SUMMARY
Prominent subtypes of the genetic disorder, Hermansky-Pudlak Syndrome, result from defects in a mysterious protein complex, BLOC-3. New work identifies BLOC-3 as a guanine nucleotide exchange factor for two Rab GTPases previously implicated in lysosome-related organelle biogenesis.
Lysosome-related organelles (LROs) comprise a group of specialized, tissue-specific subcellular compartments that derive from the endocytic pathway [1]. They include melanosomes - pigment organelles of skin and ocular melanocytes, retinal pigment epithelia (RPE), and other ocular cells - and additional organelles that regulate thrombosis, lung plasticity, immunity and other processes. Some LROs are malformed in Hermansky-Pudlak syndrome (HPS), a group of genetic diseases characterized by partial albinism, excessive bleeding, and often a lethal lung fibrosis [2]. The nine known genes that are mutated in HPS encode subunits of protein complexes that are thought to regulate membrane trafficking during LRO biogenesis. For example, the AP-3 complex (defective in HPS2), Biogenesis of Lysosome-related Organelles Complex (BLOC)-1 (defective in HPS7-9), and BLOC-2 (defective in HPS3, 5 and 6) regulate delivery of distinct cohorts of cargoes from early endosomes to nascent melanosomes [3]. Similarly, the small GTPase RAB38 - mutagenized in a rat HPS model - functions in a partially redundant pathway with the highly similar RAB32 to regulate melanosome cargo delivery [4]. However, BLOC-3 – composed of the products of genes defective in HPS1 and 4, the most prominent and lethal HPS forms – had no known role in cargo transport until now. In this issue, Gerondopoulos et al [5] show that BLOC-3 functions as a guanine nucleotide exchange factor (GEF) for RAB32 and RAB38 and is required for melanosome cargo delivery and consequent pigmentation in a cultured melanocyte model.
The HPS1 and 4 subunits of BLOC-3 lack obvious structural domains but share limited regions of sequence homology with Mon1 and Ccz1, respectively [6, 7]. Mon1 and Ccz1 comprise a GEF (GEFs activate RAB proteins by facilitating their release of GDP and association with GTP) for the yeast orthologue of RAB7, a small GTPase that regulates late endosome maturation and fusion with lysosomes [8]. Gerondopoulos et al purified recombinant human CCZ1/MON1 and HPS1/4 complexes either from bacteria or following overexpression in cultured cells, and screened them for GEF activity on a panel of endosomal Rabs. Whereas CCZ1/MON1 exchange activity was limited to RAB7, HPS1/HPS4 was active exclusively for RAB32 and RAB38. Moreover, when overexpressed with HPS1 in HeLa cells, a mitochondrially restricted form of HPS4 preferentially recruited GFP-tagged RAB32 or RAB38 – but not the related RAB7 or RAB9 – to mitochondria. Activity in both assays required both HPS1 and HPS4. Finally, depletion of HPS1 or HPS4 by siRNA in a pigmented human melanoma cell line, MNT-1, resulted in the mislocalization of RAB32. These data provide strong evidence that BLOC-3 is a selective GEF for RAB32 and RAB38 [5].
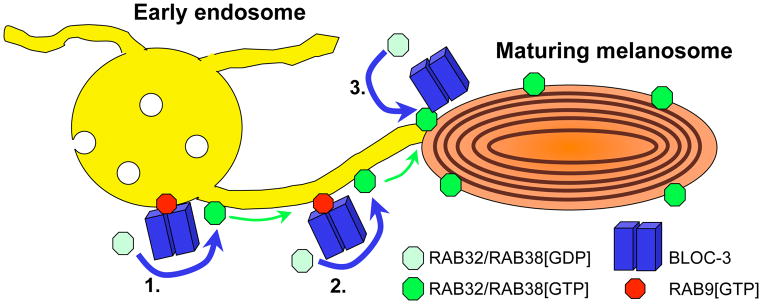
The identification of BLOC-3 as a RAB32/38 GEF makes two predictions. First, it should overlap sites of active RAB32/38 in cells. Consistently, HPS4 was detected in structures adjacent to GFP-RAB32-containing melanosomes by immunofluorescence microscopy in MNT-1 cells [5]. These data support ultrastructural studies that localized RAB38 primarily to melanosomes but also to nearby tubulovesicular structures [4], and BLOC-3 to similar tubulovesicular structures [9]. Intriguingly, melanosomes in melanocytic cells make transient contacts with closely apposed domains of recycling endosomes via tubular intermediates, providing conduits for melanosome cargo delivery [10]. The data of Gerondopoulos et al would be consistent with a model in which BLOC-3 activates RAB32/38 on these domains or the tubular intermediates, thereby positioning the Rabs to facilitate dynamic interactions between the tubules and melanosomes and perhaps additional subsequent processes from the melanosomes themselves (Figure 1, steps 1 and 2). An exciting speculation is that BLOC-3 might be recruited to endosomal domains by active RAB9[GTP], a BLOC-3 binding partner [11], in a Rab cascade for melanosome biogenesis would parallel those involved in endosome maturation and secretion.
Figure 1. Model for BLOC-3 and RAB32/38 function in melanosome biogenesis.
Specialized domains of early endosomes (yellow, left) approximate maturing melanosomes (orange, right) and make tubular connections that are required for cargo transfer from endosomes to melanosomes. BLOC-3 (blue bars) functions as a GEF to facilitate activation of RAB32 and/or RAB38 from the GDP-bound state (pale green octagon) to the GTP-bound state (bright green octagon). This might occur on the endosome (step 1) or on the tubular connections (step 2); BLOC-3 might be recruited to these sites by active RAB9 (red octagon). Once activated, RAB32 and/or RAB38 likely facilitate targeting and/or fusion of tubular connections with melanosomes, facilitating cargo transfer. RAB32 and RAB38 accumulate on melanosomes either by accompanying cargoes during transfer from endosomes or, alternatively or additionally, following activation by a pool of BLOC-3 on melanosomes (step 3). The melanosomal pool of Rabs likely facilitates downstream steps in melanosome maturation.
The second prediction is that BLOC-3 depletion should inactivate the Rabs and phenocopy RAB32/38 depletion. The new data also support this prediction. In the Rab38 mutant chocolate mouse, skin and choroidal melanocytes harbor small malformed melanosomes, causing pigment dilution in the eyes and coat [12]. Additional depletion of RAB32 from chocolate melanocytes causes further pigment loss, mature melanosome depletion and missorting/ degradation of the melanogenic enzymes Tyrosinase and TYRP1 [4]. Consistently, siRNA-mediated depletion of either HPS1 or HPS4 in MNT-1 cells also induced pigment loss, mature melanosome depletion, non-pigmented early stage melanosome accumulation, and TYRP1 mislocalization [5]. These data suggest that BLOC-3 is required for RAB32/38 function in MNT-1 cells.
Does defective RAB32/38 function explain the hypopigmentation in HPS1 and HPS4? Not exactly. While the RPE and ear and tail skin are severely hypopigmented in the pale ear and light ear mouse models of HPS1 and 4, hair pigmentation is largely unaffected [13, 14], contrasting with the obvious coat color dilution in chocolate mice [12]. The melanin content and mature melanosome numbers in pale ear and light ear hair bulb melanocytes (responsible for hair pigmentation) are only slightly diminished relative to controls [15], and melanocytes from the choroid or hair bulb harbor enlarged, highly pigmented melanosomes [14]. This contrasts with the small melanosomes in Rab38 mutant chocolate melanocytes [12] and severe pigment loss upon concomitant depletion of RAB32 [4]. How can these findings be reconciled with Gerondopoulos et al [5]?
Several potential explanations for these disparate data suggest interesting complexities in the roles of BLOC-3 and RAB32/38 that warrant further investigation. Importantly, not all pigment cells are created equal. Unlike the hair bulb melanocytes in HPS1 model pale ear mice, interfollicular dermal melanocytes (responsible for skin pigmentation), like the RPE, are severely hypopigmented and have reduced Tyrosinase enzyme activity [16], similar to BLOC-3-depleted MNT-1 cells [5]. The most straightforward potential explanation is that hair bulb and choroidal melanocytes specifically express a redundant GEF for RAB32, RAB38 or both that is not expressed by the developmentally distinct interfollicular melanocytes and RPE. It will thus be critical to test the disposition of RAB32/38 in distinct BLOC-3-deficient pigment cell types, and perhaps seek additional GEF activities in hair bulb melanocytes. The enlarged melanosomes in BLOC-3-deficient hair bulb melanocytes likely reflect interference with a second Rab-dependent pathway, downstream of cargo delivery, that also requires BLOC-3 GEF activity (Figure 1, step 3). This pathway might stimulate membrane retrieval from melanosomes or antagonize melanosome fusion with other organelles, and could be regulated either by RAB32 or RAB38 themselves or by another Rab target of BLOC-3 GEF activity. Melanocytes express several poorly characterized tissue-restricted Rabs (e.g. see [17]); although most are more distantly related to Rab32/38 than those tested by Gerondopoulos et al [5, 18], a more extended analysis of Rab specificity is warranted. With recombinant BLOC-3 and Rabs in hand, it should be possible to test these speculative models using in vitro assays and purified melanosome preparations.
The RAB32/38 GEF activity of BLOC-3 likely explains additional LRO defects observed in HPS1 and 4 patients. For example, HPS1, HPS4 or RAB38 mutant animals have enlarged lamellar bodies in type II lung epithelial cells and defective surfactant secretion [19, 20], likely underlying the lethal lung fibrosis that afflicts most HPS1 and 4 patients. A similar BLOC-3/ RAB38-dependent cargo delivery pathway from endosomes likely operates in these cells. Testing this model and identifying affected cargoes will be of utmost importance to begin to develop therapies for the most lethal symptoms in HPS patients.
References
- 1.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-golgi compartments into specialisation. Curr Opin Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei ML. Hermansky–Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 3.Sitaram A, Marks MS. Mechanisms of protein delivery to melanosomes in pigment cells. Physiology. 2012 doi: 10.1152/physiol.00043.2011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 an Rab32 control early post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012 doi: 10.1016/j.cub.2012.09.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman-Sommer M, Grynberga M, Kucharczyka R, Rytkaa J. The CHiPS domain. Ancient traces for the Hermansky Pudlak Syndrome. Traffic. 2005;6:534–538. doi: 10.1111/j.1600-0854.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 7.Kinch LN, Grishin NV. Longin-like folds identified in CHiPS and DUF254 proteins: vesicle trafficking complexes conserved in eukaryotic evolution. Protein Sci. 2006;15:2669–2674. doi: 10.1110/ps.062419006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordmann M, Cabrera M, Perz A, Bröcker C, Ostrowicz C, Engelbrecht-Vandré S, Ungermann C. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol. 2010;20:1654–1659. doi: 10.1016/j.cub.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Oh J, Liu ZX, Feng GH, Raposo G, Spritz RA. The Hermansky-Pudlak syndrome (HPS) protein is part of a high molecular weight complex involved in biogenesis of early melanosomes. Hum Mol Genet. 2000;9:375–385. doi: 10.1093/hmg/9.3.375. [DOI] [PubMed] [Google Scholar]
- 10.Delevoye C, Hurbain I, Tenza D, Sibarita JB, Uzan-Gafsou S, Ohno H, Geerts WJC, Verkleij AJ, Salamero J, Marks MS, et al. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol. 2009;187:247–264. doi: 10.1083/jcb.200907122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloer DR, Rojas R, Ivan V, Moriyama K, van Vlijmen T, Murthy N, Ghirlando R, van der Sluijs P, Hurley JH, Bonifacino JS. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J Biol Chem. 2010;285:7794–7804. doi: 10.1074/jbc.M109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loftus SK, Larson DM, Baxter LL, Antonellis A, Chen YA, Wu XS, Jiang Y, Bittner M, Hammer JA, III, Pavan WJ. Mutation of melanosome protein RAB38 in chocolate mice. Proc Natl Acad Sci USA. 2002;99:4471–4476. doi: 10.1073/pnas.072087599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner JM, Wildenberg SC, Keiper NM, Novak EK, Rusiniak ME, Swank RT, Puri N, Finger JN, Hagiwara N, Lehman AL, et al. The mouse pale ear (ep) mutation is the homologue of human Hermansky-Pudlak syndrome. Proc Natl Acad Sci USA. 1997;94:9238–9243. doi: 10.1073/pnas.94.17.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki T, Li W, Zhang Q, Karim A, Novak EK, Sviderskaya EV, Hill SP, Bennett DC, Levin AV, Nieuwenhuis HK, et al. Hermansky-Pudlak syndrome is caused by mutations in HPS4, the human homolog of the mouse light-ear gene. Nature Genet. 2002;30:321–324. doi: 10.1038/ng835. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen T, Novak EK, Kermani M, Fluhr J, Peters LL, Swank RT, Wei ML. Melanosome morphologies in murine models of Hermansky-Pudlak syndrome reflect blocks in organelle development. J Invest Dermatol. 2002;119:1156–1164. doi: 10.1046/j.1523-1747.2002.19535.x. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen T, Wei ML. Hermansky-Pudlak HPS1/pale ear gene regulates epidermal and dermal melanocyte development. J Invest Dermatol. 2007;127:421–428. doi: 10.1038/sj.jid.5700566. [DOI] [PubMed] [Google Scholar]
- 17.Hoek K, Rimm DL, Williams KR, Zhao H, Ariyan S, Lin A, Kluger HM, Berger AJ, Cheng E, Trombetta ES, et al. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270–5282. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]
- 18.Diekmann Y, Seixas E, Gouw M, Tavares-Cadete F, Seabra MC, Pereira-Leal JB. Thousands of Rab GTPases for the cell biologist. PLoS Comput Biol. 2011;7:e1002217. doi: 10.1371/journal.pcbi.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guttentag SH, Akhtar A, Tao JQ, Atochina E, Rusiniak ME, Swank RT, Bates SR. Defective surfactant secretion in a mouse model of Hermansky-Pudlak syndrome. Am J Respir Cell Mol Biol. 2005;33:14–21. doi: 10.1165/rcmb.2004-0293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osanai K, Higuchi J, Oikawa R, Kobayashi M, Tsuchihara K, Iguchi M, Huang J, Voelker DR, Toga H. Altered lung surfactant system in a Rab38-deficient rat model of Hermansky-Pudlak syndrome. Am J Lung Cell Mol Physiol. 2010;298:L243–L251. doi: 10.1152/ajplung.00242.2009. [DOI] [PubMed] [Google Scholar]