Abstract
We reported previously that both subtypes of estrogen receptors, ERα and ERβ, are expressed by human urothelial cells and mediate estrogen-induced cell proliferation in these cells. The aim of this study was to determine the extent to which each ER subtype contributes to urothelial cell proliferation and their possible involvement in the regulation of the cell cycle. We compared the expression of ERα and ERβ mRNAs and protein quantitatively in primarily cultured human bladder urothelial cells obtained from six individuals with three immortalized urothelial (E6, E7, and UROtsa) and two bladder cancer cell lines (HTB-9 and T24). We found that all these cells express similar levels of ERβ, but immortalized and cancer cells express much higher amounts of ERα than primary cells. Higher levels of ERα mRNA were also observed in the biopsies of bladder transitional cell carcinoma compared with sample from the same bladder unaffected by tumor. Using the ERα-selective agonist PPT, the ERβ-selective agonist DPN, and specific small interfering RNA against ERα or ERβ, we found that ERβ predominantly mediates estrogen-induced G1/S transition and cell proliferation in the primary urothelial cells. By contrast, ERα predominantly mediates estrogen-induced G1/S transition and cell proliferation in bladder cancer cell lines. Furthermore, we found that 17β-estradiol (E2) rapidly induces phosphorylation of extracellular signal-regulated kinases, but U0126, a mitogen-activated protein kinase kinase (MEK) inhibitor, does not affect E2-induced urothelial cell proliferation. E2 up-regulated cyclin D1 and cyclin E expression in both the primary and bladder cancer cells, and the cancer cells have higher cyclin D1 and cyclin E expression during G0/G1 phases. Our data suggest that estrogen exerts its effects through different ER subtypes in urothelial cells. Increased expression of ERα may contribute to early induction of cyclin D1 and cyclin E during the cell cycle in bladder cancer cells.
Introduction
Estrogen hormones, particularly 17β-estradiol (E2), are potent regulators of cell growth in a wide array of tissues. These effects are mediated primarily through binding to the two major estrogen receptors (ER) subtypes, ERα and ERβ, that are thought to be ligand-activated transcription factors. Ligand binding induces a conformational change within the ER that may stimulate high-affinity binding to specific estrogen response elements or regulate transcription at promoter elements by directly binding heterologous transcription factors through protein–protein interactions (Sabbah et al. 1999, Kushner et al. 2000). Recently, extensive studies have shown that estrogen may induce rapid non-genomic effects mediated by ERs or other unrelated estrogen-binding sites residing in or near the cell plasma membrane through activation of signaling pathways such as mitogen-activated protein kinase (MAPK) or interaction with diverse growth factors (Manavathi & Kumar 2006, Song & Santen 2006).
ERα and ERβ are encoded by unique genes located on different chromosomes and exhibit overlapping tissue distribution and function. Under normal conditions, ERα predominates in the anterior pituitary, mammary glands, uterus, vagina, cervix, and liver (Koike et al. 1987), whereas ERβ is primarily detected in the ovary, thyroid, skin, spleen, lungs, hypothalamus, gastrointestinal tract, thymus, and urogenital tract (Mosselman et al. 1996). ERα and ERβ display a high degree of conservation in the central DNA-binding domain and the carboxyl-terminal ligand-binding domain, with 97 and 60% amino acid homologies respectively, but have considerable divergence in the amino-terminal (18% homology; Enmark et al. 1997). Hence, ERα and ERβ exhibit a similar, but not identical, binding affinity profile for different ligands. Recently, several compounds with relatively high selectivity for each ER subtypes have been produced (Stauffer et al. 2000, Meyers et al. 2001).
Epidemiological evidence and animal studies indicate that estrogen plays an important role in cell proliferation and survival in tumor-prone tissues responsive to hormones, such as the uterus and mammary glands. These tissues usually have overall higher levels of ERα than ERβ. Studies have shown that ERα expression in many breast cancer cell lines is up-regulated, and estrogen-induced cell proliferation is mediated by ERα in these cells. Meanwhile, ERβ has been shown to be down-regulated, and overexpression of ERβ in these cells reduced cell proliferation with a concomitant decrease in many regulatory components of the cell cycle (Strom et al. 2004, Helguero et al. 2005). Transient reduction of ERα using small interfering RNA (siRNA) significantly suppressed cell cycle progression and cell proliferation in breast cancer cells (Liu et al. 2006). Transfection of HeLa cells with ERα increased cyclin D1 expression, while ERβ overexpression decreased cyclin D1 (Liu et al. 2002). These observations have led to the controversial hypothesis that an important physiological role of ERβ is to inhibit ERα actions and the loss of ERβ contributes to the development of cancer (Helguero et al. 2005). Suggested mechanisms have focused on the less conserved amino-terminal A/B domains. Unlike ERα, ERβ only has a weak activation function (AF)-1 but contains a repressor domain that may inhibit the overall transcriptional activity of the receptor (Paech et al. 1997, Hall & McDonnell 1999).
Urothelium refers to the specialized epithelial cells that line the renal pelvis, ureters, bladder, and urethra. It is one of the slowest growing epithelia in mammals under normal conditions. However, when stimulated appropriately, urothelium can undergo tremendous growth, particularly during wound healing and carcinogenesis (Walker 1960, Cohen & Ellwein 1993). More than 90% of bladder cancers arise in the urothelium, and bladder cancer is the fifth most frequently diagnosed cancer (Jemal et al. 2006). Several lines of evidence have linked estrogen to bladder carcinogenesis. Although men are thrice more likely to develop bladder cancer than women, when data are corrected for the influence of smoking, women appear to face an even greater risk than men (Castelao et al. 2001). It is interesting to note that epidemiological studies of risk factors for bladder cancer in pet dogs (a species that doesn’t smoke) found one- to threefold higher risk in females (Kelsey et al. 1998). High levels of ERβ mRNA and protein are expressed in the epithelium of the whole urogenital tract of both males and females, but expression of ERα is much lower (Hess et al. 1997, Makela et al. 2000). However, high levels of ERα have been found in a large number of human bladder transitional cell carcinomas (Kaufmann et al. 1998). Neonatal estrogenization of male rats increased expression of ERα and induced urogenital carcinogenesis (Prins et al. 1998). The purposes of the current study were to compare expression of ERα and ERβ mRNAs and protein in normal human bladder urothelial cells with that in different immortalized urothelial and bladder tumor cell lines and test the hypothesis that the ratio of ERα to ERβ is lower in primary human urothelial than bladder cancer cells. Using ERα or ERβ selective agonists and siRNA selectively against ERα or ERβ, we also investigated their individual involvement in estrogen-induced cell cycle progression and cell proliferation.
Materials and methods
Cell culture and tissue specimens
All experimental protocols were reviewed and approved by the Health Sciences Human Subjects Committee of the University of Wisconsin. Normal human bladder urothelial cells (HBUC) were primarily cultured from discarded bladder mucosal layer obtained during surgeries performed at the University of Wisconsin Hospital. None of the bladders from which urothelial cells were obtained were affected by tumor or inflammation. Cells were plated on Petri dishes coated with type I rat tail collagen in Ham’s F-12+ nutrient mixtures containing 2% fetal bovine serum (FBS) and used between passages 4 and 6. Ureteral urothelial cells immortalized with viral constructs containing human papillomavirus E6 or E7 were also maintained in F-12+ medium and cells passaged <35 times were used. HBUC immortalized with viral construct containing the SV40 large T antigen (UROtsa) were kindly provided by Dr Petia Simeonova (National Institute for Occupational Safety and Health, Morgantown, WV, USA) and maintained in Dulbecco’s Modified Eagle’s medium (DMEM) containing 5% FBS. Bladder tumor cell lines HTB-9 and T24 were purchased from The American Type Culture Collection (Manassas, VA, USA) and maintained in DMEM containing 5% FBS. All cultures were placed in a humidified incubator at 37 °C in 5% CO2. All medium and supplements were purchased from Invitrogen. Phenol red-free medium and charcoal-treated FBS were used in all experiments. Paraffin-imbedded samples of primary bladder carcinomas and matched samples of histologically normal tissues from the same bladder were obtained from six patients diagnosed with transitional cell carcinoma of the urinary bladder (grades 2–4) at the University of Wisconsin Hospital. Samples of total RNA were available from primary bladder carcinomas and matched samples of histologically normal tissues from the same bladders from ten patients diagnosed with transitional cell carcinoma of the urinary bladder (grades 2–4) at the University of Wisconsin Hospital. None of the patients had received systemic therapy. Bladder cancer tissue arrays with self-matching normal adjacent tissues were purchased from US Biomax (Rockville, MD, USA).
RNA interference
Sets of four siGENOME duplexes specifically designed against ERα or ERβ were synthesized by Dharmacon (Lafayette, CO, USA). The duplex from each set that had the most significant inhibitory effects on urothelial cell proliferation was used in the remainder of this study. For siERα, the sense sequence used was 5′-UAUCUGAACCGUGUGGGAGUU-3′. For siERβ, the sense sequence used was 5′-CAAGAAGAUU-CCCGGCUUUUU-3′. Transfection of siRNA was performed at a concentration of 100 nM using Oligofectamine (Invitrogen) when cells achieved 30–50% confluence. Control studies to evaluate the specificity of siRNAs were performed using scrambled non-targeting siCONTROL siRNA (siCon, Dharmacon) under identical conditions. To quantify cell transfection efficiency, a cytotoxic siTOX (Dharmacon) was co-transfected into cells, and singly dispersed cell viability was determined by trypan blue exclusive assay 24 h after transfection. siGLO RISC-Free fluorescent-labeled siRNA (Dharmacon) was also co-transfected into cells to serve as both a negative control and an efficiency marker. Nuclei were identified with Hoechst 33342 (Invitrogen) staining. More than 90% transfection efficiency was reproducibly achieved in all experiments.
Total RNA isolation and PCR analysis
Total RNA was isolated from cultured cells using QIAshredder and RNeasy Mini kit (Qiagen) or tissues using TRIzol reagent (Invitrogen) following the manufacturer’s protocol. The cDNA pool was synthesized from 0.5 to 1 μg RNA using the MMLV Reverse Transcriptase kit (Promega). Conventional PCR for ERα or ERβ gene was performed using the HotStarTaq kit (Qiagen) in a Peltier Thermal Cycler (MJ Research, Waltham, MA, USA). Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was determined as a reference gene. Primers for amplification were as follows. For ERα: forward –ACAGCATGAAGTGCAAGAACGTGG and reverse – TGGCTGTTCCCAACAGAAGACAGA; for ERβ: forward – TCGGCAGACCACAAGCCCAAA and reverse – CCACACTTCACCATTCCCAC; and for GAPDH: forward – TCATCATCTCTGCCCCCTCTG and reverse – TCATCATCTCTGCCCCCTCTG. Quantitative real-time PCR was performed using SYBR Green Master Mix in a 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Experiments were performed in duplicate for each data point, and the abundance of human ribosomal protein S26 mRNA was determined as a reference gene. Primers for amplification were as follows. For ERα: forward – AGATCTTCGACATGCTGCTGGCTA and reverse – AGATCTTCGACATGCTGCTGGCTA; for ERβ: forward – ACACCTGGGCACCTTTCTCC TTTA and reverse – TCTTGCTTCACACCAGG GACTCTT; and for S26: forward – ACACTAGGAA CGCATTTCCACCCT and reverse – GGCTTC AAGAACGGCAACTCACTT.
Protein extraction and immunoblotting
Cells were lysed in M-PER Mammalian Protein Extraction Reagent (Pierce, Rockford, IL, USA) containing protease inhibitors (Roche). Supernatant was collected by centrifugation at 10 000 g for 10 min at 4 °C, and the protein content was measured using the BCA Protein Assay kit (Pierce). Thirty microgram extracts were electrophoresed on SDS-PAGE gels and transferred to nitrocellulose membranes (Bio-Rad). Antibodies used were mouse anti-human cyclin D1 (1:1000, HD11, sc-246), mouse anti-human cyclin E (1:1000, HE12, sc-247), mouse anti-human proliferating cell nuclear antigen (PCNA; 1:5000, PC-10, sc-56), rabbit anti-human ERα (1:5000, MC-20, sc-542), rabbit anti-human ERβ (1:5000, H-150, sc-8974), mouse anti-human p-ERK (1:5000, E-4, sc-7383), rabbit anti-human ERK1 (1:5000, K-23, sc-94), and mouse anti-human α-tubulin (1:5000, B-7, sc-5286), all from Santa Cruz (Santa Cruz, CA, USA) and diluted in TBST (50 mM Tris, 150 mM NaCl, 0.1% Tween 20 (pH 7.5)) containing 2% BSA. The membranes were blocked with SuperBlock (Pierce) for 2 h at room temperature and incubated with primary antibodies overnight at 4 °C. They were then incubated at room temperature for 2 h in 1:10 000 alkaline phosphatase-conjugated or horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies (Santa Cruz) in TBST. Chemiluminescent signal was revealed with the ECL or CDPstar kit (Amersham) and detected by exposure to X-ray film. Band intensity was quantified with ImageJ (NIH) and normalized to that of α-tubulin in the same sample. Experiments were repeated at least four times.
Immunohistochemical staining
Slides of formalin-fixed and paraffin-embedded transitional cell carcinomas of the urinary bladder tissue were subjected to steam heat for 25 min in 10 mM citrate buffer (pH 6.0) for antigen retrieval after deparaffinization and dehydration. The antibodies used were mouse anti-ERα (aa 120–170, 1:200; Chemicon, Temecula, CA, USA), rabbit anti-human ERβ (1:500, H-150; Santa Cruz), and normal rabbit serum (1:500, sc-2338; Santa Cruz) served as negative control and mouse breast tumor tissue served as positive control. Specific staining was developed using VECTASTAIN ABC and DAB kits (Vector, Burlingame, CA, USA). 1:200 Goat anti-rabbit antibody was used as secondary antibody and diaminobenzidine was used as a chromogen.
3H-Thymidine incorporation assay
Cells were aliquoted into 96-well tissue culture plates at a density of about 5000 cell/well and transiently transfected with 100 nM siRNAs. After serum starvation for 24 h, 1–100 nM E2, (Sigma), 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT; Tocris, Ellisville, MO, USA), or 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN; Tocris) was added to the wells in the absence or presence of 100 nM ICI 182 780 (Tocris, added 2 h prior to agonist treatments). Ten hours later, 0.5 μCi [methyl-3H] thymidine (Amersham) was added to each well. After 6 h, DNA was precipitated by 10% trichloroacetic acid (TCA) and washed with 70% ethanol. The acid-insoluble material was then dissolved with 0.5 M NaOH and collected on UniFilter-96 GF/C plates (Perkin–Elmer, Wellesley, MA, USA). Thirty microliters of Micro-Scint 20 were added into each well, and the radioactivity was counted with a TOPCount Microplate Scintillation Counter (Packard, Meriden, CT, USA). DPM (disintegrations per minute) was calculated by count per minute (CPM)/efficiency (~20–30%).
Flow cytometry assay and fluorescence-activated cell sorting (FACS)
Cells were harvested with trypsinization and stained in 20 μg/ml Hoechst 33342. Fluorescence intensity was measured with a Becton-Dickinson LSRII triple laser benchtop cytometer (BD PharMingen, San Diego, CA, USA) to estimate the proportion of cells in different phases of the cell cycle. Data were analyzed with BD Cell Quest software. Live cells were also sorted into G0/G1, S, and G2/M phase cells according to DNA content using a BD FACSCalibur cytometer, lysed in M-PER (Pierce), and expression of cyclins was assessed by immunoblotting.
Statistical analysis
All results are expressed as means±S.E.M. and accompanied by the number of observations. Data were obtained from groups containing four to six replicates. Paired Student’s t-test was used to compare self-matched samples. Comparisons among treatments over time or within groups and between multiple groups were performed by one-way or two-way ANOVA using SAS/STAT (SAS Institute Inc., Cary, NC, USA). Fisher’s LSD and Tukey’s multiple tests were used to determine post hoc significance. P<0.05 was considered statistically significant.
Results
ERα and ERβ are differentially expressed in primary urothelial, immortalized urothelial, and bladder tumor cells
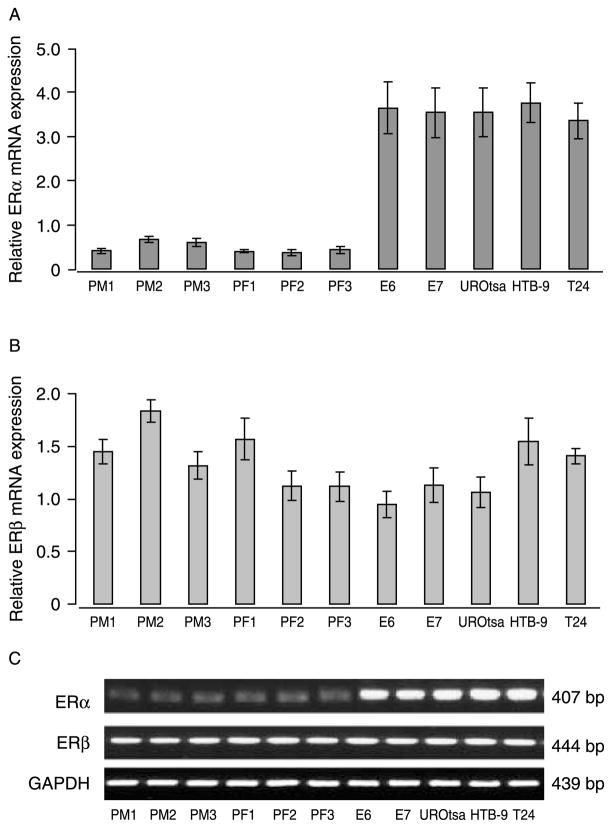
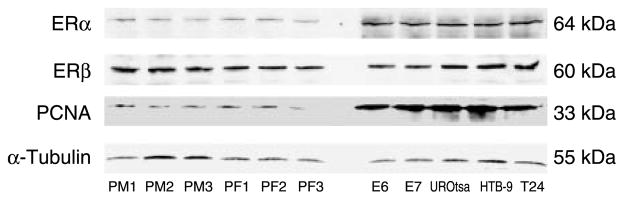
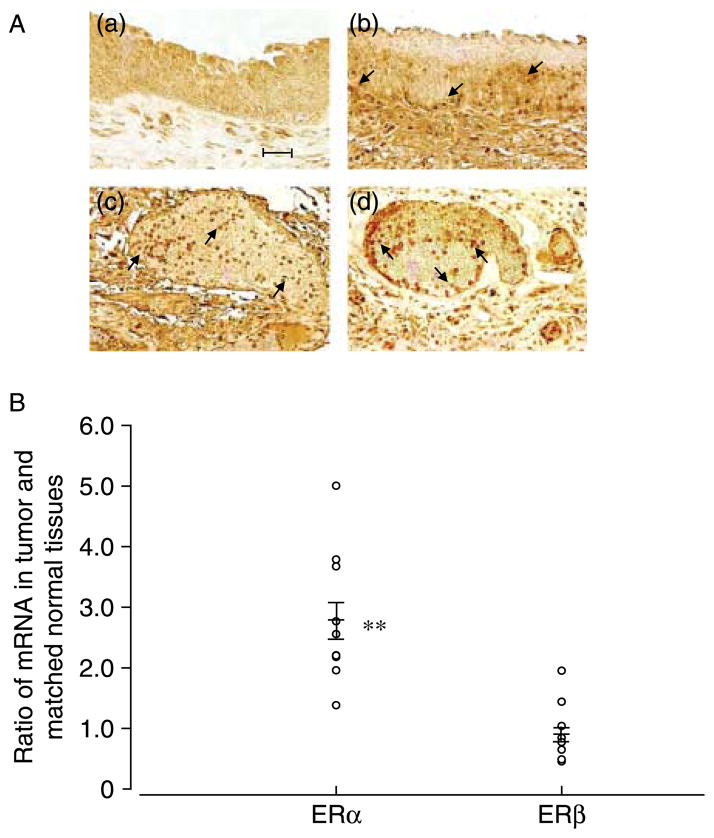
In general, we found that primary urothelial cells grow more slowly than immortalized urothelial or bladder tumor cells. The typical population-doubling time of early passages of HBUC is about 3–5 days, whereas the population-doubling time of immortalized or tumor cells is <1 day (Table 1). To investigate whether there are differences in ER expression among these cell types, we examined ERα and ERβ mRNA transcripts in total RNA preparations from three female donors (PF1–3), three male donors (PM1–3), as well as E6, E7, and UROtsa immortalized cells and bladder tumor cell lines originally from a male patient (HTB-9) or a female patient (T24) by quantitative real-time PCR. ERα mRNA expression was significantly higher (by six- to seven-fold) in immortalized urothelial or bladder tumor cells than in primary cells (Fig. 1A). By contrast, all cells expressed similar levels of ERβ mRNA (Fig. 1B). These observations were consistent with findings using conventional PCR (Fig. 1C). Immunoblotting for ERα and ERβ proteins in whole-cell lysates of these cells demonstrated a similar pattern of ER expression as seen in mRNA levels (Fig. 2). These data showed that ERα and ERβ are differentially expressed among primary, immortalized, and bladder cancer cells. Consistent with the observation that immortalized and cancer cells grow faster in culture, blots of PCNA showed that levels are significantly higher in immortalized urothelial and bladder tumor cells than in primary cells (Fig. 2). In formalin-fixed and paraffin-embedded bladder carcinomas and bladder cancer tissue arrays, both histologically normal and those tissues identified as transitional cell carcinomas stained positively for ERα and ERβ (Fig. 3A). Real-time PCR analysis of RNA obtained from ten biopsies of bladder transitional cell carcinoma and matched sample from the same bladder unaffected by tumor showed that abundance of ERα mRNA was significantly greater (2.77-fold, P=0.000017 by paired t-test) in tumor than in normal tissues. By contrast, tumor and normal tissues express similar levels of ERβ mRNA (paired t-test P=0.49; Fig. 3B).
Table 1.
Population-doubling time for human urothelial cells under normal culture conditions
| Cell type | Passage | Doubling time under normal culture conditions (h) |
|---|---|---|
| Human urothelial cells primarily cultured from ureter | 1–3 | 48–60 |
| Human urothelial cells primarily cultured from ureter | 4–7 | 24–36 |
| Human urothelial cells primarily cultured from bladder | 1–3 | 60–90 |
| Human urothelial cells primarily cultured from bladder | 4–7 | 36–48 |
| E6, E7, and urotsa immortalized cells, HTB-9 | All | 24 |
| T24 cells | All | 16 |
Figure 1.
ERα and ERβ mRNAs are differentially expressed in primary, immortalized, and tumor urothelial cells. Total cellular RNA was extracted from primarily cultured human bladder urothelial cells PM1–PM3 (male donors), PF1–PF3 (female donors), human ureteral epithelial cells immortalized with human papillomavirus E6 or E7 (E6, E7), or human bladder urothelial cells immortalized with the SV40 large T antigen (UROtsa), and human bladder tumor cell lines HTB-9 and T24. Quantitative real-time PCR was performed using different specific primers and quantified from threshold cycle. Results are normalized to S26 ribosome RNA expression in the same sample. (A) The ratios of ERα/S26 mRNA in these cells are shown. Columns and vertical bars represent the mean±S.E.M. of four separate experiments. (B) The ratios of ERβ/S26 mRNA in the above cells. (C) Conventional RT-PCR for ERα, ERβ, and GAPDH in these cells. Agarose gel representative of four separate experiments is shown.
Figure 2.
ERα, ERβ, and PCNA protein are differentially expressed in primary, immortalized, and tumor urothelial cells. Whole-cell lysates from primarily cultured human bladder urothelial cells, immortalized human ureteral epithelial cell lines E6 and E7, immortalized human bladder urothelial cells UROtsa, and human bladder tumor cell lines HTB-9 and T24 were blotted for human ERα, ERβ, PCNA, and α-tubulin. Blots representative of four separate experiments are shown.
Figure 3.
ERα and ERβ are differentially expressed in bladder carcinomas. (A) Immunohistochemical staining of transitional bladder carcinomas for ERα and ERβ (revealed by diaminobenzidine). Representative images of staining for ERα and ERβ from six randomly selected patients with self-matched normal tissues are shown. (a) ERα in normal urothelium, (b) ERβ in normal urothelium, (c) ERα in tumor tissue, (d) ERβ in tumor tissue. Scale bars, 50 μm. (B) The ratio of mRNA expression determined by real-time PCR in tumor tissue to self-matched normal tissue in ten randomly selected patients. Abundance of ERα mRNA was significantly greater (2.77-fold) in neoplastic relative to histologically normal tissues (paired t-test P=0.000017), but abundance of ERβ mRNA was similar in both tissues (paired t-test P=0.49). **P<0.01.
ERα and ERβ expression can be selectively reduced by siRNA
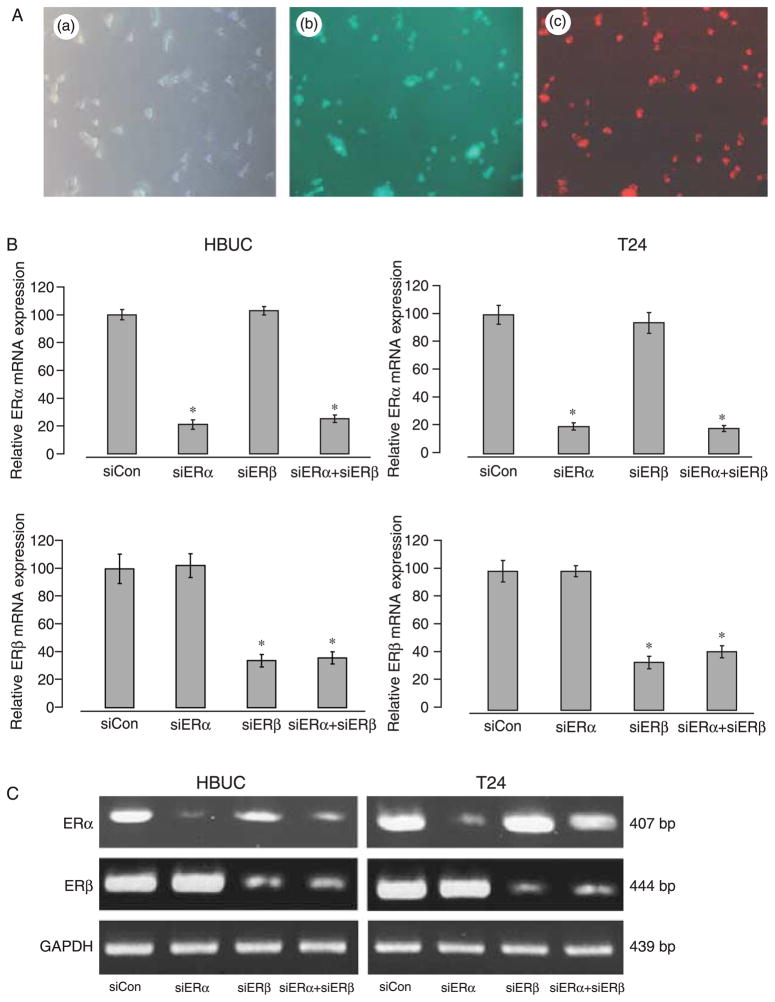
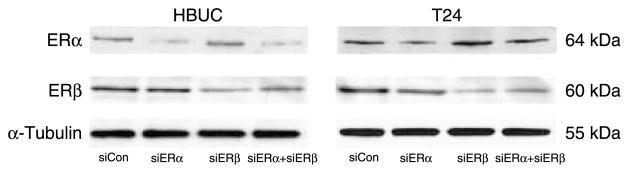
siRNAs designed to selectively suppress ERα or ERβ were used to test the hypothesis that ERα and ERβ have different effects in mediating estrogen-induced urothelial cell proliferation. Non-transfected cells, cells treated with only Oligofectamine, and cells transfected with non-targeting siRNA were used in all the experiments to control for non-specific effects of the transfection protocol. The specificity and efficiency of siRNAs were tested by transient co-transfection of cells with same amount (100 nM) of non-targeting siCon, a cytotoxic siTOX, or fluorescence-labeled siGLO RISC-Free with siERα or siERβ. More than 90% transfection efficiency and nuclear and peri-nuclear localization of siRNAs was confirmed by staining cells with Hoechst 33342, allowing observation of nuclei (Fig. 4A). Quantitative real-time PCR confirmed that ERα mRNA was selectively inhibited by siERα by ~80% (Fig. 4B), and ERβ mRNA was selectively inhibited by siERβ by ~70% in both primary urothelial and T24 cells 24 h after transfection, and these observations were consistent with findings using conventional PCR (Fig. 4C). Immunoblotting for ERα and ERβ protein levels in whole-cell lysates of these cells demonstrated that ERα protein was selectively reduced by siRNA against ERα by 50–60%, and ERβ protein was selectively reduced by siERβ by 60–80% (Fig. 5). Treatment with siERα did not affect the expression of ERβ and vice versa. These results showed that ERα or ERβ mRNA and protein can be selectively suppressed in urothelial cells by specific siRNAs with high efficiency.
Figure 4.
siRNA decreases ER mRNA expression in primary urothelial and bladder tumor cells. (A) T24 cells were co-transfected with 100 nM siERα, siERβ, and siGLO. (a) Cell viability, (b) fluorescence of nuclei stained with Hoechst 33342, and (c) fluorescence of siRNAs in the same cells. (B) Cells were transiently transfected with 100 nM siCon, 100 nM siERα, 100 nM siERβ, or 100 nM siERα+100 nM siERβ. The ratio of ERα/S26 and ERβ/S26 mRNAs are shown. Data represent the mean±S.E.M. of four different primary cultures or four separate cDNA preparations from T24 cells. *P<0.05 compared with siCon treated same type of cells. (C) Conventional RT-PCR for ERα, ERβ, and GAPDH in these cells. A gel representative of four separate experiments is shown.
Figure 5.
siRNA decreases ER protein expression in primary urothelial and bladder tumor cells. Cells were transfected with 100 nM siCon, 100 nM siERα, 100 nM siERβ, or 100 nM siERα+100 nM siERβ. Whole-cell lysates were blotted for ERα, ERβ, and α-tubulin. Blots representative of four separate experiments are shown.
Cell proliferation in response to selective ligands
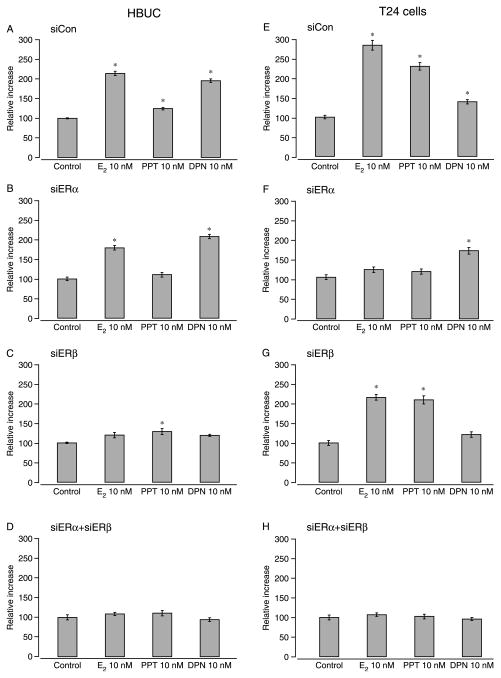
Using urothelial cells transiently transfected with siERα or siERβ, we evaluated the effects of E2, the ERα-selective ligand PPT, and the ERβ-selective ligand DPN on cell proliferation using the 3H-thymidine incorporation assay following 16-h incubation with each compound in the presence or absence of the ER antagonist ICI 182 780. Concentration–response curves were generated for each reagent and demonstrated that treatment with 10 nM E2, PPT, or DPN; 100 nM ICI 182 780; or 100 nM siRNAs were most effective (data not shown). In primary cells transfected with only siCon, 10 nM E2, PPT, or DPN induced significant increases in DNA synthesis (11.4, 24.2, and 95.5% respectively, n=6; Fig. 6A). In primary cells transfected with only siERα, the increase induced by E2 or PPT was reduced to 79.2 and 10.5% respectively. By contrast, DPN caused a higher increase in DNA synthesis (107.9%; Fig. 6B) relative to siCon-transfected cells. When cells were transfected with only siERβ, the increase induced by E2 or PPT was significantly reduced to 19.9 and 28.7% respectively. The effect of DPN on DNA synthesis (18.9%) in these cells was not significant (Fig. 6C). In primary cells co-transfected with siERα and siERβ, effects of E2, PPT, and DPN were further reduced (Fig. 6D). The specific effects of DPN or siERβ on DNA synthesis in primary cells indicate that ERβ predominantly mediates estrogen-induced cell proliferation in these cells.
Figure 6.
siERβ and siERα inhibits estrogen-induced cell proliferation in primary urothelial and bladder cancer cells respectively. Cells were transfected with 100 nM siCon, 100 nM siERα, 100 nM siERβ, or 100 nM siERα+100 nM siERβ following serum starvation for 24 h. Cells were then treated with 10 nM 17β-estradiol, 10 nM PPT, or 10 nM DPN for 16 h and labeled with 0.5 μCi [methyl-3H] thymidine. DNA was harvested with TCA and quantified using a scintillation counter to determine DPM. Relative increases in DNA were calculated by setting the average of vehicle-treated cells in each group to 100. Columns and vertical bars represent the mean±S.E.M. of six replicates. *P<0.05 compared with vehicle-treated cells.
In T24 cells transfected with only siCon, E2 or PPT induced significantly greater increases in DNA synthesis (183 and 129% respectively, n=6) than did DPN (38.8%; Fig. 6E). In T24 cells transfected with only siERα, the increase caused by E2 or PPT was significantly reduced to 18 and 13.4% respectively. By contrast, DPN caused a higher increase in DNA synthesis (63.5%) than it did in siCon-transfected T24 cells (Fig. 6F). When T24 cells were transfected with only siERβ, the increase induced by E2 or PPT were 115.6 and 109.1% respectively. The effect of DPN on DNA synthesis (17.3%) was not significant in these cells (Fig. 6G). In T24 cells co-transfected with siERα and siERβ, the effects of E2, PPT, and DPN were abolished (Fig. 6H). The specific effects of PPT or siERα on DNA synthesis in T24 cells indicate that ERα predominantly mediates estrogen-induced cell proliferation in these cells. In all of these cells, ICI 182 780 abolished proliferative effects of estrogen to levels comparable with that in control cells (i.e., not exposed to E2; data not shown). These results are consistent with patterns of ER expression in these cells and indicate that both ER subtypes contribute to cell proliferation in various urothelial cells.
Estrogen induction of G/S phase transition in primary urothelial and bladder cancer cells
The capacity of estrogen to stimulate cell cycle progression has been investigated extensively in breast cancer cells and has focused on inducing cell progress through G1 phase. To study the capacity of estrogen to induce entry into S phase in urothelial cells, we determined the percentages of cells in G0/G1, S, and G2/M phases in serum-starved primary urothelial or T24 cells treated with 10 nM E2, PPT, or DPN alone or in combination with 100 nM ICI 182 780 for 6 or 16 h. Treatment for 6 h had minimal effects, and the results of 16-h treatments from three separate experiments are summarized in Table 2. E2 caused a significant accumulation of cells in the S phase with a concomitant decrease in the proportion of those in the G0/G1 and G2/M phases in both primary (cells in S phase increased from 24.8 to 40.9%) and bladder tumor cells (cells in S phase increased from 32.3 to 45.1%). ICI 182 780 reduced E2-induced G1/S transition to the levels in control cells. Only DPN, but not PPT, increased the percentage of primary urothelial cells entering into S phase to 40%. In T24 cells, PPT induced a greater proportion of cell entry into S phase than did DPN (44.6 and 38.9% respectively). We also found in primary cells, the effect of DPN was blocked by siERβ, while in T24 cells the effect of PPT was blocked by siERα, and the effect of DPN was blocked by siERβ similar to the effects of ICI 182 780. These data are consistent with cell proliferation results and suggest that both ERα and ERβ mediate estrogen-induced G1/S transition in urothelial cells.
Table 2.
Changes in cell cycle phase distribution following estrogen treatments
| Primary HBUC | % DNA
|
T24 cells | % DNA
|
||||
|---|---|---|---|---|---|---|---|
| G0/G1 | S | G2/M | G0/G1 | S | G2/M | ||
| Control | 68.9 | 24.8 | 6.4 | Control | 65.7 | 32.3 | 2.0 |
| E2 10 nM | 59.1 | 40.9 | 0.1 | E2 10 nM | 54.8 | 45.1 | 0.1 |
| ICI 100 nM | 71.2 | 22.4 | 6.4 | ICI 100 nM | 64.0 | 33.5 | 2.5 |
| E2+ICI | 67.5 | 21.6 | 10.9 | E2+ICI | 59.6 | 32.7 | 7.7 |
| PPT 10 nM | 62.5 | 27.4 | 10.2 | PPT 10 nM | 55.4 | 44.6 | 0.0 |
| DPN 10 nM | 60.0 | 40.0 | 0.0 | DPN 10 nM | 57.5 | 38.9 | 3.6 |
| siControl | 63.7 | 24.7 | 11.6 | siControl | 65.2 | 34.8 | 0.0 |
| siERα 25 nM | 66.5 | 17.7 | 15.7 | siERα 25 nM | 66.7 | 27.4 | 5.9 |
| siERβ 25 nM | 64.8 | 20.3 | 14.9 | siERβ 25 nM | 65.2 | 30.8 | 4.0 |
| siERα+PPT | 65.4 | 20.2 | 14.4 | siERα+PPT | 65.2 | 30.7 | 4.1 |
| siERβ+DPN | 62.3 | 25.5 | 12.2 | siERβ+DPN | 64.3 | 31.2 | 4.5 |
Representative results from three separate experiments. Cells were treated with estrogen or combinations of agonists and antagonists in the absence or presence of siRNAs for 16 h and stained with Hoechst 33342. The proportion of live diploids in G0/G1, S, and G2/M phases of the cell cycle was determined with a BD LSR II cytometer. Data were analyzed by BD Cell Quest.
E2-induced urothelial cell proliferation is independent of ERK signaling
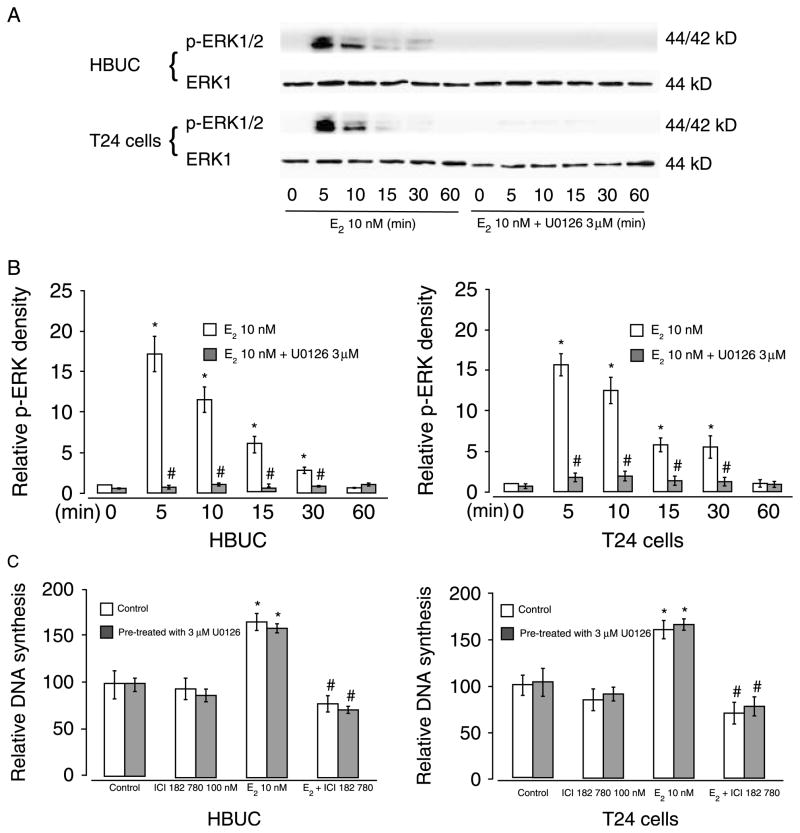
After finding that estrogen affects DNA synthesis and cell cycle progression, we investigated whether the proliferative effects of estrogen are mediated via non-genomic signaling pathways entailing MAPK. Treatment with 10 nM E2 rapidly induced a significantly increase in phosphorylation of ERK (5–30 min), and this was abolished by U0126, a specific MEK pathway inhibitor (Fig. 7A and B). However, in the presence of 3 μM U0126, E2 still induced a significant increase in DNA synthesis in both HBUC and T24 cells (Fig. 7C). These results suggest that E2-induced urothelial cell proliferation is not altered by MAPK-mediated non-genomic effects of estrogen.
Figure 7.
E2 induce ERK phosphorylation but this is independent of E2-induced cell proliferation. Cells were seeded at equal density, serum starved for 24 h, and then treated with 10 nM E2 for up to 60 min in the presence or absence of 3 μM U0126. (A) Whole-cell extracts were blotted for p-ERK and ERK-1. Blots representative of four separate experiments are shown. (B) Expression of p-ERK at different time points. The intensity of p-ERK bands was normalized to the intensity of ERK-1 in the same sample. *P<0.05. (C) [3H] thymidine incorporation into E2-treated cells for 16 h in the presence or absence of 3 μM U0126. *P<0.05 compared with vehicle-treated cells. #P<0.05 compared with E2-treated cells.
Cyclin D1 and cyclin E are induced by estrogen in urothelial cells
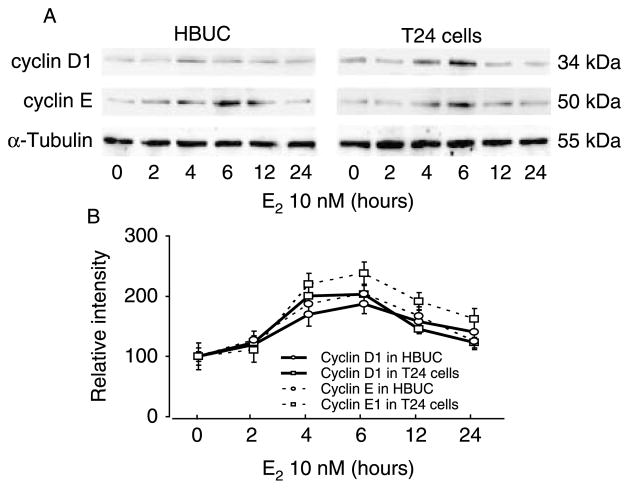
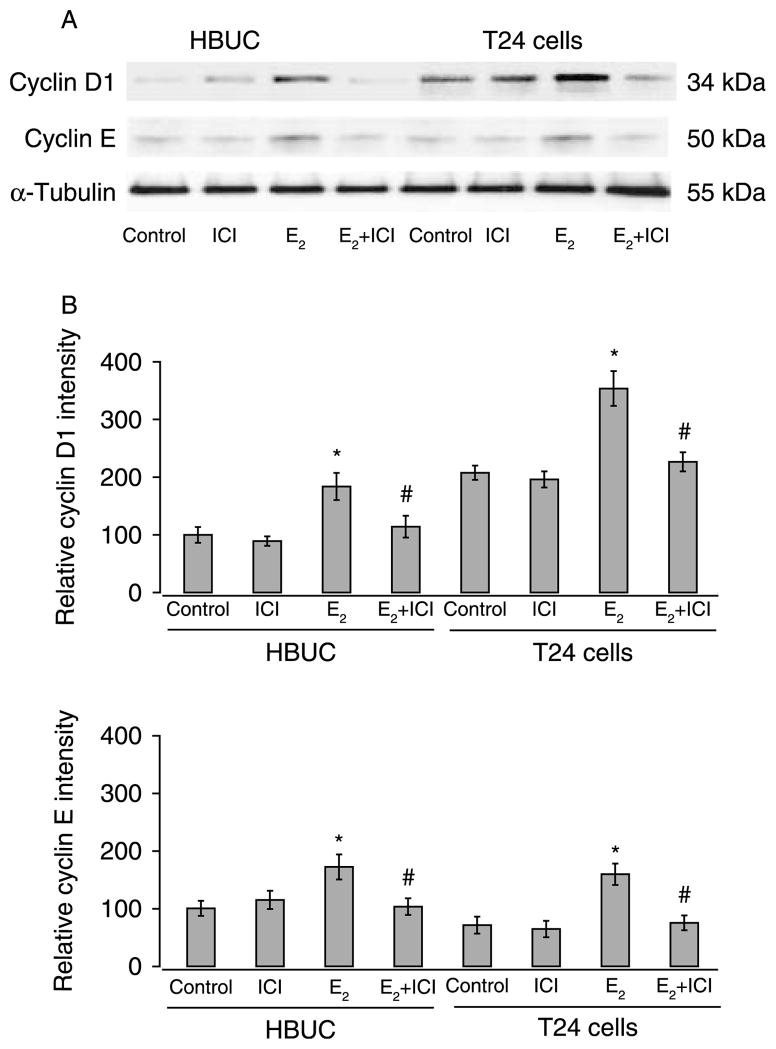
Expression of major subtypes of cyclins that govern G1/S transition, cyclin A, cyclin B1, cyclin D1, cyclin E, and cyclin H was assessed by immunoblotting after treatment of serum-starved primary urothelial or T24 cells with E2 for time intervals varying from 0 to 24 h. Only cyclin D1 and cyclin E protein levels were significantly increased. These reached maximal values after 4–6 h of E2 stimulation and subsequently declined but remained relatively high for up to 12 h (Fig. 8). In the absence of E2, cyclin D1 was found to be expressed at higher levels (approximately twofold) in T24 than in primary urothelial cells, but both cell types expressed similar level of cyclin E. Both cyclin D1 and cyclin E proteins were significantly increased (~60–90%) in primary and T24 cells after treatment with 10 nM E2 for 6 h. This increased expression was completely abolished by ICI 182 780 (Fig. 9). These data suggest that estrogens exert their effects on cell cycle progression through up-regulation of both cyclin D1 and cyclin E in both primary urothelial and bladder cancer cells. Treatment of primary urothelial cells with 10 nM E2 in the presence or absence of 3 μM U0126 resulted in a similar increase in cyclin D1 and cyclin E, indicating that the effects of estrogen on expression of cyclin D1 and cyclin E were not dependent upon ERK phosphorylation (data not shown).
Figure 8.
Induction of cyclin D1 and cyclin E by E2 in primary urothelial and bladder tumor cells in a time-dependent manner. Cells were seeded at equal density, serum starved for 24 h, and then treated with 10 nM E2 for up to 24 h. Whole-cell lysates were blotted for cyclin D1, cyclin E, and α-tubulin. (A) Blots representative of four separate experiments are shown. (B) Expression of cyclin D1 and cyclin E at different time points in E2-treated cells. The average of normalized intensity from control cells was arbitrarily set at 100%. Each point and vertical bars represent the mean±S.E.M. of four separate experiments. P<0.05 at 4, 6, and 12 h compared with vehicle-treated cells.
Figure 9.
E2 stimulates cyclin D1 and cyclin E expression in primary urothelial and bladder tumor cells. Cells were seeded at equal density, serum starved for 24 h, and then treated with 10 nM E2, 100 nM ICI 182 780, or a combination of the two compounds. Whole-cell lysates were blotted for cyclin D1, cyclin E, and α-tubulin. (A) Blots representative of four separate experiments are shown. (B) Relative intensity of cyclin D1 and cyclin E. The average density of normalized control samples was arbitrarily set at 100%. Columns and vertical bars represent the mean±S.E.M. of four separate experiments. *P<0.05 compared with control, and #P<0.05 compared with cells in the absence of ICI 182 780.
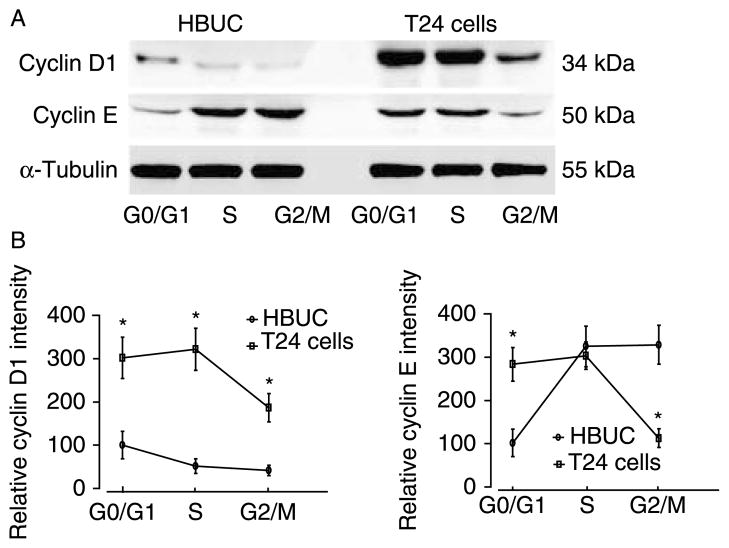
To investigate the question of how abundance of cyclin D1 and cyclin E correlate with different cell phases, live primary urothelial and T24 cells were sorted into G0/G1, S, and G2/M phases, and the expression of cyclin D1 and cyclin E proteins in whole-cell extracts was determined using immunoblotting. In the presence or absence of treatment with 10 nM E2, relatively higher levels of cyclin D1 were detected only at G0/G1 phase in primary cells, and tumor cells expressed significantly higher levels of cyclin D1 in both G0/G1 and S phase cells. Cyclin E was expressed at higher levels in primary urothelial cells in S and G2/M phases. However, in T24 cells, cyclin E was expressed at higher levels during G0/G1 and S phases (Fig. 10). These results suggest that higher constitutive and induced expression of cyclin D1 and cyclin E during G0/G1 and S phases may contribute to accelerated growth of bladder tumor cells.
Figure 10.
Cyclin D1 and cyclin E are differentially expressed in primary and bladder tumor cells at different phases of the cell cycle. Cells were stained with Hoechst 33342, and live diploids at G0/G1, S, and G2/M phases of the cell cycle were collected into separate tubes with a BD FACSCalibur. Whole-cell lysates from 2000 sorted cells were sequentially blotted for cyclin D1, cyclin E, and α-tubulin. Blots representative of three separate experiments are shown in (A). The relative abundance of cyclin D1 or cyclin E at different cell phases is shown in (B) by arbitrarily setting the intensity of primary cells at G0/G1 phases at 100%. Data represent the mean±S.E.M. of three separate experiments. *P<0.05.
Discussion
Estrogen-induced cell proliferation is intimately linked with the development and progression of a number of human cancers, notably breast, uterine, and ovarian cancers. Previous studies in these tissues found that ERβ expression declines when cells undergo malignant transformation, and this causes an increased ERα/ERβ ratio (Pujol et al. 1998, Foley et al. 2000, Roger et al. 2001). In the present study, we demonstrate that the increase in the ERα/ERβ mRNA or protein ratio in bladder cancer cells was rather due to a significant increase in ERα expression. Both ERα and ERβ may mediate estrogen-induced cell proliferation through activation of cyclin D1 and cyclin E, and increased expression of ERα may contribute to dysregulated growth of bladder cancer cells.
The data presented here suggest that ERα expression in immortalized and tumor urothelial cells is significantly higher than in primary cells, but that all of these urothelial cells expressed similar levels of ERβ. This result was consistent with the analysis of mRNA from bladder samples from patients with transitional cell carcinoma. Our finding that immortalized and tumor urothelial cells also express significantly higher levels of PCNA than primary cells supports the concept that immortalization, malignant transformation, recurrence, and the development of muscle invasion of human bladder tumor are accompanied by increased expression of PCNA (Syrigos et al. 2004). One study reported that bladder cancers express high levels of ERβ but low levels of ERα; however, this report considered tumors with 10% or fewer cells expressing ERα as negative for ERα (Shen et al. 2006). Thus, low expression of ERα was equaled with no expression. We observed that both histologically normal bladder tissues and tissues from bladders affected with transitional cell carcinoma expressed ERα and ERβ, but we did not attempt to quantify relative expression of ERα and ERβ by immunohistochemistry. Analysis of total RNA extracted from histologically normal and neoplastic tissues from bladders affected by transitional cell carcinoma indicated that both tissues expressed similar abundance of ERβ mRNA but that neoplastic tissues expressed approximately threefold more ERα mRNA than histologically normal tissue from the same bladder. This observation is consistent with our findings in bladder cancer cell lines and immortalized urothelial cells. These data do not prove a causal relationship between increased expression of ERα and malignant transformation of urothelial cells, but at the very least they do provide evidence of temporal association of this change with the presence of cancer of the bladder.
Several studies have shown that ERα is cancer promoting, while ERβ is protective by repressing ERα activation (Lindberg et al. 2003, Helguero et al. 2005). However, these observations are not free of controversy. The HC11 cell line used by Helguero et al. (2005) is a particular mouse mammary cell line reflecting the situation in late pregnancy and lactation, during which mammary glands are resistant to the proliferative effects of estrogen (Shyamala & Ferenczy 1982). Although Lindberg et al. (2003) suggested that there is a ‘ying–yang’ relationship between ERα and ERβ, it is interesting to note that in their experiments, ERβ was able to mediate estrogen-induced gene transcription in the absence of ERα. ERβ expression was induced in carcinogen-transformed human breast epithelial cells, and higher grade cancerous transformed cells expressed higher levels of ERβ (Hu et al. 1998). In invasive breast tumors from postmenopausal women, there was a trend for ERβ expression to increased cell proliferation and worse prognosis (O’Neill et al. 2004). ERβ expression was also found to be associated with increased expression of cell proliferation markers Ki67 and cyclin A in human breast cancers (Jensen et al. 2001). High intake of dietary plant estrogen has been associated with a low incidence of breast cancer. In light of previous hypothesis, this might be attributed to the fact that most phytoestrogens have higher affinity for ERβ than ERα. Curiously, at least two epidemiological studies showed that high dietary soy intake increased the risk of bladder cancer (Sun et al. 2002, 2004). These experimental and pathological studies indicate that ERβ may also contribute to initiation and progression of tumorigenesis. Therefore, the hypothesis that ERα and ERβ have opposite effects on cell proliferation and apoptosis and that repression of ERα is the major physiological function of ERβ should not be extrapolated generally to all tissues, especially potential estrogen target tissues that predominantly express ERβ such as normal urothelium.
Many investigations of estrogen-induced cell proliferation have focused on recruitment of noncycling cells into the cell cycle and induction of cycling cells to enter, progress, and exit the G1 phase. Progression through the G1 phase is under the control of cyclin-dependent kinases (Cdks) that are activated by two major types of cyclins: D and E. There is a strong correlation between cyclin D and cyclin E expression and increased ERα levels in breast cancer cells (Musgrove et al. 1993, Prall et al. 1998). Enhanced expression of cyclin D1 is also frequently (15% of cases) observed in bladder cancers (Hall & Peters 1996) and appears to be an early event in bladder transitional cell carcinogenesis (Shin et al. 1997). In MCF-7 breast cancer cells, overexpression of cyclin D1 is sufficient to mimic estrogen action (Prall et al. 1998) and cyclin D1 antisense or microinjection of cyclin D1 antibody blocked estrogen-induced entry into the cell cycle. The blockade imposed by antiestrogens in MCF-7 cells can be overcome by cyclin D1 overexpression (Lukas et al. 1996). These results indicate that cyclin D1 is necessary for estrogen action. Estrogen also activates formation of the cyclin E-cdk2 complex in MCF-7 cells preceding entry into S phase (Prall et al. 1998). We found that both cyclin D1 and cyclin E were induced by E2 in primary and tumor urothelial cells. During G1 phase, the cancer cells constitutively expressed much higher levels of cyclin D1 and cyclin E than primary cells. Our results suggest that both cyclin D1 and cyclin E play important roles in mediating E2-induced cell cycle progression. The higher expression of cyclin D1 and cyclin E during G1/S phases may contribute to dysregulated growth in tumor cells. Our results are not in agreement with studies showing that ERα and ERβ have opposing actions on expression of cyclins, in which ERα is reported to be an activator, while ERβ is repressive (Liu et al. 2002, Helguero et al. 2005). Estrogen-activated non-genomic pathways, particularly MAPK, have been shown to play an important role in cell proliferation of non-reproductive tissues (Mendelsohn 2002, Song & Santen 2006). It has been observed in other cell types that estrogen may induce G1/S transition via non-genomic pathways entailing activation of PI3/akt and Ras/MAPK cascades that result in increased expression of cyclin D1 and decreased nuclear localization of p27 respectively (Castoria et al. 2004). Our data indicate that estrogen-dependent activation of ERK does not have this effect on urothelial cells. However, there is an ever-increasing number of proposed non-genomic effects of estrogen on cellular function, and while the time course of cellular response to estrogen seems to make this unlikely, our experiments certainly do not completely exclude the possibility of a role for non-genomic pathways in the response of urothelial cells to estrogen.
In summary, we observed that ERα expression is significantly greater in immortalized urothelial and bladder cancer cells than in primary urothelial cells, but all of these cells expressed similar levels of ERβ. Abundance of ERα mRNA was greater in neoplastic than histologically normal tissues when matched tissue samples from patients with transitional cell carcinomas were compared. Our results suggest that both ERα and ERβ contribute to estrogen-induced G1/S phase progression and cell proliferation in urothelial cells, increased ERα expression may induce early cyclin D1 and cyclin E expression, and result in dysregulated cell proliferation in bladder cancer cells.
Acknowledgments
We thank Dr Petia Simeonova at the National Institute for Occupational Safety and Health (Morgantown, WV, USA) for providing UROtsa cells. This work was supported by grants from the National Institutes of Health (R01 DK57258 and R01 DK066349 to D E B).
Footnotes
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Castelao JE, Yuan JM, Skipper PL, Tannenbaum SR, Gago-Dominguez M, Crowder JS, Ross RK, Yu MC. Gender- and smoking-related bladder cancer risk. Journal of National Cancer Institute. 2001;93:538–545. doi: 10.1093/jnci/93.7.538. [DOI] [PubMed] [Google Scholar]
- Castoria G, Migliaccio A, Di Domenico M, Lombardi M, de Falco A, Varricchio L, Bilancio A, Barone MV, Auricchio F. Role of atypical protein kinase C in estradiol-triggered G1/S progression of MCF-7 cells. Molecular and Cellular Biology. 2004;24:7643–7653. doi: 10.1128/MCB.24.17.7643-7653.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Ellwein LB. Use of cell proliferation data in modeling urinary bladder carcinogenesis. Environmental Health Perspectives. 1993;101:111–113. doi: 10.1289/ehp.93101s5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. Journal of Clinical Endocrinology and Metabolism. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor beta in malignant human colon. Cancer Research. 2000;60:245–248. [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Advances in Cancer Research. 1996;68:67–108. doi: 10.1016/s0065-230x(08)60352-8. [DOI] [PubMed] [Google Scholar]
- Helguero LA, Faulds MH, Gustafsson JA, Haldosen LA. Estrogen receptors alfa (ERα) and beta (ERβ) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene. 2005;24:6605–6616. doi: 10.1038/sj.onc.1208807. [DOI] [PubMed] [Google Scholar]
- Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, Greene GL. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. Journal of Andrology. 1997;18:602–611. [PubMed] [Google Scholar]
- Hu YF, Lau KM, Ho SM, Russo J. Increased expression of estrogen receptor beta in chemically transformed human breast epithelial cells. International Journal of Oncology. 1998;12:1225–1228. doi: 10.3892/ijo.12.6.1225. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA: A Cancer Journal for Clinicians. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Jensen EV, Cheng G, Palmieri C, Saji S, Makela S, Van Noorden S, Wahlstrom T, Warner M, Coombes RC, Gustafsson JA. Estrogen receptors and proliferation markers in primary and recurrent breast cancer. PNAS. 2001;98:15197–15202. doi: 10.1073/pnas.211556298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann O, Baume H, Dietel M. Detection of oestrogen receptors in non-invasive and invasive transitional cell carcinomas of the urinary bladder using both conventional immunohistochemistry and the tyramide staining amplification (TSA) technique. Journal of Pathology. 1998;186:165–168. doi: 10.1002/(SICI)1096-9896(1998100)186:2<165::AID-PATH155>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Kelsey JL, Moore AS, Glickman LT. Epidemiologic studies of risk factors for cancer in pet dogs. Epidemiologic Reviews. 1998;20:204–217. doi: 10.1093/oxfordjournals.epirev.a017981. [DOI] [PubMed] [Google Scholar]
- Koike S, Sakai M, Muramatsu M. Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic Acids Research. 1987;15:2499–2513. doi: 10.1093/nar/15.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. Journal of Steroid Biochemistry and Molecular Biology. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a ‘ying yang’ relationship between ERalpha and ERbeta in mice. Molecular Endocrinology. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price RH, Jr, Pestell RG, Kushner PJ. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. Journal of Biological Chemistry. 2002;277:24353–24360. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- Liu W, Konduri SD, Bansal S, Nayak BK, Rajasekaran SA, Karuppayil SM, Rajasekaran AK, Das GM. Estrogen receptor-alpha binds p53 tumor suppressor protein directly and represses its function. Journal of Biological Chemistry. 2006;281:9837–9840. doi: 10.1074/jbc.C600001200. [DOI] [PubMed] [Google Scholar]
- Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Molecular and Cellular Biology. 1996;16:6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela S, Strauss L, Kuiper G, Valve E, Salmi S, Santti R, Gustafsson JA. Differential expression of estrogen receptors alpha and beta in adult rat accessory sex glands and lower urinary tract. Molecular and Cellular Endocrinology. 2000;164:109–116. doi: 10.1016/s0303-7207(00)00233-1. [DOI] [PubMed] [Google Scholar]
- Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. Journal of Cellular Physiology. 2006;207:594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. American Journal of Cardiology. 2002;90:3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure–activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. Journal of Medicinal Chemistry. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Letters. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Musgrove EA, Hamilton JA, Lee CS, Sweeney KJ, Watts CK, Sutherland RL. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Molecular and Cellular Biology. 1993;13:3577–3587. doi: 10.1128/mcb.13.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill PA, Davies MP, Shaaban AM, Innes H, Torevell A, Sibson DR, Foster CS. Wild-type oestrogen receptor beta (ERβ1) mRNA and protein expression in Tamoxifen-treated post-menopausal breast cancers. British Journal of Cancer. 2004;91:1694–1702. doi: 10.1038/sj.bjc.6602183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Prall OW, Rogan EM, Musgrove EA, Watts CK, Sutherland RL. c-Myc or cyclin D1 mimics estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry. Molecular and Cellular Biology. 1998;18:4499–4508. doi: 10.1128/mcb.18.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Marmer M, Woodham C, Chang W, Kuiper G, Gustafsson JA, Birch L. Estrogen receptor-beta messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology. 1998;139:874–883. doi: 10.1210/endo.139.3.5827. [DOI] [PubMed] [Google Scholar]
- Pujol P, Rey JM, Nirde P, Roger P, Gastaldi M, Laffargue F, Rochefort H, Maudelonde T. Differential expression of estrogen receptor-alpha and -beta messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Research. 1998;58:5367–5373. [PubMed] [Google Scholar]
- Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Research. 2001;61:2537–2541. [PubMed] [Google Scholar]
- Sabbah M, Courilleau D, Mester J, Redeuilh G. Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. PNAS. 1999;96:11217–11222. doi: 10.1073/pnas.96.20.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen SS, Smith CL, Hsieh JT, Yu J, Kim IY, Jian W, Sonpavde G, Ayala GE, Younes M, Lerner SP. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer. 2006;106:2610–2616. doi: 10.1002/cncr.21945. [DOI] [PubMed] [Google Scholar]
- Shin KY, Kong G, Kim WS, Lee TY, Woo YN, Lee JD. Overexpression of cyclin D1 correlates with early recurrence in superficial bladder cancers. British Journal of Cancer. 1997;75:1788–1792. doi: 10.1038/bjc.1997.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamala G, Ferenczy A. The nonresponsiveness of lactating mammary gland to estradiol. Endocrinology. 1982;110:1249–1256. doi: 10.1210/endo-110-4-1249. [DOI] [PubMed] [Google Scholar]
- Song RX, Santen RJ. Membrane initiated estrogen signaling in breast cancer. Biology of Reproduction. 2006;75:9–16. doi: 10.1095/biolreprod.105.050070. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. Journal of Medicinal Chemistry. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. PNAS. 2004;101:566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CL, Yuan JM, Arakawa K, Low SH, Lee HP, Yu MC. Dietary soy and increased risk of bladder cancer: the Singapore Chinese Health Study. Cancer Epidemiology, Biomarkers and Prevention. 2002;11:1674–1677. [PubMed] [Google Scholar]
- Sun CL, Yuan JM, Wang XL, Gao YT, Ross RK, Yu MC. Dietary soy and increased risk of bladder cancer: a prospective cohort study of men in Shanghai, China. International Journal of Cancer. 2004;112:319–323. doi: 10.1002/ijc.20384. [DOI] [PubMed] [Google Scholar]
- Syrigos KN, Karapanagiotou E, Harrington KJ. The clinical significance of molecular markers to bladder cancer. Hybridoma and Hybridomics. 2004;23:335–342. doi: 10.1089/hyb.2004.23.335. [DOI] [PubMed] [Google Scholar]
- Walker BE. Renewal of cell populations in the female mouse. American Journal of Anatomy. 1960;107:95–105. doi: 10.1002/aja.1001070202. [DOI] [PubMed] [Google Scholar]