largest volumes (ddH20 and/or buffers) and add any enzymes the last.
A smear product in the 100-1500 base pair range should be visible. Make sure the genomic DNA is fully restricted, so no high-weight DNA molecules are present (Fig. 1d-f).
The efficiency of the ligation step cannot be verified on the gel.
Prepare the agarose gels by boiling for 2 min 0.75 g agarose in 50 ml TBE buffer. Let the solution to cool to 70 °C and add 1 μl ethidium bromide (attention! Highly carcinogenic).
Sephadex purification of the AFLP products leads to a reduction of primer-dimers and a better resolution in the range 50-500 bp. In the protocol presented the purification step is necessary to remove from the selective products the inert red dye that has been introduced with the Red Taq polymerase. Other purification protocols can be also used.
Packing of the Sephadex filter and filtering of the PCR products must be performed at the same rotation speed.
6. References
- 1.Vos P, Hogers R, Bleeker M, et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachem CWB, van der Hoeven RS, de Bruijn SM, et al. Visualization of differential gene expression using a novel method of RNA fingerprinting based on AFLP: analysis of gene expression during potato tuber development. The Plant Journal. 1996;9:745–753. doi: 10.1046/j.1365-313x.1996.9050745.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn E. From library screening to microarray technology: strategies to determine gene expression profiles and to identify differentially regulated genes in plants. Ann. Bot.-London. 2001;87:139–155. doi: 10.1006/anbo.2000.1314. [DOI] [PubMed] [Google Scholar]
- 4.Donson J, Fang Y, Espiritu-Santo G, et al. Comprehensive gene expression analysis by transcript profiling. Plant Molecular Biology. 2002;48:75–97. [PubMed] [Google Scholar]
- 5.Breyne P, Dreesen R, Cannoot B, et al. Quantitative cDNA-AFLP analysis for genome-wide expression studies. Molecular Genetics and Genomics. 2003;269:173–179. doi: 10.1007/s00438-003-0830-6. [DOI] [PubMed] [Google Scholar]
- 6.Paun O, Fay MF, Soltis DE, Chase MW. Genetic and epigenetic alterations after hybridization and genome doubling. Taxon. 2007;56:649–656. [PMC free article] [PubMed] [Google Scholar]
- 7.Baurens F-C, Bonnot F, Bienvenu D, et al. Using SD-AFLP and MSAP to assess CCGG methylation in the banana genome. Plant Molecular Biology Reporter. 2003;21:339–348. [Google Scholar]
- 8.Ainouche ML, Baumel A, Salmon A, Yannic G. Hybridization, polyploidy and speciation in Spartina (Poaceae) New Phytologist. 2003;161:165–172. [Google Scholar]
- 9.Salmon A, Ainouche ML, Wendel JF. Genetic and epigenetic consequences of recent hybridization and polyploidy in Spartina (Poaceae) Molecular Ecology. 2005;14:1163–1175. doi: 10.1111/j.1365-294X.2005.02488.x. [DOI] [PubMed] [Google Scholar]
- 10.Paun O, Bateman RM, Fay MF, et al. Stable epigenetic effects impact adaptation in allopolyploid orchids (Dactylorhiza: Orchidaceae) Molecular Biology and Evolution. 2010;27:2465–2473. doi: 10.1093/molbev/msq150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonin A, Bellemain E, Bronken Eidesen P, et al. How to track and assess genotyping errors in population genetic studies. Molecular Ecology. 2004;13:3261–3273. doi: 10.1111/j.1365-294X.2004.02346.x. [DOI] [PubMed] [Google Scholar]