Abstract
Context
Oral bisphosphonate use has increased dramatically in the USA. Recent case reports have suggested a link between bisphosphonate use and esophageal cancer, but this is yet to be robustly investigated.
Objective
To investigate the association between bisphosphonate use and esophageal cancer.
Design, setting and participants
Data were extracted from the UK General Practice Research to compare the incidence of esophageal and gastric cancer in a cohort of patients treated with oral bisphosphonates between January 1996 and December 2006 to a control cohort not treated with these drugs. Cancers were identified from relevant Read\OXMIS codes in the patient’s clinical files. Cox proportional hazards modelling was used to calculate hazard ratios (HRs) and 95% CIs for esophageal and gastric cancer risk in bisphosphonate users compared to non bisphosphonate users, with adjustment for potential confounders.
Main outcome measure
The HR for the risk of esophageal and gastric cancer in the bisphosphonate users compared to the non bisphosphonate users.
Results
There were 41,826 members in each cohort; 81% female, mean (SD) age, 70.0 (11.4) years, excluding patients with under 6 months follow-up. 116 esophageal or gastric cancers (79 esophageal) occurred in the bisphosphonate cohort and 115 (72 esophageal) in the control cohort. Mean follow-up time was 4.5 and 4.4 years in the bisphosphonate and control cohorts, respectively. There was no difference in combined esophageal and gastric cancer risk between the cohorts for any bisphosphonate use; adjusted HR (95% CI), 0.96 (0.74, 1.25) or esophageal risk alone; adjusted HR (95% CI), 1.07 (0.77, 1.49). There was also no difference in esophageal or gastric cancer risk by level of bisphosphonate intake.
Conclusion
This large study does not provide evidence for an increased risk of esophageal (or gastric) cancer in persons using oral bisphosphonates.
Keywords: Bisphosphonates, esophageal cancer, gastric cancer, epidemiology
INTRODUCTION
Bisphosphonates inhibit osteoclast-mediated bone resorption and are mainly used to prevent or treat osteoporosis, especially in post-menopausal women. Bisphosphonate use has increased dramatically in recent years in the USA and other western populations1–2, and are now commonly prescribed in elderly women e.g. in 2005 approximately 10% of UK women over 70 received a bisphosphonate prescription.3
Esophagitis is a well recognized complication of oral bisphosphonate use 4–5, but a recent report suggested that bisphosphonates may increase esophageal cancer risk.6 Between October 1995 and May 2008, 23 cases of esophageal cancer in patients using alendronate were reported to the USA Food and Drug Administration. Primarily, these cases occurred in elderly women, were in the lower oesophagus and were adenocarcinomas. Thirty one cases were also reported in Europe and Japan, mostly in elderly women using alendronate. Although these data suggest a link between oral bisphosphonates and esophageal cancer, information was not available to determine whether cancer rates were higher than expected in elderly women. Also, the median time to cancer diagnosis was 2.1 and 1.3 years in the USA and European/Japanese cases respectively, which may be too short for cancer development unless a predisposing condition, such as Barrett’s esophagus with dysplasia, is already present.7
A subsequent report, which examined national registers of fracture patients in Denmark, showed a decreased, rather than an increased, risk of esophageal cancer in patients treated with bisphosphonates compared to matched untreated patients, but this study had very short follow-up (median 2.2 years) and only included participants who had sustained fractures.8 The incidence rate ratio (IRR) of esophageal cancer among persons receiving oral bisphosphonates within the US Medicare programme was also not increased compared to persons receiving other osteoporosis medications and was similar to esophageal cancer rates within the Surveillance, Epidemiology and End Results (SEER) Registry, although the 95% confidence intervals around the IRRs were wide and consistent with an almost 5-fold increase in risk.9 Neither study adjusted for potential confounders associated with bisphosphonate usage and esophageal cancer risk such as smoking, alcohol intake and BMI and only basic information was provided on bisphosphonate exposure.
Large studies with appropriate comparison groups, adequate follow-up, robust characterization of bisphosphonate exposure and information on relevant confounders are required to determine whether bisphosphonates increase esophageal cancer risk. We undertook such a study within the UK General Practice Research Database (UKGPRD).
METHODS
The UKGPRD is the world’s largest computerised database of anonymised longitudinal patient records and includes 500 general practices comprising about 6% of the UK population. Participating practices follow protocols to record and transfer data10 and are assessed for completeness, continuity and plausibility. Practices meeting predefined standards are registered as ‘up to standard’ (UTS). The information recorded includes demographic information, clinical diagnoses, referral and hospital discharge information and details of all prescriptions issued. Read and Oxford Medical Information System (OXMIS) codes are used to classify medical diagnoses. The high quality of GPRD prescription and diagnosis information has been documented.11 Ethical approval for all observational research using GPRD data has been obtained from a Multicentre Research Ethics Committee (MREC).
Study population
We undertook a cohort study within UTS practices of the UKGPRD in which we compared esophageal and gastric cancer incidence in a cohort of all patients receiving a prescription for oral bisphosphonates (between 1/1/1996 to 31/12/2006) to the incidence in an age-sex matched control cohort. The date of first oral bisphosphonate prescription was taken as the index date. Each bisphosphonate user was matched to a single control (who was allocated their index date) and who was randomly selected from individuals of the same gender, year of birth and General Practice, regardless of bisphosphonate use (to avoid removing patients from the control cohort who received bisphosphonates for treatment of cancer-related osteoporosis/metastasis, thereby artificially reducing the risk of cancer in the control cohort). Therefore, some controls may have been prescribed a bisphosphonate but such subjects did not then participate in the study as bisphosphonate users. Participants were excluded if they were aged under 40 years on their index date or if they had a cancer diagnosis (excluding non-melanoma skin cancer) in the 3 years prior to their index date.
Cancers were identified from relevant Read/OXMIS codes in the patient’s clinical files. All cancer codes recorded for potential esophageal and gastric cancer cases were examined and only patients with consistently recorded codes for these cancers were accepted. The date of the first recorded esophageal or gastric cancer code was considered the diagnosis date. Subjects with codes for Barrett’s esophagus and gastro-esophageal reflux disease were also identified.
Classification of bisphosphonate exposure
All prescriptions for oral bisphosphonates were identified. Data on the preparations prescribed, the date of prescription and the number of packs/tablets prescribed were extracted and converted to daily defined doses (DDDs). The DDD system is a validated measure of drug consumption maintained by the World Health Organisation (http://www.whocc.no/atcddd/atcsystem.html). It is the assumed average maintenance dose per day of a drug used for its main indication in adults, which for oral bisphosphonates is the prevention or treatment of osteoporosis. In the bisphosphonate cohort, the total number of DDDs of oral bisphosphonates received was divided by the number of days of follow-up and categorized by approximate tertiles into high, medium and low use. The bisphosphonate cohort was also subdivided according to whether the first oral bisphosphonate received was nitrogen containing (e.g. alendronate, risedronate and ibandronate), alendronate or a non-nitrogen containing bisphosphonate (e.g. etidronate, tiludronate and clodronate). The total dose of nitrogen containing bisphosphonates or alendronate received was categorised as for all bisphosphonate use.
Data extraction relating to potential confounders
Data on smoking, alcohol consumption and BMI (opportunistically collected within the GPRD) in the 3-year period before the index date were extracted; where several records were available, that closest to the index date was used. Data on use of hormone replacement therapy (HRT), non steroidal anti-inflammatory drugs (NSAIDs), H2-receptor antagonists (H2RAs) and Proton Pump Inhibitors (PPIs) prior to the index date were also extracted.
Statistical analysis
The expected number of esophageal and gastric cancers was determined in the control cohort using the person years of follow-up in the cohort and the age and sex-specific incidence rates from England in 2005 (http://www.statistics.gov.uk/). A standardised incidence ratio (SIR) was then calculated and exact methods used to produce 95% confidence intervals (95%CIs).
The main survival analysis was conducted on the time from index date to the first esophageal or gastric cancer diagnosis with the first six months of follow-up removed for every participant, as cancer incidence in this time period is unlikely to be due to bisphosphonate usage. Participants were censored at the first of the following outcomes: date of death, date of other cancer diagnosis, date of leaving GP practice or date of last data download from GP practice by GPRD. Kaplan-Meier curves were plotted to investigate survival in the two groups and to check the assumption of proportional hazards. Cox’s proportional hazards model was used to calculate hazard ratios (HR) and 95%CIs and to adjust for confounding variables. Confounders with missing data were included using a missing data category, also a complete case analysis was conducted (not shown, as estimates were little altered). To investigate dose response, separate analyses were conducted only including time after the bisphosphonate user had received a specified numbers of DDDs (i.e. 182, 365, 730 and 1440 DDDs equivalent to a 6 month, 1 year, 2 year and 4 year supply respectively). In these analyses the start of follow-up for each bisphosphonate user and their matched control was moved from the index date to the date at which the bisphosphonate user had received the specified number of DDDs. Similar analyses were conducted for nitrogen containing bisphosphonates and alendronate. As the control cohort may have included some bisphosphonate users, a re-analysis was conducted removing such individuals from the control cohort.
Sample size calculation
Prior to conducting the study, using incidence rates from England in 2005 (http://www.statistics.gov.uk/) we estimated there would be around 60 cases of esophageal cancer in the control cohort, allowing over 80% power to detect a 60% increase in esophageal cancer incidence in the bisphosphonate cohort.12
RESULTS
Data was received from GPRD for 46,036 oral bisphosphonate users and 46,036 matched controls. 314 cohort members had esophageal or gastric cancer codes recorded during follow-up. In 27 cases (8.6%) the codes were inconsistent and the diagnosis was not accepted, leaving a total of 287 incident esophageal or gastric cancer diagnoses (0.34% of the combined cohorts); 181 esophageal cancer (92 in the control cohort) and 106 gastric cancers (57 in the control cohort). Data on cancer histological subtypes was unavailable. The SIRs of esophageal and gastric cancer in the control cohort were 1.18 (95%CI 0.95, 1.45) and 0.70 (95%CI 0.53, 0.91) respectively, showing under-recording of gastric cancer. The combined esophageal and gastric cancer SIR was 0.94 (95%CI 0.79, 1.10). These SIRs suggest that tumours arising at the gastro-esophageal junction or in the gastric cardia may have been classified as esophageal cancers rather than gastric cancers. Our principal analysis was therefore for gastric and esophageal cancers combined.
41,826 bisphosphonate users had at least 6 months of follow-up and further analyses were restricted to these patients and their matched controls. 81% of both cohorts were female and the mean age (SD) was 70.0 (11.4) years (Table 1). Mean (SD) follow-up was 4.5 (2.6) years and 4.4 (2.6) years in the bisphosphonate and control cohorts, respectively, and both had a maximum follow-up period of 12.9 years. All of the bisphosphonate cohort and 9% of the control cohort received at least one prescription for oral bisphosphonates during the follow-up period. The mean (SD) number of bisphosphonate DDDs prescribed per day in the bisphosphonate and control cohorts during this period was 0.59 (0.49) and 0.03 (0.16), respectively. Data on BMI was available for 48.3% of the bisphosphonate and 41.9% of the control cohort members: mean BMI was higher in the control cohort than the bisphosphonate cohort, 27.1 Kg/m2 compared to 25.5 Kg/m2. There were only small differences in smoking and alcohol status between the cohorts. Ever use of HRT, NSAIDs, PPIs and H2RAs before the index date was higher in the bisphosphonate cohort than in the control cohort.
Table 1.
Participant characteristics in bisphosphonate cohort and matched control cohort (including only individuals with more than 6 months follow-up).
| Characteristic | Bisphosphonate cohort |
Matched control cohort |
||
|---|---|---|---|---|
| n (%) | Mean (sd) | % (n) | Mean (sd) | |
| Age | 41,826 (100%) | 70.0 (11.4) | 41,826 | 70.0 (11.4) |
| Sex | ||||
| Male | 7,777 (19%) | 7,777 (19%) | ||
| Female | 34,049 (81%) | 34,049 (81%) | ||
| Any bisphosphonate prescription (during follow-up period) |
41,826 (100%) | 3,705 (9%) | ||
| Bisphosphonate in DDDs per day (during follow-up period) |
41,826 | 0.59 (0.49) | 41,826 | 0.03 (0.16) |
| Follow-up (years) | 41,826 (100%) | 4.5 (2.6) | 41,826 (100%) | 4.4 (2.6) |
| BMI | 20,199 (48%) | 25.5 (2.25) | 17,513 (42%) | 27.1 (2.25) |
| Missing | 21,627 (52%) | 24,313 (58%) | ||
| Smoking | ||||
| Never | 12,609 (30%) | 11,871 (28%) | ||
| Ex | 6,916 (17%) | 5,689 (14%) | ||
| Current | 4,328 (10%) | 3,531 (8%) | ||
| Missing | 17,973 (43%) | 20,735 (50%) | ||
| Alcohol | ||||
| Never | 3,619 (9%) | 3,178 (8%) | ||
| Ex | 534 (1%) | 369 (1%) | ||
| Current | 11,146 (27%) | 10,406 (25%) | ||
| Missing | 26,527 (63%) | 27,873 (67%) | ||
| HRT prescription (ever, before index date) |
10,281 (25%) | 7,774 (19%) | ||
| NSAID prescription (ever, before index date) |
34,113 (82%) | 29,287 (70%) | ||
| PPI prescription (ever, before index date) |
12,961 (31%) | 8,847 (21%) | ||
| H2 prescription (ever, before index date) |
14,495 (35%) | 9,098 (22%) | ||
| Barretts esophagus diagnosis (ever, before index date) |
198 (0.5%) | 145 (0.4%) | ||
| GERD diagnosis (ever, before index date) |
5,016 (12%) | 3,657 (9%) | ||
Any bisphosphonate use
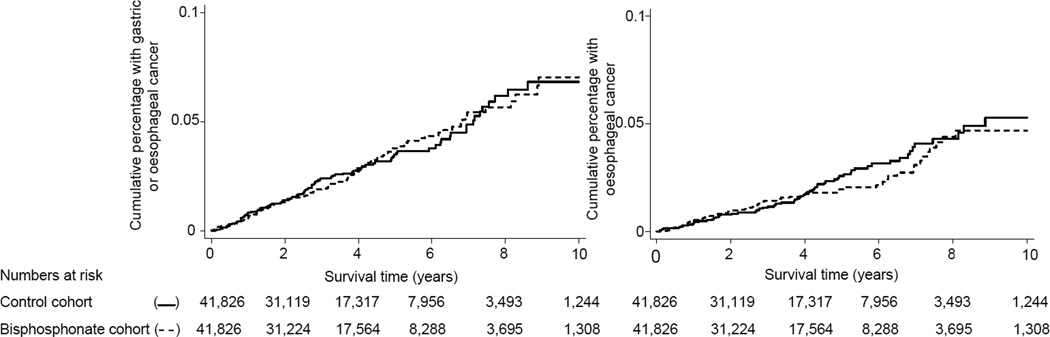
Figure 1 shows the cumulative incidences of combined esophageal and gastric cancers and esophageal cancers in the cohorts. There was no difference in combined esophageal and gastric cancer risk between the cohorts before or after adjustments for potential confounders; HR (95% CI) 1.00 (0.77, 1.29) and 0.96 (0.74, 1.25) respectively, Table 2. Similarly, there was no difference in esophageal cancer risk between the two cohorts; adjusted HR (95% CI) 1.07 (0.77, 1.49), Table 3. Tables 2 and 3 also show that after receiving specified amounts of bisphosphonate DDDs there was no evidence of an increase in esophageal and gastric cancer risk (or esophageal cancer risk). For instance, after receiving 365 bisphosphonate DDDs (equivalent to a 1 year supply) the risk of esophageal and gastric cancer (or esophageal cancer) were similar in the bisphosphonate and control cohorts: unadjusted HR (95% CI) 0.94 (0.64, 1.39) and 0.88 (0.55, 1.43), respectively . Table 3 also shows no increase in esophageal and gastric cancer risk (or esophageal cancer risk) in the groups with higher use of bisphosphonates based on DDDs per day. There was no association between any bisphosphonate use and risk of these cancers when members of the control cohort who were prescribed bisphosphonates subsequent to the index date (and their matched bisphosphonate cohort member) were excluded from the analysis, adjusted HRs (95% CI), 0.92 (0.70, 1.21) and 1.01 (0.72, 1.42) for risk of esophageal and gastric cancer and esophageal cancer only, respectively. When, in order to maximise follow-up, we restricted the analysis to patients whose date of first receipt of bisphosphonates was before 1/1/2000 (and their matched controls), the adjusted HRs (95% CI) for esophageal and gastric cancer risk, and esophageal cancer risk, were 1.19 (0.69, 2.05) and 1.23 (0.66, 2.30), respectively, for any bisphosphonate use. This analysis included 7,082 members (17%) from each cohort and mean (SD) follow-up was 6.8 (3.7) years.
Figure 1.
Time to esophageal and gastric cancer or esophageal cancer only in the bisphosphonate (—) and contol (--) cohort.
Table 2.
Esophageal and gastric cancer incidence in the bisphosphonate and control cohorts.
| Bisphosphonate cohort | Matched control cohort | Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|---|---|
| Cases | Person years | Cases | Person years | Hazard ratio (95%CI) | P | Hazard ratio (95%CI) | P | |
| Any bisphosphonate | 116 | 165,400 | 115 | 163,479 | 1.00 (0.77, 1.29) | 0.98 | 0.96 (0.74,1.25) | 0.77 |
| After cumulative bisphosphonate prescriptions greater than‡ | ||||||||
| 183 DDDs (~6 month supply) | 75 | 104,678 | 75 | 104,104 | 1.00 (0.72, 1.37) | 0.98 | 1.01 (0.73, 1.40) | 0.96 |
| 365 DDDs (~1 year supply) | 50 | 73,364 | 53 | 73,171 | 0.94 (0.64, 1.39) | 0.76 | 0.98 (0.66, 1.45) | 0.90 |
| 730 DDDs (~2 year supply) | 28 | 40,326 | 29 | 40,491 | 0.97 (0.58, 1.63) | 0.91 | 0.96 (0.56, 1.63) | 0.87 |
| 1095 DDDs (~3 year supply) | 16 | 22,813 | 17 | 22,891 | 0.95 (0.48, 1.87) | 0.88 | 0.90 (0.44, 1.81) | 0.76 |
| Total bisphosphonate intake during follow-up† (in DDDs per day) | ||||||||
| Low use (>0 – 0.24) | 48 | 62,922 | 45 | 63,648 | 1.08 (0.72, 1.62) | 0.71 | 0.95 (0.63, 1.45) | 0.83 |
| Medium use (0.24 – 0.89) | 35 | 58,161 | 36 | 55,334 | 0.93 (0.58, 1.48) | 0.74 | 0.96 (0.59, 1.54) | 0.86 |
| High use (≥ 0.89) | 33 | 44,316 | 34 | 44,497 | 0.98 (0.60, 1.58) | 0.92 | 0.96 (0.59, 1.58) | 0.89 |
| Nitrogen containing bisphosphonate first prescribed |
71 | 106,480 | 77 | 106,412 | 0.92 (0.67, 1.27) | 0.63 | 0.91 (0.65, 1.27) | 0.59 |
| After cumulative nitrogen containing bisphosphonate prescriptions greater than‡: | ||||||||
| 365 DDDs (~1 year supply) | 48 | 70,251 | 51 | 69,935 | 0.94 (0.63, 1.39) | 0.75 | 0.98 (0.65, 1.47) | 0.92 |
| 730 DDDs (~2 year supply) | 27 | 39,022 | 29 | 39,187 | 0.94 (0.55, 1.58) | 0.81 | 0.92 (0.54, 1.58) | 0.77 |
| Alendronate first prescribed | 55 | 81,369 | 67 | 80,837 | 0.82 (0.57, 1.17) | 0.27 | 0.79 (0.55, 1.15) | 0.22 |
| After cumulative alendronate containing bisphosphonate prescriptions greater than‡: | ||||||||
| 365 DDDs (~1 year supply) | 34 | 52,308 | 45 | 51,741 | 0.75 (0.48, 1.17) | 0.20 | 0.75 (0.48, 1.19) | 0.23 |
| 730 DDDs (~2 year supply) | 22 | 28,898 | 28 | 28,04 | 0.79 (0.45, 1.38) | 0.40 | 0.76 (0.43, 1.35) | 0.35 |
| Non-nitrogen containing bisphosphonate first prescribed |
45 | 58,920 | 38 | 57,068 | 1.14 (0.74, 1.76) | 0.55 | 1.04 (0.67, 1.62) | 0.87 |
Adjusted for BMI, alcohol, smoking, HRT prescription (before index date), NSAID prescription (before index date), Barretts esophagus diagnosis (before index date), GERD diagnosis esophagus (before index date), H2 (before index date), PPIs (before index date).
In bisphosphonate cohort.
Person years and cancer cases occurring after the date of specified bisphosphonate prescriptions received for each bisphosphonate cohort member and their matched control.
Table 3.
Esophageal (only) cancer incidence in the bisphosphonate and control cohorts.
| Bisphosphonate cohort | Matched control cohort | Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|---|---|
| Cases | Person years | Cases | Person years | Hazard ratio (95%CI) | Cases | Person years | ||
| Any bisphosphonate | 79 | 165,400 | 72 | 163,480 | 1.08 (0.79, 1.49) | 0.63 | 1.07 (0.77, 1.49) | 0.67 |
| After cumulative bisphosphonate prescriptions greater than‡: | ||||||||
| 183 DDDs (~6 month supply) | 51 | 104,676 | 49 | 104,104 | 1.04 (0.70, 1.53) | 0.86 | 1.05 (0.70, 1.57) | 0.82 |
| 365 DDDs (~1 year supply) | 31 | 73,364 | 35 | 73,170 | 0.88 (0.55, 1.43) | 0.62 | 0.92 (0.56, 1.51) | 0.74 |
| 730 DDDs (~2 year supply) | 22 | 40,326 | 22 | 40,492 | 1.00 (0.56, 1.81) | 0.99 | 0.98 (0.53, 1.81) | 0.95 |
| 1095 DDDs (~3 year supply) | 15 | 22,813 | 14 | 22,891 | 1.08 (0.52, 2.23) | 0.84 | 1.01 (0.48, 2.12) | 0.99 |
| Any bisphosphonate intake during follow-up† (in DDDs per day) | ||||||||
| Low use (>0 – 0.24) | 35 | 62,922 | 27 | 63,648 | 1.31 (0.80, 2.17) | 0.29 | 1.24 (0.74, 2.09) | 0.41 |
| Medium use (0.24 – 0.89) | 24 | 58,162 | 23 | 55,334 | 0.98 (0.55, 1.74) | 0.94 | 1.03 (0.57, 1.86) | 0.92 |
| High use (≥ 0.89) | 20 | 44,316 | 22 | 44,497 | 0.91 (0.50, 1.67) | 0.78 | 0.90 (0.48, 1.68) | 0.74 |
| Nitrogen containing bisphosphonate first prescribed | 44 | 106,480 | 47 | 106,412 | 0.94 (0.62, 1.41) | 0.75 | 0.96 (0.63, 1.47) | 0.86 |
| After cumulative nitrogen containing bisphosphonate prescriptions greater than‡: | ||||||||
| 365 DDDs (~1 year supply) | 30 | 70,251 | 34 | 69,935 | 0.88 (0.54, 1.44) | 0.61 | 0.93 (0.56, 1.54) | 0.78 |
| 730 DDDs (~2 year supply) | 22 | 39,022 | 22 | 39,187 | 1.01 (0.56, 1.82) | 0.99 | 0.98 (0.53, 1.80) | 0.95 |
| Alendronate first prescribed | 33 | 81,369 | 42 | 80,837 | 0.78 (0.50, 1.23) | 0.29 | 0.77 (0.48, 1.23) | 0.27 |
| After cumulative alendronate containing bisphosphonate prescriptions greater than‡ | ||||||||
| 365 DDDs (~1 year supply) | 22 | 52,308 | 31 | 51,741 | 0.70 (0.41, 1.21) | 0.20 | 0.68 (0.39, 1.19) | 0.18 |
| 730 DDDs (~2 year supply) | 19 | 28,898 | 21 | 28,904 | 0.91 (0.49, 1.68) | 0.75 | 0.85 (0.45, 1.61) | 0.62 |
| Non-nitrogen containing bisphosphonate first prescribed | 35 | 58,920 | 25 | 57,068 | 1.35 (0.81, 2.25) | 0.25 | 1.25 (0.73, 2.12) | 0.37 |
Adjusted for BMI, alcohol, smoking, HRT prescription (before index date), NSAID prescription (before index date), Barretts esophagus diagnosis (before index date), GERD diagnosis esophagus (before index date), H2 (before index date), PPIs (before index date).
In bisphosphonate cohort.
Person years and cancer cases occurring after the date of specified prescriptions received for each bisphosphonate cohort member and their matched control.
Bisphosphonate subtypes
Table 2 also shows no association between the risk of esophageal and gastric cancer or esophageal cancer only and nitrogen containing bisphosphonates (adjusted HRs 0.91 and 0.96), aldendronate (adjusted HRs 0.79 and 0.77, respectively) or non-nitrogen containing bisphosphonates. Similarly, there was no evidence of an association with risk of these cancers after receiving over 1 year (or 2 years) of prescriptions for either nitrogen containing bisphosphonates or alendronate.
Cancer risk in cohort members with a history of gastro-esophageal reflux disease (GERD) or Barrett’s esophagus
5,016 (12%) and 3,657 (9%) members of the bisphosphonate cohort and control cohorts, respectively, had GERD codes recorded prior to their index date (Table 1). The association between GERD and incidence of esophageal and gastric cancer, or esophageal cancer only, did not differ between the bisphosphonate and control cohort (P for interaction term = 0.74 and 0.99, respectively). Specifically, GERD diagnosis was associated with a 49% increase in the incidence of esophageal and gastric cancer (HR =1.49, 95%CI 0.85, 2.61) in the bisphosphonate cohort and a 69% increase in the control cohort (HR=1.69, 95%CI 1.06, 2.71), with similar increases in risk seen for esophageal cancer only. 198 (0.47%) and 145 (0.35%) members of the bisphosphonate cohort and control cohorts, respectively, had Barrett’s esophagus codes recorded prior to their index date (Table 1). Only one of these (in the control cohort) developed esophageal or gastric cancer.
COMMENT
In this study we found no difference in the incidence of esophageal and gastric cancer (or esophageal cancer alone) in a large cohort of mainly elderly women exposed to oral bisphosphonates compared to an age- and sex-matched unexposed population. There was no increase in the risk of these cancers in patients who had ever been prescribed bisphosphonates, those who had been prescribed nitrogen containing bisphosphonates, alendronate or non-nitrogen containing bisphosphonates. There was also no association with cancer risk by duration of use of these drugs. Esophageal and gastric cancer incidence in patients with a history of GERD was not different in those exposed to bisphosphonates compared to those not exposed to these drugs.
Strengths of our study were its large size, substantial period of follow-up, and the use of recorded prescription data rather than self reported drug use, which may misclassify exposure. Additionally, underestimation of bisphosphonate usage would seem unlikely as these drugs cannot be obtained without prescription in the UK. However, based on the confidence intervals of the hazard ratios, a modest (<30%) increase in risk of esophageal and gastric cancer in bisphosphonate users cannot be excluded and a modest protective effect (20–25% decrease in risk) is also possible. Data from preclinical studies indicate that bisphosphonates, especially nitrogen-containing bisphosphonates, may affect tumor proliferation, invasion and angiogenesis, potentially reducing cancer risk.13–16
Our findings agree with recent brief reports from studies showing no increase in esophageal cancer risk in users of oral bisphosphonates 8–9 but these studies included few events, had short follow-up periods and did not consider potential confounders.
Our study also has several limitations. As exposure was determined from recorded prescriptions, overestimation of usage is possible as compliance with bisphosphonate prescribing is known to be suboptimal.17 A further weakness was the ascertainment of cancer incidence as our GPRD data was not linked to cancer registries, therefore we relied on relevant diagnostic codes from patients’ clinical files. Some inaccuracy is therefore inevitable, although the recording of cancer outcomes within GPRD has been shown to be high18 and, in this dataset, less than 10% of cases with an esophageal or gastric cancer had inconsistently recorded cancer codes. The lack of information on histological subtype of esophageal cancers is also a weakness and it is possible an association with either esophageal adenocarcinoma or squamous cell carcinoma was obscured . However, we did not see an increased risk of esophageal/gastric cancer in patients with a prior history of GERD who received bisphosphonates compared to those who did not receive these drugs. Too few patients had a history of Barrett’s esophagus to examine esophageal or gastric cancer rates in this subgroup, which is predisposed to esophageal adenocarcinoma. Another limitation of our study was the relatively high proportion of missing data on potential confounders. It is possible that residual confounding by poorly measured or unmeasured confounders may have masked an association between the use of bisphosphonates and esophageal and gastric cancer risk but the estimates seen in an analysis involving only subjects with complete data on confounders were not different from those in the principal analyses.
In conclusion, this study does not provide evidence for an increased risk of esophageal (or gastric) cancer in persons using oral bisphosphonates. These drugs should not be withheld, on the basis of possible esophageal cancer risk, from patients with a genuine clinical indication for their use.
Acknowledgements
This study is based in part on data from the Full Feature General Practice Research Database obtained under licence from the UK Medicines and Healthcare Products Regulatory Agency. However, the interpretation and conclusions contained in this study are those of the authors alone. Access to the GPRD database was funded through the Medical Research Council’s licence agreement with MHRA. The authors would like thank Mr Maciej Domanski, QUB, for data management and Professor Carmel Hughes, QUB, for commenting on the manuscript
Funding/Support and Role of Sponser: Access to the GPRD database was funded through the Medical Research Council’s licence agreement with MHRA. However, the interpretation and conclusions contained in this study are those of the authors alone.
Footnotes
Conflicts of Interest and Financial Disclosures: None.
Data Access and Responsibility: Dr Cardwell had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions:
Study concept and design: Abnet, Cardwell, Murray.
Acquisition of data: Cardwell, Murray.
Analysis and interpretation of data: Abnet, Cantwell, Cardwell, Murray.
Drafting of the manuscript: Cardwell, Murray.
Critical revision of the manuscript for important intellectual content: Abnet, Cantwell, Cardwell, Murray.
Statistical analysis: Cardwell.
References
- 1.Usher C, Teeling M, Bennett K, Feely J. Effect of clinical trial publicity on HRT prescribing in Ireland. Eur J Clin Pharmacol. 2006;62(4):307–310. doi: 10.1007/s00228-005-0083-x. [DOI] [PubMed] [Google Scholar]
- 2.Udell JA, Fischer MA, Brookhart MA, Solomon DH, Choudhry NK. Effect of the Women's Health Initiative on osteoporosis therapy and expenditure in Medicaid. J Bone Miner Res. 2006;21(5):765–771. doi: 10.1359/jbmr.060119. [DOI] [PubMed] [Google Scholar]
- 3.Watson J, Wise L, Green J. Prescribing of hormone therapy for menopause, tibolone, and bisphosphonates in women in the UK between 1991 and 2005. Eur J Clin Pharmacol. 2007;63(9):843–849. doi: 10.1007/s00228-007-0320-6. [DOI] [PubMed] [Google Scholar]
- 4.Ryan JM, Kelsey P, Ryan BM, Mueller PR. Alendronate-induced esophagitis: case report of a recently recognized form of severe esophagitis with esophageal stricture--radiographic features. Radiology. 1998;206(2):389–391. doi: 10.1148/radiology.206.2.9457190. [DOI] [PubMed] [Google Scholar]
- 5.de Groen PC, Lubbe DF, Hirsch LJ, et al. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335(14):1016–1021. doi: 10.1056/NEJM199610033351403. [DOI] [PubMed] [Google Scholar]
- 6.Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360(1):89–90. doi: 10.1056/NEJMc0808738. [DOI] [PubMed] [Google Scholar]
- 7.Hofbauer LC, Miehlke S. More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360(17):1790–1792. [PubMed] [Google Scholar]
- 8.Abrahamsen B, Eiken P, Eastell R. More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360(17):1789–1782. doi: 10.1056/NEJMc096026. [DOI] [PubMed] [Google Scholar]
- 9.Solomon DH, Patrick A, Brookhart MA. More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360(17):1789–1790. [PubMed] [Google Scholar]
- 10.Hollowell J. The General Practice Research Database: quality of morbidity data. Popul Trends. 1997;(87):36–40. [PubMed] [Google Scholar]
- 11.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302(6779):766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breslow NE, Day NE. Statistical methods in cancer research: Volume II - The design and analysis of cohort studies. Oxford: Oxford University Press; 1987. [PubMed] [Google Scholar]
- 13.Shipman CM, Rogers MJ, Apperley JF, Russell RG, Croucher PI. Bisphosphonates induce apoptosis in human myeloma cell lines: a novel anti-tumour activity. Br J Haematol. 1997;98(3):665–672. doi: 10.1046/j.1365-2141.1997.2713086.x. [DOI] [PubMed] [Google Scholar]
- 14.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J. Clin Invest. 2004;114(5):623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coxon JP, Oades GM, Kirby RS, Colston KW. Zoledronic acid induces apoptosis and inhibits adhesion to mineralized matrix in prostate cancer cells via inhibition of protein prenylation. BJU Int. 2004;94(1):164–170. doi: 10.1111/j.1464-4096.2004.04831.x. [DOI] [PubMed] [Google Scholar]
- 16.Guise TA. Antitumor effects of bisphosphonates: promising preclinical evidence. Cancer Treat Rev. 2008;34(Suppl 1):S19–S24. doi: 10.1016/j.ctrv.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18(8):1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 18.Jick H, Jick S, Derby LE, et al. Calcium-channel blockers and risk of cancer. Lancet. 1997;349(9051):525–528. doi: 10.1016/S0140-6736(97)80084-0. [DOI] [PubMed] [Google Scholar]