Abstract
Rats can discriminate simple shapes visually, even if they are moved around, made smaller, or partially covered up; the strategy they use may help shed light on human brain mechanisms for discriminating complex features, such as faces.
Look at any two people, places or objects, and you can almost immediately tell if they appear the same or different from one another. Indeed, the remarkable speed with which we can discern things based on their appearance suggests that our brains evolved specific circuitries for realizing ‘same versus different’ by vision. Despite the significance of object discrimination for day-to-day survival, the neural circuits and mechanisms that enable us to distinguish even the most basic shapes still remain unclear. A new study reported in this issue of Current Biology from Vermaercke and Op de Beeck [1] provides exciting new insights into how the brain might distinguish visual shapes under both clear and uncertain conditions.
Rodents as Emerging Models for Studying Visual Perception
What tools are available to probe the mechanisms underlying shape discrimination? Vision has long been studied using psychophysics and neuronal activity recordings, mainly in monkeys, cats and humans. That approach continues to provide insights into the structures, cell types and receptive field properties in the visual pathway [2], and the neural correlates of certain aspects of visual perception, such as directional motion [3]. In recent years, however, new genetic tools have raised the opportunity not just to record from, but also to label and control the activity of highly defined sets of neurons [4,5]. Because these genetic tools are most easily applied to rodents, the field of visual neuroscience is now rapidly expanding to include rats and mice as staple models for probing the circuits underlying perception and decision-making [6].
There are obvious barriers to studying vision in rodents, not the least of which is that rodents view the world at much lower resolution than do primates [6,7]. Nonetheless, recent experiments have shown that rodent visual circuits bear many similarities to those of larger species [6,8–10]. Indeed, many neurons in the primary visual cortex of mice (V1) have receptive fields that are as highly tuned as those found in primate V1;mice just need to see larger stimuli in order to activate those neurons [10]. Are the boundaries on studying visual perception in rodents therefore simply a matter of scale? Or could it be that rodents are fundamentally limited in terms of their ability to carry out complex ‘higher order’ tasks? Moreover, given that the ultimate goal is to understand how humans make sense of the visual world, it is crucial to determine not just if rodents can perform complex visual discriminations, but the strategies they use.
A High-Throughput Paradigm for Probing Shape Recognition
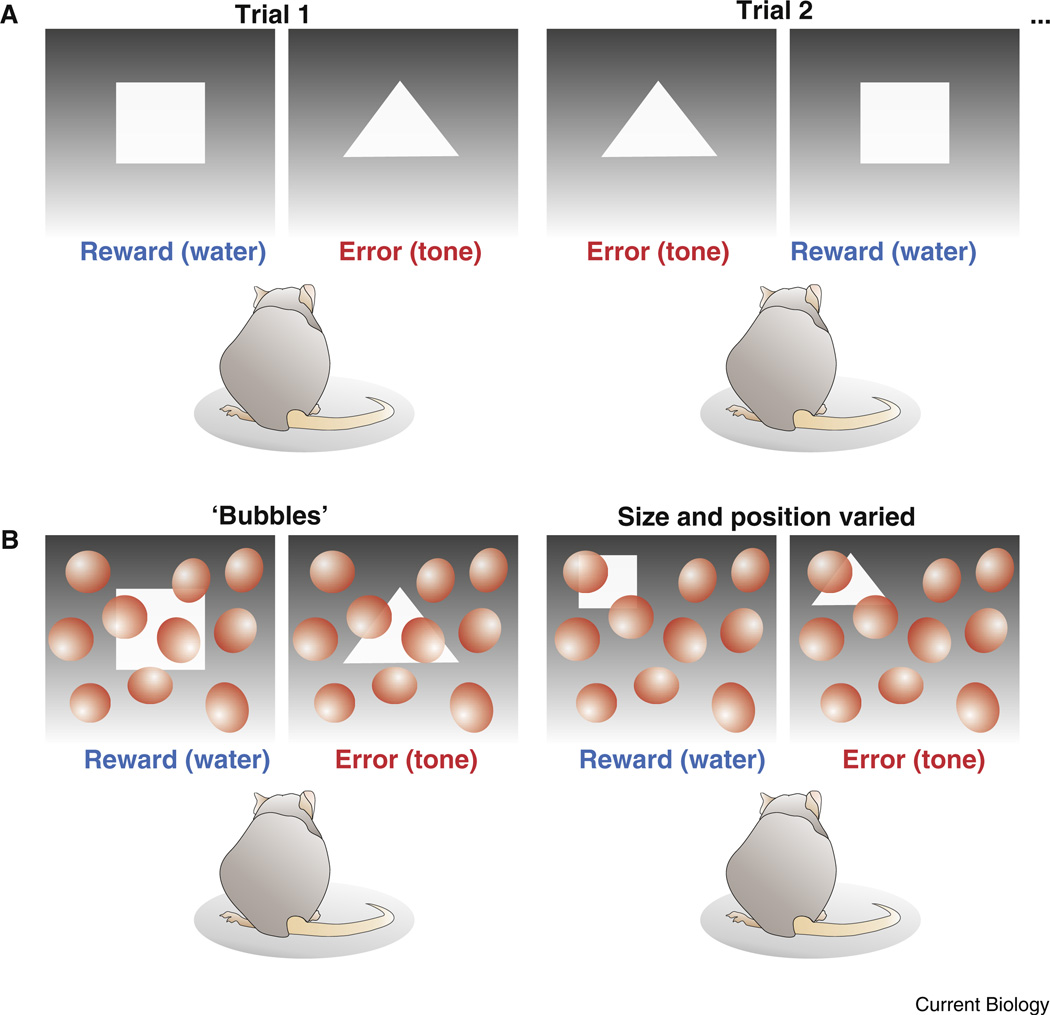
In their paper in this issue, Vermaercke and Op de Beeck [1] report a paradigm for testing shape recognition in rodents. A water-deprived rat was placed into a chamber viewing two screens: one displaying a triangle, and the other displaying a square. In order to receive a water reward, the rat was required to touch a sensor near the screen displaying the square. Rats quickly learned this task, which is somewhat similar to tasks used in previous studies [11]. A key strength of this paradigm is that, because of their highly motivated state (the rats were thirsty) and the short time required to access reward (the chamber was small), the rats performed thousands of trials over a relatively short period of time. That provided a high-throughput, quantifiable platform for probing the strategies underlying shape discrimination.
‘Bubbles’ Reveal Where and How Rats Discern Shapes
Once they established that rats could perform the discrimination task, Vermaercke and Op de Beeck [1] explored which features of the visual stimuli the rats used to distinguish triangles from squares. Round masks called ‘bubbles’ were introduced to various portions of the visual stimuli such that, on any given trial, parts of both the square and triangle were occluded from view (Figure 1). After the experiment, the authors analyzed the statistics of the images that led to errors (trials when the rat went to the screen displaying the triangle) versus the statistics of the images that led to reward (trials when the rat went to the screen displaying the square). By ‘reverse correlating’ the image statistics with trial performance in this way, the authors were able to determine which portions of the visual stimuli were most relevant for shape discrimination.
Figure 1. Shape recognition testing in rodents.
(A) Top panels display the basic stimuli and experimental design whereby a rat has to visually discern a triangle from a square in order to receive a water reward. The reward source is coupled to the location of the screen displaying the square, which varies randomly from trial to trial. (B) Lower panels illustrate the ‘bubbles’ paradigm in which the test shapes are partially occluded and in some cases are also moved and/or made smaller. In the actual experiment, achromatic Gaussian blobs, rather than red balloons, were used as ‘bubbles’. See [1] for details.
Vermaercke and Op de Beeck [1] found that rats relied more heavily on the information in the lower portion of the display. As the authors aptly note, this strategy deviates from the ‘ideal observer’ scenario [12] — a psychophysical metric based on the theoretically optimal strategy of evaluating every pixel in the visual scene. Thus, rats use only a portion of the visual information available to them in order to discriminate shapes.
Rats Distinguish Shapes Irrespective of Position
One could imagine that rats do not really compare visual shapes per se, but rather that they adopt a simple strategy based on local differences in screen brightness, such as “the square is displayed on the screen that is brighter at the bottom”. Vermaercke and Op de Beeck [1] tested this idea by analyzing the set of trials when bubbles occluded the lower parts of the image. Interestingly, the authors found that, when the lower field was covered, the rats adapted their strategy to compare the upper visual field instead. Thus, rats are not cemented to a fixed spatial regime for discriminating shapes. They may indeed have a simple, reflexive strategy that relies on lower field analysis, but as Vermaercke and Op de Beeck [1] show, rats can adopt new visual search and shape comparison strategies if that is what is required to satisfy their thirst.
Scale-Invariant Shape Discrimination
Can rats learn the difference between a square and a triangle even if those shapes are made smaller or moved to different locations within the display? In a second set of experiments (called ‘Phase II’), the rats were tested for their ability to recognize shapes that were reduced to half of their initial size, and randomly placed within each display. As Vermaercke and Op de Beeck [1] point out, this required the rats to shift from a simple strategy of comparing matched locations in the two screens to a ‘higher-order’ strategy involving local comparisons of pixel content. In other words, the rats had to decide whether a given dark or light portion of the screen belonged to the shape or not. The rats’ performance initially dropped under these conditions but, with time, they learned to identify the triangle and the square nested among the bubbles, and discriminate them from one another. This is particularly intriguing because position and size invariance are visual challenges that humans confront every day as they search for physical objects in space.
Rats versus Humans
Another unique strength of the Vermaercke and Op de Beeck [1] study is that the authors directly compared rat versus human psychophysical performance. After adjusting the task to account for differences in spatial resolution, and so forth, human subjects were asked to distinguish squares and triangles masked behind bubbles. Some interesting differences quickly became apparent. First, humans never showed a bias towards particular portions of the display. Second, humans scanned and compared most of the visible area of the square and the triangle, indicating they act closer to the ideal observer model. Third, whereas none of the rats ever achieved >80% correct trials, humans achieved near perfect performance. The authors point out, however, that in a water-escape paradigm [13] rats too can exhibit near perfect discriminations, indicating that motivational state must be taken into account when comparing the performance thresholds of rodents and humans.
Implications for Understanding Visual Circuits
The new findings described by Vermaercke and Opde Beeck [1] extend on those from previous studies [11,13–16] by exploring complex aspects of shape discrimination, its context dependence, and the underlying strategies that rats follow. Their work also provides an important step forward toward the goal of linking specific cell types and circuits with higher order visual perceptions. One can now imagine combining the psychophysics paradigm described here with a head-fixed or mobile imaging protocol [16–18], to directly monitor and control [4,5] the activity of the brain circuits hypothesized to mediate shape recognition. These are truly exciting times for studying visual perception in rodents. As Vermaerke and Op de Beeck [1] rigorously show, rodents not only see, they can also perform discrimination tasks that parallel the visual challenges humans face every day. The general neural circuit mechanisms of shape perception are therefore within reach.
References
- 1.Vermaercke B, Op de Beeck H. A multivariate approach reveals the behavioral templates underlying visual discrimination in rats. Curr. Biol. 2012;22:50–55. doi: 10.1016/j.cub.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 2.Werner JS, Chalupa LM. The Visual Neurosciences. Cambridge: MIT Press; 2004. [Google Scholar]
- 3.Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- 4.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huberman AD, Niell CM. What can mice tell us about how vision works? Trends Neurosci. 2011;34:464–473. doi: 10.1016/j.tins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 8.Krahe TE, El-Danaf RN, Dilger EK, Henderson SH, Guido W. Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. J. Neurosci. 2011;31:17437–17448. doi: 10.1523/JNEUROSCI.4370-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao E, DeAngelis GC, Burkhalter A. Parallel input channels to mouse primary visual cortex. J. Neurosci. 2010;30:5912–5926. doi: 10.1523/JNEUROSCI.6456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J. Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoccolan D, Oertelt N, DiCarlo JJ, Cox DD. A rodent model for the study of invariant visual object recognition. Proc. Natl. Acad. Sci. USA. 2009;106:8748–8753. doi: 10.1073/pnas.0811583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisler WS. Contributions of ideal observer theory to vision research. Vision Res. 2011;51:771–781. doi: 10.1016/j.visres.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas RM, Neve A, Quittenbaum JP, Alam NM, Prusky GT. Perception of visual motion coherence by rats and mice. Vision Res. 2006;46:2842–2847. doi: 10.1016/j.visres.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Meier P, Reinagel P. Rat performance on visual detection task modeled with divisive normalization and adaptive decision thresholds. J. Vis. 2011;11 doi: 10.1167/11.9.1. 10.1167/11.9.1. [DOI] [PubMed] [Google Scholar]
- 15.Busse L, Ayaz A, Dhruv NT, Katzner S, Saleem AB, Scholvinck ML, Zaharia AD, Carandini M. The detection of visual contrast in the behaving mouse. J. Neurosci. 2011;31:11351–11361. doi: 10.1523/JNEUROSCI.6689-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andermann ML, Kerlin AM, Reid RC. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front. Cell Neurosci. 2010;4 doi: 10.3389/fncel.2010.00003. 10.3389/fncel.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rochefort NL, Narushima M, Grienberger C, Marandi N, Hill DN, Konnerth A. Development of direction selectivity in mouse cortical neurons. Neuron. 2011;71:425–432. doi: 10.1016/j.neuron.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]