Abstract
Abnormalities in structural and functional connectivity have been reported in autism spectrum disorders (ASD) across a wide age range. However, developmental changes in white matter microstructure are poorly understood. We used a cross-sectional design to determine whether white matter abnormalities measured using diffusion tensor imaging (DTI) were present in adolescents and adults with ASD and whether age-related changes in white matter microstructure differed between ASD and typically developing (TD) individuals. Participants included 28 individuals with ASD and 33 TD controls matched on age and IQ and assessed at one time point. Widespread decreased fractional anisotropy (FA), and increased radial diffusivity (RaD) and mean diffusivity (MD) were observed in the ASD group compared to the TD group. In addition, significant group-by-age interactions were also observed in FA, RaD, and MD in all major tracts except the brain stem, indicating that age-related changes in white matter microstructure differed between the groups. We propose that white matter microstructural changes in ASD may reflect myelination and/or other structural differences including differences in axonal density/arborization. In addition, we suggest that white matter microstuctural impairments may be normalizing during young adulthood in ASD. Future longitudinal studies that include a wider range of ages and more extensive clinical characterization will be critical for further uncovering the neurodevelopmental processes unfolding during this dynamic time in development.
Keywords: autism, white matter, DTI, age, interaction
1.0 Introduction
Autism symptom presentation and severity is heterogeneous and varies throughout development. In addition to the core features of autism spectrum disorder (ASD), children and adolescents with ASD often develop maladaptive behaviors including irritability, hyperactivity, aggression, depression, and anxiety, among others (Anderson et al., 2011). During the adolescent period, individuals with ASD appear to be at a higher risk for developing seizures and behavior problems and psychiatric symptoms may increase (Gillberg and Steffenberg, 1987). Limited information is available about changes in autism symptoms from childhood to early adulthood, but emerging evidence suggests that core-ASD symptoms may be milder in adulthood than during early development (Boelte and Poustka, 2000; Gilchrist et al., 2001; Piven et al., 1996). These behavioral improvements with age may be a result of maturation and the stabilization of disease processes. However, very little is known about age-related changes in brain structure and function in the period from adolescence through adulthood in ASD.
As with manifestations of autism symptomatology, structural and functional brain abnormalities can change across the lifespan. For example, enlarged brain volume has been reported in young children with ASD (Courchesne et al., 2001; Hazlett et al., 2005; Sparks et al., 2002), particularly in early childhood, despite normal head circumference at birth (Courchesne et al., 2003; Dawson et al., 2007; Dementieva et al., 2005; Hazlett et al.). By later childhood or adolescence, brain enlargement seems to resolve (Aylward et al., 2002; Courchesne, 2004; Hardan et al., 2003; Redcay and Courchesne, 2005) and many earlier observed morphological differences are no longer apparent; however, an abundance of evidence suggests that brain function has not completely normalized. Brain imaging studies of adolescent and adult individuals with autism spectrum disorders (ASD) suggest that abnormalities in neural circuitry and connectivity are present (for review, see Williams and Minshew, 2007).
Volumetric studies of ASD have shown that some brain regions are disproportionately enlarged (Aylward et al., 2002; Courchesne et al., 2001; Hazlett et al., 2005; Kemper and Bauman, 1998; Redcay and Courchesne, 2005; Sparks et al., 2002) and grow out of synchrony with other brain regions (Hardan et al., 2006; Langen et al., 2007). Increased brain size in ASD during early development has implicated abnormalities of both grey matter (Friedman et al., 2006; Petropoulos et al., 2006) white matter, particularly in the superficial/radiate white matter regions of the cerebrum (Herbert et al., 2004) and in the frontal lobes (Carper et al., 2002). Although these white matter volumetric findings do not directly support abnormal connectivity among brain structures in autism, the abnormal growth patterns are consistent with this consideration.
Functional imaging studies have provided further, indirect evidence of connectivity abnormalities in adolescents and adults with ASD. Studies utilizing functional connectivity (fcMRI) techniques have identified abnormal connectivity between brain regions involved in mediating complex language, selective attention, visuomotor coordination, emotion perception and executive functioning tasks (see, e.g. Just et al., 2004; Just et al., 2006; Kana et al., 2006; Kleinhans et al., 2008; Koshino et al., 2005; Mizuno et al., 2006; Mostofsky et al., 2009; Rudie et al., in press; Welchew et al., 2005). In most studies, under-connectivity has been reported, suggesting reduced within-network efficiency (Muller et al., 2011). However, it is important to note that a growing literature has reported over-connectivity in ASD (see, e.g. Mizuno et al., 2006; Monk et al., 2010; Welchew et al., 2005), which may reflect inadequate synaptic pruning or other downstream effects (Muller et al., 2011).
Diffusion tensor imaging (DTI) studies provide complementary indirect evidence of abnormal white matter structural connectivity. Although most studies to date have documented widespread reductions in white matter integrity in ASD compared to controls, a few notable exceptions exist. A report of 7 children with autism between 18 and 40 months of age found increased fractional anisotropy (FA) in the genu and splenium of the corpus callosum, left posterior limb of the internal capsule, and left forceps minor (Ben Bashat et al., 2007); reduced FA was observed in the left corticospinal tracts. Similarly, in a conference presentation, Courchesne and colleagues reported increased FA in the superior longitudinal fasciculus, forceps minor, uncinate fasciculus, and the corpus callosum in children with autism between 13 and 43 months (Solso et al., 2011). In a sample with a slightly broader age-range (1.5 – 5.8 years), only the genu and body of the corpus callosum were found to retain significantly increased FA values (Weinstein et al., 2011). A recent longitudinal DTI study has largely confirmed the postulated transient nature of white matter integrity measurement in very young children with autism. Wolff et al (2012) reported increased FA in 6 month-old infants who were later diagnosed with autism in the body of the corpus callosum, left fornix, left inferior longitudinal fasciculus, right posterior limb of the internal capsule, and left uncinate. Notably, the trend in all fiber tracts except the left anterior thalamic radiation was in the direction of increased FA in the 6-month-old children with autism compared to high-risk but typically developing peers. At twelve months, although the comparisons did not reach statistical significance, the same pattern of globally increased FA was observed, still with the exception of the anterior thalamic radiation bilaterally. However, by 24 months of age, the trend reversed, with decreased FA becoming the dominant pattern across all fiber tracts. Although the available data on very young children is still limited, it seems clear that once children with autism reach 3–4 years of age, increased FA is no longer evident (but see Cheng et al., 2010; Cheung et al., 2009; Ke et al., 2009). Instead, studies of young children whose approximate age range is 2.5–9 years of age (with a mean of 5) reported reduced FA in the uncinate fasciculus, inferior fronto-occipital fasciculus, arcuate fasciculus, right cingulum, and the corpus callosum (Kumar et al., 2010), and in the short association fibers of the frontal lobe (Sundaram et al., 2008), and no regions with increased FA. The pattern of white matter abnormalities becomes more widespread with older children (aged 6–14), who show reduced FA in frontal corona radiata (Barnea-Goraly et al., 2011), corpus callosum (Barnea-Goraly et al., 2011; Brito et al., 2009), internal and external capsules(Barnea-Goraly et al., 2011; Brito et al., 2009), uncinate fasciculus (Poustka et al., in press), superior longitudinal fasciculus (Barnea-Goraly et al., 2011; Fletcher et al., 2010; Poustka et al., 2012), cingulate gyrus (Barnea-Goraly et al., 2011), temporal lobes (Barnea-Goraly et al., 2011; Cheung et al., 2009; Ke et al., 2009), parietal lobes(Barnea-Goraly et al., 2011), prefrontal white matter (Cheung et al., 2009; Ke et al., 2009) right corticospinal tract (Brito et al., 2009), and the cerebellum (Brito et al., 2009; Cheung et al., 2009). Despite a normalization of brain volume in adolescents and adults with ASD, white matter abnormalities persist. Studies of individuals in the adolescent and adult age range have reported reduced FA in the superior longitudinal fasciculus (Bloemen et al., 2010; Cheng et al., 2010; Groen et al., 2011; Jou et al., 2011; Noriuchi et al., 2010; Shukla et al., 2010), inferior longitudinal fasciculus (Bloemen et al., 2010; Groen et al., 2011; Shukla et al., 2010), left posterior limb of internal capsule (Cheng et al., 2010; Shukla et al., 2010), right inferior cerebellar peduncle (Cheng et al., 2010), corona radiata(Groen et al., 2011), corpus callosum (Bloemen et al., 2010; Jou et al., 2011; Noriuchi et al., 2010; Shukla et al., 2010), fronto-occipital fasciculus (Bloemen et al., 2010; Jou et al., 2011; Noriuchi et al., 2010; Shukla et al., 2010), left dorsolateral prefrontal cortex (Noriuchi et al., 2010), right temporal pole (Noriuchi et al., 2010), cingulum (Bloemen et al., 2010; Shukla et al., 2010), anterior limb of internal capsule (Shukla et al., 2010), corticospinal tract (Bloemen et al., 2010; Shukla et al., 2010), anterior thalamic radiation (Bloemen et al., 2010; Shukla et al., 2010) and the uncinate fasciculus (Bloemen et al., 2010). Although fewer reports are available, mean diffusivity (MD) appears to be consistently higher in individuals with autism across various age ranges, or no different from controls. Increased MD was reported in the corpus callosum (Alexander et al., 2007; Brito et al., 2009; Groen et al., 2011; Shukla et al., 2010), corona radiata (Groen et al., 2011), anterior and posterior limb of the internal capsule (Groen et al., 2011; Shukla et al., 2010), middle cerebellar peduncle (Groen et al., 2011), thalamus and thalamic radiations (Groen et al., 2011; Shukla et al., 2010), inferior and superior longitudinal and fronto-occipital fasciculus (Groen et al., 2011; Shukla et al., 2010), temporal lobes (Lee et al., 2007), cingulum, corticospinal tract (Shukla et al., 2010), external capsule(Shukla et al., 2010), and uncinate fasciculus (Shukla et al., 2010). Two studies found no significant differences in MD (Barnea-Goraly et al., 2011; Weinstein et al., 2011).
Evidence is beginning to accrue that abnormal brain connections may underlie functional abnormalities and their concomitant behavioral abnormalities in ASD (Belmonte et al., 2004; Cherkassky et al., 2006; Courchesne and Pierce, 2005; Courchesne et al., 2007; Just et al., 2004; Muller, 2007). It has been suggested that altered levels of brain activation and underconnectivity could be secondary to abnormal development of gray matter, white matter, or both (Just et al., 2004; Just et al., 2006). A series of neuropathological studies have provided clues to the neurobiological basis of reduced connectivity including ongoing neuroinflammatory processes in the frontal lobes and cerebellum (Vargas et al., 2005) and abnormally small and densely packed minicolumns (Buxhoeveden et al., 2006; Casanova et al., 2006). Casanova and colleagues propose that abnormalities in minicolumnar development combined with larger than normal brain size contribute to neural circuit dysfunction in individuals with ASD (Casanova and Tillquist, 2008). This aberrant neurodevelopmental pattern has been hypothesized to result in abnormally increased local cortical connectivity but reduced long-distance reciprocal connectivity (Casanova et al., 2006; Casanova and Tillquist, 2008; Courchesne and Pierce, 2005). Further, a recent postmortem study of white matter in ASD reported fewer long distance axons, thinner axons, and excessive axonal branching in adults with ASD (Zikopoulos and Barbas, 2010). Overall, the growing DTI literature in ASD suggests that poor white matter integrity likely contributes to impaired communication across brain regions.
Many advances have been made in characterizing brain changes during the early childhood period, yet little is known about the pathophysiological mechanisms that contribute to ongoing, but potentially remitting or diminishing autism symptoms during adolescence and adulthood. In our current study, we used DTI to investigate white matter integrity in a cross-sectional sample of high functioning adolescents and adults with ASD, compared to typically developing (TD) controls, using a conservative, whole-brain analytic approach. In addition to characterizing differences between the two diagnostic groups, we focused on age-related changes in an effort to understand ongoing developmental processes in ASD. We hypothesized 1) that the ASD group would have reduced white matter integrity compared to an age and IQ matched TD group. Based on previous literature, we further predicted 2) that white matter integrity would show evidence of normalizing with age in the ASD group. We also hypothesized 3) that age-related rates of change would be significantly different between the TD and ASD groups.
2.0 RESULTS
2.1 Group comparison between ASD and TD
Compared with the TD group, ASD participants demonstrated widely distributed reduced white matter integrity characterized by increased MD and RaD and reduced FA values. No significant group differences were found in AxD. Regions with reduced FA included association fibers (cingulum, fornix, stria terminalis, sagittal stratum, superior fronto-occipital fasciculus, superior longitudinal fasiculus, uncinate fasciculus), brainstem tracts (inferior, middle, and superior cerebellar peduncle, medial lemniscus, and pontine crossing tract), the corpus callosum, tapetum, and all projection fibers (anterior and superior corona radiata, anterior and posterior limb and retrolenticular internal capsule, external capsule, cerebral peduncle, and corticospinal tract, posterior thalamic radiation). Increased MD was observed in the cingulum, sagittal stratum, superior longitudinal fasiculus, body and splenium of the corpus callosum, tapetum, and several projection fibers (external capsule, posterior corona radiata, posterior limb and retrolenticula part of the internal capsule, posterior thalamic radiation, and superior corona radiata). Increased RaD was observed in association fibers (cingulum, fornix, stria terminalis, sagittal stratum, superior longitudinal fasciculus, uncinate fasciculus), the corpus callosum and tapetum, and several projection fibers (anterior and posterior corona radiata, cerebral peduncle, corticospinal tract, external capsule, posterior limb and retrolenticular part of the internal capsule, posterior thalamic radiation, and middle cerebellar peduncle). See tables 2–4 for specific locations and descriptive statistics. Additional cerebral white matter regions are reported in supplementary material.
Table 2.
Regions showing decreased fractional anisotropy in ASD
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| White Matter Region | side | p (max) | x | y | z | # of voxels |
| JHU Atlas lables | ||||||
| Association Fibers | ||||||
| Cingulum (cingulate gyrus) | L,R | .001 | −10 | −24 | 32 | 402 |
| Fornix | I | .005 | 0 | −1 | 14 | 97 |
| Fornix / Stria terminalis | R,L | .004 | 29 | −28 | −5 | 351 |
| Sagittal stratum | R,L | .002 | 40 | −37 | −12 | 637 |
| Superior fronto-occipital fasciculus | L,R | .007 | −21 | −3 | 19 | 25 |
| Superior longitudinal fasciculus | R,L | .001 | 37 | −56 | 15 | 1661 |
| Uncinate fasciculus | R,L | .004 | 35 | −1 | −12 | 72 |
| Brainstem Tract | ||||||
| Middle cerebellar peduncle | I | .028 | 5 | −19 | −31 | 232 |
| Pontine crossing tract | I | .037 | −7 | −31 | −28 | 150 |
| Medial lemniscus | L,R | .037 | −4 | −34 | −27 | 148 |
| Superior cerebellar peduncle | L,R | .037 | −7 | −34 | −23 | 194 |
| Commissural Fibers | ||||||
| Body of corpus callosum | I | .001 | −13 | −30 | 29 | 2441 |
| Genu of corpus callosum | I | .003 | 6 | 24 | 13 | 1267 |
| Splenium of corpus callosum | I | .001 | −18 | −39 | 27 | 2029 |
| Tapetum | R | .005 | 33 | −42 | 8 | 23 |
| Projection Fibers | ||||||
| Anterior corona radiata | L,R | .003 | −15 | 37 | 2 | 1420 |
| Anterior limb of internal capsule | L,R | .004 | 23 | 23 | 2 | 379 |
| Cerebral peduncle | L,R | .003 | 17 | −20 | −12 | 510 |
| Corticospinal tract | L,R | .025 | 6 | −23 | −32 | 436 |
| External capsule | L,R | .002 | −29 | −19 | 13 | 1004 |
| Posterior corona radiata | L,R | .001 | −20 | −37 | 32 | 917 |
| Posterior limb of internal capsule | L,R | .001 | −27 | −25 | 18 | 925 |
| Posterior thalamic radiation | L,R | .001 | 35 | −58 | 1 | 1804 |
| Retrolenticular part of internal capsule | L,R | .001 | −34 | −38 | 10 | 920 |
| Superior corona radiata | L,R | .001 | −21 | −31 | 41 | 662 |
Note. R = right, L = left, I = interhemispheric. Regions are labled according to the peak p value within that region. When both R and L are listed, the bolded side indicates the peak p value and location, the # of voxels includes both sides combined.
Table 4.
Regions showing increased radial diffusivity in ASD
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| White Matter Region | side | p (max) | x | y | z | # of voxels |
| JHU atlas lables | ||||||
| Association_Fibers | ||||||
| Cingulum (cingulate gyrus) | L,R | .001 | 12 | −47 | 25 | 308 |
| Cingulum (hippocampus) | R | .040 | 25 | −35 | −10 | 45 |
| Fornix / Stria terminalis | R | .014 | 35 | −14 | −13 | 29 |
| Sagittal stratum | L,R | .001 | −39 | −44 | −8 | 365 |
| Superior longitudinal fasciculus | L,R | .001 | −43 | −50 | 2 | 1746 |
| Uncinate fasciculus | L,R | .009 | −34 | −2 | −21 | 30 |
| Commissural_Fibers | ||||||
| Body of corpus callosum | I | .001 | −14 | −30 | 29 | 2060 |
| Genu of corpus callosum | I | .004 | 6 | 24 | 13 | 687 |
| Splenium of corpus callosum | I | .001 | −18 | −40 | 26 | 1844 |
| Tapetum | R | .004 | 32 | −42 | 12 | 12 |
| Projection_Fibers | ||||||
| Anterior corona radiata | L,R | .004 | 18 | 17 | 29 | 913 |
| Anterior limb of internal capsule | R | .040 | 22 | 22 | 1 | 5 |
| External capsule | L,R | .001 | −28 | −21 | 15 | 376 |
| Posterior corona radiata | L,R | .001 | −20 | −38 | 32 | 1138 |
| Posterior limb of internal capsule | L,R | .001 | −24 | −21 | 8 | 558 |
| Posterior thalamic radiation | L,R | .001 | 35 | −64 | −3 | 1696 |
| Retrolenticular part of internal capsule | L,R | .001 | −40 | −35 | −1 | 663 |
| Superior corona radiata | L,R | .001 | 21 | −32 | 40 | 973 |
Note. R = right, L = left, I = interhemispheric. Regions are labled according to the peak p value within that region. When both R and L are listed, the bolded side indicates the peak p value and location, the # of voxels includes both sides combined.
2.2 Correlations with autism severity
There were no significant positive or negative correlations found between the ADOS severity scores and FA, MD, RaD, or AxD in the ASD group (p > .05, corrected for multiple comparisons). However, the trends were in the expected direction. With a liberal, uncorrected threshold of p < .05, FA showed a negative correlation with ADOS severity in the body of the corpus callosum, MD showed a positive correlation with ADOS severity in the genu of the corpus callosum, and RaD showed a positive relationship with ADOS severity in the entire corpus callosum and the right anterior corona radiata. No statistical trend was observed between AxD and ADOS severity.
2.3 Correlations with age
In the ASD group, age was negatively associated with MD, AxD, and RaD; no significant age association was observed with FA. In the TD group, age was negatively associated with FA, MD, and AxD, and positively correlated with RaD. In the TD group, FA was found to decrease with age in several association fibers (cingulate gyrus, hippocapmpus, fornix, sagittal stratum, superior fronto-occipital fasciculus, and the superior longitudinal fasciculus), brain stem tracts (middle and superior cerebellar peduncle and medial lemniscus), the corpus callosum and tapetum, and several projection fibers (anterior, superior, and posterior corona radiata, internal capsule, external capsule, posterior thalamic radiation, cerebral peduncle). (See supplementary tables). Major white matter tracts in which MD decreased with age in both the ASD and TD groups included the stria terminalis, sagittal stratum, superior fronto-occipital fasciculus, uncinate fasciculus, the body and genu of the corpus callosum, the anterior and superior corona radiata, anterior and posterior limb of the internal capsule, external capsule, and the cerebellar peduncle. The ASD group showed additional regions in which MD decreased over the age-span of the evaluated subjects, including the cingulum, splenium of the corpus callosum, the tapetum, posterior corona radiata, posterior thalamic radiation, and the retrolenticular part of the internal capsule. Major white matter tracts in which AxD decreased with age in both the ASD and TD groups included the cingulum, stria terminalis, sagittal stratum, superior fronto-occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus, the corpus callosum, the tapetum, anterior, superior and posterior corona radiata, anterior, retrolenticular, and posterior limb of internal capsule, cerebral peduncle, external capsule, and posterior thalamic radiation. There were several additional major tracts that showed a negative correlation between AxD and age in the TD group only, including the middle, inferior, and superior cerebellar peduncle, pontine crossing tract, and medial lemniscus, and the corticospinal tract. The ASD group showed a negative age relationship while the TD group showed a positive age relationship with RaD for a number of tracts, including the stria terminalis, sagittal stratum, superior longitudinal fasciculus, the body and splenium of the corpus callosum, and the cerebral peduncle. Notably, the ASD group showed an age-related decrease in RaD in the cingulum, uncinate fasciculus, genu of the corpus callosum, tapetum, anterior corona radiata, and anterior limb of internal capsule whereas no significant age effects were detected for these regions in the TD group.
2.4 Diagnostic group by age interaction
Significant diagnostic group by age interaction effects were observed in FA, MD, and RaD as shown in Table 5. FA values generally decreased in the TD group and slightly increased in the ASD group as a function of age in the cingulate gyrus, sagittal stratum, the corpus callosum, tapetum, corona radiata, internal capsule, external capsule, cerebral peduncle, and the posterior thalamic radiation. The significant interaction term in MD reflected a positive age correlation in the TD group and a negative age correlation in the ASD group in the cingulum, superior longitudinal fasciculus, the body and splenium of the corpus callosum, the posterior thalamic radiation, and the posterior and superior corona radiata. Similarly, the significant interaction term in RaD reflected a positive age correlation in the TD group and a negative age correlation in the ASD group in the following regions: cingulum, stria terminalis, sagittal stratum, superior longitudinal fasciculus, the corpus callosum, tapetum, corona radiata, cerebral peduncle, external capsule, internal capsule, and the posterior thalamic radiation. No group by age interaction was found in AxD.
Table 5.
Regions showing a significant group by age interaction effects
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| White Matter Region | side | p (max) | x | y | z | # of voxels |
| Age by Dx interaction in FA | ||||||
| Association Fibers | ||||||
| Cingulum (cingulate gyrus) | L,R | .021 | 12 | −46 | 25 | 117 |
| Sagittal stratum | L,R | .012 | −40 | −34 | −13 | 255 |
| Superior longitudinal fasciculus | L,R | .012 | −42 | −47 | 2 | 454 |
| Commissural_Fibers | ||||||
| Body of corpus callosum | I | .011 | −12 | −30 | 27 | 910 |
| Genu of corpus callosum | I | .022 | −16 | 22 | 24 | 171 |
| Splenium of corpus callosum | I | .011 | −17 | −41 | 25 | 838 |
| Tapetum | L,R | .029 | −28 | −53 | 17 | 11 |
| Projection_Fibers | ||||||
| Anterior corona radiata | L,R | .017 | −17 | 40 | −4 | 426 |
| Anterior limb of internal capsule | L | .029 | −11 | 9 | −4 | 23 |
| Cerebral peduncle | L | .044 | −16 | −13 | −7 | 25 |
| External capsule | L,R | .011 | −28 | −22 | 18 | 38 |
| Posterior corona radiata | L,R | .010 | −27 | −27 | 19 | 876 |
| Posterior limb of internal capsule | L,R | .010 | −27 | −25 | 17 | 364 |
| Posterior thalamic radiation | L,R | .011 | −38 | −47 | 1 | 861 |
| Retrolenticular part of internal capsule | L,R | .011 | −27 | −28 | 18 | 421 |
| Superior corona radiata | L,R | .010 | −25 | −23 | 20 | 615 |
| Age by Dx interaction in MD | ||||||
| Association Fibers | ||||||
| Cingulum (cingulate gyrus) | L | .028 | −10 | −48 | 25 | 29 |
| Superior longitudinal fasciculus | L | .022 | −34 | −40 | 32 | 228 |
| Commissural Fibers | ||||||
| Body of corpus callosum | I | .028 | −16 | −30 | 31 | 37 |
| Splenium of corpus callosum | I | .020 | −18 | −37 | 31 | 133 |
| Projection Fibers | ||||||
| Posterior corona radiata | L | .020 | −28 | −61 | 19 | 216 |
| Posterior thalamic radiation | L | .020 | −27 | −60 | 17 | 215 |
| Superior corona radiata | L | .022 | −20 | −29 | 40 | 117 |
| Age by Dx interaction in RaD | ||||||
| Association Fibers | ||||||
| Cingulum (cingulate gyrus) | L,R | .005 | −11 | −34 | 35 | 225 |
| Fornix / Stria terminalis | L,R | .007 | −32 | −22 | −8 | 119 |
| Sagittal stratum | L,R | .007 | −40 | −34 | −14 | 371 |
| Superior longitudinal fasciculus | L,R | .005 | −37 | −40 | 19 | 601 |
| Commissural Fibers | ||||||
| Body of corpus callosum | I | .005 | −15 | −30 | 30 | 796 |
| Genu of corpus callosum | I | .024 | 17 | 20 | 25 | 1 |
| Splenium of corpus callosum | I | .004 | −17 | −35 | 29 | 783 |
| Tapetum | L,R | .011 | 28 | −39 | 20 | 10 |
| Projection Fibers | ||||||
| Anterior corona radiata | R | .016 | 19 | 16 | 32 | 77 |
| Cerebral peduncle | L | .037 | −17 | −23 | −5 | 1 |
| External capsule | L,R | .005 | −28 | −22 | 18 | 160 |
| Posterior corona radiata | L,R | .004 | −20 | −36 | 38 | 1028 |
| Posterior limb of internal capsule | L,R | .005 | −27 | -26 | 17 | 269 |
| Posterior thalamic radiation | L,R | .004 | −29 | −70 | 11 | 1016 |
| Retrolenticular part of internal capsule | L,R | .005 | −28 | −33 | 14 | 633 |
| Superior corona radiata | L,R | .004 | −19 | −27 | 37 | 760 |
3.0 DISCUSSION
Our study demonstrated that white matter structural integrity is altered across all major tracts in high functioning adolescents and adults with ASD compared to age and IQ matched TD individuals. The major findings were reduced FA and increased RaD in the ASD group, as well as a number of age-related interactional effects. This work adds to a limited number of studies that have investigated white matter integrity in ASD, and demonstrates convincingly that white matter microstructure is atypical in ASD and appears to follow an abnormal developmental trajectory during adolescence and adulthood.
Due to the vast number of affected areas, we focused on the major white matter tracts implicated in ASD and detailed in the John’s Hopkins University (JHU) white matter atlas (Mori et al., 2005). Additional areas that showed statistically significant results are reported in the supplementary information section. The JHU atlas classifies white matter tracts into four groups. The projection tracts connect cortical and subcortical grey matter, the association tracts connect cortical areas, the commissural tracts connect the left and right hemispheres, and the brain stem tracts are the five major white matter tracts that can be reconstructed in the brainstem (Mori et al., 2005). We found reduced FA in the ASD group in all white matter tracts included in the JHU atlas with the exception of the bilateral hippocampus tracts (association fibers), the right inferior cerebellar peduncle (brain stem tract), and the left tapetum (commissural fibers). The majority of white matter tracts with reduced FA also had corresponding increases in RaD. Our study is consistent with most previous DTI studies of children and adults with ASD, although we found considerably more widespread abnormalities than had been previously reported. The most consistently reported findings in the extant literature have been abnormal FA in the cingulate gyrus (Barnea-Goraly et al., 2011; Bloemen et al., 2010; Jou et al., 2011; Pardini et al., 2009; Shukla et al., 2010; Thakkar et al., 2008; Weinstein et al., 2011), inferior fronto-occipital fasciculus (Bloemen et al., 2010; Cheng et al., 2010; Jou et al., 2011; Noriuchi et al., 2010; Shukla et al., 2010), superior longitudinal fasciculus (Barnea-Goraly et al., 2011; Bloemen et al., 2010; Cheng et al., 2010; Cheung et al., 2009; Jou et al., 2011; Noriuchi et al., 2010; Poustka et al., in press; Shukla et al., 2010), the corpus callosum (Barnea-Goraly et al., 2011; Ben Bashat et al., 2007; Bloemen et al., 2010; Brito et al., 2009; Cheon et al., 2011; Kumar et al., 2010; Noriuchi et al., 2010; Shukla et al., 2010; Weinstein et al., 2011), and the posterior limb of the internal capsule (Barnea-Goraly et al., 2011; Ben Bashat et al., 2007; Brito et al., 2009; Cheng et al., 2010; Shukla et al., 2010). A few studies (Bloemen et al., 2010; Cheon et al., 2011; Kumar et al., 2010; Poustka et al., in press) in addition to ours have found reduced FA in the uncinate fasciculus, the tract that connects the orbital aspect of the frontal lobe to the temporal pole and terminates in the amygdala. The uncinate fasciculus is thought to be involved in processing information about the emotional significance of stimuli and the generation of emotional expression (Schmahmann and Pandya, 2006). However, white matter abnormalities in ASD are widespread, affecting all major neural systems. See Table 6 for a summary of FA in autism, organized according to the age of the sample.
Table 6.
Summary of FA results in DTI studies of ASD, organized by age range.
| Group size | Group Age, mean (SD) yr | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Study | Method | Diagnosis | Autism | Control | Autism | Control | FA | Location |
| Wolff et al. 2012 | Tractography | Autism | 28 | 64 | 0.57 (0.07) | 0.56 (0.07) | ↑ | l-fx, l-ilf, l-unc, body of the cc, r-plic |
| Wolff et al. 2012 | Tractography | Autism | 17 | 49 | 1.06 (0.06) | 1.06 (0.05) | ↓ | l-atr |
| Wolff et al. 2012 | Tractography | Autism | 17 | 33 | 2.04 (0.05) | 2.06 (0.07) | ↓ | l-alic, l-atr |
| Bashat et al. 2007 | ROI | Autism | 7 | 18 | 1.80 – 3.30* | 9.60** | ↑↓ | Increase: gcc, scc, l-plic, l-fminor Decrease: l-cst-sl2 |
| Weinstein et al. 2011 | Whole-brain | Autism | 21 | 26 | 3.30 (1.10) | 3.30 (1.20) | ↑ | gcc, body of the cc, l-slf, cg |
| Sundaram et al. 2008 | Tractography | Autism, PDD-NOS, Asperger's disorder | 50 | 16 | 4.79 (2.43) | 6.84 (3.45) | ↓ | short association fibers |
| Kumar et al. 2010 | ROI | Autism, PDD-NOS, Asperger's disorder | 32 | 16 | 5.00** | 4.60** | ↓ | r-unc, l-slf, r-cg, cc |
| Sivaswamy et al. 2010 | ROI | Autism, PDD-NOS, Asperger's disorder | 27 | 16 | 5.00** | 5.90** | ↑ | r-mcp |
| Ke et al. 2009 | Whole-brain | Autism | 12 | 10 | 8.75 (2.26) | 9.40 (2.07) | ↑↓ | Increase: r-middle temporal gyrus, r-sub-gyral frontal lobe, l-sub-lobar Decrease: l-middle frontal gyrus, l-stg, l-inferior frontal gyrus |
| Cheung et al. 2009 | Whole-brain | Autism | 13 | 14 | 9.30 (2.60) | 9.90 (2.50) | ↑↓ | Increase: r-slf, l-fmajor Decrease: l-frontal orbital cortex BA47, r-precentral gyrus BA4, frontal pole BA11, r-fusiform gyrus BA19, r-unc, l-middle temporal gyrus BA20 |
| Brito et al. 2009 | ROI | Autism | 8 | 8 | 9.53 (1.83) | 9.57 (1.36) | ↓ | anterior body of the cc, r-cst, r-plic, l-scp, mcp |
| Poustka et al. in press | ROI and tractography | ASD | 18 | 18 | 9.70 (2.10) | 9.70 (1.90) | ↓ | r-slf, unc |
| Barnea-Goraly et al. 2011 | Whole-brain | ASD | 13 | 11 | 10.50 (2.00) | 9.60 (2.10) | ↓ | medial prefrontal white matter, acr, gcc, anterior forceps of the cc, body of the cc, ec, slf, mid/posterior cingulate gyrus, stg, temporo-parietal junction, fronto-parietal centrum semiovale |
| Jou et al. 2011 | Whole-brain | ASD | 15 | 8 | 10.90 (3.70) | 11.50 (2.60) | ↓ | cg, ifo, ilf, slf, unc, atr, cst, fmajor, fminor |
| Cheon et al. 2011 | ROI | Asperger's disorder, PDD-NOS | 17 | 17 | 11.00 (2.10) | 10.20 (2.00) | ↓ | atr, cc, l-unc, ilf |
| Ameis et al. in press | Whole-brain | Autism, Asperger's disorder | 19 | 16 | 12.40 (3.10) | 12.30 (3.60) | ns | whole brain |
| Shukla et al. 2010 | Whole-brain | Autism, Asperger's disorder | 26 | 24 | 12.80 (0.60) | 13.00 (0.60) | ↓ | ilf, ifo, slf, cg, gcc, body of the cc, scc, plic, alic, cst, atr |
| Jou et al. 2011 | ROI | Autism, PDD-NOS, Asperger's disorder | 10 | 10 | 13.06 (3.85) | 13.94 (4.23) | ↓ | anterior radiation of the cc, cg, body of the cc, l-slf, l-ifo, ilf |
| Cheng et al. 2010 | Whole-brain | ASD | 25 | 25 | 13.71 (2.54) | 13.51 (2.20) | ↑↓ | Increase: r-slf, r-scr, l-insula, r-atr, r-plic, r-ifo, mcp Decrease: r-slf, l-plic, r-icp |
| Noriuchi et al. 2010 | Whole-brain | ASD | 7 | 7 | 13.96 (2.68) | 13.36 (2.74) | ↓ | white matter around the r-anterior cingulate cortex, l-dorsolateral prefrontal cortex, r-temporal pole, r-amygdala, r-slf, l-posterior superior-temporal sulcus, anterior cc, r-fronto-occipital fasciculus |
| Fletcher et al. 2010 | ROI | High-functioning autism | 10 | 10 | 14.25 (1.92) | 13.36 (1.34) | ns | slf |
| Groen et al. 2011 | Whole-brain | Autism | 17 | 25 | 14.40 (1.60) | 15.50 (1.80) | ↓ | slf, ilf, l-corona radiata |
| Bode et al. 2011 | Whole-brain | ASD | 27 | 26 | 14.70 (1.60) | 14.50 (1.50) | ↑ | opt, r-ifo |
| Lee et al. 2007 | ROI | Autism, PDD-NOS | 43 | 34 | 16.20 (6.70) | 16.40 (6.00) | ↓ | white matter of the stg, temporal stem |
| Alexander et al. 2007 | ROI | Autism, PDD-NOS, Asperger's disorder | 43 | 34 | 16.23 (6.70) | 16.44 (5.97) | ↓ | gcc, scc, total cc |
| Knaus et al. 2010 | tractography | ASD | 7 | 15 | 16.83 (2.35) | 14.43 (2.47) | ns | slf |
| Pardini et al. 2009 | Whole-brain & ROI | Autism | 10 | 10 | 19.70 (2.83) | 19.90 (2.64) | ↓ | l-orbitofrontal cortex, anterior cingulate, medial frontal gyrus, inferior frontal gyrus, r-superior frontal gyrus |
| Langen et al. 2007 | tractography | Autism | 21 | 22 | 25.57 (6.08) | 28.45 (6.39) | ↓ | putamen tract |
FA is a measure of the ratio of restricted diffusion to restrained diffusion and is thought to be related to intracellular diffusion along axonal microtubules, diffusion constrained to the myelin-cell membrane border or diffusion directed by the outer sheath of the myelin complex (Song et al., 2005). Reduced FA indicates potential abnormalities in white matter tissue integrity and has been reported in several neurological diseases such as multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, Huntington’s disease and traumatic brain injury(see e.g., Weaver et al., 2009). RaD is a measure of diffusion perpendicular to the long axis of the white matter tract. Although controversy exists as to how this measure should be interpreted (Wheeler-Kingshott and Cercignani, 2009), evidence suggests that changes in RaD are associated with the process of myelination and demeylination, as opposed to axonal degeneration (Song et al., 2005). One report showed strong correlations between histological markers of axonal tissue and AxD diffusivity values in an animal model of spinal cord injury and between myelin concentrations and RaD diffusivity values in an animal model of multiple sclerosis (Budde et al., 2007). The combined evidence stemming from this and other animal work suggests that diffusivity values can differentiate between axonal damage and myelin loss. Specifically, the loss of axons within white matter decreases AxD values but does not affect RaD, while the loss of myelin increases RaD values but does not affect AxD values (Deboy et al., 2007; Harms et al., 2006; Song et al., 2002). However, it is important to exhibit caution in interpreting changes in RaD and AxD, because when crossing fibers are present, changes in RaD can be confused with changes in AxD and vice-a-versa (Wheeler-Kingshott and Cercignani, 2009).
In neurodevelopmental disorders such as ASD, interpreting group differences in DTI scalars presents additional challenges. Unlike in degenerative diseases or when a loss of white matter integrity is secondary to aging, in ASD white matter is expected to have formed abnormally and be subjected to unknown, ongoing developmental processes. Volumetric studies have shown that white matter development is atypical in ASD. Young children with ASD appear to have excessive white matter (Carper et al., 2002; Hazlett et al., 2005; Herbert et al., 2004) and, potentially, increased FA values (Ben Bashat et al., 2007; Weinstein et al., 2011; Wolff et al., 2012). However, morphological differences disappear by adolescence and adulthood while impairment is still detectable in the white matter microstructure. The mechanism for the transient, exuberant growth followed by a premature plateau (in gross morphology) is unknown. Furthermore, direct evidence of white matter microstructural abnormalities is extremely limited. However, recently, Zikopoulos and Barbas (2010) undertook a post mortem investigation of the fine structure of myelinated axons in the prefrontal cortex of five adults with ASD aged 30–44. They found a decrease in the number of long distance axons in the white matter and an excessive number and higher density of short and medium range axons under the anterior cingulate cortex. Further analysis of this area revealed that increased density was due to increased branching along the nodes of Ranvier in the superficial white matter. In the white matter below the orbital frontal cortex there was decreased myelin thickness, independent of axonal diameter. Although this work was based on a very small sample size, our findings of abnormalities in RaD, but not AxD, would be consistent with postmortem evidence of abnormal myelin rather than axonal injury. Reduced FA, as well as increased RaD values, would be expected in the presence of reduced long distance axons with increased branching and thinner myelin. Thus, at this time, it does not appear that white matter abnormalities in ASD can be attributed to frank demyelination.
A major goal of this study was to look at age-related differences in DTI from adolescence through adulthood in ASD. Although white matter microstructural abnormalities in ASD have been discussed previously, the developmental trajectory of such abnormalities has been largely understudied. We found a number of significant age-by-diagnosis interaction effects for the association fibers, commissural fibers, and projection fibers. In general, FA, MD, and RaD levels appeared to be normalizing to TD levels over time in the ASD group. During adolescence, differences in the DTI scalars are pronounced and indicate robust differences in white matter microstructure. However, during early adulthood, the ASD group’s FA, MD, and RaD values were similar to and heading in the direction that would suggest equivalent white matter integrity as the TD group. Because of the limited number of older participants that we have in this study and the limitations inherent in interpreting DTI data, it is premature to suggest that white matter integrity is improving in the ASD group over this age range. However, the age-related pattern identified in this study is not consistent with accelerated aging processes which affect myelin integrity. Future studies that look at age effects in a across a broader span of older subjects may be able to more specifically characterize the developmental processes occurring in ASD.
Age by diagnosis interaction effects were widespread throughout the white matter, yet notably absent in the brain stem tracts. In addition, the ASD groups did not show correlations between age and white matter integrity in any of the brain stem tracts. This is in contrast to the TD group, for whom age was negatively correlated with FA and AxD in the middle and superior cerebellar peduncles and the medial lemniscus. A negative correlation with AxD only was observed in the pontine crossing tract and inferior cerebellar peduncle. While the ASD group showed microstructural abnormalities in the brainstem, consistent with myelin abnormalities, such processes appear to be static in the ASD brain throughout adolescence and early adulthood. This is in stark contrast to the TD group, wherein regional declines in measures of white matter integrity were observed.
There are several limitations to consider when evaluating the current study. We sought to investigate white matter microstructural abnormalities in ASD and their relationship to age. Although we report our results in terms of the white matter tracts, it is important to keep in mind that the TBSS is a voxelwise approach, not a tractography approach, and the overlap with tracts is inferred based on the JHU atlas template. Also, because of our coarse spatial resolution (relative to axonal diameter), one voxel may contain multiple white matter tracts. Second, our ASD sample was comprised almost exclusively of high functioning male adolescents and young adults. Thus, it is not certain whether the findings reported here are generalizable to the entire spectrum of clinical presentations, levels of functioning, females, and age groups. It is quite possible that younger and lower functioning individuals with ASD may have a different developmental course and/or level of white matter abnormalities. Third, because this was a cross-sectional study, we cannot rule out the role of cohort effects on our findings. However, our results suggest that follow-up longitudinal studies of white matter integrity are warranted in this age range. In addition, future studies should consider the role of interaction effects when statistically controlling for age when comparing ASD and TD groups. Our study indicates that investigating age as an independent variable may yield important information about the pathophysiology of ASD.
In conclusion, our study found widespread white matter abnormalities in high functioning adolescents in ASD across all major white matter tracts. Since differences were primarily in RaD, not AxD, we proposed that white matter abnormalities in ASD may be related to myelin dysfunction. In addition, increased RaD may reflect differences in axonal density and/or arborization. Although this current study does not support the presence of axonal thinning as reported in post-mortem work (Zikopoulos and Barbas, 2010), higher resolution techniques such as High Angular Resolution Diffusion Imaging, which produced modeling of crossing fibers may be a more sensitive approach for detecting axonal pathology. Dramatic developmental changes in white matter appear to be occurring during the transition period between adolescence and adulthood, although the trajectory of this change is significantly different between the ASD and TD groups. It is notable that impairments are most pronounced in adolescence, a period of time that may be characterized by increases in comorbid psychiatric difficulties and, in some cases, the onset of seizures. From adolescence to adulthood, white matter scalars appear to normalize to levels that overlap with typical developing peers. It is possible that the apparent improvements in autism symptom expression (and/or maladaptive behaviors) during adulthood are associated with improvements in white matter integrity. Additional studies utilizing a longitudinal design that includes a wider range of ages and more extensive clinical characterization would be useful for further exploration of the neurodevelopmental processes unfolding during this dynamic time in development.
4.0 Experimental Procedure
4.1 participants
Twenty-eight individuals with ASD and 33 TD controls participated in the DTI study. Data from five TD participants were excluded due to an incidental finding on MRI (n =1), a clinically significant elevation (moderate to severe range) on our social anxiety measure (n=1), or excessive artifacts (n = 3). Data from three individuals with ASD were excluded for excessive artifacts (n =2) or because of distortions caused by the participant’s braces (n =1). All participants were able to tolerate the MRI scanning protocol without sedation. The included ASD group (n=25; females = 9; mean age = 21.29 ± 5.66; range = 13.72 – 35.59) was composed of 11 individuals with autistic disorder, 9 individuals with Asperger’s disorder, and 5 individuals with pervasive developmental disorder-not otherwise specified (PDD-NOS) based on expert clinical judgment utilizing DSM-IV criteria (American Psychiatric Association, 1994). Diagnoses were confirmed with the Autism Diagnostic Interview-Revised (ADI-R, Lord et al., 1994) and the Autism Diagnostic Observation Schedule (ADOS, Lord et al., 2000). All participants under 18 years of age are part of an ongoing longitudinal study at the University of Washington Autism Center and received the ADI-R and ADOS at ages 3–4, 6, 9, and 14 years of age. Adult ASD participants were administered the ADOS as part of their current research visit. Most adults were administered and ADI-R as part of the current research visit; however, when a prior ADI-R was available, the ADI-R was not repeated in order to minimize participant and family burden. TD participants (n = 28; female = 6; mean age = 21.31 ± 7.269; range = 13.58 – 40.92) were screened for current or past psychiatric disorders, history of a developmental learning disability, and contraindications to MR imaging. The ASD and control groups did not significantly differ on age (t =−.011; df = 51, p = .992) or full-scale IQ (t =−.787; df = 51, p = .435). Clinical and demographic information is reported in table 1.
Table 1.
Included participant charateristics
| ASD (n=25) | TD (n = 28) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p value | |
| Age | 21.29 | (5.66) | 21.31 | (7.27) | .99 |
| Full Scale IQa | 109.88 | (16.94) | 113.25 | (14.24) | .44 |
| Verbal IQa | 106.16 | (21.00) | 111.14 | (13.78) | .31 |
| Nonverbal IQa | 110.72 | (14.25) | 111.96 | (14.39) | .75 |
| ADOS subscales | |||||
| Communication | 4.08 | (1.96) | |||
| Social | 7.36 | (2.33) | |||
| ADOS severity | 6.44 | (1.39) | |||
| ADI-R subscales | |||||
| Communication | 15.36 | (5.24) | |||
| Social | 19.76 | (6.25) | |||
| Repetitive Behavior | 5.56 | (2.33) | |||
Based on Differential Abilities Scale for participants age 13–17 and the WASI for participants age 18– 40
ADOS = Autism Diagnostic Observation Schedule; ADI-R = Autism Diagnostic Interview-Revised
This study was approved by the University of Washington Human Subjects Institutional Review Board and written, informed consent was obtained from all study participants.
4.2 Data Acquisition
MRI scans were collected on a 3T Philips Achieva MR system (version 1.5, Philips Medical Systems, Best, The Netherlands) with dual Quasar gradients (80mTm–1 with a slew rate of 110mTms–1 or 40mTm–1 at a slew rate of 220mTms–1) using an 8-channel SENSE head coil. A T1-weighted MPRAGE (magnetization prepared-rapid gradient echo; TR=7.7 ms; TE=3.7 ms; flip angle = 8; FOV=220 mm; matrix 200×200; 180 slices; acquisition voxel size (mm) = 1.00/1.00/1.00; reconstruction voxel size (mm) 0.86/0.86/1.00; TFE shots=144; TFE durations=1633.0; Inversion delay (TI) 823.8 ms; slice orientation axial, fold-over direction RL; REST slab 57.1 mm slice thickness) volume was collected for registration and anatomical localization.
The DTI scan consisted of a single-shot echo-planar sequence with the following parameters: TR/TE/flip angle: 10. 5 s/63 ms/90°, matrix size of 128×128, FoV of 240×240, 2 mm slice thickness, 72 slices. Diffusion weighting consisted of 32 non-colinear gradient directions, a non-diffusion weighted b0 map and a b-factor set at 1000 s/mm2. The B0 field map was acquired using a fast field echo sequence (TR=200 ms; TE1=4.6 ms; TE2 = 5.6 ms; flip angle=30°; FOV=220 mm) with a matrix size of 64 × 64 (in-plane resolution=3.44×3.44 mm). Thirty-eight axial slices covering the entire brain (slice thickness = 3.5mm, 0 mm gap) were acquired during each image. Scan duration = 53 s. The B0 field map was reconstructed by subtracting the phase images from the two TE image acquisitions. The output contained a magnitude map and a B0 map.
4.3 DTI Processing and Statistical Analysis
All DTI data were preprocessed offline using FDT (fMRIB’s Diffusion Toolbox; http://www.fmrib.ox.ac.uk/fsl/fdt/index.html). The raw DTI images were visually inspected following eddy current correction by a rater blinded to diagnosis in order to identify DTI studies that had artifacts, including “venetian blinds,” “checkers,” large intensity differences in any of the slices, wrapping, or motion artifacts (as per above, 2 ASD and 3 TD studies were excluded on this basis). Studies containing any of the artifacts on more than 10 gradient directions were excluded. For DTI studies with 10 or fewer artifact-contaminated gradient directions, the bad gradient directions were removed from the dataset and the bvec file was modified to reflect these changes. In our sample, an average of 1.7 (SD=2.2) directions were removed from the scans of the ASD group and 2.1 (SD = 3.3) directions were removed from the scans of the TD group (p = .632). Head motion and eddy current correction was conducted with affine registration to a reference volume. Using the field maps, B0-field inhomogeneity-induced geometric distortion was then corrected with PRELUDE (Phase Region Expanding Labeller for Unwrapping Discrete Estimates; [78]) and FUGUE (fMRIB's Utility for Geometrically Unwarping EPIs; http://www.fmrib.ox.ac.uk.offcampus.lib.washington.edu/fsl/fugue/). Diffusion tensors were estimated at each voxel using FDT. From these maps, λ1, λ2 and λ3, mean diffusivity (MD), and fractional anisotropy (FA) indices were calculated. In addition, axial diffusivity (AxD; the magnitude of the primary eigenvalue) and radial diffusivity (RaD; the mean of the two eigenvalues that describe width and depth) maps were computed. Tract Based Spatial Statistics (TBSS) was used to delineate the white matter tracts and warp individual maps to the FMRIB58_FA standard-space image. Voxelwise statistics were performed using Randomise with threshold-free cluster-enhancement (TFCE, Salimi-Khorshidi et al., 2011) using 5000 permutations. Between-group contrasts were applied to each DTI scalar map (FA, MD, RaD, AxD) independently, controlling for gender. In addition, we investigated the relationship between age and the DTI scalars for the ASD and TD group and tested the group by age interaction, controlling for IQ and gender. As a follow-up to the interaction model, we ran a correlational analyses between age and the DTI indices for the ASD and TD groups separately, controlling for IQ and gender. Lastly, in the ASD group, we tested the relationship between ADOS severity and all DTI scalars, controlling for gender and IQ. Significance for all analyses was set at p < 0. 05, whole brain family-wise-error-corrected.
We identified affected white matter structures using the John’s Hopkins University (JHU) ICBM-DTI-81 white matter atlas (Mori et al., 2005) which parcellates the brain into 50 white matter tracts. Using software developed in our laboratory, each voxel in the corrected statistical map, thresholded at p < .05, was assigned to a JHU tract. For each tract, the total number of significant voxels was calculated and the voxel with the highest p-value and its corresponding MNI coordinates was identified. These summary statistics are reported in the Results tables. For completeness, we also used the Talairach atlas to label white matter not included in the JHU atlas. The results of the white matter regions outside the JHU atlas are reported in the supplementary information section.
Supplementary Material
Figure 1.
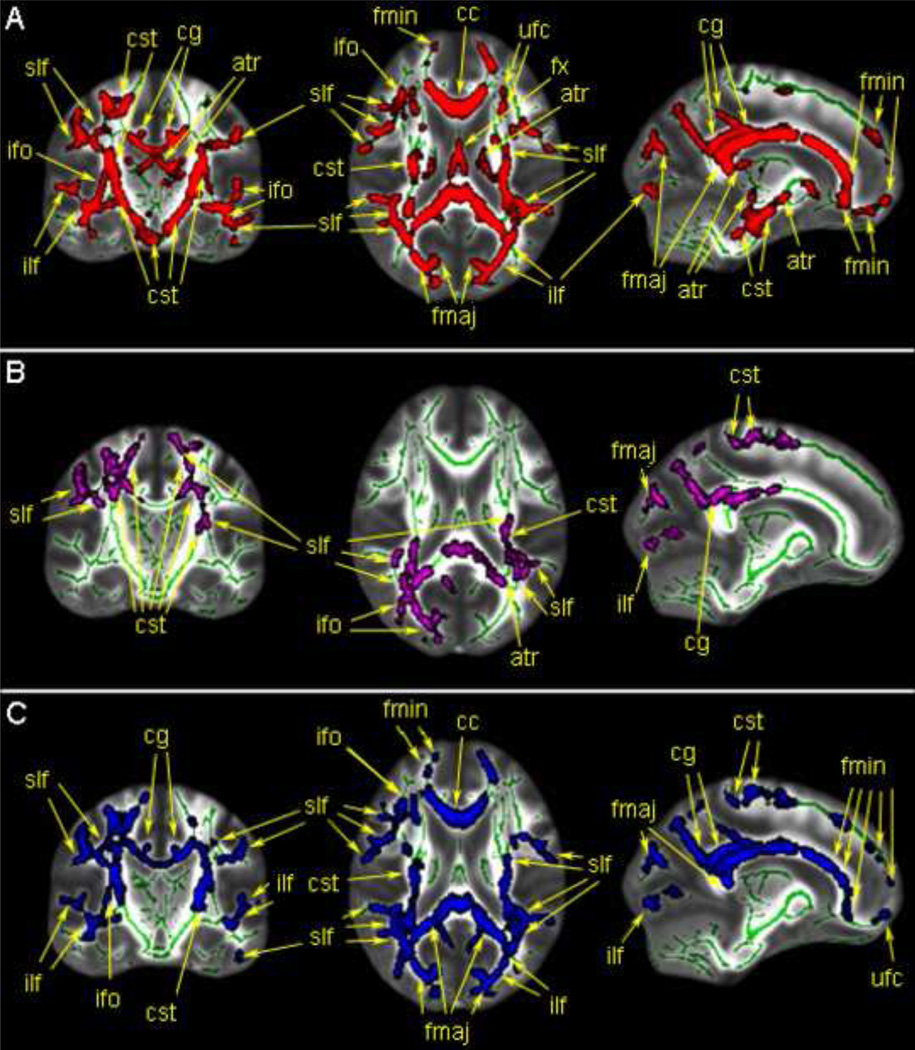
Regions with significant between-group difference in FA (A.), MD (B.) and RaD(C). The TBSS skeleton is shown in green over the FA template brain. Areas in red indicate white matter regions where FA values were significantly lower in the ASD group compared to the TD group. The purple areas indicate white matter regions where MD values were significantly higher in the ASD group compared to the TD group. The blue areas indicate white matter regions where the RaD values were significantly higher in the ASD group compared to the TD group. Regions are labeled according the JHU atlas, using standard abbreviations. Additional statistical information is available in Tables 2–4.
Figure 2.
Regions with a significant interaction effect between age and diagnosis for FA (A), MD (B), and RaD (C) along with scatter plots to illustrate the directionality of the interaction effect. Each DTI scalar is overlaid on the slices that were the most representative of the results (x = −28, y = 27, z = 23). Red areas indicate voxels where FA values showed a significant interaction effect. Purple areas indicate voxels where MD values showed a significant interaction effect. Blue areas indicate voxels where RaD showed a significant interaction effect. Scatter plots were created by computing a mean DTI scalar value for each participant, which was obtained by averaging the z-score of all voxels for that participant included in the mask. The mask was defined by the voxels showing the significant interaction effect. The top scatter plot is FA, the middle scatter plot is MD, and the bottom scatter plot is RaD. Additional statistical information is provided in Table 5.
Table 3.
Regions showing increased mean diffusivity in ASD
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| White Matter Region | side | p (max) | x | y | z | # of voxels |
| JHU Atlas lables | ||||||
| Association Fibers | ||||||
| Cingulum (cingulate gyrus) | L,R | 0.0184 | 11 | −47 | 26 | 76 |
| Sagittal stratum | L,R | 0.035 | 36 | −53 | −4 | 24 |
| Superior longitudinal fasciculus | L,R | 0.0172 | 34 | −29 | 35 | 775 |
| Commissural Fibers | ||||||
| Body of corpus callosum | I | 0.0176 | 13 | −28 | 28 | 410 |
| Splenium of corpus callosum | I | 0.0168 | 26 | −54 | 14 | 768 |
| Tapetum | R | 0.0178 | 29 | −50 | 18 | 3 |
| Projection Fibers | ||||||
| External capsule | L | 0.0408 | −30 | −17 | 13 | 18 |
| Posterior corona radiata | L,R | 0.0168 | 28 | −57 | 20 | 911 |
| Posterior limb of internal capsule | L | 0.0374 | −27 | −25 | 18 | 41 |
| Posterior thalamic radiation | L,R | 0.0172 | 29 | −57 | 18 | 577 |
| Retrolenticular part of internal capsule | L,R | 0.0184 | 31 | −38 | 16 | 158 |
| Superior corona radiata | L,R | 0.0176 | 25 | −24 | 31 | 566 |
Note. R = right, L = left, I = interhemispheric. Regions are labled according to the peak p value within that region. When both R and L are listed, the bolded side indicates the peak p value and location, the # of voxels includes both sides combined.
Highlights.
Widespread, robust white matter microstructural abnormalities are present in ASD.
Impairments are observed in FA, MD, and radial diffusivity in ASD.
Abnormal myelination, excessive branching, or thinning may be present in ASD.
Age-by-Dx interaction effects suggest normalization may occur in adults with ASD.
Acknowledgements
This work was supported by NINDS/NIH 5K01NS059675 and NICHD/NIH 5P50HD055782. We would like to thank Drs. Paul Borghesani and Kurt Weaver for their input on background and interpretation of DTI scalars and Dr. Edith Sullivan for her input on the DTI preprocessing pipeline and methods for evaluating data quality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, Bigler ED, Lainhart JE. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Vol. IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Anderson DK, Maye MP, Lord C. Changes in Maladaptive Behaviors From Midchildhood to Young Adulthood in Autism Spectrum Disorder. American Journal on Intellectual and Developmental Disabilities. 2011;116:381–397. doi: 10.1352/1944-7558-116.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Lotspeich LJ, Reiss AL. Similar white matter aberrations in children with autism and their unaffected siblings: a diffusion tensor imaging study using tract-based spatial statistics. Arch Gen Psychiatry. 2011;67:1052–1060. doi: 10.1001/archgenpsychiatry.2010.123. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Even A, Levy Y, Ben Sira L. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Bloemen OJ, Deeley Q, Sundram F, Daly EM, Barker GJ, Jones DK, van Amelsvoort TA, Schmitz N, Robertson D, Murphy KC, Murphy DG. White matter integrity in Asperger syndrome: a preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res. 2010;3:203–213. doi: 10.1002/aur.146. [DOI] [PubMed] [Google Scholar]
- Boelte S, Poustka A. Diagnosis of autism: the connection between current and historical information. Autism. 2000;4:382–390. [Google Scholar]
- Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Jr, Rodrigues Lde S, Gasparetto EL, Calcada CA. Diffusion tensor imaging findings in school-aged autistic children. J Neuroimaging. 2009;19:337–343. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57:688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Semendeferi K, Buckwalter J, Schenker N, Switzer R, Courchesne E. Reduced minicolumns in the frontal cortex of patients with autism. 2006;Vol. 32:483–491. doi: 10.1111/j.1365-2990.2006.00745.x. ed.^eds. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol (Berl) 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Tillquist CR. Encephalization, emergent properties, and psychiatry: a minicolumnar perspective. Neuroscientist. 2008;14:101–118. doi: 10.1177/1073858407309091. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010;50:873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Kim YS, Oh SH, Park SY, Yoon HW, Herrington J, Nair A, Koh YJ, Jang DP, Kim YB, Leventhal BL, Cho ZH, Castellanos FX, Schultz RT. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A Diffusion Tensor Imaging study. Brain Research. 2011;1417:77–86. doi: 10.1016/j.brainres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TK, Ho TP, McAlonan GM. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry. 2009;50:1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. Jama. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brain development in autism: early overgrowth followed by premature arrest of growth. Ment Retard Dev Disabil Res Rev. 2004;10:106–111. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Dawson G, Munson J, Webb SJ, Nalty T, Abbott R, Toth K. Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biol Psychiatry. 2007;61:458–464. doi: 10.1016/j.biopsych.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboy CA, Zhang J, Dike S, Shats I, Jones M, Reich DS, Mori S, Nguyen T, Rothstein B, Miller RH, Griffin JT, Kerr DA, Calabresi PA. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain. 2007 doi: 10.1093/brain/awm122. [DOI] [PubMed] [Google Scholar]
- Dementieva YA, Vance DD, Donnelly SL, Elston LA, Wolpert CM, Ravan SA, DeLong GR, Abramson RK, Wright HH, Cuccaro ML. Accelerated head growth in early development of individuals with autism. Pediatr Neurol. 2005;32:102–108. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, Alexander AL, Bigler ED, Lange N, Lainhart JE. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010;51:1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Dawson G, Petropoulos H, Dager SR. Gray and white matter brain chemistry in young children with autism. Arch Gen Psychiatry. 2006;63:786–794. doi: 10.1001/archpsyc.63.7.786. [DOI] [PubMed] [Google Scholar]
- Gilchrist A, Green J, Cox A, Burton D, Rutter M, Le Couteur A. Development and current functioning in adolescents with Asperger syndrome: a comparative study. J Child Psychol Psychiatry. 2001;42:227–240. [PubMed] [Google Scholar]
- Gillberg C, Steffenberg S. Outcome and prognostic factors in infantile autism and similar conditions: A population based study of 46 cases followed through puberty. Journal of Autism and Developmental Disorders. 1987;17:273–287. doi: 10.1007/BF01495061. [DOI] [PubMed] [Google Scholar]
- Groen WB, Buitelaar JK, van der Gaag RJ, Zwiers MP. Pervasive microstructural abnormalities in autism: a DTI study. J Psychiatry Neurosci. 2011;36:32–40. doi: 10.1503/jpn.090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Kilpatrick M, Keshavan MS, Minshew NJ. Motor performance and anatomic magnetic resonance imaging (MRI) of the basal ganglia in autism. J Child Neurol. 2003;18:317–324. doi: 10.1177/08830738030180050801. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Adams J, Gilbert AR, Keshavan MS, Minshew NJ. Abnormal brain size effect on the thalamus in autism. Psychiatry Res. 2006 doi: 10.1016/j.pscychresns.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Harms MP, Kotyk JJ, Merchant KM. Evaluation of white matter integrity in ex vivo brains of amyloid plaque-bearing APPsw transgenic mice using magnetic resonance diffusion tensor imaging. Exp Neurol. 2006;199:408–415. doi: 10.1016/j.expneurol.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, Vachet C, Piven J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 68:467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS., Jr Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Mateljevic N, Kaiser MD, Sugrue DR, Volkmar FR, Pelphrey KA. Structural Neural Phenotype of Autism: Preliminary Evidence from a Diffusion Tensor Imaging Study Using Tract-Based Spatial Statistics. AJNR Am J Neuroradiol. 2011 doi: 10.3174/ajnr.A2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and Anatomical Cortical Underconnectivity in Autism: Evidence from an fMRI Study of an Executive Function Task and Corpus Callosum Morphometry. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, Zhou Z, Ruan Z, Lu Z, Tao G, Liu Y. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. J Neuropathol Exp Neurol. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sundaram SK, Sivaswamy L, Behen ME, Makki MI, Ager J, Janisse J, Chugani HT, Chugani DC. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cereb Cortex. 2010;20:2103–2113. doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- Langen M, Durston S, Staal WG, Palmen SJ, van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry. 2007;62:262–266. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Johnson M, Morgan J, Miller JN, McMahon WM, Lu J, Jeong EK, Lainhart JE. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, DiLavore P, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule--Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Muller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Monk CS, Weng SJ, Wiggins JL, Kurapati N, Louro HM, Carrasco M, Maslowsky J, Risi S, Lord C. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci. 2010;35:105–114. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher L, Van Zijl P. MRI Atlas of Human White Matter. Vol. Amsterdam: Elsevier; 2005. [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but How? A Survey of Functional Connectivity MRI Studies in Autism Spectrum Disorders. Cereb Cortex. 2011;21:2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, Tobimatsu S, Kamio Y. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res. 2010;1362:141–149. doi: 10.1016/j.brainres.2010.09.051. [DOI] [PubMed] [Google Scholar]
- Pardini M, Garaci FG, Bonzano L, Roccatagliata L, Palmieri MG, Pompili E, Coniglione F, Krueger F, Ludovici A, Floris R, Benassi F, Emberti Gialloreti L. White matter reduced streamline coherence in young men with autism and mental retardation. Eur J Neurol. 2009;16:1185–1190. doi: 10.1111/j.1468-1331.2009.02699.x. [DOI] [PubMed] [Google Scholar]
- Petropoulos H, Friedman SD, Shaw DW, Artru AA, Dawson G, Dager SR. Gray matter abnormalities in autism spectrum disorder revealed by T2 relaxation. Neurology. 2006;67:632–636. doi: 10.1212/01.wnl.0000229923.08213.1e. [DOI] [PubMed] [Google Scholar]
- Piven J, Harper J, Palmer P, Arndt S. Course of behavioral change in autism: a retrospective study of high-IQ adolescents and adults. J Am Acad Child Adolesc Psychiatry. 1996;35:523–529. doi: 10.1097/00004583-199604000-00019. [DOI] [PubMed] [Google Scholar]
- Poustka L, Jennen-Steinmetz C, Henze R, Vomstein K, Haffner J, Sieltjes B. Fronto-temporal disconnectivity and symptom severity in children with autism spectrum disorder. World J Biol Psychiatry. 2012;13:269–280. doi: 10.3109/15622975.2011.591824. [DOI] [PubMed] [Google Scholar]
- Poustka L, Jennen-Steinmetz C, Henze R, Vomstein K, Haffner J, Sieltjes B. Fronto-temporal disconnectivity and symptom severity in children with autism spectrum disorder. World J Biol Psychiatry. doi: 10.3109/15622975.2011.591824. in press. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Rudie JD, Shehzad Z, Hernandez LM, Colich NL, Bookheimer SY, Iacoboni M, Dapretto M. Reduced Functional Integration and Segregation of Distributed Neural Systems Underlying Social and Emotional Information Processing in Autism Spectrum Disorders. Cereb Cortex. doi: 10.1093/cercor/bhr171. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Nichols TE. Adjusting the effect of nonstationarity in cluster-based and TFCE inference. Neuroimage. 2011;54:2006–2019. doi: 10.1016/j.neuroimage.2010.09.088. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Pandya D. Fiber Pathways of the Brain. Vol. New York: Oxford University Press, Inc.; 2006. [Google Scholar]
- Shukla DK, Keehn B, Muller RA. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry. 2010;52:286–295. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solso S, Thompson W, Campbell K, Ahrens-Barbeau C, Stoner R, Carter C, Weinfeld M, Spendlove S, Young J, Mayo M, Kuperman J, Hagler D, Theilmann R, Eyler L, Pierce K, Courchesne E, Dale AM. International Meeting for Autism Research. Vol. California, USA: San Diego; 2011. Abnormally Accelerated Development of Higher-Order Long-Distance Cerebral Tracts In ASD Infants and Toddlers. ed.^eds. [Google Scholar]
- Song S-K, Yoshino J, Le TQ, Lin S-J, Sun S-W, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion Tensor Imaging of Frontal Lobe in Autism Spectrum Disorder. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Weaver KE, Richards TL, Liang O, Laurino MY, Samii A, Aylward EH. Longitudinal diffusion tensor imaging in Huntington's Disease. Exp Neurol. 2009;216:525–529. doi: 10.1016/j.expneurol.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, Tarrasch R, Eksteine PM, Hendler T, Ben Bashat D. Abnormal white matter integrity in young children with autism. Hum Brain Mapp. 2011;32:534–543. doi: 10.1002/hbm.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, Bullmore E. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biol Psychiatry. 2005;57:991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About "axial" and "radial" diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Williams DL, Minshew NJ. Understanding autism and related disorders: what has imaging taught us? Neuroimaging Clin N Am. 2007;17:495–509. doi: 10.1016/j.nic.2007.07.007. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J, Gu H, Gerig G, Elison J, Styner M, Gouttard S, Botteron K, Dager S, Dawson G, Estes A, Evans A, Hazlett H, Kostopoulos P, McKinstry R, Paterson S, Schultz R, Zwaigenbaum L, Piven J. Differences in White Matter Fiber Tract Development Present from 6 to 24 Months in Infants with Autism. American Journal of Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Changes in Prefrontal Axons May Disrupt the Network in Autism. J. Neurosci. 2010;30:14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.