Abstract
Objectives
To evaluate associations between mid- and late-life obesity and risk of dementia.
Design
Prospective cohort followed 5.4 years from 1992/4 through 1999.
Setting
Community-dwelling sample in four US sites recruited from Medicare eligibility files.
Participants
2,798 adults without dementia, mean age 74.7 years, 59.1% women, participating in the Cardiovascular Health Cognition Study completing a magnetic resonance image, measured for height and weight at baseline (late-life) and self-reporting weight at age 50 (mid-life). Body mass index (BMI) was calculated at both times.
Main Outcome Measures
Dementia, Alzheimer’s disease (AD) and vascular dementia (VaD) classified by a multidisciplinary committee using standardized criteria.
Results
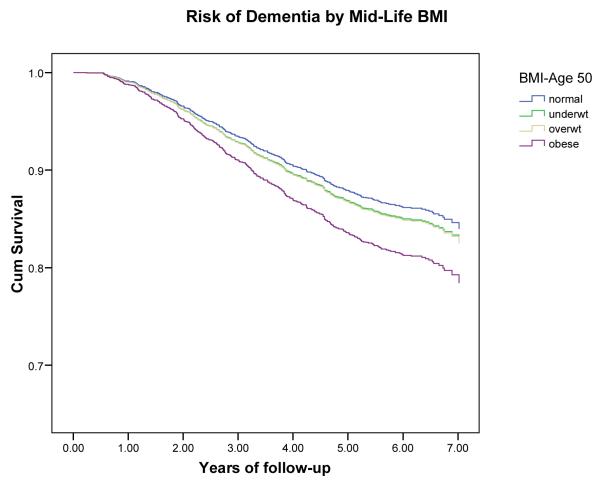
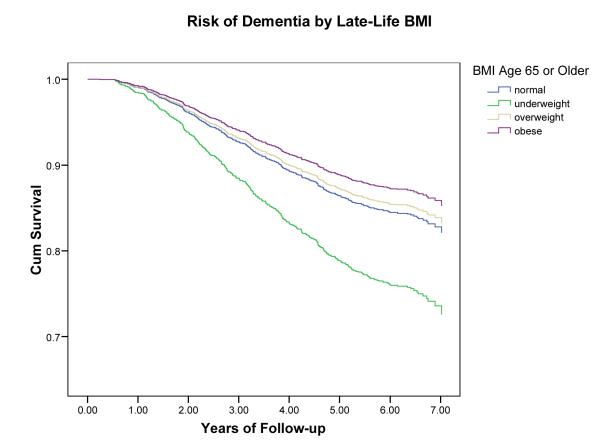
Classification resulted in 480 persons with incident dementia, 245 with AD (no VaD) and 213 with VaD (with or without AD). In evaluations of mid-life obesity, an increased risk of dementia was found for obese (BMI >30) compared to normal (BMI 20-25) persons adjusted for demographics (HR: 1.39, 95% CI: 1.03-1.87) and for caradiovascularl risk factors (HR: 1.36, 95% CI: 0.94-1.95). The risk estimates reversed in assessments of late-life BMI. Underweight persons (BMI < 20) had an increased risk of dementia (HR: 1.62, 95% CI: 1.02-2.64) while being overweight (BMI 25-30) was not associated (HR: 0.92, 95% CI: 0.72-1.18) and being obese reduced the risk of dementia (HR: 0.63, 95% CI: 0.44-0.91) compared to those with normal BMI.
Conclusions
These results help explain the “obesity paradox” as differences in dementia risk over time are consistent with physical changes in the trajectory toward disability.
INTRODUCTION
Dementia prevalence will quadruple by 2047 (1). Obesity, hyperinsulinemia, and diabetes are increasing worldwide (2-5). High adiposity predicts hyperinsulinemia and diabetes (6), both risk factors for dementia (7-12). While high adiposity in middle age appears to be related to greater dementia risk (13-14), studies exploring this association in the elderly are conflicting (15-18). Several reasons may explain these inconsistencies: (a) body mass index (BMI) may not be a good measure of adiposity in the elderly (19), (b) the association between high BMI and outcomes may be attenuated with age (20), and (c) low BMI is a marker of weight loss, frailty and pre-clinical dementia (21,22).
The term “obesity paradox” was coined after several studies reported excess weight, traditionally considered detrimental for health, to predict survival in the elderly (23-25). The Cardiovascular Health Study (CHS) allowed us to examine the obesity paradox in dementia as CHS collected BMI from at both mid- and late-life. In addition, adiposity was clinically measured several ways in late life. We sought to evaluate the associations between mid- and late-life BMI and the risk of dementia, Alzheimer’s disease (AD), and vascular dementia (VaD).
METHODS
CHS, a multi-site observational study of 5,888 adults age 65 years and older (26), was initiated in 1989. The CHS recruited 5201 participants during its initial wave from Medicare eligibility lists in four U.S. communities: Forsyth County, NC, Washington County, MD, Sacramento County, CA, and Pittsburgh, PA (27). In 1992/93 687 African-Americans were recruited. From its baseline (1989/90) until 1998/99, up to ten annual clinic visits were completed. Data collected at these examinations each year included demographics, anthropometry, vital signs, cognitive function, psychosocial interviews, depression, medical history, and physical function. Phlebotomy was performed for laboratory analyses. Surveillance and collection of events data is ongoing (28). All participants completed an informed consent and Institutional Review Board approvals were received at all sites. A separate DNA consent was obtained for genetic studies.
In 1998/99, dementia was classified in 3,602 CHS participants as a part of the CHS Cognition Study (29,30). Inclusion into the Cognition cohort required completion of a cranial MRI and the Modified Mini-Mental Status Exam or 3MSE in 1992/94. These participants were screened using data collected at the visit closest to the MRI to identify those at higher risk who were asked to return to the clinic for additional cognitive testing. An individual was considered to be at high risk of dementia if he/she had previously scored less than 80 or had a decrease of 5 or more points on the 3MSE administered at previous exams, a previous Telephone Interview for Cognitive Status (TICS) score <28 or an Informant Questionnaire on Cognitive Decline in the Elderly (IQCode) score of > 3.6, incident stroke, or currently residing in a nursing home. A battery of neuropsychiatric tests was administered to those agreeing to return to the clinic or to receive a home-visit using the following exams: the America version of the National Reading test, Raven’s Couloured Progressive Matrices, California Verbal Learning Test, Rey-Osterreith figure, Immediate and Delayed Recall, modified Boston Naming test, Verbal fluency test, Block design (modified from the Wechsler Adult Intelligence Scale-revised), Stroop Neuropsychological Screening Test, Trail Making, Digit Spans, and the Baddeley & Papagno Divided Attention Task. Methods to evaluate persons who declined the neuropsychiatric battery or were no longer living included a medical record review of all hospitalizations, questionnaires sent to his/her personal physician, and standardized interviews by phone with the participants (if living) or a designated informant (TICS, Neuropsychiatric Inventory, and/or IQCode). In addition, all prospectively collected data from the CHS’s inception were reviewed to provide additional information on cognitive decline over the ten years of follow-up including repeated measures of the 3MSE, Digit Symbol Substitution Test (DSST), Benton Visual Retention Test (BVRT), Trails A and B, CESD-Depression, medications inventory, Activities of Daily Living (ADL), Instrumental Activities of Daily Living (IADLs), other physical function measures (gait speed, balance tests, grip strength, etc), and documentation of hospitalized medical events such as strokes, myocardial infarctions, etc. All data were compiled into packets for review during the classification process.
Dementia classification was completed by consensus of neurologists and psychiatrists using data from the neuropsychiatric tests or by other data as noted above for deceased participants or those unable to come into the clinic. Cranial MRIs were used for classification of dementia subtype. AD was classified by National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer Disease and Related Disorders Association criteria (31). VaD was classified by State of California Alzheimer’s Disease Diagnostic and Treatment Centers (ADDTC) criteria (32). Dementia onset was determined by review of the longitudinal data collected during the 10 years of study follow-up and by family input using the NPI. If date of onset was determined to be prior to entry into the cognition cohort, the participant was determined to have prevalent dementia at baseline.
Anthropometric measurements in late-life were collected in-person at the clinic visit occurring closest to the CHS MRI. These measures included standing height measured in cm, weight measured in kg, waist and hip circumference measured in cm in 1992/93 (baseline for the Cognition Study). Mid-life weight, however, relied upon self-report of weight at age 50 collected in the Medical History form. Mid-life BMI was estimated using this self-report of participants’ “usual” weight at age 50 years and height measured at study baseline. BMI was calculated as weight divided by height squared (kg/m2), and WHR, as the ratio of waist to hip circumference. BMI was also categorized into four groups as follows: underweight (< 20), normal (20-25), overweight (>25-30) and obese (> 30) based on recommendations for older adults (33). Time to dementia was calculated in days from entry into the Cognition cohort until dementia onset, death, or July 1, 1999 (end of dementia follow-up).
Covariates examined included self-reported age, race (white vs non-white), gender and education (in years); diabetes was ascertained by American Diabetes Association definition. Hypertension was defined as a systolic blood pressure above 140 or diastolic over 190 mm/Hg. Coronary heart disease was based upon a history of myocardial infarction, angina, coronary bypass surgery, or angioplasty. Total cholesterol, C-reactive protein, interleukin-6, and apolipoprotein E (ApoE) genotype were assayed by the CHS Central Laboratory (34). Smoking status was self-reported (current, previous or never). Ankle-Arm Index was calculated using blood pressure at the brachial artery and ankle (35).
Of the 3,602 participants in the CHS Cognition Study, 227 participants with prevalent dementia at the MRI and 577 with mild cognitive impairment (MCI) were excluded. We calculated descriptive statistics for demographics and comorbidities by category of BMI. Chi-square tests and analyses of variance determined bivariate differences. The sample size for these analyses included 2,798 persons, 480 classified with dementia and 2,318 persons without dementia throughout follow-up. Cox proportional hazards regression estimated the risk of dementia associated with BMI at mid- and late-life as continuous and categorical variables. We also examined WHR as an exposure. Models were adjusted for demographics (age, gender, race, and education), cardiovascular and dementia risk factors including history of hypertension, diabetes, coronary heart disease, total cholesterol, ankle-arm blood pressure, C-reactive protein, Interleukin-6, smoking, kilocalories expended per week, and ApoE genotype. For dementia subtype, persons were censored at onset of VaD in models evaluating AD and for AD in models of VaD. All analyses were done using SPSS (version 13.0, Chicago, IL).
RESULTS
Of the 2,798 participants included in analyses, 480 were classified with incident dementia over an average of 5.4 years of follow-up. Of these, 245 were determined to have pure AD (AD without vascular dementia), 62 with pure vascular dementia (VaD without AD) and 151 with both AD and VaD, or mixed dementia. Due to the low number of cases with pure VaD, these were combined with mixed dementia to provide 213 cases for VaD-specific models. Age of participants ranged from 65 through 97 years with a mean age of 74.7 years (SD 4.8); 59% were women; 16% were African-American. Less than one-third of the sample (n=920) had a normal BMI at baseline. While only 117 were underweight (4.2%), 1,207 (43.1%) were overweight and 554 (19.8%) were obese.
Table 1 presents characteristics of the study sample by BMI category. BMI was related to primary demographics (age, gender, race and education), other risk factors for cardiovascular cardiovascular disease (Ankle-Arm Index) and measures of inflammation (C-reactive protein and Interleukin-6). BMI was not related to a history of coronary heart disease or the presence of the APOE-ε4 allele.
Table 1.
Selected characteristics of 2,798 participants in the Cardiovascular Health Cognition Study by categories of baseline body mass index (BMI).
| Characteristic* | BMI Category* | ||||
|---|---|---|---|---|---|
| Underweight (< 20) |
Normal (20 - 25) |
Overweight (>25 - 30) |
Obese (> 30) |
||
| N | 117 | 920 | 1207 | 554 | p** |
| N (%) or mean (SD) |
N (%) or mean (SD) |
N (%) or mean (SD) |
N (%) or mean (SD) |
||
| Age in years (mean/SD) | 76.1 (5.3) | 75.0 (5.0) | 74.4 (4.8) | 73.6 (4.3) | <.001 |
| Gender | |||||
| Female | 91 (5.5) | 555 (33.6) | 635 (38.4) | 372 (22.5) | <.001 |
| Male | 26 (2.3) | 365 (31.8) | 572 (50.0) | 182 (15.9) | |
| Race | |||||
| White | 107 (4.3) | 875 (35.0) | 1056 (42.1) | 465 (18.6) | <.001 |
| Non-White | 10 (3.4) | 45 (15.3) | 151 (51.1) | 89 (30.2) | |
| Education | |||||
| LT HS | 26 (4.3) | 160 (26.8) | 263 (44.0) | 149 (24.9) | |
| HS Graduate | 33 (4.1) | 258 (32.1) | 353 (43.9) | 160 (19.9) | <.001 |
| Some College | 23 (3.3) | 235 (33.5) | 299 (42.6) | 145 (20.6) | |
| College Graduate | 34 (4.9) | 267 (38.8) | 292 (42.4) | 96 (13.9) | |
| Smoking Status | |||||
| Never | 63 (5.0) | 401 (31.7) | 539 (42.7) | 260 (20.6) | |
| Former | 28 (2.2) | 415 (32.2) | 586 (45.5) | 259 (20.1) | <.001 |
| Current | 26 (10.6) | 103 (41.9) | 82 (33.3) | 35 (14.2) | |
| History of Diabetes | |||||
| Normal | 108 (5.1) | 779 (36.5) | 911 (42.7) | 334 (15.7) | |
| Impaired Fasting Glucose | 5 (1.7) | 65 (21.6) | 135 (44.8) | 96 (31.9) | <.001 |
| Diabetes | 3 (0.8) | 75 (21.3) | 153 (43.3) | 122 (34.6) | |
| History of Hypertension | |||||
| Normal | 65 (5.3) | 478 (38.9) | 516 (42.0) | 169 (13.8) | |
| Borderline | 16 (4.5) | 123 (34.9) | 149 (42.4) | 64 (18.2) | <.001 |
| Hypertension | 36 (3.0) | 319 (26.2) | 542 (44.4) | 321 (26.4) | |
| History of Coronary Heart Disease |
21 (3.8) | 187 (34.2) | 231 (42.2) | 108 (19.7) | .88 |
| Ankle Arm Index | |||||
| > 1.0 | 80 (3.5) | 723 (32.0) | 1000 (44.3) | 456 (20.2) | |
| 1.0-0.9 | 21 (8.2) | 100 (39.1) | 91 (35.5) | 44 (17.2) | .001 |
| < 0.9 | 15 (5.6) | 96 (35.5) | 109 (40.4) | 50 (18.5) | |
| Total Cholesterol (mean/SD) | 197.2 (38.4) | 206.6 (36.8) | 211 2 (38.6) | 211.8 (37.4) | <.001 |
| C-Reactive Protein (mean/SD) |
4.49 (13.27) | 4.08 (7.43) | 4.68 (8.28) | 6.79 (9.59) | <.001 |
| Interleukin 6 (mean/SD) | 1.72 (1.59) | 1.72 (1.30) | 2.01 (1.77) | 2.43 (2.35) | <.001 |
| ApoE Genotype | |||||
| Presence of ε4 allele | 33 (5.5) | 204 (33.7) | 259 (42.8) | 109 (18.0) | .32 |
| No ε4 allele | 79 (4.1) | 626 (32.1) | 851 (43.7) | 391 (20.1) | |
Details and definitions of characteristics provided in the Methods.
Row percentages are shown across BMI; p-values are based on chi-square tests for categorical variables and analysis of variance for continuous variables.
Higher mid-life BMI was not associated with lower dementia risk (Table 2) using BMI as a continuous variable adjusted for demographics and cardiovascular risk factors ( HR per BMI unit kg/m2: 1.01, 95% CI: 0.98-1.04). However, in the categorical models, being obese was associated with a 40% increased risk of dementia adjusted for demographics (HR: 1.39, 95% CI: 1.03-1.87) although the association was attenuated in the fully adjusted model (HR:1.36, 0.94-1.95). The relationships were similar for AD and VaD. Being underweight at mid-life was not associated with dementia, AD or VaD.
Table 2.
Mid-life Body mass index (BMI) estimated at age 50 years and risk of dementia, Alzheimer’s disease (AD) and vascular dementia (VaD) in 2,798 participants of the CHS Cognition Study 1992-99.
| Outcome | Risk Factor | N | Adjusted for demographics1 |
p | Adjusted for demographics + CVD2 |
p |
|---|---|---|---|---|---|---|
| Dementia/ Normal |
Hazard Ratio (95% Confidence Interval) |
Hazard Ratio (95% Confidence Interval) |
||||
| Dementia | BMI Age 50 (kg/m2) |
461/2245 | 1.02 (0.99-1.05) | .17 | 1.01 (0.98-1.04) | .58 |
| BMI-categorical | .19 | .41 | ||||
| Underweight | 15/77 | 1.09 (0.64-1.85) | .74 | 1.20 (0.66-2.17) | .55 | |
| Normal | 200/1063 | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| Overweight | 188/904 | 1.10 (0.90-1.35) | .35 | 1.01 (0.83-1.35) | .67 | |
| Obese | 58/203 | 1.39 (1.03-1.87) | .03 | 1.36 (0.94-1.95) | .10 | |
| AD3 | BMI (kg/m2) | 236/2245 | 0.99 (0.96-1.03) | .76 | 1.00 (0.95-1.04) | .84 |
| BMI-categorical | .60 | .66 | ||||
| Underweight | 11/81 | 1.46 (0.78-2.73) | .24 | 1.47 (0.70-3.09) | .30 | |
| Normal | 103/1160 | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| Overweight | 96/996 | 1.12 (0.84-1.48) | .44 | 1.04 (0.74-1.47) | .81 | |
| Obese | 26/235 | 1.17 (0.75-1.81) | .48 | 1.25 (0.74-2.11) | .40 | |
| VaD4 | BMI (kg/m2) | 206/2245 | 1.04 (1.00-1.08) | .06 | 1.02 (0.97-1.07) | .56 |
| BMI-categorical | .17 | .72 | ||||
| Underweight | 4/88 | 0.70 (0.26-1.90) | .48 | 0.87 (0.31-2.40) | .78 | |
| Normal | 89/1174 | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| Overweight | 85/1007 | 1.10 (0.82-1.49) | .52 | 1.00 (0.70-1.44) | .98 | |
| Obese | 28/233 | 1.57 (1.02-2.42) | .04 | 1.33 (0.78-2.29) | .30 |
adjusted for age, race, gender, years of education
adjusted for age, race, gender, years of education + CRP + IL-6 + hypertension status + diabetes status + CHD + total cholesterol + AAI + smoking status + kcal expended/wk + ApoE4
Alzheimer’s disease without the presence of Vascular dementia using NINCDS criteria (possible or probable)
Vascular dementia with or without the presence of AD using ADDTC criteria (possible or probable).
In contrast, an inverse relationship between late-life BMI as a continuous variable and incident dementia was found independent of demographics (hazard ratio per BMI unit kg/m2: 0.97, 95% CI: 0.95-0.99) (Table 3). The association remained significant when adjusted for cardiovascular and dementia risk factors (HR: 0.95, 95% CI: 0.92-0.98). Adjusted for all covariates, being underweight (BMI < 20) increased the risk of dementia by 60% (HR: 1.62, 95% CI: 1.02-2.64) while being overweight (BMI >25-30) was not associated (HR: 0.90, 95% CI: 0.70-1.16) and being obese (BMI >30) was associated with a reduced risk of dementia (HR: 0.63, 95% CI: 0.44-0.91)) compared to being normal (BMI 20-25). Results for dementia subtype were similar to those for total dementia. One difference was that higher estimates were produced in the models assessing VaD, suggesting that underweight is a greater risk for VaD than AD.
Table 3.
Late-Life Body Mass Index measured at age 65 or older and risk of dementia, Alzheimer’s disease and vascular dementia in 2,798 participants of the CHS Cognition Study 1992-1999.
| Dementia | Risk Factor | N | Adjusted for demographics1 |
p | Adjusted for demographics + CVD2 |
p |
|---|---|---|---|---|---|---|
| Dementia/ Normal) |
Hazard Ratio (95% Confidence Interval) |
Hazard Ratio (95% Confidence Interval) |
||||
| Total | BM (kg/m2) | 480/2311 | 0.97 (0.95-.99) | .004 | 0.95 (0.92-0.98) | <.001 |
| BMI-categorical | .01 | .005 | ||||
| Underweight | 31/85 | 1.62 (1.10-2.39) | .01 | 1.62 (1.02-2.64) | .04 | |
| Normal | 168/752 | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| Overweight | 203/1004 | 0.93 (0.76-1.15) | .51 | 0.92 (0.72-1.18) | .50 | |
| Obese | 78/472 | 0.81 (0.61-1.07) | .13 | 0.63 (0.44-0.91) | .01 | |
| Alzheimer’s3 | BMI (kg/m2) | 245/2546 | 0.96 (0.93-0.99) | .009 | 0.95 (0.91-0.99) | .008 |
| BMI-categorical | .02 | .05 | ||||
| Underweight | 17/99 | 1.53 (0.91-2.58) | .11 | 1.42 (0.74-2.70) | .29 | |
| Normal | 94/826 | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| Overweight | 93/1114 | 0.76 (0.57-1.02) | .07 | 0.74 (0.52-1.05) | .10 | |
| Obese | 41/509 | 0.70 (0.48-1.03) | .07 | 0.58 (0.36-0.96) | .03 | |
| Vascular4 | BMI (kg/m2)I | 213/2570 | 0.98 (0.94-1.01) | .17 | 0.95 (0.91-0.99) | .02 |
| BMI-categorical | .15 | .03 | ||||
| Underweight | 14/102 | 1.89 (1.06-3.37) | .03 | 2.15 (1.11-4.19) | .02 | |
| Normal | 67/853 | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| Overweight | 98/1109 | 1.15 (0.84-1.57) | .39 | 1.20 (0.83-1.76) | .33 | |
| Obese | 34/516 | 0.98 (0.64-1.50) | .92 | 0.72 (0.41-1.27) | .26 |
adjusted for age, race, gender, years of education
adjusted for age, race, gender, years of education + CRP + IL-6 + hypertension status + diabetes status + CHD + total cholesterol + AAI + smoking status + ApoE4
Alzheimer’s disease without the presence of Vascular dementia using NINCDS criteria (possible or probable_
Vascular dementia with or without the presence of AD using ADDTC criteria (possible or probable)..
Significant associations were not found between WHR in late life and dementia, AD, or VaD (data not shown). While the hazard ratio was close to 1.0 for the association between WHR and dementia adjusting for demographics, the point estimate fell below 1.0 when adjusted for CVD risk factors (HR: 0.71, 95% CI: 0.15 - 3.27). However, confidence intervals were wide and the association was not significant. Results were similar by dementia subtype.
DISCUSSION
The ability to evaluate BMI at two ages in the CHS cohort provides insight into differences found in other studies and the obesity paradox. We found that while mid-life obesity was related to higher dementia risk, BMI after age 65 was inversely related. The greatest dementia risk was found in underweight individuals at older ages. These findings suggest that the predictive ability of BMI changes over time. High BMI in middle age has been found to be associated with higher dementia risk (13,14). Higher BMI at ages 70, 75 and 79 years has also been found to predict dementia (17), although there have been reports of no association (16), of lower BMI related to higher AD risk (15), and of a U-shaped relation between BMI and dementia at older ages (18). These conflicting findings could be explained by different age groups in different studies; those conducted in middle age show a relation of high BMI to increased dementia risk, while those in older populations differ. While the association we found at mid-life may be related to conditions such as hypertension emerging in middle-age, the association of high BMI to cardiovascular and total mortality may be attenuated in older age groups, where high BMI becomes a predictor of decreased mortality (20). However, difference we found may possibly be due to the decreased value of BMI as a measure of adiposity in the oldest old. In addition, as associations we reported at mid- and late-life involved the same individuals, our findings may also have been influenced by changes in BMI with age, or they may reflect differences in the importance of exposure to high adiposity in middle-age versus old age.
The curvilinear associations found in our results for late-life BMI are similar to those found by Sturman, et al., in a biracial community (36). This U-shaped association has been reported in other outcomes in older adults (37) and helps to explain the paradoxical findings between BMI and dementia. Low BMI related to worse outcomes is usually ascribed to conditions associated with weight loss. Higher BMI related to worse outcomes is usually interpreted as evidence of the consequences of obesity. Our study had the advantage of having BMI measures at both mid- and late-life in the same persons, and results are consistent with the body of literature showing that BMI in mid-life is a predictor of dementia while it is not at older ages. Our study also supports the notion that important exposures that are related to a higher risk of dementia often occur in middle age, which is not assessed in most studies of aging. Thus, it is important to assess mid-life exposures in studies of aging, either by enrolling participants at an earlier age, which has obvious logistical and cost difficulties, or by including proxy measures of mid-life exposures, such as subclinical markers or self reported measures.
Aging is characterized by lean body mass loss and adipose tissue increase without weight gain, which is not captured by BMI. Thus, traditional adiposity measures are less useful in the elderly (38). Because measurements such as BMI may be less accurate in assessing obesity in the elderly, alternative anthropometric tools could be used. The highest quintile of sagittal abdominal diameter measured in mid-life was associated with a three-fold increased risk of dementia (39). Waist circumference and WHR have been proposed as better adiposity measures in the elderly (19,37,38). One study in New York City found that elevated waist circumference was related to higher dementia risk in persons aged 65 to 76 years, not in those over 76 years of age, and to a higher risk of vascular dementia in all age groups (18). We found no associations for WHR in old age.
Weight loss occurs with comorbidities at older ages and is often reflective of poor health. Weight loss, along with psychological, behavioral, and mobility problems, is one of the principal manifestations of AD (40). Weight loss may predate dementia onset by as much as 10 years (41). We found that while higher BMI at mid-life may increase the risk of dementia, when measured after age 65, increased BMI may actually be a marker for decreased dementia risk.
While the large sample and well-characterized CHS data are strengths in this study, there are several limitations. Although we have treated BMI at mid- and late-life equally, mid-life weight was collected by self-report and recall bias may have occurred. We did not have height at age 50, and mid-life BMI was calculated using height at the CHS Cognition Study baseline. As height may be lost with aging, our mid-life BMI estimate may be biased. However, elevated weight is protective against bone (42) and height loss. Thus, biases in this study would be most relevant to underweight participants. The greater misclassification inherent in BMI at mid-life (versus late-life) may also have influenced results found in these models. Thus it is possible that the relatively weak associations found for mid-life BMI understate the true relationships that would be observed in a cohort recruited at middle age and followed through late life. We should also note here that the methods for ascertaining dementia and date of onset used in the CHS Cognition Study were non-traditional and may have resulted in misclassification. However, this would have attenuated models toward the null and not changed overall conclusions found here. Finally, results from this study are only relevant to those who live beyond age 65 without dementia and generalizations should only be made to this group. Similarly, as nutritional status is related to both morbidity and mortality in the elderly, competing risks must be taken into consideration.
The associations between mid- and late-life BMI and risk of dementia reported here are consistent with physical changes in the trajectory towards disability and frailty. These results reinforce the necessity of monitoring weight loss closely in older adults. These data also suggest the value of modified classification of “overweight” in the elderly because being overweight (as compared to obese) may confer the same risk as normal weight for some diseases including dementia. Clarification of consequences of intentional versus unintentional weight loss is also needed to help guide clinical recommendations for older adults.
Figure 1.
Risk of dementia by BMI at mid-life (age 50).
Figure 2.
Risk of dementia by BMI at late-life (age 65 or older).
ACKNOWLEDGMENTS
The research reported in this article was supported by grant 5 R01 AG15928-02 from the National Institute on Aging, and contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
REFERENCES
- 1.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler WC, Narayan KM, Hanson RL, et al. Preventing non-insulin-dependent diabetes. Diabetes. 1995;44:483–488. doi: 10.2337/diab.44.5.483. [DOI] [PubMed] [Google Scholar]
- 3.Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21(Suppl 3):C11–14. doi: 10.2337/diacare.21.3.c11. [DOI] [PubMed] [Google Scholar]
- 4.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 5.Mann J. Stemming the tide of diabetes mellitus. Lancet. 2000;356:1454–1455. doi: 10.1016/S0140-6736(00)02862-2. [DOI] [PubMed] [Google Scholar]
- 6.Reaven GM, Laws A. Insulin resistance: the metabolic syndrome X. Humana Press; Totowa, New Jersey: 1999. [Google Scholar]
- 7.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 8.Peila R, Rodriguez BL, White LR, Launer LJ. Fasting insulin and incident dementia in an elderly population of Japanese-American men. Neurology. 2004;63:228–233. doi: 10.1212/01.wnl.0000129989.28404.9b. [DOI] [PubMed] [Google Scholar]
- 9.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peila R, Rodriguez BL, Launer LJ. Type 2 Diabetes, APOE Gene, and the Risk for Dementia and Related Pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 11.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 12.Leibson CL, Rocca WA, Hanson VA, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 13.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 14.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005 doi: 10.1136/bmj.38446.466238.E0. bmj.38446.466238.E466230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nourhashemi F, Deschamps V, Larrieu S, et al. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 16.Stewart R, Masaki K, Xue Q-L, et al. A 32-Year Prospective Study of Change in Body Weight and Incident Dementia: The Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 17.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-Year Follow-up of Overweight and Risk of Alzheimer Disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 18.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 20.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 21.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63:1312–7. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 22.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–7. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 23.Freedman DM, Ron E, Ballard-Barbash R, Doody MM, Linet MS. Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond) 2006;30:822–9. doi: 10.1038/sj.ijo.0803193. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;82:909–10. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, Borhani NO, Enright PE, et al. The Cardiovascular Health Study: Design and Rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 27.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 28.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 29.Lopez OL, Kuller LH, Fitzpatrick AL, et al. Evaluation of dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 30.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. Feb. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 31.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 32.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992 Mar;42(3 Pt 1):473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]; Current Controlled Trials in Cardiovascular Medicine. 2002;3:1–8. doi: 10.1186/1468-6708-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diehr P, Newman AB, Jackson SA, Kuller L, Powe N. Weight-modification trials in older adults: what should the outcome measures be? Curr Control Trials Cardiovasc Med. 2002;3:1. doi: 10.1186/1468-6708-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clinical Chemistry. 1995;41:264–270. [PubMed] [Google Scholar]
- 35.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in The Cardiovascular Health Study. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 36.Sturman MT, Mendes de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008;70:360–367. doi: 10.1212/01.wnl.0000285081.04409.bb. [DOI] [PubMed] [Google Scholar]
- 37.Allison DB, Faith MS, Heo M, Kotler DP. Hypothesis concerning the U-shaped relation between body mass index and mortality. Am J Epidemiol. 1997;146:339–349. doi: 10.1093/oxfordjournals.aje.a009275. [DOI] [PubMed] [Google Scholar]
- 38.Stevens J, Cai J, Juhaeri, Thun MJ, Williamson DF, Wood JL. Consequences of the use of different measures of effect to determine the impact of age on the association between obesity and mortality. Am J Epidemiol. 1999;150:399–407. doi: 10.1093/oxfordjournals.aje.a010019. [DOI] [PubMed] [Google Scholar]
- 39.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson FP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. doi: 10.1212/01.wnl.0000306313.89165.ef. (epub March 26, 2008, 10.1212/01.2nl.000306313.891.ef. [DOI] [PubMed] [Google Scholar]
- 40.Gillette Guyonnet S, Abellan Van Kan G, Alix E, et al. IANA (International Academy on Nutrition and Aging) Expert Group: weight loss and Alzheimer’s disease. J Nutr Health Aging. 2007;11:38–48. [PubMed] [Google Scholar]
- 41.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69:739–46. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 42.Zhai G, Hart DJ, Valdes AM, Kato BS, Richards JB, Hakim A, Spector TD. Natural history and risk factors for bone loss in postmenopausal Caucasia women: a 15 year follow-up population-based study. Osteoporosis Int. 2008 doi: 10.1007/s00198-008-0562-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]