Abstract
Pre-neoplastic lesions (ACF, aberrant-crypt-foci; Hp, hyperplastic/dysplastic polyps) are believed to be precursors of sporadic colorectal-tumors (Ad, adenomas; AdCA, adenocarcinomas). ACF/Hp likely originate due to abnormal growth of colonic-crypts in response to aberrant queues in the microenvironment of colonic-crypts. Thus identifying factors which regulate homeostatic vs aberrant proliferation/apoptosis of colonocytes, especially stem/progenitor cells, may lead to effective preventative/treatment strategies. Based on this philosophy, role of growth-factors/peptide-hormones, potentially available in the circulation/microenvironment of colonic-crypts is being examined extensively. Since the time gastrins were discovered as trophic (growth) factors for gastrointestinal-cells, the effect of gastrins on the growth of normal/cancer cells has been investigated, leading to many discoveries. Seminal discoveries made in the area of gastrins and colon-cancer, as it relates to molecular pathways associated with formation of colonic tumors will be reviewed, and possible impact on diagnostic/preventative/treatment strategies will be discussed.
Keywords: Akt, cancer-stem-cells, β-catenin, colonic-crypts, colon-carcinogenesis, curcumin
Introduction
Gastrin-gene was cloned (1,2) and sequence of full-length progastrin identified (1) in the early 1980’s. Enzymatic steps involved in processing of pre-progastrin (product of gastrin-mRNA) into processing-intermediates (non-amidated) and mature (amidated) gastrins (G17/G34) were identified (3). Meal ingestion leads to release of gastrins from antral G cells, which stimulate acid secretion into the lumen of the stomach. In the 1980’s, C-terminal-amidation of gastrin-like peptides (G17/G34/CCK8) was defined as a critical step for measuring maximum acid response from parietal cells; non-amidated progastrins were believed to be biologically inactive (4). However, we now know that non-amidated progastrins (PG) are expressed and secreted by many cancer cells including colon-cancer-cells (5). Progastrins are biologically active and exert potent proliferative/anti-apoptotic effects on normal colonic crypt cells and significantly increase tumorigenic/metastatic potential of colonic-tumor cells in vitro and in vivo (6). Sustained hyperproliferation is a risk factor for colon-carcinogenesis (7). Elevated levels of circulating progastrins in animal-models cause hyperproliferation of colonic-crypts, increase stem-cell-populations, and increase the risk of colon carcinogenesis in response to DNA damaging agents (6,8–12) or mutation of tumor-suppressor-genes (Apc/p53) (13,14). Sustained hyperplastic/dysplastic growth of colonic-crypts within a focal area of colons, resulting in formation of ACF/Hp, are the earliest lesions one can diagnose. Multiplicity and size of hyperplastic-polyps are associated with the risk for developing adenomas/adenocarcinomas in patients with sporadic CRCs (15,16). Genetic/epigenetic molecular pathways associated with Hp-Ads-AdCA sequence of colon-cancer progression has been defined within 3–4 broad categories (Fig. 1), based on presence of chromosomal-instability (CIN), microsatellite-instability (MSI) and CpG-island-methylator-phenotype (CIMP) (17). Expression of gastrin-gene/progastrin, while widespread in adenocarcinomas (18), may be detected in pre-neoplastic lesions (19,20). Some of the molecular pathways associated with colon-carcinogenesis reportedly increase gastrin-gene expression (21,22), which may explain increasing expression of progastrin during hyperplasia-adenoma-carcinoma stages. Thus, totality of literature to-date, implicates a role of endocrine/paracrine/autocrine progastrins during different stages of colon-carcinogenesis. Endocrine/paracrine progastrins may play a role during formation of hyperplastic-growths in a subset of patients positive for sustained levels of circulating gastrins/progastrins (as in hypergastrinemia). The growth of colonic tumors maybe addicted to autocrine-progastrins in a small subset of patients (23), but for majority of the patients autocrine-progastrins may mainly impact progression of the disease. Thus targeting endocrine/autocrine progastrins may help in preventing formation of hyperplastic-growths and/or reducing resistance to chemotherapeutic agents. Targeting receptor/signaling-pathways of progastrins may also provide some benefit. Literature in the past two decades that supports the concepts introduced in here are presented diagrammatically in Fig. 1, and described below.
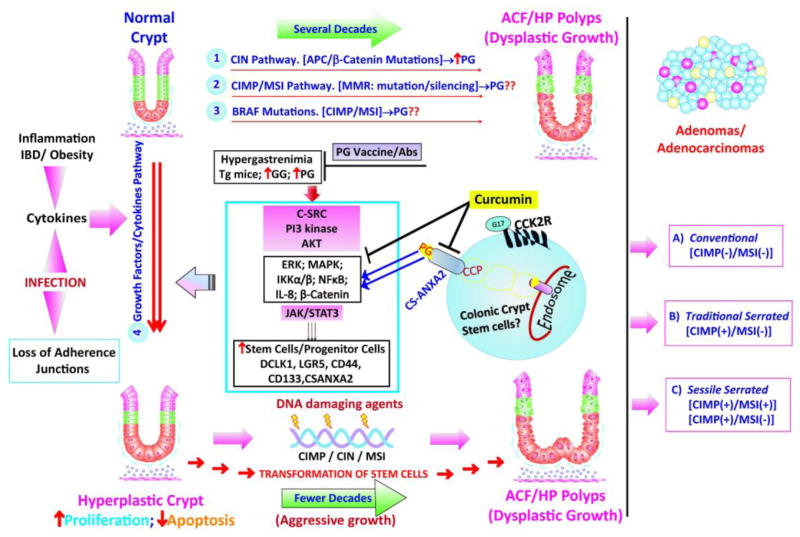
Figure 1. Hypothetical Model of Molecular Pathways Associated with Development of Sporadic Hyperprolifative/Dysplastic/Adenomatous growths in the Colons: Role of Endocrine/Autocrine Progastrins.
athways 1–3 represent genetic/epigenetic mechanisms believed to be associated with the development of sporadic colonic growths/tumors in humans and rodent models of investigation (reviewed in 17). At least three sub-types of sporadic Adenomas have been described, based on phenotype and associated genotype (in terms of MSI/CIMP/CIN (aneuploidy)) (reviewed in 17). Many of these genetic/epigenetic changes, associated with pathways 1–3, can potentially increase the expression of autocrine progastrins (PG) in the colonic growths and serum of patients with CRCs, as described in the text. In addition a 4th pathway, termed growth factor/cytokine pathway, can significantly increase the risk for developing sporadic colonic growths, in response to DNA damaging agents and/or the indicated genetic/epigenetic changes, leading perhaps to more aggressive growths in a shorter time-frame. Risk factors, believed to be associated with the 4th pathway are indicated and may include: 1) elevated levels of endocrine/paracrine progastrins, as in Hypergastrenemic patients; 2) elevated cytokines/growth factors associated with inflammation/obesity/specific infections, as discussed in the text. The 4th pathway, associated with a sustained increase in the associated risk factors, likely results in sustained elevation of the indicated intracellular-kinases/transcription-factors, resulting in hyperproliferation of the affected colonic-crypts and possible transformation of the colonic-stem-cells, significantly increasing the risk for developing dysplastic/neoplastic growths. Abbreviations of key words used in here are described in the text. The role of specific molecules/pathways presented in here, were derived from a large number of reports in literature, referred to in the text.
Processing-intermediates of gastrin (progastrins) are autocrine/endocrine growth-factors for colon-cancer/intestinal-cells
Structure-activity studies in late 1980’s suggested that C-terminal amidation was not critical for displacing binding of 3H-G17 to colon-cancer cells (24). This report led to investigation of possible growth effects of non-amidated-gastrins (G-Gly, glycine-extended gastrins; PG, full-length progastrins1-80). G-Gly was soon established as a mitogenic growth factor for many normal and cancer cells (5,6,25–27). Simultaneously, several laboratories reported that gastrin-gene is variably expressed by colon-cancer-cells/adenocarcinomas (5,6,28), and that colon-cancers mainly secrete unprocessed/partially-processed progastrins (3,5,6,29,30). Significant levels of progastrins were reported in patients positive for CRCs (31–33), which were attenuated on surgical-removal of the tumors or by inhibiting tumor-growth (31,33), confirming CRC tumors as source of circulating progastrins. Availability of specific anti-PG-antibodies has now confirmed expression of progastrins in colonic adenomas/adenocarcinomas, including Hp-polyps (19,20).
The discovery that gastrin-gene products are not processed by colon-cancers led investigators to generate recombinant-full-length-progastrin (rhPG) (34,35) or transgenic (Tg) mice. Tg-mice either constitutively overexpressed G-Gly/hPG (MTI/G-Gly/hGAS) (36,37), or overexpressed hPG in the intestines, in response to fatty-acid-binding-proteins (Fabp-PG mice) (8–10); mice overexpressing amidated-gastrins in response to insulin (INS-GAS mice) were also generated (36). With the help of rhPG and Tg-mice, it became evident that G-Gly/PG were potent proliferative/anti-apoptotic factors for immortalized cell lines, in vitro (27,34,35,38), and for colonic-crypts in vivo, resulting in hyperproliferation of colonic-crypts (10,36,37). The C-terminal flanking peptide of PG (CTFP) has also been recently reported to be biologically active (3); its role in colon-carcinogenesis remains to be examined.
Some colon-cancers may be addicted to autocrine progastrins
In the mid-1990’s it was discovered that down-regulation of gastrin-gene expression in colon-cancer cell lines resulted in attenuating growth/tumorigenic potential of >60% cell lines (23,39). However, a complete loss in tumorigenic-potential was measured in <10% of the cell-lines, suggesting that growth of a small percentage of colonic-tumors may be addicted to autocrine-progastrins. Since then an autocrine role of gastrin-gene-products has been confirmed by several laboratories (40,41). Antibodies derived against PG attenuate growth of gastrin dependent colon-cancer-cells, while anti-G-Gly-antibodies are less effective (42).
Circulating progastrins may be a risk factor for CRC
Elevation of endogenous gastrins/progastrins in rodent models, including transgenic-mice (described above) caused an increase in colon-carcinogenesis in response to the carcinogen, AOM (8–12,43–44). However experimental hypergastrinemia or elevation of G-Gly/PG in Tg-mice, in the absence of carcinogens, did not result in colon-carcinogenesis (45), suggesting that progastrins/gastrins are not carcinogenic. The total number/multiplicity of ACF were significantly higher in hGAS/Fabp-PG Tg-mice (overexpressing PG) compared to that in INS-GAS/wild-type mice (expressing amidated-gastrins), which correlated with size and number of colonic adenomas/adenocarcinomas developed in these mice, in response to AOM (8,9). Thus circulating G-Gly/PG can potentially function as co-carcinogens and increase the risk of crypt-fission/colon-carcinogenesis, while amidated-gastrins, alone, may be ineffective. Animal studies strongly suggest that hypergastrinemia in itself may not initiate colon-carcinogenesis, but elevation of non-amidated-gastrins in the presence of DNA damaging agents enhance colon-carcinogenesis (Fig. 1). Processed forms of gastrins are generally present in the circulation. However in patients with hypergastrinemia, due to many different etiologies (including, Zollinger-Ellison (ZE) syndrome), elevated levels of circulating progastrin are detected (5,6,46,47), which can potentially increase the risk of tumor development in response to DNA damaging agents.
Importantly, proliferative/anti-apoptotic/co-carcinogenic effects of PG were significantly enhanced in proximal colons of Tg-mice; distal colons were much less responsive (8–10). In a recent study, however, proximal growths were not observed in AOM treated Tg mice (11). While elevated levels of PG may increase the risk of proximal colon-carcinogenesis, insulin-like growth-factors (IGFs) increase the risk of mid-gut to distal colon-carcinogenesis in transgenic-mice overexpressing IGF-II (48). Differences in the incidence of proximal vs distal CRCs have been reported in relation to age, gender, hereditary cancer syndromes (49). Silencing of mismatched repair (MMR) genes are significantly higher in proximal vs distal sporadic CRCs (50). Gene expression profiles of right vs left colons are significantly different during embryonic/post-natal development (51), which may explain differences in susceptibility for developing proximal vs distal colon-cancers, in response to activation of specific growth factors/molecular pathways.
Mechanisms mediating proliferative/anti-apoptotic effects of progastrins on intestinal/colon-cancer cells
Having discovered biological effects of progastrins by the turn of 21st century, the past decade has been devoted to delineating receptor/signaling mechanisms mediating growth effects of progastrins.
Target genes of Gastrins/Progastrins
Using the method of differential display it was discovered that cytochrome-oxygenaseVb (CoxVb) sub-unit of holoenzyme COX, was specifically up-regulated by autocrine/endocrine progastrins (52). Cox is the terminal enzyme complex of electron-transfer-chain, involved in ATP generation (53). Mammalian Cox is composed of three major catalytic subunits (CoxI-III), encoded by mitochondrial genome. In addition ten smaller regulatory subunits are encoded by nuclear genome to form the complex Cox-enzyme (53). CoxVb is up-regulated during hypoxia, relevant to tumorigenesis. Thus significant differences in expression of specific subunits of Cox-enzyme, as reported in cancer vs benign/normal cells (discussed in 52), in response to estrogens/growth-factors, such as PG, may help the cells to meet high energy requirements of rapidly proliferating cells.
It was also discovered that CoxVb binds cytochrome c (52). Down-regulation of gastrin-gene in colon-cancer cells reduced CoxVb levels, resulting in an influx of cytochrome c into the cytosol from mitochondria and activation of pro-apoptotic enzymes, caspases 9 and 3 (52). Treatment of Chinese-hamster-ovary-cancer cells with cAMP elevating-agents, reduced Cox activity and released cytochrome c into the cytosol (54), mimicking the results with PG. Thus anti-apoptotic effects of endocrine/autocrine PG peptides (37,52), may be mediated by elevation of CoxVb/ATP associated with loss of cytochrome c release in the presence of pro-apoptotic agents, such as AOM. Activation of p65NFκB also mediates anti-apoptotic effects, resulting in hyperproliferation/colon-carcinogenesis of colonic-crypts, as described below. Many other target genes are up-regulated in response to signaling-pathways activated by gastrins/progastrins, including c-Myc, ornithine-decarboxylase, cyclin D1, cyclooxygenase-2 (Cox-2), VEGF, Heparin-binding-EGF, as described previously (3,55–58).
Intracellular signaling pathways
Pathways mediating proliferative/anti-apoptotic effects of PG/G-Gly, were examined by treating cells/mouse-models in vitro/in vivo, with the peptides, or by examining colonic-crypts from wild-type (WT) vs Tg (overexpresing PG) mice. Based on these investigations, we now know that crosstalk between several signaling-pathways mediates proliferative/anti-apoptotic effects of PG/G-Gly as diagrammatically presented in Fig. 1. Early on, role of Src-kinases was investigated. Of the nine Src-family-members, colonic-epithelial-cells/colon-cancers only express pp60c-Src and pp62c-Yes (discussed in 59). Down-regulation of pp60c-Src resulted in loss of malignant-potential of colon-cancer cells. Since Src-proteins are not over-expressed or mutated in colon-cancers, it was concluded that Src-proteins are probably activated by endogenous mitogen(s). To investigate this possibility, intestinal-epithelial-cells were stimulated with gastrin/progastrins; the peptides significantly activated c-Src, but not c-Yes, in the order of PG>G-Gly>G17, similar to their growth potencies (59). Thus the addition of C and N-terminal amino-acids to G17, increased the biological potency of PG-peptides Micro-injection with anti-c-Src-Abs, confirmed a role of c-Src in mediating growth effects of PG/G17 in intestinal-cells (59). Importantly, inhibitory effects of micro-injecting anti-Src-antibodies, was only observed in response to PG, but not in response to IGFs or fetal-calf-serum (59). Since down-regulation of gastrin-gene in gastrin-dependent colon-cancer-cells, attenuated c-Src-kinase activity, it is speculated that autocrine progastrins may activate Src kinases in colorectal-cancers, imparting a growth potential to the cells. Since these early findings, cross-talk between several signaling-pathways/transcription-factors (c-Src/PI3K/Akt/JAKs/STAT3/ERKs/MAPK/NFκB/Wnt/β-catenin/Notch) have been identified, which may be involved in mediating proliferative/anti-apoptotic effects of endocrine/autocrine progastrins (3,27,60,61). Some of the more critical pathways are discussed in more detail below.
Patients with CRC-tumors, positive for high levels of activated p65NFκB, were reported to have high levels of circulating PG/IL-8; treatment with cyclooxygenase-2 inhibitors significantly decreased gastrin-gene expression/NFκB-activation and reduced serum levels of IL-8/PG (33). These findings suggested an important role of NFκB in mediating PG effects. NFκB activation was confirmed to be critically required for mediating growth effects of PG in vitro and in vivo, downstream of p38MAPK/ERKs/IKKα/β activation (62,63); activation of PI3K/Akt may also play an important role (62).
β-catenin is also activated in response to endocrine/autocrine PG (64,65). Recent findings suggest that β-catenin activation maybe downstream of NFκB, in-vitro and in-vivo, via direct inhibition of GSK3β Tyr216 phosphorylation (resulting in reduced activation of GSK3β) (66). PG-mediated activation of β-catenin results in increased expression of Jagged-1 (Notch ligand) (65), and several stem-cell-markers (discussed below), amplifying the role of PG in tumorigenesis (Fig. 1).
Inflammation (due to IBD/obesity) (67,68) and infectious agents such as citrobacter-rodentium (CR), increase the risk for developing colonic-tumors (69). Significant synergistic effects of PG and CR infection were measured on hyperproliferation of colonic-crypts in mice, in relation to up-regulation of ERKs/NFκB/β-catenin (70). Thus growth-factors such as PG may synergize with infectious/inflammatory agents and further increase the risk of colon-carcinogenesis (Fig. 1).
Amidated/non-amidated-gastrins apparently bind ferric ions, which may be required for measuring proliferative effects of progastrins, but not amidated-gastrins (71).
Receptor mechanisms mediating biological effects of PG/G-Gly
Biochemical/pharmacological/physiological evidence strongly suggested that novel receptor mechanisms, distinct from CCK1R/CCK2R, mediate growth effects of non-amidated-gastrins (5,6,61,72).
Cell lines, which do not express detectable levels of CCK2R, were responsive to proliferative/anti-apoptotic effects of progastrins (6,35,38,52,72–74). Unlike anti-apoptotic effects of PG/G-Gly(26,38,62,63), pro-apoptotic effects of amidated-gastrins, mediated by wtCCK2R (75,76) have been reported. While proliferative effects of gastrins/progastrins on colonic-crypts/colon-cancer cells have been reported (as described above), detectable levels of wtCCK2R have not been measured on these cells (6,77). Elevated amidated-gastrins in INS-GAS mice had significantly different effects compared to elevated progastrins in hGAS mice (8,9,75), strongly suggesting that CCK2R do not mediate progastrin effects.
While, amidated-gastrins and cholecystokinin (CCK) bind CCK2R with high affinity, G-Gly/PG do not (25,62,72). Therefore, in the quest for finding the novel receptor(s) for non-amidated-gastrins, several biochemical methods were used. CCK2R-independent binding of progastrin with unknown glycosaminoglycan proteins on colonic-crypt/intestinal-epithelial-cells was reported (78). Using cross-linking methods, a 36KDa protein with high-affinity for PG>G-Gly>G17 was identified (79). Using advanced proteomics, it was discovered that AnnexinA2 represents the novel 36KDa, non-conventional ‘receptor protein’ for progastrins/gastrins (74). Binding of progastrin with annexinA2 was confirmed by co-immunoprecipitation and solution binding assays (74). Mesenchymal cells around colonic-crypts may express CCK2R. Therefore isolated colonic-crypts, free of mesenchymal cells, were used to confirm binding of PG to ANXA2 on colonic crypt cells of mice overexpressing progastrin (63). A recent report suggests that CCK2R may be required for measuring growth effects of PG on colonic-crypts of Tg-mice (11). Reports also suggest that progastrins may up-regulate expression of CCK2R (13), as previously reported for gastrins (80). Since no laboratory has demonstrated binding/co-localization of progastrin with CCK2R, in vitro or in vivo, it is possible that CCK2R, expressed by mesenchymal cells, may indirectly mediate growth effects of PG/ANXA2.
AnnexinA2 is a multi-functional protein, which binds acidic phospholipids and actin and is involved with intracellular trafficking and kinase activation (81). ANXA2 has a C-terminal core domain and an N-terminal function domain, which binds several ligands with high affinity, including PG (81). Unlike CCK2R-antibodies, ANXA2-antibodies blocked growth effects of PG (62). ANXA2 expression was required for measuring growth effects of autocrine-PG (74). Since AnnexinA2-Antibodies attenuated growth effects of PG, it suggested that PG perhaps binds cell-surface-associated AnnexinA2 (CS-ANXA2). Presence of CS-ANXA2 has been reported on many cancer cells (discussed in 81–83). Functional significance of CS-ANXA2 in proliferation and metastasis of cancer-cells is becoming increasingly evident (discussed in 83). CS-ANXA2 was recently confirmed on intestinal/colon-cancer/HEK-293 cells, all responsive to growth effects of PG (83–85). Importantly, PG in association with ANXA2 was rapidly internalized via clathrin-mediated-endocytosis (CME) (83,84), and it was discovered that internalization of ANXA2/PG complexes was required for measuring PG mediated activation of signaling-kinases (83). TM601, a synthetic peptide which binds CS-ANXA2 on endothelial and tumor cells, is also internalized and promotes neo-angiogenesis (86). PG induces hyperproliferation of proximal colonic-crypts, positive for internalized ANXA2/PG complexes; non-responsive distal-crypts were negative for internalized ANXA2/PG (63), suggesting distal-crypt cells may lack CS-ANXA2, thus resulting in the differential effects of PG on proximal vs distal colons.
Since ANXA2 molecules lack transmembrane domain(s), mechanisms mediating externalization/internalization of ANXA2/PG remain speculative. ANXA2 may be anchored to the surface of cell membranes by ANXA2-receptors (R) (87), which is essential for metastasis of prostate cancer cells (discussed in 83). Exosomes, secreted by cancer cells, contain Annexins, and may represent the source of CS-ANXA2 and soluble ANXA2 measured in the conditioned medium and serum of cancer cells/patients (discussed in 83). Exosomal ANXA2 was recently reported to be internalized by raft-mediated pathways in cancer cells and traffic to endosomes (88), providing strong evidence that extracellular-ANXA2 can be internalized in cells by many different pathways. We now know that PG binding of CS-ANXA2 also internalizes CS-ANXA2 via CME (83).
CME of many peptide/receptor complexes is required for activation of downstream signaling pathways, including MAPK/NFκB (discussed in 83). Effective signal transduction depends on internalization, as opposed to short term signaling from cell surface (discussed in 83). Targeting activated receptors to clathrin-dependent-endocytosis, rather than non-clathrin-endocytosis, was reported to be necessary for sustained signaling and growth response (discussed in 83). Since sustained activation of NFκB, in response to PG, was required for measuring growth effects of PG on pancreatic-cancer-cells (62), it appears likely that CME of PG may mediate the sustained activation of NFκB (Fig. 1).
Effect of progastrins on normal/cancer stem-cell-populations
Several cancer/normal stem-cell-markers, including CD44/CD133/Lgr5 are target genes of β-catenin/Tcf/Lef transcriptional-factors (89,90). Since β-catenin is activated in response to progastrins, a possible elevation of stem-cell-markers was examined in colon-cancer cells and colonic-crypts of mice in response to autocrine/endocrine progastrins (41,84,85). Putative stem-cell-markers, CD44, DCLK1, Lgr5, CD133 were up-regulated by endocrine progastrins, in vitro and in vivo (11,13,41,84,85). An important role of CS-ANXA2 in PG-mediated elevation of stem-cell-markers was confirmed in vitro and in vivo (84). PG/G-Gly was co-expressed with CD133/CD44/Lgr5 in colonic-tumors from patients, suggesting stem-cell populations may express PG-peptides (41). Down-regulation of gastrin-gene expression in DLD1 cells significantly reduced activation of Akt/STAT3 and expression of CD133, resulting in loss of tumorigenic potential of the cells in vivo (41). As described below, overexpression of gastrin-gene/PG in HEK-293 cells (HEKmGAS-cells) resulted in significantly increasing % stem-cell populations co-expressing DCLK1/CD44/CS-ANXA2, in association with increased expression of MMPs, compared to that in control HEK-C cells, which may have contributed to imparting tumorigenic/metastatic potential to HEKmGAS cells in vivo and in the formation of amorphous-spheroids in vitro (85). Almost all CD44(+)HEKmGAS cells, growing either as 2D-cultures/3D-spheroids or xenografts co-expressed CS-ANXA2, and a large % co-expressed DCLK1 (85). It is thus possible that CD44/CS-ANXA2 in PG expressing stem-cells (positive for DCLK1) facilitates growth/metastasis of transformed cells. Thus, down-regulation of either PG/ANXA2/DCLK1/CD44 may attenuate proliferative/tumorigenic/metastatic potential of transformed/cancer cells.
It is becoming increasingly evident that targeting cancer-stem-cells (CSCs)/tumor-initiating-cells (TICs) may prevent relapse of the cancer disease. Selectively targeting CSCs/TICs is being investigated, but this field is as yet in its infancy. It is believed that small populations of neoplastic cells in a tumor are capable of self-renewal and re-population, and thus developing strategies for targeting CSCs is clinically significant. It is believed that CSCs share many features with adult tissue stem-cells, including self-renewal and differentiation (91); however unlike normal stem-cells, CSCs lose the ability to produce multi-lineage differentiated cells. Investigations so far suggest that progastrin mediated signaling pathways up-regulate expression of stem-cell-markers within normal colonic-crypts and transformed epithelial cells (11,41,84,85), giving further credence to the concept that CSCs arise from adult tissue stem-cells (91). Thus preventing an increase in the expression of stem-cell-markers/stem-cell-populations in adult tissues may represent an ideal method of preventing progression of cancer disease.
Gastrin-gene expression is increased by molecular pathways associated with colon carcinogenesis
Gastrin-gene expression is up-regulated at the transcriptional level in response to growth-factors, such as EGF, TRH, and VIP in transformed endocrine (pituitary/insulinoma) cell lines (92,93). It is likely that gastrin-gene is de-repressed in transformed endocrine cells and transcription-factors involved in up-regulating gastrin expression in normal antral-G cells are also functional in transformed endocrine cells. In gastrointestinal mucosal cells, expression of peptide-hormones (such as gastrin) is normally silenced in the epithelial cells; peptide-hormones are expressed only in neuroendocrine cells. Mechanism(s) that allow de-repression of gastrin-gene during colon-carcinogenesis in transformed colonic-epithelial-cells remain unknown. But once the gastrin-gene has been de-repressed, several growth-factors and signaling-pathways have been shown to increase gastrin-gene expression resulting in the expression and secretion of progastrins in colonic-tumors. Two signaling pathways, constitutively up-regulated during colon-carcinogenesis, as a result of either activating (Kras, β-catenin) or loss of function (Apc) mutations, can potentially up-regulate gastrin-gene expression (13,14), providing the molecular basis for the observed increase in gastrin-gene expression during colon-carcinogenesis.
Constitutive over-expression of gastrin-gene/PG in embryonic cells, HEK-293, resulted in transforming the cells and imparting tumorigenic/metastatic potential to the cells (85), suggesting that over-expression of gastrin-gene, itself, can function as an oncogene in specific cell lineages (such as stem-cells/endocrine-cells) which may explain the formation of gastric carcinoid tumors in patients with ZE syndrome. It remains to be determined if formation of a subset of gastrin-dependent colonic-tumors, is perhaps due to constitutive over-expression of gastrin-gene in these tumors.
Prevention of autocrine/endocrine/co-carcinogenic effects of progastrins, using dietary agents
There is a large body of evidence demonstrating that many natural dietary products have significant chemopreventive effects, which lends itself to a safe approach for developing preventative and/or therapeutic strategies (94). Plant phenolics (curcumin) significantly reduces Apc-associated intestinal-carcinogenesis in Min/+ mice while flavonoids (quercetin) are ineffective (95). Curcumin (diferuloylmethane; 1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is the major pigment in turmeric powder and has anti-inflammatory/anti-oxidant properties (94,96). Curcumin inhibits chemically induced carcinogenesis during initiation and/or post-initiation phases (97). Chemopreventative activity of curcumin was also demonstrated during promotion/progression phases of colon-carcinogenesis (98). Activation of NFκB is a crucial event both in inflammation and cancer (99). Inhibition of NFκB plays an important role in curcumin induced apoptosis (94). Inhibitory effects of curcumin are mediated through IκB/NFκB pathway in intestinal-cells and colon-cancer cells (100,101). Curcumin inhibits cell migration of human colon-cancer-cells through inhibition of NFκB/p65, COX-2 and MMP-2 expression. Constitutive activation of NFκB has been observed in colorectal-cancer-cells but not in normal-colorectal-epithelial cells (102). In summary, curcumin suppresses NFκB activation and down-regulates expression of NFκB regulated gene products involved in survival (Bcl2, Bcl-xL, XIAP and cIAP-1), proliferation (COX2, cyclin D1 and c-myc), angiogenesis (VEGF and IL-8), invasion (MMP-9) and metastasis (ICAM-1, VCAM-1 and ELAM-1) (102). Since NFκB activation is critically required for mediating PG effects (62,63), it was not surprising that curcumin significantly inhibited growth effects of autocrine-PG in IEC-18 (100), and HCT-116 (103).
Inhibitory effects of curcumin were compared on intestinal-epithelial-cells, stably overexpressing either PG or IGF-II (100). IGF-II-expressing-clones were resistant to apoptotic effects of curcumin, while PG-expressing-clones demonstrated apoptotic death (100). Overexpression of Hsp70 (104), BCL-XL and ku70 (105) reduces inhibitory effects of curcumin. Curcumin was less effective against growth of HCT-116 cells expressing autocrine-PG versus HCT-116 cells down-regulated for PG-expression (100). However, IEC-18 cells, overexpressing IGF-II, were re-sensitized to pro-apoptotic effects of curcumin in cells inhibited for p38MAPK activation (100), suggesting that additional inhibitory strategies can be used to augment the non-toxic inhibitory effects of dietary agents.
Curcumin inhibits ligand-induced activation of EGF (106). Treatment with curcumin+oxaliplatin significantly inhibits growth of colon-cancer-cells, associated with decreased expression and activation of EGFR/HER-2/HER-3 (107). Since PG/gastrin-peptides up-regulate EGFR (6), it is possible that partial growth effects of PG are indirectly mediated via up-regulation of EGFR. Potent inhibitory effects of curcumin on PG-stimulated growth may therefore be additionally mediated via inhibition of EGFR-mediated pathways. Curcumin can potentially regulate physical properties of cell-membranes (108), which may account for the loss of ligand-activated EGFR signaling (106) and inhibition of PG-binding/internalization to CS-ANXA2 (unpublished data from our lab); however, curcumin did not inhibit binding of IGFs to IGF-I-R. Thus curcumin may inhibit interaction of PG/CS-ANXA2, which may additionally contribute to curcumin-mediated attenuation of elevated pp38MAPK, in response to PG, but not IGF-II (100).
Diagnostic/therapeutic implications of a role of autocrine/endocrine progastrins on colon-carcinogenesis
Since a majority of colonic-tumors express autocrine-gastrins, down-regulating gastrin-gene expression with either nanoparticles, loaded with siRNA against gastrin-gene, or by inducing anti-sense gastrin-mRNA expression with the help of viral-vectors ( for gene-therapy) can be used in patients positive for gastrin-dependent (addicted) colon-cancers. A patent describing gene therapy for targeting autocrine gastrins was issued (39), but has not progressed to clinical trials. Since patients positive for CRC secrete progastrins into the circulation, and high levels of amidated-gastrins may also be growth-promoting factors, a vaccine (G17DT) was developed for targeting gastrins. G17DT-conjugate contained nine amino-acids from N-terminal end of G-17, conjugated to diphtheria-toxoid. In animal models G17DT reduced growth and metastatic spread of several GI tumors (reviewed in 6). However, even though G17DT vaccinations were relatively safe and generated high titers of G17-antibodies, the vaccine did not augment the effects of chemotherapy in patients with advanced GI-cancers, in phase-II trials (109). A vaccine to target full length progastrins was recently developed (42), and was 100% effective in attenuating growth-promoting-effects of autocrine/paracrine progastrins in animal models (110); however the vaccine/antibody approach for targeting endocrine/autocrine progastrins has not reached clinical-trials as yet. Radio-labeled peptide-ligands for membrane-receptors are being developed for diagnostic/therapeutic purposes. Since CS-ANXA2 binds PG- peptides with high-affinity, labeled-PG-peptide (FAM-PG26) was used to determine if it homes to primary/metastatic tumors, in vivo, in mouse-models. Accumulation of FAM-PG26 was localized to focal areas of primary/metastatic tumors, which reflects overexpression of CS-ANXA2 at these sites, confirmed by ANXA2 staining (85). Thus labeled/conjugated molecules with high-affinity for CS-ANXA2 may be useful for diagnosing/treating CRC and other epithelial cancers, positive for elevated levels of CS-ANXA2. Serum/tumor levels of progastrin can also be used as prognostic markers, as recently reported (20).
Rationally designed personalized strategies are required for treating cancer. Results described above heighten the need to examine inhibitory efficacy of dietary-agents, in the presence of physiologically/pathologically relevant endocrine/autocrine growth-factors. Patients positive for high levels of autocrine-growth-factors, are likely to be less responsive to inhibitory effects of curcumin; tumors positive for expression of autocrine growth-factors may require combinatorial treatments for reducing upstream signaling pathways, including MAPK/NFκB/β-catenin/Notch, for reasons described above. Recent studies also suggest that curcumin can potentially inhibit growth of cancer-stem-cell-populations (111,112). In preliminary studies, we measured inhibitory effects of curcumin on cancer-stem-cell-populations, expressing DCLK1 and CD44 (103), strongly supporting this possibility.
Thus in order to capitalize on our growing understanding of mechanisms by which autocrine/endocrine progastrins increase the risk of colon-carcinogenesis, and increase the tumorigenic/metastatic potential of colonic-tumors, several strategies can be developed to either, 1) target gastrin-gene-expression by cancer-cells, 2) target PG-peptides using antigen-specific vaccinations, 3) develop tools to inhibit binding of progastrins with CS-ANXA2, 4) develop toxic-ligands for binding CS-ANXA2 to specifically target colonic-tumors, 5) develop combinatorial treatment strategies with dietary-agents and/or inhibitors of critical signaling molecules.
Conclusions
Based on the investigations within the past 2 decades, several important, paradigm shifting concepts have taken root in the field of gastrins and colon-cancer: 1) non-amidated-gastrins are biologically active and exert proliferative/anti-apoptotic/hyperproliferative effects on colonic-epithelia-cells; 2) colonic-growths increasingly express gastrin-gene as the disease progresses through hyperplasia-adenoma-carcinoma sequence; 3) colonic-tumors mainly express full-length progastrins and to a lesser extract G-Gly, with a small percentage expressing amidated-gastrins; 4) a subset of colonic-tumors may be addicted to autocrine-progastrins; 5) elevated levels of non-amidated, but not amidated, gastrins may increase the risk of proximal colon-carcinogenesis via specific molecular pathways, in patients positive for other risk factors. Additional concepts, more recently introduced include: 1) endocytosis of progastrins, bound to CS-ANXA2, maybe required for measuring biological effects of PG-peptides; 2) several oncogenic pathways mediate growth/co-carcinogenic effects of progastrins; 3) colon-cancers expressing high levels of progastrins/CS-ANXA2 may be at a higher risk for developing metastatic growths; 3) constitutive over-expression of progastrins may transform stem-cells; 4) transformed stem-cells, as in colonic-tumors, may co-express CS-ANXA2/PG with DCLK1/CD133/CD44; 5) progastrins may augment the risk of colon-carcinogenesis in patients who are also positive for other risk factors such as obesity and inflammatory diseases.
Acknowledgments
P. Singh is supported by NIH grant RO1-CA97959-(9). The authors thank Cheryl Simmons for her valuable secretarial help in preparing the manuscript and Claiborne Fant for helping with the graphic design of the figure.
Abbreviations
- ACF
Aberrant-crypt-foci
- Ad
adenomas
- AdCA
adenocarcinomas
- CS-ANXA2
Cell-Surface-AnnexinA2
- CD44
cluster-of-differentiation-44
- CD133
cluster-of-differentiation-133
- CIMP
CpG-Island-Methylator-Phenotype
- CIN
chromosomal-instability
- CRC
colorectal-cancer
- CoxVb
cytochrome c oxidase subunit Vb
- DCLK1
Doublecortin-Ca+2/Calmodulin-Kinase-like-protein
- ERKs
extracellular-signal-regulated kinases
- Hp-polyps
hyperplastic-polyps
- JAK1/2
Janus-Kinase
- Lgr5
leucine-rich-repeat-containing-G-protein-coupled-receptor-5
- MSI
microsatellite-instability
- NFκB
nuclear-factor-kappa-B
- p38MAPK
p38-mitogen-activated-protein-kinase
- PG/G-Gly
Progastrin/Glycine-extended-gastrin
- PI3K
Phosphoinositide-3-Kinase
- cSrc-kinase
Rous Sarcoma viral gene encoded kinase
- STAT3
Signal-transducer-and-activator-of-transcription-3
Footnotes
Disclosure
P. Singh: patent regarding vaccine against Prograstrin pending (technology associated with the vaccine has been licensed by BioRéalités SAS, with licensing fees received by University of Texas Medical Branch); S. Sarkar: none; C. Kantara: none; C. Maxwell: none.
Contributor Information
Pomila Singh, Email: posingh@utmb.edu.
Shubhashish Sarkar, Email: shsarkar@utmb.edu.
Carla Kantara, Email: cakantar@utmb.edu.
Carrie Maxwell, Email: camaxwel@utmb.edu.
References
- 1.Lund T, Olsen J, Rehfeld JF. Cloning and sequencing of the bovine gastrin gene. Mol Endocrinol. 1989;3(10):1585–8. doi: 10.1210/mend-3-10-1585. [DOI] [PubMed] [Google Scholar]
- 2.Kang SC, Agarwal KL, Yoo OJ. Molecular cloning and sequence analysis of cDNA coding for canine gastrin. Biochem Int. 1989;18(3):631–6. [PubMed] [Google Scholar]
- 3*.Kovac S, Shulkes A, Baldwin GS. Peptide processing and biology in human disease. Curr Opin Endocrinol Diabetes Obes. 2009;16(1):79–85. doi: 10.1097/MED.0b013e3283202555. In this review article, the authors have described in detail the steps involved in enzymatic processing of pro-peptide hormones including progastrins, and have also reviewed the biological activity of the pro-peptide and processed forms, as currently known. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh JH. Gastrins. In: Walsh JH, Dockray GJ, editors. Gut peptides: biochemistry and physiology. New York: Raven; 1994. pp. 75–121. [Google Scholar]
- 5.Singh P, Dai B, Wu H, Owlia A. Role of autocrine and endocrine gastrin-like peptides in colonic carcinogenesis. Curr Opin Gastroenterol. 2000;16(1):68–77. doi: 10.1097/00001574-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Rengifo-Cam W, Singh P. Role of progastrins and gastrins and their receptors in GI and pancreatic cancers: targets for treatment. Curr Pharm Des. 2004;10(19):2345–58. doi: 10.2174/1381612043383999. [DOI] [PubMed] [Google Scholar]
- 7.Lipkin M. Biomarkers of increased susceptibility to gastrointestinal cancer: new application to studies of cancer prevention in human subjects. Cancer Res. 1988;48(2):235–45. [PubMed] [Google Scholar]
- 8.Singh P, Velasco M, Given R, Varro A, Wang TC. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology. 2000 Jul;119(1):162–71. doi: 10.1053/gast.2000.8527. [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Velasco M, Given R, Wargovich M, Varro A, Wang TC. Mice overexpressing progastrin are predisposed for developing aberrant colonic crypt foci in response to AOM. Am J Physiol Gastrointest Liver Physiol. 2000;278(3):G390–9. doi: 10.1152/ajpgi.2000.278.3.G390. [DOI] [PubMed] [Google Scholar]
- 10.Cobb S, Wood T, Ceci J, Varro A, Velasco M, Singh P. Intestinal expression of mutant and wild-type progastrin significantly increases colon carcinogenesis in response to azoxymethane in transgenic mice. Cancer. 2004;100(6):1311–23. doi: 10.1002/cncr.20094. [DOI] [PubMed] [Google Scholar]
- 11*.Jin G, Ramanathan V, Quante M, Baik GH, Yang X, Wang SS, Tu S, Gordon SA, Pritchard DM, Varro A, Shulkes A, Wang TC. Inactivating cholecystokinin-2 receptor inhibits progastrin-dependent colonic crypt fission, proliferation, and colorectal cancer in mice. J Clin Invest. 2009 Sep;119(9):2691–701. doi: 10.1172/JCI38918. Epub 2009 Aug 3. The paper describes a possible role of cholecystokinin-type 2 receptor (CCK2R) in mediating growth effects of PG. However, in a letter to the editor, P. Singh and S. Sarkar cited several reports in literature, which strongly disagrees with this conclusion. The letter is published along with the article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ottewell PD, Varro A, Dockray GJ, Kirton CM, Watson AJ, Wang TC, Dimaline R, Pritchard DM. COOH-terminal. 26-amino acid residues of progastrin are sufficient for stimulation of mitosis in murine colonic epithelium in vivo. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):G541–9 E. doi: 10.1152/ajpgi.00268.2004. [DOI] [PubMed] [Google Scholar]
- 13.Ramanathan V, Jin G, Westphalen CB, Whelan A, Dubeykovskiy A, Takaishi S, Wang TC. P53 gene mutation increases progastrin dependent colonic proliferation and colon cancer formation in mice. Cancer Invest. 2012;30(4):275–86. doi: 10.3109/07357907.2012.657814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson SA, Smith AM. Hypergastrinemia promotes adenoma progression in the APC(Min-/+) mouse model of familial adenomatous polyposis. Cancer Res. 2001;61(2):625–31. [PubMed] [Google Scholar]
- 15.Warner AS, Glick ME, Fogt F. Multiple large hyperplastic polyps of the colon coincident with adenocarcinoma. Am J Gastroenterol. 1994;89(1):123–5. [PubMed] [Google Scholar]
- 16.Huang EH, Whelan RL, Gleason NR, Maeda JS, Terry MB, Lee SW, Neugut AI, Forde KA. Increased incidence of colorectal adenomas in follow-up evaluation of patients with newly diagnosed hyperplastic polyps. Surg Endosc. 2001;15(7):646–8. doi: 10.1007/s004640000389. [DOI] [PubMed] [Google Scholar]
- 17*.Kang GH. Four molecular subtypes of colorectal cancer and their precursor lesions. Arch Pathol Lab Med. 2011 Jun;135(6):698–703. doi: 10.5858/2010-0523-RA.1. In this review article the author describes the four molecular pathways, believed to be associated with the development of colorectal growths/cancers, based on literature. Some of these pathways are diagrammatically presented in Fig. 1 of the current article. [DOI] [PubMed] [Google Scholar]
- 18.Smith AM, Watson SA. Gastrin and gastrin receptor activation: an early event in the adenoma-carcinoma sequence. Gut. 2000;47(6):820–4. doi: 10.1136/gut.47.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh P, Deng X, Rengifo-Cam W, Raju G. Relative Levels of gastrin and CCK2R-RNA in fresh biopsies of hyper-proliferative and adenomatous polyps from the colon of patients. Gastroenterology. 2005;128(4 Supplement2) [Google Scholar]
- 20*.Do C, Bertrand C, Palasse J, Delisle MB, Shulkes A, Cohen-Jonathan-Moyal E, Ferrand A, Seva C. A new biomarker that predicts colonic neoplasia outcome in patients with hyperplastic colonic polyps. Cancer Prev Res (Phila) 2012;5(4):675–84. doi: 10.1158/1940-6207.CAPR-11-0408. Data presented in this paper from a large group of patients strongly suggests that progastrin expression by hyperplastic colonic growths can be used as a prognostic biomarker. High levels of PG expression may predict a worse prognosis for the patient, and require closer surveillance. [DOI] [PubMed] [Google Scholar]
- 21.Chakladar A, Dubeykovskiy A, Wojtukiewicz LJ, Pratap J, Lei S, Wang TC. Synergistic activation of the murine gastrin promoter by oncogenic Ras and beta-catenin involves SMAD recruitment. Biochem Biophys Res Commun. 2005;336(1):190–6. doi: 10.1016/j.bbrc.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 22.Koh TJ, Bulitta CJ, Fleming JV, Dockray GJ, Varro A, Wang TC. Gastrin is a target of the beta-catenin/TCF-4 growth-signaling pathway in a model of intestinal polyposis. J Clin Invest. 2000;106(4):533–9. doi: 10.1172/JCI9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh P, Owlia A, Varro A, Dai B, Rajaraman S, Wood T. Gastrin gene expression is required for the proliferation and tumorigenicity of human colon cancer cells. Cancer Res. 1996 Sep 15;56(18):4111–5. [PubMed] [Google Scholar]
- 24.Singh P, Kumar S, Townsend CM, Thompson JC. Structural requirements for the binding of gastrin to mouse colon cancer cells. Surgical Forum. 1987;38:171–3. [Google Scholar]
- 25.Seva C, Dickinson CJ, Yamada T. Growth-promoting effects of glycine-extended progastrin. Science. 1994;265(5170):410–2. doi: 10.1126/science.8023165. [DOI] [PubMed] [Google Scholar]
- 26.Ogunwobi OO, Beales IL. Glycine-extended gastrin stimulates proliferation and inhibits apoptosis in colon cancer cells via cyclo-oxygenase-independent pathways. Regul Pept. 2006;134(1):1–8. doi: 10.1016/j.regpep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Beales IL, Ogunwobi OO. Glycine-extended gastrin inhibits apoptosis in Barrett’s oesophageal and oesophageal adenocarcinoma cells through JAK2/STAT3 activation. J Mol Endocrinol. 2009;42(4):305–18. doi: 10.1677/JME-08-0096. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin GS, Zhang QX. Measurement of gastrin and transforming growth factor alpha messenger RNA levels in colonic carcinoma cell lines by quantitative polymerase chain reaction. Cancer Res. 1992;52(8):2261–7. [PubMed] [Google Scholar]
- 29.Van Solinge WW, Nielsen FC, Friis-Hansen L, Falkmer UG, Rehfeld JF. Expression but incomplete maturation of progastrin in colorectal carcinomas. Gastroenterology. 1993;104(4):1099–107. doi: 10.1016/0016-5085(93)90279-l. [DOI] [PubMed] [Google Scholar]
- 30.Singh P, Xu Z, Dai B, Rajaraman S, Rubin N, Dhruva B. Incomplete processing of progastrin expressed by human colon cancer cells: role of noncarboxyamidated gastrins. Am J Physiol. 1994;266(3 Pt 1):G459–68. doi: 10.1152/ajpgi.1994.266.3.G459. [DOI] [PubMed] [Google Scholar]
- 31.Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109(4):1142–53. doi: 10.1016/0016-5085(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 32.Siddheshwar RK, Gray JC, Kelly SB. Plasma levels of progastrin but not amidated gastrin or glycine extended gastrin are elevated in patients with colorectal carcinoma. Gut. 2001;48(1):47–52. doi: 10.1136/gut.48.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konturek PC, Rembiasz K, Burnat G, Konturek SJ, Tusinela M, Bielanski W, Rehfeld J, Karcz D, Hahn E. Effects of cyclooxygenase-2 inhibition on serum and tumor gastrins and expression of apoptosis-related proteins in colorectal cancer. Dig Dis Sci. 2006;51(4):779–87. doi: 10.1007/s10620-006-3206-z. [DOI] [PubMed] [Google Scholar]
- 34.Baldwin GS, Hollande F, Yang Z, Karelina Y, Paterson A, Strang R, Fourmy D, Neumann G, Shulkes A. Biologically active recombinant human progastrin(6-80) contains a tightly bound calcium ion. J Biol Chem. 2001;276(11):7791–6. doi: 10.1074/jbc.M009985200. [DOI] [PubMed] [Google Scholar]
- 35.Singh P, Lu X, Cobb S, Miller BT, Tarasova N, Varro A, Owlia A. Progastrin1-80 stimulates growth of intestinal epithelial cells in vitro via high-affinity binding sites. Am J Physiol Gastrointest Liver Physiol. 2003;284(2):G328–39. doi: 10.1152/ajpgi.00351.2002. [DOI] [PubMed] [Google Scholar]
- 36.Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98(8):1918–29. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh TJ, Dockray GJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Wang TC. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103(8):1119–26. doi: 10.1172/JCI4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H, Owlia A, Singh P. Precursor peptide progastrin reduces apoptosis of intestinal epithelial cells and upregulates cytochrome c oxidase Vb levels and synthesis of ATP. Am J Physiol. 2003;285(6):G1097–1110. doi: 10.1152/ajpgi.00216.2003. [DOI] [PubMed] [Google Scholar]
- 39.Singh P. Inhibition of endogenous gastrin expression for treatment of colorectal cancer. Patent#5, 786, 213. Texas, U.S.A.:
- 40.Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology. 1997;113(5):1576–88. doi: 10.1053/gast.1997.v113.pm9352860. [DOI] [PubMed] [Google Scholar]
- 41*.Ferrand A, Sandrin MS, Shulkes A, Baldwin GS. Expression of gastrin precursors by CD133-positive colorectal cancer cells is crucial for tumour growth. Biochim Biophys Acta. 2009;1793(3):477–88. doi: 10.1016/j.bbamcr.2009.01.004. The paper describes expression of progastrins by colorectal cancer stem cells for the first time. The autocrine role of gastrin gene expression in dictating tumorogenic potential of colon cancer cells was also confirmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Singh P. Immunogenic Compositions Comprising Progastrin and Uses Thereof’. Patent # US 7,854,932. Texas, U.S.A.: issued on December 21st 2010. This patent describes the generation of monoclonal antibodies and a specific vaccine for targeting growth/co-carcinogenic effects of progastrin peptides, in vivo.
- 43.Williamson RC, Bauer FL, Oscarson JE, Ross JS, Malt RA. Promotion of azoxymethane-induced colonic neoplasia by resection of the proximal small bowel. Cancer Res. 1978;38(10):3212–7. [PubMed] [Google Scholar]
- 44.Karlin DA, McBath M, Jones RD, Elwyn KE, Romsdahl MM. Hypergastrinemia and colorectal carcinogenesis in the rat. Cancer Lett. 1985;29(1):73–8. doi: 10.1016/0304-3835(85)90125-9. [DOI] [PubMed] [Google Scholar]
- 45.Pinson DM, Havu N, Sztern MI, Mattsson H, Looney GA, Kimler BF, Hurwitz A. Drug-induced hypergastrinemia: absence of trophic effects on colonic carcinoma in rats. Gastroenterology. 1995;108(4):1068–74. doi: 10.1016/0016-5085(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 46.Bardram L. Progastrin in serum from Zollinger-Ellison patients. An indicator of malignancy? Gastroenterology. 1990;98(6):1420–6. doi: 10.1016/0016-5085(90)91071-d. [DOI] [PubMed] [Google Scholar]
- 47.Smith AM, Varro A, Watson SA, Hardcastle JD. Progastrin and glycine extended gastrin-17 are overexpressed in pernicious anaemia patients. Gastroenterology. 1998;114(Supplement 1):A1181. [Google Scholar]
- 48.Diehl D, Oesterle D, Elmlinger MW, Hoeflich A, Wolf E, Lahm H. IGF-II transgenic mice display increased aberrant colon crypt multiplicity and tumor volume after 1,2-dimethylhydrazine treatment. J Carcinog. 2006;5:24. doi: 10.1186/1477-3163-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iacopetta B. Are there two sides to colorectal cancer. Int J Cancer. 2002;101:403–8. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe T, Kobunai T, Toda E, Yamamoto Y, Kanazawa T, Kazama Y, Tanaka J, Tanaka T, Konishi T, Okayama Y, Sugimoto Y, Oka T, Sasaki S, Muto T, Nagawa H. Distal colorectal cancers with microsatellite instability (MSI) display distinct gene expression profiles that are different from proximal MSI cancers. Cancer Res. 2006;66(20):9804–8. doi: 10.1158/0008-5472.CAN-06-1163. [DOI] [PubMed] [Google Scholar]
- 51.Glebov OK, Rodriguez LM, Nakahara K, Jenkins J, Cliatt J, Humbyrd CJ, DeNobile J, Soballe P, Simon R, Wright G, Lynch P, Patterson S, Lynch H, Gallinger S, Buchbinder A, Gordon G, Hawk E, Kirsch IR. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12(8):755–62. [PubMed] [Google Scholar]
- 52.Wu H, Rao GN, Dai B, Singh P. Autocrine gastrins in colon cancer cells Up-regulate cytochrome c oxidase Vb and down-regulate efflux of cytochrome c and activation of caspase-3. J Biol Chem. 2000;275(42):32491–8. doi: 10.1074/jbc.M002458200. [DOI] [PubMed] [Google Scholar]
- 53.Capaldi RA, Marusich MF, Taanman JW. Mammalian cytochrome-c oxidase: characterization of enzyme and immunological detection of subunits in tissue extracts and whole cells. Methods Enzymol. 1995;260:117–32. doi: 10.1016/0076-6879(95)60134-1. [DOI] [PubMed] [Google Scholar]
- 54.Yang WL, Iacono L, Tang WM, Chin KV. Novel function of the regulatory subunit of protein kinase A: regulation of cytochrome c oxidase activity and cytochrome c release. Biochemistry. 1998;37(40):14175–80. doi: 10.1021/bi981402a. [DOI] [PubMed] [Google Scholar]
- 55.Narayan S, Rajakumar G, Prouix H, Singh P. Estradiol is trophic for colon cancer: Effect on ornithine decarboxylase and c-myc mRNA. Gastroenterology. 1992;103:1823–32. doi: 10.1016/0016-5085(92)91441-6. [DOI] [PubMed] [Google Scholar]
- 56.Wang JY, McCormack SA, Viar MJ, Johnson LR. Secretin inhibits induction of ornithine decarboxylase activity by gastrin in duodenal mucosa and IEC-6 cells. Am J Physiol. 1994;267(2 Pt 1):G276–84. doi: 10.1152/ajpgi.1994.267.2.G276. [DOI] [PubMed] [Google Scholar]
- 57.Clarke PA, Dickson JH, Harris JC, Grabowska A, Watson SA. Gastrin enhances the angiogenic potential of endothelial cells via modulation of heparin-binding epidermal-like growth factor. Cancer Res. 2006;66(7):3504–12. doi: 10.1158/0008-5472.CAN-05-0280. [DOI] [PubMed] [Google Scholar]
- 58.Bertrand C, Kowalski-Chauvel A, Do C, Résa C, Najib S, Daulhac L, Wang TC, Ferrand A, Seva C. A gastrin precursor, gastrin-gly, upregulates VEGF expression in colonic epithelial cells through an HIF-1-independent mechanism. Int J Cancer. 2010;126(12):2847–57. doi: 10.1002/ijc.25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown D, Yallampalli U, Owlia A, Singh P. pp60c-Src Kinase mediates growth effects of the full-length precursor progastrin1-80 peptide on rat intestinal epithelial cells, in vitro. Endocrinology. 2003;144(1):201–11. doi: 10.1210/en.2002-220501. [DOI] [PubMed] [Google Scholar]
- 60.Grabowska AM, Watson SA. Role of gastrin peptides in carcinogenesis. Cancer Lett. 2007;257(1):1–15. doi: 10.1016/j.canlet.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Ferrand A, Wang TC. Gastrin and cancer: a review. Cancer Lett. 2006;238(1):15–29. doi: 10.1016/j.canlet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 62.Rengifo-Cam W, Umar S, Sarkar S, Singh P. Antiapoptotic effects of progastrin on pancreatic cancer cells are mediated by sustained activation of nuclear factor-{kappa}B. Cancer Res. 2007;67(15):7266–74. doi: 10.1158/0008-5472.CAN-07-1206. [DOI] [PubMed] [Google Scholar]
- 63*.Umar S, Sarkar S, Cowey S, Singh P. Activation of NF-kappaB is required for mediating proliferative and antiapoptotic effects of progastrin on proximal colonic crypts of mice, in vivo. Oncogene. 2008;27(42):5599–611. doi: 10.1038/onc.2008.169. A critical role of p65NFκB for mediating anti-apoptotic and growth effects of progastrins on proximal colonic crypts of mice is described, in relation to co-localization of AnnexinA2 with PG in colonic crypt cells. Related literature regarding importance of NFκB signaling pathways in cancer cells is discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Pannequin J, Bonnans C, Delaunay N, Ryan J, Bourgaux JF, Joubert D, Hollande F. The wnt target jagged-1 mediates the activation of notch signaling by progastrin in human colorectal cancer cells. Cancer Res. 2009;69(15):6065–73. doi: 10.1158/0008-5472.CAN-08-2409. The cross-talk between the Wnt and Notch signaling pathway in mediating the growth effects of progastrins on human colorectal cancers is discussed. [DOI] [PubMed] [Google Scholar]
- 65.Pannequin J, Delaunay N, Buchert M, Surrel F, Bourgaux JF, Ryan J, Boireau S, Coelho J, Pélegrin A, Singh P, Shulkes A, Yim M, Baldwin GS, Pignodel C, Lambeau G, Jay P, Joubert D, Hollande F. Beta-catenin/Tcf-4 inhibition after progastrin targeting reduces growth and drives differentiation of intestinal tumors. Gastroenterology. 2007;133(5):1554–68. doi: 10.1053/j.gastro.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 66*.Umar S, Sarkar S, Wang Y, Singh P. Functional cross-talk between beta-catenin and NFkappaB signaling pathways in colonic crypts of mice in response to progastrin. J Biol Chem. 2009;284(33):22274–84. doi: 10.1074/jbc.M109.020941. The cross-talk between NFκB and β-catenin signaling pathways in mediating growth effects of progastrins on colonic crypts in mice is discussed. A novel mechanism by which β-catenin is activated downstream of NFκB activation in colonic crypts of transgenic mice, overexpressing progastrins was reported in this paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67*.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140(6):1807–16. doi: 10.1053/j.gastro.2011.01.057. The review article discusses literature which demonstrates a strong association between IBD and inflammation with colorectal cancer. [DOI] [PubMed] [Google Scholar]
- 68*.Hayakawa Y, Hirata Y, Nakagawa H, Sakamoto K, Hikiba Y, Otsuka M, Ijichi H, Ikenoue T, Tateishi K, Akanuma M, Ogura K, Yoshida H, Ichijo H, Omata M, Maeda S. Apoptosis signal-regulating kinase. 1 regulates colitis and colitis-associated tumorigenesis by the innate immune responses. Gastroenterology. 2010;138(3):1055–67. e1–4. doi: 10.1053/j.gastro.2009.11.015. In this paper the authors reported a risk for developing colonic tumors in mouse models of colitis. Infection with Citrobacter Rodentium was used as one of the models for inducing colitis like symptoms. [DOI] [PubMed] [Google Scholar]
- 69*.Na SY, Myung SJ. Obesity and colorectal cancer. Korean J Gastroenterol. 2012;59(1):16–26. doi: 10.4166/kjg.2012.59.1.16. The risk of developing colorectal cancers in obese patients is reviewed in this article. [DOI] [PubMed] [Google Scholar]
- 70.Umar S, Singh P. Synergistic Effects of Progastrin (PG) and Citrobacter Rodentium (CR) on the growth of proximal and distal colonic epithelial cells in FVB/N mice: Associated changes in the levels of β-Catenin, NF-κB and ERK1/2. Gastroenterology. 2008;134(4: Supplement 1):A-295. [Google Scholar]
- 71.Kovac S, Anderson GJ, Baldwin GS. Gastrins, iron homeostasis and colorectal cancer. Biochim Biophys Acta. 2011;1813(5):889–95. doi: 10.1016/j.bbamcr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh P, Owlia A, Espeijo R, Dai B. Novel gastrin receptors mediate mitogenic effects of gastrin and processing intermediates of gastrin on Swiss 3T3 fibroblasts. Absence of detectable cholecystokinin (CCK)-A and CCK-B receptors. J Biol Chem. 1995;270(15):8429–38. doi: 10.1074/jbc.270.15.8429. [DOI] [PubMed] [Google Scholar]
- 73.Bold RJ, Ishizuka J, Townsend CM, Jr, Thompson JC. Gastrin stimulates growth of human colon cancer cells via a receptor other than CCK-A or CCK-B. Biochem Biophys Res Commun. 1994;202(3):1222–6. doi: 10.1006/bbrc.1994.2061. [DOI] [PubMed] [Google Scholar]
- 74.Singh P, Wu H, Clark C, Owlia A. Annexin II binds progastrin and gastrin-like peptides, and mediates growth factor effects of autocrine and exogenous gastrins on colon cancer and intestinal epithelial cells. Oncogene. 2007;26(3):425–40. doi: 10.1038/sj.onc.1209798. [DOI] [PubMed] [Google Scholar]
- 75.Przemeck SM, Varro A, Berry D, Steele I, Wang TC, Dockray GJ, Pritchard DM. Hypergastrinemia increases gastric epithelial susceptibility to apoptosis. Regul Pept. 2008;146(1–3):147–56. doi: 10.1016/j.regpep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Sebens Müerköster S, Rausch AV, Isberner A, Minkenberg J, Blaszczuk E, Witt M, Fölsch UR, Schmitz F, Schäfer H, Arlt A. The apoptosis-inducing effect of gastrin on colorectal cancer cells relates to an increased IEX-1 expression mediating NF-kappa B inhibition. Oncogene. 2008;27(8):1122–34. doi: 10.1038/sj.onc.1210728. [DOI] [PubMed] [Google Scholar]
- 77.Reubi JC, Schaer JC, Waser B. Cholecystokinin(CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997;57(7):1377–86. [PubMed] [Google Scholar]
- 78.Dubeykovskiy A, Nguyen T, Dubeykovskaya Z, Lei S, Wang TC. Flow cytometric detection of progastrin interaction with gastrointestinal cells. Regul Pept. 2008;151(1–3):106–14. doi: 10.1016/j.regpep.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chicone L, Narayan S, Townsend CM, Jr, Singh P. The presence of a 33–40 KDa gastrin binding protein on human and mouse colon cancer. Biochem Biophys Res Commun. 1989 Oct 16;164(1):512–9. doi: 10.1016/0006-291x(89)91749-x. [DOI] [PubMed] [Google Scholar]
- 80.Speir GR, Takeuchi K, Peitsch W, Johnson LR. Mucosal gastrin receptor VII up- and downregulation. Am J Physiol. 1982;242(3):G243–9. doi: 10.1152/ajpgi.1982.242.3.G243. [DOI] [PubMed] [Google Scholar]
- 81.Singh P. Role Annexin-II in GI cancers: interaction with gastrins/progastrins. Cancer Letter. 2007;252(1):19–35. doi: 10.1016/j.canlet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ortiz-Zapater E, Peiró S, Roda O, Corominas JM, Aguilar S, Ampurdanés C, Real FX, Navarro P. Tissue plasminogen activator induces pancreatic cancer cell proliferation by a non-catalytic mechanism that requires extracellular signal-regulated kinase 1/2 activation through epidermal growth factor receptor and annexin A2. Am J Pathol. 2007;170(5):1573–84. doi: 10.2353/ajpath.2007.060850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83*.Sarkar S, Kantara C, Singh P. Clathrin mediates endocytosis of progastrin and activates MAPKs: role of cell surface annexin A2. Am J Physiol Gastrointest Liver Physiol. 2012;302(7):G712–22. doi: 10.1152/ajpgi.00406.2011. The important role of endocytosis in measuring biological effects of progastrins on intestinal cells is described. The paper describes the methods used to confirm the association of progastrins with cell surface associated AnnexinA2 (CS-ANXA2). Role of CS-ANXA2 in tumorigenic/metastatic potential of cancer cells is reviewed. Critical role of clathrin mediated endocytosis in biological effects of ligand-membrane receptors is also discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84*.Sarkar S, Swiercz R, Kantara C, Hajjar KA, Singh P. Annexin A2 mediates up-regulation of NF-κB, β-catenin, and stem cell in response to progastrin in mice and HEK-293 cells. Gastroenterology. 2011;140(2):583–595. e4. doi: 10.1053/j.gastro.2010.08.054. Data demonstrating a critical role of CS-ANXA2 in up-regulating NFκB/β-catenin/stem cell markers in response to progastrins, in vitro and in vivo, using ANXA2 knock-out mice are presented. A novel finding was that activation of both NFκB and β-catenin was required for measuring maximum growth effects in response to progastrins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85*.Sarkar S, Kantara C, Ortiz I, Swiercz R, Kuo J, Davey R, Escobar K, Ullrich R, Singh P. Progastrin overexpression imparts tumorigenic/metastatic potential to embryonic epithelial cells: Phenotypic differences between transformed and nontransformed stem cells. Int J Cancer. 2012;131(7):E1088–99. doi: 10.1002/ijc.27615. Several novel concepts are presented in this paper. Transformation of embryonic cells by constitutive expression of gastrin gene in the cell, is described for the first time. Significant differences in the phenotype of transformed versus non-transformed stem cells were identified, which could be used as a biomarker for assessing tumorogenic potential of cells in the future. The paper also describes a specific ligand for diagnosing CRCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86*.Kesavan K, Ratliff J, Johnson EW, Dahlberg W, Asara JM, Misra P, Frangioni JV, Jacoby DB. Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. J Biol Chem. 2010;285(7):4366–74. doi: 10.1074/jbc.M109.066092. The authors have reported an important role of CS-ANXA2 in mediating metastatic potential of cancer cells, and the development of a peptide ligand (TM601) for blocking the effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105(2):370–80. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88*.Valapala M, Vishwanatha JK. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem. 2011;286(35):30911–25. doi: 10.1074/jbc.M111.271155. The authors have described the mechanisms by which annexinA2 is trafficked extra-cellularly within exosomes from cancer cells, and endocytotic mechanisms by which exosomes are re-\nternalized. These findings provide possible mechanisms by which activated oncogenic molecules in transformed cells can be exported via exosomes and potentially ‘infect’ other cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wielenga V, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers R, Pals S. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154(2):515–523. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 91.Takebe N, Ivy SP. Controversies in cancer stem cells: targeting embryonic signaling pathways. Clin Cancer Res. 2010;16(12):3106–12. doi: 10.1158/1078-0432.CCR-09-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Godley JM, Brand SJ. Regulation of the gastrin promoter by epidermal growth factor and neuropeptides. Proc Natl Acad Sci U S A. 1989;86(9):3036–40. doi: 10.1073/pnas.86.9.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brand SJ, Wang TC. Gastrin gene expression and regulation in rat islet cell lines. J Biol Chem. 1988;263(32):16597–603. [PubMed] [Google Scholar]
- 94*.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269(2):199–225. doi: 10.1016/j.canlet.2008.03.009. The authors have compiled a vast amount of literature in this field which describes the chemopreventive effects of curcumin against growth and metastasis of cancer cells, in relation to signaling pathways targeted by curcumin. [DOI] [PubMed] [Google Scholar]
- 95.Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL, Bertagnolli MM. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21(5):921–7. doi: 10.1093/carcin/21.5.921. [DOI] [PubMed] [Google Scholar]
- 96*.Sarkar FH, Li Y, Wang Z, Padhye S. Lesson learned from nature for the development of novel anti-cancer agents: implication of isoflavone, curcumin, and their synthetic analogs. Curr Pharm Des. 2010;16(16):1801–12. doi: 10.2174/138161210791208956. In this article, the authors have similarly reviewed the literature which describes the inhibitory effects of curcumin and isoflavones, and their usefulness in treating cancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995 Jan 15;55(2):259–66. [PubMed] [Google Scholar]
- 98.Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59(3):597–601. [PubMed] [Google Scholar]
- 99.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 100*.Singh P, Sarkar S, Umar S, Rengifo-Cam W, Singh AP, Wood TG. Insulin-like growth factors are more effective than progastrin in reversing proapoptotic effects of curcumin: critical role of p38MAPK. Am J Physiol Gastrointest Liver Physiol. 2010;298(4):G551–62. doi: 10.1152/ajpgi.00497.2009. Methods used to investigate apoptotic effects of curcumin, in relation to several signaling pathways, in the presence or absence of autocrine growth factors, relevant to colon cancers, are described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marín YE, Wall BA, Wang S, Namkoong J, Martino JJ, Suh J, Lee HJ, Rabson AB, Yang CS, Chen S, Ryu JH. Curcumin downregulates the constitutive activity of NF-kappaB and induces apoptosis in novel mouse melanoma cells. Melanoma Res. 2007;17(5):274–83. doi: 10.1097/CMR.0b013e3282ed3d0e. [DOI] [PubMed] [Google Scholar]
- 102.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14(7):2128–36. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 103.Kantara C, Ortiz I, Sarkar S, Ullrich R, Singh P. Targeting Caner Stem Cells (CSCs) with Curcumin±RNAi against Stem-Cell markers, In Vitro and In Vivo: Effect on oncogenic-pathways. Gastroenterology. 2012;142(5, Supplement 1):S-331. [Google Scholar]
- 104.Rashmi R, Kumar S, Karunagaran D. Ectopic expression of Hsp70 confers resistance and silencing its expression sensitizes human colon cancer cells to curcumin-induced apoptosis. Carcinogenesis. 2004;25(2):179–87. doi: 10.1093/carcin/bgh001. [DOI] [PubMed] [Google Scholar]
- 105.Rashmi R, Kumar S, Karunagaran D. Ectopic expression of Bcl-XL or Ku70 protects human colon cancer cells (SW480) against curcumin-induced apoptosis while their down-regulation potentiates it. Carcinogenesis. 2004;25(10):1867–77. doi: 10.1093/carcin/bgh213. [DOI] [PubMed] [Google Scholar]
- 106.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, Majumdar AP. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122(2):267–73. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 108.Barry J, Fritz M, Brender JR, Smith PE, Lee DK, Ramamoorthy A. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin. J Am Chem Soc. 2009;131(12):4490–8. doi: 10.1021/ja809217u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ajani JA, Hecht JR, Ho L, Baker J, Oortgiesen M, Eduljee A, Michaeli D. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer: the GC4 study. Cancer. 2006;106(9):1908–16. doi: 10.1002/cncr.21814. [DOI] [PubMed] [Google Scholar]
- 110.Singh P, Singh G. Development of Progastrin (PG) Specific Monoclonal Antibodies (Mabs) and PG Specific Vaccine for Attenuating Growth Factor Effects of Autocrine and Endocrine PG-Like Peptides on Colon Cancer Cells and Colon Carcinogenesis, Respectively. Gastroenterology. 2007;132(Supplement 2):A-400, 69–70, 2007. [Google Scholar]
- 111*.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122(3):777–85. doi: 10.1007/s10549-009-0612-x. Methods used to investigate inhibitory effects of curcumin on breast cancer stem cells are described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112*.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl Oncol. 2009;2(4):321–8. doi: 10.1593/tlo.09193. Methods used to examine inhibitory effects of curcumin, in the presence or absence of chemotherapeutic agents, on colon cancer stem cells are described. [DOI] [PMC free article] [PubMed] [Google Scholar]