Abstract
Current influenza vaccines are primarily targeted to induce immunity to the influenza virus strain-specific hemagglutinin antigen and are not effective in controlling outbreaks of new pandemic viruses. An approach for developing universal vaccines is to present highly conserved antigenic epitopes in an immunogenic conformation such as virus-like particles (VLPs) together with an adjuvant to enhance the vaccine immunogenicity. In this review, the authors focus on conserved antigenic targets and molecular adjuvants that were presented in VLPs. Conserved antigenic targets that include the hemagglutinin stalk domain, the external domain of influenza M2 and neuraminidase are discussed in addition to molecular adjuvants that are engineered to be incorporated into VLPs in a membrane-anchored form.
Keywords: adjuvant, HA stalk domain, influenza, M2, NA, universal vaccine, VLPs
Influenza virus causes 17,000–51,000 deaths in the USA and 250,000–500,000 deaths worldwide annually; a global pandemic could kill millions [1,2]. Vaccination is the most cost-effective public health measure to prevent disease and mortality caused by influenza virus infection [2]. The envelope of influenza virus contains two major surface glycoproteins: the receptor-binding hemagglutinin (HA) and the sialic acid receptor-cleaving neuraminidase (NA) proteins [3,4]. Human influenza viruses are continually evolving, resulting in numerous variants with distinct antigenic surface glycoprotein properties. Current influenza vaccines consist of virus chemically inactivated with formalin or β-propiolactone and further detergent-treated to produce soluble forms of the viral surface antigens. In addition, a live attenuated influenza vaccine FluMist™ (MedImmune) is licensed for intranasal delivery in humans [5]. A limitation of current vaccines based on immunity to HA is that the antigenic regions of HA are highly susceptible to continual changes by mutations in circulating epidemic virus strains [6,7]. The HA proteins of influenza A viruses are divided into 16 subtypes and are further categorized into two major phylogenetic groups based on the distinct structures in the HA2 stalk domain: Group 1 (subtypes H1, H2, H5, H6, H8, H9, H11, H12, H13 and H16) and Group 2 (subtypes H3, H4, H7, H10, H14 and H15 [8]). Influenza vaccines are updated annually to match the antigenicity of the virus strains predicted to circulate in the coming winter season, and current vaccines are not effective in preventing emerging new pandemic strains with distinct HA antigenicity. Therefore, developing broadly cross-protective vaccines is a high priority.
Several new approaches are being applied to develop universal influenza A vaccines inducing broadly cross-protective immunity against conserved antigenic targets such as the HA stalk domain, the extracellular domain of M2 (M2e) and NA. These conserved antigenic targets are designed to be presented in a carrier system or conjugated to adjuvant molecules. In particular, virus-like particles (VLPs) are similar to the virus in structure and morphology, and thus can be a promising platform for presenting surface proteins in a highly immunogenic form. VLPs are produced without viral genomes (Figure 1), representing a high safety feature. The viral glycoproteins are unmodified by fixatives and presented in a membrane-anchored form mimicking their native conformation. VLPs are effective in stimulating APCs such as dendritic cells (DCs) as well as in inducing both B- and T-cell responses [9–14]. VLPs, similar to the virus in structure, are able to induce CD4 T-cell proliferation and cytotoxic T-cell immune responses [13,15]. In particular, influenza VLP vaccine was demonstrated to stimulate CD8 T cells via DC-mediated antigen cross-presentation, probably through the MHC class I pathway [13].
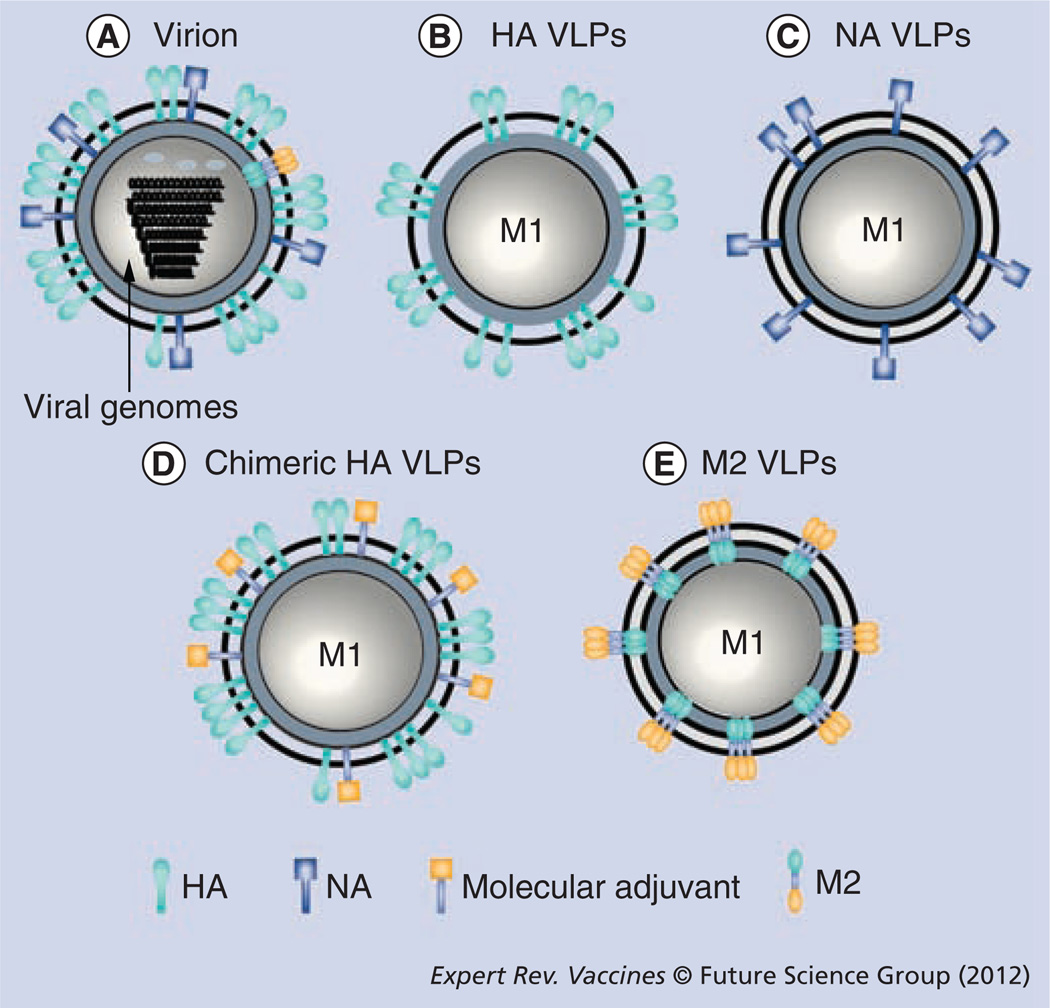
Figure 1. Schematic diagrams of influenza virions and virus-like particles.
(A) Influenza virion showing surface proteins, HA, NA, ion channel protein M2 and viral nucleoproteins. (B) HA VLPs: influenza VLPs containing matrix protein M1 and HA. (C) NA VLPs: influenza VLPs containing matrix protein M1 and NA. (D) Chimeric HA VLPs: influenza VLPs containing matrix protein M1, HA and a molecular adjuvant such as GM-CSF, CD40 ligand or flagellin as immune-stimulating molecules. (E) M2 VLPs: influenza VLPs containing matrix proteins M1 and M2. The matrix protein (M1) is thought to be positioned along the inner surface of the membrane and stabilizes the particle [147].
HA: Hemagglutinin; NA: Neuraminidase; VLP: Virus-like particle.
The HA antigen is incorporated into influenza virions at higher levels than NA or M2 and is known to be immune-dominant; thus, immune responses to other antigens such as NA are relatively low [16,17]. In this regard, VLP technology has an advantage by expressing fewer immunogenic components separately (Figure 1). Thus, NA and M2 antigens presented separately on VLPs can be more immunogenic by avoiding an HA immune-dominant component in the vaccines [16,17]. In addition, adjuvant molecules can be directly incorporated into VLP vaccines [18–21]. Here, the current progress in developing universal influenza vaccines using different platforms for presenting conserved target antigens is reviewed and discussed. These conserved target antigens include the HA stalk domain, M2e or NA (Figures 1 & 2). Influenza VLP vaccines presenting conserved influenza antigenic targets as well as adjuvant molecules in a membrane-anchored form have been receiving more attention and are discussed in comparison with other vaccine platforms.
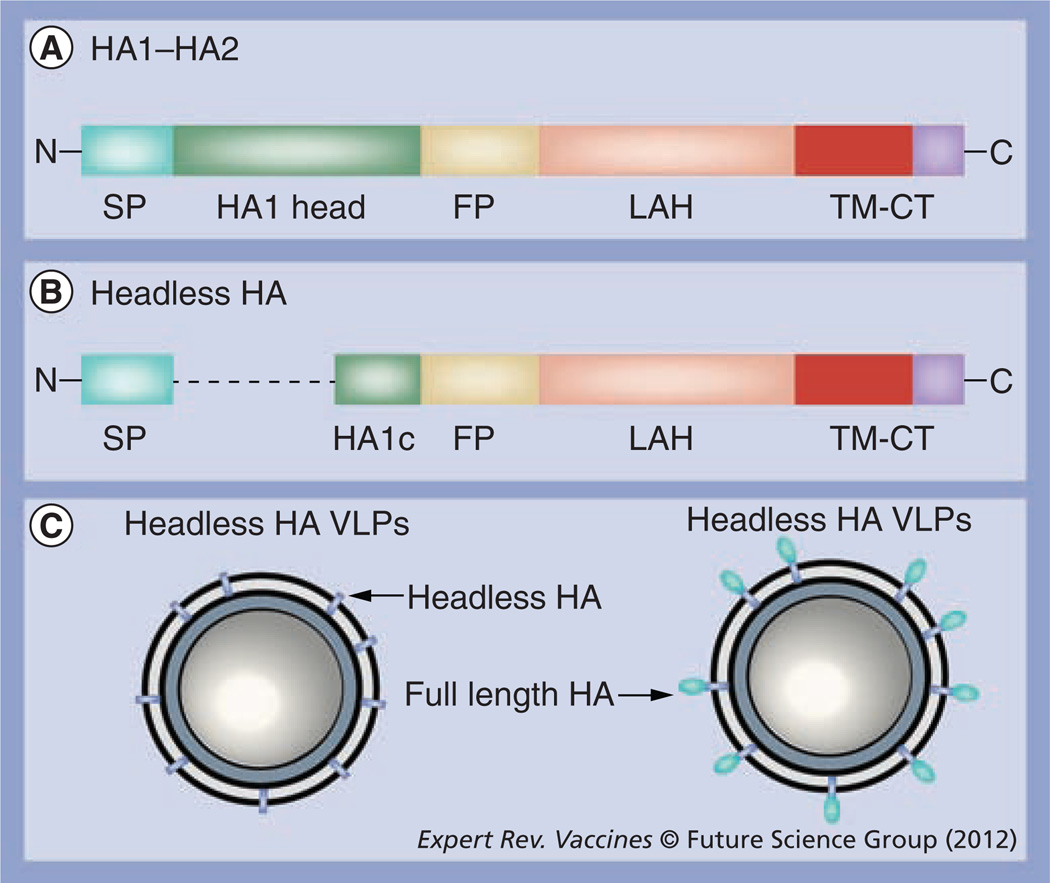
Figure 2. HA2 stalk domain and virus-like particles.
(A) HA1–HA2 subunits: the HA1 subunit contains the globular head domain (HA1 head), a major antigenic target of current vaccination. The HA2 subunit contains the FP, the LAH domain and TM-CT. The FP domain includes 11 highly conserved amino acids in the N-terminal cleavage region and the presumed fusion peptide region of amino acids 1–38. The LAH region contains the amino acids 76–130, a region recognized by monoclonal antibody 12D1 [28]. (B) A diagram of a headless HA protein design reported by Steel et al. [42]. A SP and HA1 subunit C-terminal adjoining region (HA1c) with the globular head domain deleted are connected to the HA1 subunit for expression on the cell surfaces [42]. (C) A schematic diagram of headless HA-containing VLPs. FP: Fusion peptide; HA: Hemagglutinin; LAH: Long α-helix; SP: Signal peptide; TM-CT: Transmembrane-cytoplasmic tail; VLP: Virus-like particle.
VLPs expressing the conserved HA stalk domain
Influenza virus HA is a homotrimeric protein molecule, and each monomer consists of two disulfide-linked subunit glycoproteins, a globular head of HA1 and a stem domain composed of the N- and C-terminal parts of HA1 and all of HA2 (Figure 2) [22]. Current influenza vaccination primarily induces immunity to the globular head domain of HA. The globular head domain of the receptor-binding pocket is surrounded by variable antigenic sites contributing to the generation of numerous escape mutants, identified by monoclonal antibodies and natural variants [23,24]. The locations of four antigenic sites (A, B, C and D) are consistent with antigenic mapping studies of the HA molecule-based reactivity of distinct monoclonal antibodies [25,26]. The failure or reduced efficacy of influenza vaccination primarily results from mutations occurring in the HA1 globular head domains [25,26].
In an effort to identify conserved epitopes, recent studies have indicated the HA stalk domain as a potential target for developing universal vaccines. The sequence homology of the HA2 subunit among different subtypes is in a range of 51–80%, relatively lower compared with that of the HA1 subunit (34–59% [27]). The sequence homology of the HA2 subunit within the same subtype is even higher [27]. In particular, specific regions in the HA stalk domains were identified to be highly conserved among different subtypes of influenza viruses. A long α-helix domain in the HA2 subunit is one such part that shows a high degree of conservation in amino acid sequence and amino acid type (Figure 2). Alignment of the 76–130 amino acid region of the HA2 subunit shows this conservation among different HA subtypes including H1, H2, H3, H5 and H7 [28].
Another conserved region is the HA cleavage site that forms an extended, highly exposed loop structure on the surface that is highly conserved in most influenza A viruses (Figure 2) [29]. Particularly, the N-terminal sequence of 11 amino acids in the HA cleavage site is invariant among most influenza A virus strains and differs only by one or two amino acid replacements in influenza B virus. The maintenance of this invariant domain is likely to be required because of functional constraints for being a suitable substrate for host-encoded proteases. This cleavage domain is exposed in a loop structure in the HA precursor and is accessible to antibody on the uncleaved HA precursor expressed on the plasma membrane of infected host cells [29].
By vaccination of mice with bromelain-treated or low pH-exposed influenza virus, previous studies identified monoclonal antibodies that are reactive to the stalk region of the HA molecule [8,30–32]. Some of these monoclonal antibodies recognizing epitopes in the stalk domain were found to show weak but broadly neutralizing activity [33–35]. Specific monoclonal antibodies recognizing epitopes in the fusion peptide region were shown to inhibit the fusion step of virus entry [8,34], to neutralize different influenza viruses [36], and in some cases, to provide survival protection [35]. HA2-specific monoclonal antibodies were also used to develop a simple slot test for detecting all subtypes of influenza HA proteins after a denaturation step [37].
In contrast to many studies on monoclonal antibodies recognizing the conserved stalk domain, fewer studies were reported regarding the development of universal influenza vaccines based on conserved antigenic targets of the HA2 subunit. Vaccines utilizing HA2 subunits include the cleavage region, fusion peptide, long α-helical polypeptides and recombinant HA2 subunits vaccines (Figure 2). Synthetic polypeptides covering the HA1/HA2 cleavage region conjugated to the carrier proteins were shown to induce antibodies recognizing cleavage region peptide epitopes and weak protection of immunized mice, as shown by reduced illness and fewer deaths upon lethal challenge with influenza A virus [38]. Similarly, a peptide conjugate vaccine based on the highly conserved maturational cleavage site of the HA precursor of the influenza B virus elicited a protective immune response against lethal challenge with an antigenically different influenza B virus [39].
Recent studies demonstrate the efficacy of stalk domain-based vaccines. An HA2-based immunogen destabilizing the low-pH conformation of HA2 was expressed in Escherichia coli and used for the immunization of mice [40]. This HA2 vaccine was highly immunogenic, protecting mice against lethal challenge with a homologous virus [40]. In addition, the chemically synthesized fusion peptide (amino acids 1–38 of HA2) (Figure 2) was conjugated to the keyhole limpet hemocyanin and tested in mice as a vaccine [40,41]. Similarly, this vaccine provided survival protection against a low-dose challenge with homologous and heterologous virus in immunized mice [40,41].
The Palese laboratory described an HA2-specific monoclonal antibody, 12D1, cloned from hybridoma fusion of lymphocytes from mice repeatedly immunized with H3 subtype HA DNA vaccines and followed by H3 virus boost [33]. This 12D1 monoclonal antibody was shown to recognize a region within amino acids 76–106 of the HA2 subunit stalk domain and to broadly neutralize heterologous viruses within the H3 subtype [33]. A further study by the same group revealed that 12D1 shows maximal binding to the extended structural element of a peptide representing the whole long α-helix domain amino acids 76–130 of the HA2 subunit from the subtype H3 HA (A/Hong Kong/1/1968 [28]). Based on the stalk domain epitope identified to be recognized by 12D1 (Figure 2), a long α-helix HA2 vaccine consisting of the amino acid 76–130 polypeptide was designed and coupled to the carrier protein keyhole limpet hemocyanin [28]. Sera from mice immunized with α-helix HA2 vaccine showed substantial binding antibodies reactive to heterosubtypic virus [28]. This α-helix HA2 vaccine could provide partial protection against heterosubtypic challenge viruses (10–15 mouse LD50, A/PR8 H1N1, A/Vietnam/04 H5N1 virus) and survival protection against the homologous virus H3 subtype [28]. Although the HA2 vaccine immune sera showed significant breadth in cross-reactivity with different HA molecules, the breadth was limited to Group 2 HAs (subtypes H3, H4, H7, H10, H14 and H15 [28]).
Using recombinant genetic engineering techniques, Steel et al. expressed a modified HA molecule lacking the globular head domain [42]. This headless HA is composed of membrane-proximal portions of both the HA1 signal peptide region and HA2 subunits so that headless HA molecules are stably expressed on cell surfaces. Furthermore, this construct was intended to remove the highly immunogenic head domain. A novel approach was to incorporate the headless HA into VLPs (Figure 2). Co-expression of the HIV Gag core protein and headless HA protein by transient DNA co-transfections resulted in the production of chimeric Gag VLPs containing headless HA molecules [42]. A three-dose vaccine regimen was applied to immunize mice. Two vaccinations with DNA constructs (Gag and HA) were followed by boost with chimeric headless HA VLP vaccines (150 ng HA content) in the presence of Freund’s complete adjuvant [42]. The headless HA VLP vaccines provided protection against homologous challenge in mice with moderate body weight loss. The neutralizing activity against the homologous virus in the immune sera of mice with headless HA VLP vaccines was marginally higher than the background, but was not conclusively confirmed [42]. Nonetheless, it is important to note that headless HA (A/PR8) VLP immune sera were likely to exhibit greater reactivity to heterologous strains than the full-length HA vaccine [42]. A/Hong Kong/68 (H3N2) headless HA VLP vaccines did not induce antibodies cross-reactive to different Group 1 HAs (subtypes H1, H2, H5, H6, H8, H9, H11, H12, H13 and H16). In other studies, vaccines of influenza virions stripped of HA1 by treatment with acid or dithiothreitol were not effective in inducing cross-reactive antibodies and cross-protection [43,44]. Acid or chemical treatment to strip the HA1 part would change the remaining HA2 portion to the extended, low-pH conformation, possibly explaining the low efficacy of protection. By contrast, headless HA VLPs remain in the pre-fusion conformation, which is more likely to be in a native-like structure. Some limitations of these approaches are the low yield of headless HA VLPs produced by transient co-transfection with DNA expression constructs, use of Freund’s adjuvant and breadth due to the subtype or HA group.
VLPs expressing M2e
The M2 protein is produced by translation from a spliced mRNA derived from influenza gene segment 7, also coding for the matrix protein M1 [45]. The first nine codons of M2 are shared with those of M1, and this overlapping RNA gene seems to further contribute to the conservation of M2 amino acid sequence. M2 is a type III integral membrane protein, forming a pH-dependent proton-selective ion channel [46–48]. M2 is a specific target of anti-influenza drugs such as amantadine and rimantadine, and these drugs are known to inhibit virus uncoating and maturation [49,50]. Influenza virus enters the target cells via receptor-mediated endocytosis, and M2 lowers the pH of the virus interior by opening the H+ ion channel across the virus membrane. The low pH facilitates viral membrane fusion and protein–protein dissociation between the matrix protein and the nucleoprotein (NP) complex. The M2 protein is also known to play a role in virus morphogenesis and assembly [51–55].
The M2 protein of 96 amino acids has three structural domains: an amino-terminal extracellular domain (23 residues), a transmembrane domain (19 residues) and a cytoplasmic tail domain (54 residues). The native conformation of M2 is a tetrameric membrane protein with disulfide bonds [56,57]. M2 is expressed on infected cell surfaces at high levels [58], in a ratio of approximately two M2 per HA trimer [59]. However, M2 molecules are estimated to be present at low levels (~23–60) on each virion [60]. The amino acid sequence in M2e is highly conserved among human influenza A viruses. For example, the N-terminal epitope SLLTEVET (residues two to nine) in M2e was found to be conserved at a level of 100% among human influenza A virus isolates and approximately over 99% among all influenza A subtypes [61,62]. In other residues in M2e, there are few amino acid changes depending on the host species (human, avian, swine, equine and other hosts) where influenza viruses were isolated [61,62]. The five amino acids within the residues 10–20 of M2e (underlined residues) were observed to be host restricted: PIRNEWGCRCN (amino acids 10–20, human isolates), PTRNGWECKCS (amino acids 10–20, avian isolates) and PIRNGWECRCN (amino acids 10–20, swine isolates [62]). Owing to the low degree of variation in the M2 extracellular domain compared with the HA and NA protein, M2 is considered to provide an attractive antigenic target for developing a universal influenza vaccine.
The extracellular 23-amino acid residue of the M2 protein is a small peptide and a very weak immunogen in its native form. Anti-M2e antibodies are detected only in a fraction of infected people [63]. Even mice infected with influenza virus do not induce high levels of antibodies recognizing M2 [64]. Nonetheless, there is evidence that anti-M2 immunity can confer protection against different influenza viruses. M2e-specific monoclonal antibodies were shown to reduce the plaque size or the growth of some influenza A virus strains in vitro in a strain-specific manner [53,55,60]. The reduction in plaque size might be due to blocking a late stage of replication by M2 antibodies [53,55] as similarly observed with treatment of antibodies specific to NA [65]. Passive transfer of M2 monoclonal antibodies protected mice by lowering lung virus titers upon subsequent infection with influenza A virus [20,66]. Therefore, induction of adaptive anti-M2 immunity would be a cost-effective and practical strategy for controlling influenza epidemics or pandemics.
One concern about M2e-based influenza A vaccine is its limited efficacy. Hence, different approaches to link M2e peptide to carriers and/or use of potent adjuvants were explored. The first study on cross-protection was reported using an M2 vaccine composed of a partially purified M2-containing membrane fraction derived from the recombinant baculovirus insect-cell expression system in combination with incomplete Freund’s adjuvant [67]. This recombinant M2 vaccine provided a survival advantage to immunized mice after lethal challenge [67]. Since then, many studies have focused on developing recombinant M2e fusion constructs using a variety of carrier molecules or systems: HBV core particles [68–70], human papillomavirus L protein VLPs [71], phage Qβ-derived VLPs [72], keyhole limpet hemocyanin [73], bacterial outer membrane complex [69,74], liposomes [75] and flagellin [76]. With the inclusion of diverse adjuvants, recombinant M2e-carrier vaccines were demonstrated to provide protection against lethal challenge with H1N1, H3N2 and H5N1 influenza A viruses. In particular, different forms of M2e vaccines fused to the HBV core VLPs were shown to induce high levels of anti-M2e antibody responses [61,68,70,77,78]. This might be due to the particulate nature of the carrier molecules. VLPs as M2e antigen carriers are likely to present M2e epitopes in an ordered array and in a particulate form, which enables a strong immune response as well as increasing their stability and immunogenicity [77,79]. However, M2e-mediated protection was relatively weak or partial compared with HA-mediated protection [64]. In addition, M2e-specific antibodies were not very effective in binding to the virus [64]. It is probable that chemical or genetic fusion of M2e would not be likely to form the tetrameric structure of M2 in its native conformation.
A novel approach was pursued in an attempt to facilitate the formation and maintenance of tetrameric structure for improving the immunogenicity of M2e. Genetically linking M2e to the tetramer-forming leucine zipper domain of the yeast transcription factor GCN4 was demonstrated to form recombinant tetrameric M2e vaccines [80]. The recombinant M2e–GCN4 vaccine induced potent M2e-specific antibody responses and 100% survival protection to the vaccinated mice [80].
An alternative approach is to express M2 proteins in a membrane-anchored form mimicking the native conformation on influenza virions. It is possible that the presence of the larger and more abundant HA proteins probably suppresses M2 from interacting efficiently with immune effector cells to induce antibodies (Figures 1 & 3). Thus, a full-length M2 was presented on influenza M1 VLPs (M2 VLPs) without HA and NA using the baculovirus insect-cell expression system [81]. Mice vaccinated with M2 VLP vaccines induced higher levels of M2e-specific antibodies compared with whole inactivated influenza virus vaccination [82]. In addition, M2e-specific antibodies induced by M2 VLP vaccination were highly cross-reactive to subtypes H1N1, H3N2 and H5N1 influenza viruses [81,82]. Mice immunized with M2 VLPs survived lethal infection with different subtypes of influenza A viruses [81]. Therefore, M2 proteins presented in a membrane-anchored form on VLPs can be a promising approach to avoid the immune-dominant HA proteins (Figure 3) and are effective in inducing M2 antibodies reactive to antigenically different influenza virions.
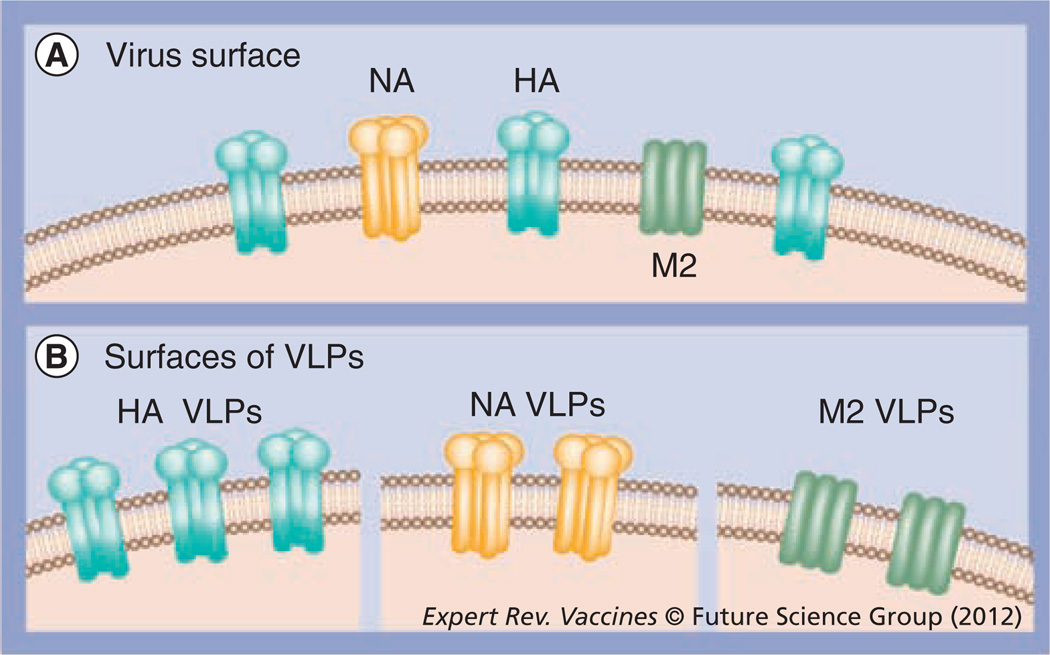
Figure 3. A model of influenza viral surface proteins and virus-like particles expressing influenza surface proteins separately.
(A) The viral surface is represented with large trimeric globular HA, tetrameric NA and small tetrameric M2. (B) Influenza VLP surface expressing influenza HA, NA or M2 protein.
HA: Hemagglutinin; NA: Neuraminidase; VLP: Virus-like particle.
M2e-based immunity alone is infection-permissive and could not eliminate disease symptoms, as also shown by most other candidate universal vaccines studied so far in animal models. A desirable universal influenza vaccine should be able to diminish morbidity and mortality. A suggestion is to use such vaccines as adjunct to current vaccination. In this regard, it was shown that the use of M2e-based vaccines as a supplement could significantly improve the efficacy of cross-protection. The addition of the M2e peptide to an aluminum-adjuvant split H3N2 virus vaccines significantly enhanced the cross-protection in mice by intraperitoneal vaccination [83]. Despite enhanced survival protection, significant morbidity as evidenced by weight loss was observed in mice that received M2e-peptide supplemented vaccines after heterosubtypic challenge infection [83]. By contrast, M2 expressed on VLPs in a membrane-anchored form was found to be highly effective in improving cross-protection when used in combination with inactivated whole viral vaccine in mice [82]. Mice that were intranasally immunized with a mixture of an inactivated virus and M2 VLP were protected from both mortality and morbidity. M2 VLP-supplemented inactivated influenza virus vaccine (A/PR/8/34, H1N1) conferred broad cross-protection to the immunized mice against lethal challenge with 2009 H1N1 pandemic virus, heterosubtypic H3N2 or H5N1 influenza viruses [82]. As a new approach to improve cross-protection, M2 VLP supplementation was extended to the seasonal influenza split vaccines as well as to the more commonly used route of intramuscular immunization (data not shown). Ultimately, clinical trials are required to validate this supplementation method as a potential universal vaccine for human use.
The protective immune mechanisms of the immune responses induced by M2 vaccination are not fully elucidated and further studies are needed. Protection by vaccination with M2e-hepatitis B core vaccine was found to be mediated by antibody-dependent, natural killer cell-mediated cytotoxicity [64]. Another M2e-carrier vaccine, C-terminal 28-kDa domain of Mycobacterium tuberculosis heat shock protein 70 (M2e-HSP70359–610), was shown to provide protection via alveolar macrophages and Fc receptor-dependent elimination of influenza A virus-infected cells [84]. It was demonstrated that protection by immune sera after M2 VLP vaccination was dependent on the presence of dendritic and macrophage cells in vivo in a mouse model as shown by depletion experiments using clodronate liposomes [81,82]. Other studies suggest that M2 antibodies can restrict the growth of influenza viruses as shown by inhibiting the plaque size or replication of in vitro cultured viruses [53,55,60,85,86]. Therefore, multiple mechanisms are likely to be involved in conferring protection by M2 vaccination.
Some recombinant M2e vaccines have been tested in clinical trials. Sanofi Pasteur Biologics Co. has tested the safety and immunogenicity of a recombinant vaccine candidate, M2e-HBc fusion protein (ACAM-FLU-A), in a Phase I trial study [201]. The ACAM-FLU-A vaccine was reported to be immunogenic and well tolerated with no significant side effects [201]. M2e-HBc carrier protein vaccine also conferred partial protection to ferrets with 70% survival from infection by the highly lethal avian H5N1 influenza strain [201]. VaxInnate reported the first Phase I clinical trial study of an M2e-flagellin fusion vaccine (STF2.4xM2e) in healthy young volunteers aged 18–49 years [87,202]. Low doses of flagellin-M2 vaccines (0.3 and 1.0 μg doses) were safe and tolerated in subjects tested. Also, two doses of these vaccines were immunogenic in 75% after the first dose and 96% after the second dose [87,202]. However, two high doses (3 and 10 μg doses) of flagellin-M2 vaccines were associated with the appearance of influenza-like symptoms in some of the subjects. Therefore, toxicity seems to be an issue at higher doses of vaccines. Development of a safer vaccine based on M2 may be advantageous and VLP-based vaccines can be an attractive approach. Also, the flagellin-HA fusion protein vaccine was shown to be immunogenic in elderly vaccinees [88]. These clinical trials encourage further studies for developing safe and effective universal influenza vaccines.
NA-containing VLPs
The NA content in current vaccines is variable and the antibody response to NA in vaccinees is low (18% seroconversion) compared with the HA response (84% [89]). The host immune response to NA is poor in primed individuals and limited by current vaccination because of two factors: a lack of sufficient immunogenic NA in the vaccine and the dominant immunogenic response to HA [16,90,91]. Antigenic drift of NA was found to be slower than the drift in HA [92,93]. The independent and slower rate of NA evolution as well as less host immunity to NA might favor the more rapid emergence of HA escape mutants [16,92]. These considerations suggest that the current influenza vaccines are not effective in inducing antibodies against NA [91]. Inhibition of NA activity either by NA host immunity or by drugs can significantly reduce the morbidity and mortality caused by influenza. Thus, the NA protein has become an important target for antiviral drugs (oseltamivir, zanamivir). For preventive efficacy and lower treatment cost, a vaccine inducing protective immunity to the less variable antigenic target NA would be desirable in addition to the immunity to HA.
Previous studies of the role of NA in vaccination against influenza include use of purified NA proteins [91,94–97], DNA plasmid [98–102] and a variety of live virus-vectored vaccines expressing NA [103–107]. The use of live vectored vaccines may have some concerns about antivector immunity and vaccine safety, particularly in young infants, elderly and immune-compromised individuals. The efficacy of DNA vaccines is relatively low, and thus multiple immunizations are required. The preparation of soluble recombinant protein vaccines is laborious and may increase the vaccine cost. Thus, the development of more affordable and effective NA vaccines should be continued.
Like other enveloped viruses, influenza virus HA and NA are major viral glycoproteins incorporated into virions in a membrane-anchored form (Figure 3). Therefore, expressing NA on VLPs in a membrane-anchored form would mimic the native conformation of NA on virions. Protective immunity of influenza VLPs containing both HA and NA was also reported [108–110]. However, NA-mediated protective immune responses by vaccination with influenza VLPs have not been well studied. Probably due to the immunodominant effects of HA in the same particles, the host immune response to NA was relatively weak, which made it difficult to investigate the protective roles of NA [16,109,110]. Thus, generation of VLPs containing NA without HA will provide a valuable vaccine modality in understanding the roles of NA in inducing protective immune responses (Figure 3).
Intranasal immunization of mice with influenza M1-derived VLPs containing NA from A/PR8 (H1N1) without HA effectively induced NA-specific immune responses [111]. The mice immunized with NA VLPs were found to have significant NA inhibition activity and to be reactive to homologous virus as well as heterosubtypic whole viral antigen (A/Philippines/82, H3N2 [111]). Importantly, NA VLPs conferred 100% protection after challenge with homologous A/PR/8/34 (H1N1) as well as heterosubtypic A/Philippines/82 (H3N2) virus, although body weight loss was observed after lethal challenge with the heterosubtypic H3N2 virus [111]. Thus, influenza VLP vaccines containing NA in a membrane-anchored conformation appear to be able to induce immunity to homologous as well as antigenically distinct influenza A virus strains.
NA-specific antibodies confer protection by a different mechanism from that of HA-specific antibodies induced by conventional vaccination, although the mechanisms of NA-immune mediated protection are not well understood. Antibodies reactive to the head domain of HA are able to neutralize and block the infection by influenza virus in an antigenically homologous or in a closely related strain-specific manner, capable of inducing sterilizing immunity, whereas NA antibodies can block the release of infectious virus from the surface of infected cells, causing virus aggregation, limiting viral spread and thus allowing the host to develop adaptive immunity [112,113]. Despite the fact that anti-NA sera do not prevent infection of cells in vitro, passive immunization reduces morbidity and mortality in mice after infection [113–115]. Immune responses to NA VLP vaccination were found to effectively control lung viral replication resulting in several thousand-fold lower titers, although they were not able to prevent the infection [111]. Less protection was observed when mice were treated with clodronate, indicating that protection by NA VLP immune sera was partially dependent on lung dendritic and macrophage cells [111]. By contrast, immune sera with high neutralizing antibodies from infected mice were completely protective independent of lung dendritic and macrophage cells [111].
Current influenza vaccines contain both HA and NA as major antigens. However, when both HA and NA are presented together in a viral particle, HA immune responses become dominant over NA in both B- and T-cell priming as a result of antigen competition (Figure 3) [16]. Therefore, a better vaccine strategy may include HA and NA as separate components to broaden the immune response against influenza. Immunity to the more slowly evolving NA should reduce the effect of antigenic changes in a potential emerging influenza virus strain. To avoid the immune-dominant effects of HA, it was shown that NA is sufficiently immunogenic when added as a separate component in a vaccine [17,97,116,117].
An alternative promising approach is to use purified NA as a supplement to standard influenza A vaccine. Chromatographically purified NA from influenza virus was used to supplement conventional monovalent-inactivated inf luenza A vaccine [94]. Supplementing the inactivated viral vaccine with purified NA protein induced high titers of antibodies to both HA and NA at equivalent levels for each antigen to those in animals immunized with either antigen alone, and resulted in greater reduction in viral replication following heterosubtypic challenge than the non-supplemented vaccine [94]. Alternative production methods such as recombinant baculovirus or yeast expression systems could be explored to reduce current egg substrate-based production time and cost. Influenza HA VLPs were superior to soluble HA protein vaccine in the induction of binding and neutralizing antibodies, in levels of long-lived memory B cells and antibody-secreting plasma cells, in levels of IFN-γ secreting T cells and in clearing lung viral loads [118]. Therefore, presenting NA on VLPs in a membrane-anchored form for a supplemental vaccine to the HA-based current influenza vaccine is expected to be more effective in broadening and enhancing cross-protection compared with the soluble subunit NA vaccines.
Adjuvant-containing VLPs
Adjuvants are being used as a means of improving the immunogenicity of weak immunogens such as M2 vaccines, which is important for developing vaccines against potential pandemic influenza virus strains. Stimulating the innate immune system is important for inducing adaptive immune responses. Viruses and other pathogens can interact with innate immune cells through pathogen-specific molecular patterns that are recognized by pattern recognition receptors (PRRs) such as Toll-like receptors, and thus are highly effective in inducing potent adaptive immunity. Binding of a PRR by its ligand or a pathogen triggers a signaling cascade inducing cytokines and chemokines. The resulting cytokine and chemokine milieu plays a significant role in developing adaptive immune responses [119–121].
APCs such as DCs or macrophage cells initially capture and process pathogen or vaccine-specific antigens, and present these antigens to T and B cells. The interaction of pathogen-associated molecular patterns (pathogen or vaccine antigens) with PRRs on the APCs stimulates their activation, resulting in the expression of costimulatory molecules (CD40, CD80/86) and cytokines. B cells have multiple roles in recognizing antigens and producing antigen-specific antibodies. B cells also express PRRs, and capture, process and present antigens to T cells. Some adjuvant molecules activate APCs and have a significant impact on enhancing T- and B-cell adaptive immune responses [122–126].
Replicating viruses are highly effective in inducing potent B-and T-cell responses, whereas many isolated recombinant proteins or T-cell epitopes are very poor in inducing immune responses unless given with strong adjuvants [127,128]. By contrast, the highly repetitive and particulate properties of VLPs are considered an attractive feature contributing to strong and long-lasting IgG responses in the absence of exogenous adjuvants [79,118, 129–131]. The highly organized form of antigens on VLPs induces strong B-cell responses [118,131]. In addition, VLPs are more effective for MHC class I and II pathway antigen presentation compared with a soluble antigen [13,118,132]. However, VLPs are less effective in inducing cytotoxic CD8 T-cell and helper CD4 T-cell responses as compared with replicating viral antigens because of their nonreplicating property [128]. In an attempt to enhance the T-cell immunogenicity, viral epitope-containing hepatitis B core VLPs that were conjugated to an adjuvant molecule (anti-CD40 antibody or CpG oligonucleotide) were found to effectively induce cytotoxic CD8 T-cell responses conferring protection in vivo in a mouse model [133,134]. Incorporating immunostimulatory molecules into VLPs would be an approach to enhance the immunogenicity of influenza VLPs, thus resulting in broader cross-protection.
VLPs with lipid envelopes need special attention in choosing an adjuvant owing to the nature of their membranes. Utilizing recombinant genetic engineering techniques, new approaches have been applied to express novel adjuvant molecules on VLPs in a membrane-anchored form (Figure 1). Chimeric HIV VLPs were generated to incorporate influenza HA into VLPs containing HIV envelope proteins, aiming to enhance mucosal immunity by using HA for binding to sialic acid present on epithelial cells on the mucosa [135,136]. Chimeric simian human immunodeficiency virus (SHIV) VLPs containing HA were shown to have significantly increased their immunogenicity even in a mouse lacking CD4 T cells [135,136]. HIV VLPs containing membrane-anchored Flt3 ligand, a DC growth factor, were also generated to target VLPs to DCs and were shown to increase DC and monocyte/macrophage populations in the spleen when administered to mice [14]. GM-CSF is clinically used to increase the immunogenicity of various antigens, probably by stimulating APCs. It was found that the chimeric simian immunodeficiency virus (SIV) VLPs containing GM-CSF were more effective in inducing SIV-specific antibody and neutralizing activities compared with standard SIV VLPs with or without equivalent amounts of soluble GM-CSF [18]. CD40 ligand is expressed on the surfaces of activated T cells and has multiple immune functions, such as enhancing DC maturation and CD8 T-cell activity and stimulating antibody isotype switching and maturation. HIV or SIV VLPs containing CD40 ligand were demonstrated to enhance DC activation, cytokine-expressing T-cell responses and antibody titers at a moderate level [18,137]. These approaches have desirable advantages because the low level of immune-enhancing molecules incorporated into VLPs are effective in enhancing immune responses and thus side effects are unlikely [14,18,137].
Flagellin is the major proinflammatory protein component of Gram-negative bacterial organisms and is a potent immune stimulator by triggering Toll-like receptor 5 on host cells [138,139]. It was found that enhancing the immunogenicity of inactivated influenza vaccines by cholera toxin adjuvant could be an approach to broaden cross-protection [140]. Thus, to develop a more effective VLP vaccine inducing broad cross-protection, flagellin derived from Salmonella bacteria was engineered to be expressed on the cell surfaces of insect cells in a membrane-anchored form by linking the ectodomain of flagellin to the transmembrane-cytoplasmic tail domain of HA [20,21,141]. This membrane-anchored flagellin was incorporated into influenza HA VLPs (derived from A/PR8/34, H1N1 subtype), resulting in chimeric HA VLPs [20,141]. Intramuscular immunization of mice with the flagellin-containing VLPs elicited significantly enhanced levels of IgG2a/2b antibody responses, cytokine secreting T-cell responses in response to both MHC I and II peptides, and higher survival protection (67%) against heterosubtypic challenge (A/Philippines/82, H3N2 subtype [20]). In addition, mice intranasally immunized with HA (H1) VLPs incorporating flagellin were fully protected (100% survival) against H3N2 heterosubtypic challenge [141]. By contrast, use of soluble flagellin as an adjuvant was not as effective at improving the protective immunity compared with the chimeric HA VLPs with membrane-anchored flagellin, as determined by both systemic and mucosal immunization studies [20,141]. In a clinical study, a recombinant HA influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI) was demonstrated to be highly immunogenic, overcoming poor immune responses in the elderly (older than 65 years [88]). In addition, a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) was shown to be safe and immunogenic in healthy adults [87]. Recombinant flagellin-adjuvanted influenza vaccines could be a promising new candidate for the prevention of influenza A disease in both young adults and the elderly. The novel approach of anchoring an immunostimulatory molecule in VLPs can target low levels of a molecular adjuvant specifically to the VLP vaccines, and enhance the vaccine immunogenicity and cross-protection.
Expert commentary & five-year view
HA-based current influenza vaccination is not fully protective and needs to be improved. Several additional antigenic targets and new approaches are being developed. The HA stalk domain contains conserved regions that can be a target for developing universal influenza vaccines. The cleavage region of the N-terminal HA2 domain contains 11 highly conserved amino acids. Also, the long α-helix region of HA2 involved in viral fusion is highly conserved among different viral subtypes. Monoclonal antibodies were identified that recognize an epitope in the conserved HA2 stalk domain and exhibit broadly neutralizing activity against different strains. However, the reactivity is HA-subtype group specific, and there is a limitation in its breadth. As an approach to develop an HA2 stalk domain-based vaccine, VLPs expressing the whole HA2 domain without the HA1 globular head are likely to be more promising than alternatives such as fusion peptide conjugate vaccines. It is desirable to develop a more immunogenic epitope design as well as scalable approaches to produce VLP vaccines expressing headless HA in a membrane-anchored form, likely to represent the native conformation. As a future direction, it is also suggested to develop insect-cell or other expression systems producing headless HA VLPs. Also, incorporating a molecular adjuvant into these headless HA VLPs will be an attractive method to enhance their immunogenicity.
M2e is a target extensively used to develop universal influenza vaccines using different platforms for presentation. Some candidates (M2e-HBc fusion protein, an M2e-flagellin fusion vaccine) are being tested in Phase I/II human clinical trials. M2 immunity is broad and highly cross-reactive among influenza A viruses when the M2e epitopes are in an immunogenic form such as presented in VLPs or conjugated to potent adjuvant molecules. However, as demonstrated in numerous efficacy studies in preclinical animal models, the strength of protection is relatively weak compared with HA-based immunity. This is evident since most animals immunized with M2 vaccine experience a certain level of morbidity as shown by significant weight loss and viral loads in the lungs. Therefore, studies to improve the efficacy of M2 vaccines should be continued. Designing multiple copies of M2e epitopes and presenting them on VLPs will be an attractive approach to present the membrane proteins in a native-like conformation, and incorporating adjuvant molecules in a membrane-anchored form as well as M2 into VLPs can enhance immunogenicity. An M2e vaccine can be used singly or can be added as a supplement to seasonal vaccines. In either approach, M2e vaccination is expected to reduce the risk of mortality when new pandemic outbreaks occur.
Current influenza vaccines contain some NA, although regulation of its content is not required and its efficacy in clinical studies remains unknown. Preclinical and clinical studies demonstrated that supplementary NA enhanced the immune responses to NA. Also, NA is under less immune pressure to mutate and thus has a slower rate of evolutionary change. New production methods for NA vaccines should be developed to reduce the cost. NA vaccines based on VLPs can provide a feasible method because the NA is presented in a membrane-anchored and immunogenic conformation, mimicking the native structure. Insect cell-derived NA vaccines were proven to be safe and immunogenic in clinical studies, and can be an attractive option for NA VLP production [111].
Most universal antigenic targets (HA2 stalk domain, M2, NA) are less immunogenic and induce relatively weak protection despite their greater breadth of cross-reactivity. Therefore, approaches to enhance their immunogenicity should be a priority for future studies. In this respect, new recombinant techniques are being utilized to develop new designs of vaccine epitopes and novel VLP vaccines. Universal antigenic targets can be engineered to present them on VLPs in a membrane-anchored form, making them highly immunogenic. In addition, molecular adjuvants such as flagellin conjugated to a conserved target (M2e) can be designed and produced in a VLP format [20,141,142]. Thus, VLPs have enormous potential for developing universal influenza vaccines, which can include several conserved targets in a particulate and immunogenic form as well as molecular adjuvants. In particular, such adjuvanted VLPs will be highly desirable since low concentrations of adjuvant molecules minimize the potential side reactions, but specifically target VLP vaccines to immune cells [14,18,20].
Approaches to combine multiple conserved antigenic targets into a vaccine will be important since these multiple targets are likely to reduce the chances of escape mutants once universal influenza vaccines are used in humans. Including T-cell epitopes in vaccines would be an advantage in broadening the cross-protection. Influenza NP is a relatively well-conserved target for inducing T-cell immunity but T-cell immunity alone would induce weak protection, whereas previous studies demonstrated that combining the NP component with M2 vaccine could provide enhanced crossprotection [73,143–146]. In addition, more attention will be needed to cover both human influenza A and B strains as well as potential avian-origin pandemic influenza viruses. M2e-like approaches including the conserved ectodomain of NB or BM2 could provide protection against multiple influenza B strains and should be further explored. Some divergence and deviation in the M2e sequence are present in the strains from swine- and avian-origin influenza viruses, and these need to be considered in designing M2e vaccines to cover potential pandemic strains. These conserved target epitopes could be delivered as separate vaccine antigens. Preclinical studies also need to be expanded to more relevant animal models. Ferrets are an important animal model for developing human influenza vaccines, and chickens and pigs as potential natural reservoirs need to be included in the efficacy tests of universal influenza vaccines. Such studies should be expanded because this is an essential part of developing universal influenza vaccines.
Key issues.
Continuous emergence of new strains cause influenza epidemics and pandemics, and require the development of effective universal influenza vaccines.
Multiple conserved epitopes for inducing broadly cross-reactive antibodies have been identified and are being developed, which include the hemagglutinin stalk domain, M2 external domain and neuraminidase proteins.
Virus-like particles (VLPs) presenting conserved epitopes in a membrane-anchored form are likely to be more immunogenic and display conserved epitopes in a virus-like conformation.
Incorporating molecular adjuvants into VLPs is desirable to specifically target adjuvants to VLP vaccines at low concentrations.
Use of influenza universal vaccines as stand-alone vaccines or as supplements needs to be considered and tested using preclinical and clinical studies.
For the development of effective universal vaccines, their evaluation in animal models is essential.
Acknowledgements
The authors would like to thank Erin-Joi Collins for her valuable assistance in preparing this manuscript.
This work was supported in part by NIH/NIAID grant AI0680003 (RW Compans), and NIH/NIAID grants AI081385 (SM Kang) and AI093772 (SM Kang).
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 Pandemic in the US and comparison with past influenza seasons. PLoS Curr. 2010 doi: 10.1371/currents.RRN1153. RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osterholm MT. Preparing for the next pandemic. N. Engl. J. Med. 2005;352(18):1839–1842. doi: 10.1056/NEJMp058068. [DOI] [PubMed] [Google Scholar]
- 3.Palese P, Compans RW. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J. Gen. Virol. 1976;33(1):159–163. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- 4.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 2004;78(22):12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belshe RB. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 2004;103(1–2):177–185. doi: 10.1016/j.virusres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Bush RM, Bender CA, Subbarao K, Cox NJ, Fitch WM. Predicting the evolution of human influenza A. Science. 1999;286(5446):1921–1925. doi: 10.1126/science.286.5446.1921. [DOI] [PubMed] [Google Scholar]
- 7.Plotkin JB, Dushoff J. Codon bias and frequency-dependent selection on the hemagglutinin epitopes of influenza A virus. Proc. Natl Acad. Sci. USA. 2003;100(12):7152–7157. doi: 10.1073/pnas.1132114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekiert DC, Bhabha G, Elsliger MA, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buonaguro L, Tornesello ML, Tagliamonte M, et al. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J. Virol. 2006;80(18):9134–9143. doi: 10.1128/JVI.00050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morón G, Rueda P, Casal I, Leclerc C. CD8a-CD11b+ dendritic cells present exogenous virus-like particles to CD8+ T cells and subsequently express CD8a and CD205 molecules. J. Exp. Med. 2002;195(10):1233–1245. doi: 10.1084/jem.20011930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aricò E, Wang E, Tornesello ML, et al. Immature monocyte derived dendritic cells gene expression profile in response to virus-like particles stimulation. J. Transl. Med. 2005;3:45. doi: 10.1186/1479-5876-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morón VG, Rueda P, Sedlik C, Leclerc C. In vivo, dendritic cells can cross-present virus-like particles using an endosome-tocytosol pathway. J. Immunol. 2003;171(5):2242–2250. doi: 10.4049/jimmunol.171.5.2242. [DOI] [PubMed] [Google Scholar]
- 13.Song H, Wittman V, Byers A, et al. In vitro stimulation of human influenza-specific CD8+ T cells by dendritic cells pulsed with an influenza virus-like particle (VLP) vaccine. Vaccine. 2010;28(34):5524–5532. doi: 10.1016/j.vaccine.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 14.Sailaja G, Skountzou I, Quan FS, Compans RW, Kang SM. Human immunodeficiency virus-like particles activate multiple types of immune cells. Virology. 2007;362(2):331–341. doi: 10.1016/j.virol.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy P, Noad R. Virus-like particles as a vaccine delivery system: myths and facts. Hum. Vaccin. 2008;4(1):5–12. doi: 10.4161/hv.4.1.5559. [DOI] [PubMed] [Google Scholar]
- 16.Johansson BE, Moran TM, Kilbourne ED. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc. Natl Acad. Sci. USA. 1987;84(19):6869–6873. doi: 10.1073/pnas.84.19.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson BE, Kilbourne ED. Dissociation of influenza virus hemagglutinin and neuraminidase eliminates their intravirionic antigenic competition. J. Virol. 1993;67(10):5721–5723. doi: 10.1128/jvi.67.10.5721-5723.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skountzou I, Quan FS, Gangadhara S, et al. Incorporation of glycosylphosphatidylinositolanchored granulocyte-macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J. Virol. 2007;81(3):1083–1094. doi: 10.1128/JVI.01692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang BZ, Liu W, Kang SM, et al. Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into virus-like particles. J. Virol. 2007;81(20):10869–10878. doi: 10.1128/JVI.00542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang BZ, Quan FS, Kang SM, Bozja J, Skountzou I, Compans RW. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J. Virol. 2008;82(23):11813–11823. doi: 10.1128/JVI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassilieva EV, Wang BZ, Vzorov AN, et al. Enhanced mucosal immune responses to HIV virus-like particles containing a membrane-anchored adjuvant. MBio. 2011;2(1):e00328–e00310. doi: 10.1128/mBio.00328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 23.Laver WG, Gerhard W, Webster RG, Frankel ME, Air GM. Antigenic drift in type A influenza virus: peptide mapping and antigenic analysis of A/PR/8/34 (HON1) variants selected with monoclonal antibodies. Proc. Natl Acad. Sci. USA. 1979;76(3):1425–1429. doi: 10.1073/pnas.76.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laver WG, Air GM, Dopheide TA, Ward CW. Amino acid sequence changes in the haemagglutinin of A/Hong Kong (H3N2) influenza virus during the period 1968–77. Nature. 1980;283(5746):454–457. doi: 10.1038/283454a0. [DOI] [PubMed] [Google Scholar]
- 25.Gerhard W, Yewdell J, Frankel ME, Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- 26.Wiley DC, Skehel JJ. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- 27.Krystal M, Elliott RM, Benz EW, Jr, Young JF, Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc. Natl Acad. Sci. USA. 1982;79(15):4800–4804. doi: 10.1073/pnas.79.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang TT, Tan GS, Hai R, et al. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc. Natl Acad. Sci. USA. 2010;107(44):18979–18984. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Lee KH, Steinhauer DA, Stevens DJ, Skehel JJ, Wiley DC. Structure of the hemagglutinin precursor cleavage site, a determinant of influenza pathogenicity and the origin of the labile conformation. Cell. 1998;95(3):409–417. doi: 10.1016/s0092-8674(00)81771-7. [DOI] [PubMed] [Google Scholar]
- 30.Webster RG, Brown LE, Jackson DC. Changes in the antigenicity of the hemagglutinin molecule of H3 influenza virus at acidic pH. Virology. 1983;126(2):587–599. doi: 10.1016/s0042-6822(83)80015-4. [DOI] [PubMed] [Google Scholar]
- 31.Vanlandschoot P, Beirnaert E, Barrère B, et al. An antibody which binds to the membrane-proximal end of influenza virus haemagglutinin (H3 subtype) inhibits the low-pH-induced conformational change and cell–cell fusion but does not neutralize virus. J. Gen. Virol. 1998;79(Pt 7):1781–1791. doi: 10.1099/0022-1317-79-7-1781. [DOI] [PubMed] [Google Scholar]
- 32.Kostolansky F, Russ G, Mucha V, Styk B. Changes in the influenza virus haemagglutinin at acid pH detected by monoclonal antibodies to glycopolypeptides HA1 and HA2. Arch. Virol. 1988;101(1–2):13–24. doi: 10.1007/BF01314648. [DOI] [PubMed] [Google Scholar]
- 33.Wang TT, Tan GS, Hai R, et al. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 2010;6(2):e1000796. doi: 10.1371/journal.ppat.1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sui J, Hwang WC, Perez S, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009;16(3):265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prabhu N, Prabakaran M, Ho HT, et al. Monoclonal antibodies against the fusion peptide of hemagglutinin protect mice from lethal influenza A virus H5N1 infection. J. Virol. 2009;83(6):2553–2562. doi: 10.1128/JVI.02165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashem AM, Van Domselaar G, Li C, et al. Universal antibodies against the highly conserved influenza fusion peptide cross-neutralize several subtypes of influenza A virus. Biochem. Biophys. Res. Commun. 2010;403(2):247–251. doi: 10.1016/j.bbrc.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Li C, Jaentschke B, Song Y, et al. A simple slot blot for the detection of virtually all subtypes of the influenza A viral hemagglutinins using universal antibodies targeting the fusion peptide. Nat. Protoc. 2010;5(1):14–19. doi: 10.1038/nprot.2009.200. [DOI] [PubMed] [Google Scholar]
- 38.Horváth A, Tóth GK, Gogolák P, et al. A hemagglutinin-based multipeptide construct elicits enhanced protective immune response in mice against influenza A virus infection. Immunol. Lett. 1998;60(2–3):127–136. doi: 10.1016/s0165-2478(97)00137-5. [DOI] [PubMed] [Google Scholar]
- 39.Bianchi E, Liang X, Ingallinella P, et al. Universal influenza B vaccine based on the maturational cleavage site of the hemagglutinin precursor. J. Virol. 2005;79(12):7380–7388. doi: 10.1128/JVI.79.12.7380-7388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bommakanti G, Citron MP, Hepler RW, et al. Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc. Natl Acad. Sci. USA. 2010;107(31):13701–13706. doi: 10.1073/pnas.1007465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staneková Z, Király J, Stropkovská A, et al. Heterosubtypic protective immunity against influenza A virus induced by fusion peptide of the hemagglutinin in comparison to ectodomain of M2 protein. Acta Virol. 2011;55(1):61–67. doi: 10.4149/av_2011_01_61. [DOI] [PubMed] [Google Scholar]
- 42. Steel J, Lowen AC, Wang TT, et al. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010;1(1) doi: 10.1128/mBio.00018-10. pii: e00018-10.. • A novel virus-like particle (VLP) design to express the headless HA2 domain.
- 43.Graves PN, Schulman JL, Young JF, Palese P. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive HA2 determinants. Virology. 1983;126(1):106–116. doi: 10.1016/0042-6822(83)90465-8. [DOI] [PubMed] [Google Scholar]
- 44.Quan FS, Li ZN, Kim MC, et al. Immunogenicity of low-pH treated whole viral influenza vaccine. Virology. 2011;417(1):196–202. doi: 10.1016/j.virol.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamb RA, Lai CJ, Choppin PW. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc. Natl Acad. Sci. USA. 1981;78(7):4170–4174. doi: 10.1073/pnas.78.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 47.Chizhmakov IV, Geraghty FM, Ogden DC, Hayhurst A, Antoniou M, Hay AJ. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukaemia cells. J. Physiol. (Lond.) 1996;494(Pt 2):329–336. doi: 10.1113/jphysiol.1996.sp021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mould JA, Drury JE, Frings SM, et al. Permeation and activation of the M2 ion channel of influenza A virus. J. Biol. Chem. 2000;275(40):31038–31050. doi: 10.1074/jbc.M003663200. [DOI] [PubMed] [Google Scholar]
- 49.Leonov H, Astrahan P, Krugliak M, Arkin IT. How do aminoadamantanes block the influenza M2 channel, and how does resistance develop? J. Am. Chem. Soc. 2011;133(25):9903–9911. doi: 10.1021/ja202288m. [DOI] [PubMed] [Google Scholar]
- 50.Lin TI, Heider H, Schroeder C. Different modes of inhibition by adamantane amine derivatives and natural polyamines of the functionally reconstituted influenza virus M2 proton channel protein. J. Gen. Virol. 1997;78(Pt 4):767–774. doi: 10.1099/0022-1317-78-4-767. [DOI] [PubMed] [Google Scholar]
- 51.Iwatsuki-Horimoto K, Horimoto T, Noda T, et al. The cytoplasmic tail of the influenza A virus M2 protein plays a role in viral assembly. J. Virol. 2006;80(11):5233–5240. doi: 10.1128/JVI.00049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCown MF, Pekosz A. The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging. J. Virol. 2005;79(6):3595–3605. doi: 10.1128/JVI.79.6.3595-3605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts PC, Lamb RA, Compans RW. The M1 and M2 proteins of influenza A virus are important determinants in filamentous particle formation. Virology. 1998;240(1):127–137. doi: 10.1006/viro.1997.8916. [DOI] [PubMed] [Google Scholar]
- 54.Roberts PC, Hughey PG, Holsinger LJ, Lamb RA, Compans RW. Effect of influenza A virus M2 protein on virus assembly and release. In: Brown LE, Hampson AW, Webster RG, editors. Options for the Control of Influenza III. Australia: Elsevier, Cairns; 1996. pp. 351–356. [Google Scholar]
- 55.Hughey PG, Roberts PC, Holsinger LJ, Zebedee SL, Lamb RA, Compans RW. Effects of antibody to the influenza A virus M2 protein on M2 surface expression and virus assembly. Virology. 1995;212(2):411–421. doi: 10.1006/viro.1995.1498. [DOI] [PubMed] [Google Scholar]
- 56.Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008;451(7178):591–595. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stouffer AL, Acharya R, Salom D, et al. Structural basis for the function and inhibition of an influenza virus proton channel. Nature. 2008;451(7178):596–599. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hughey PG, Compans RW, Zebedee SL, Lamb RA. Expression of the influenza A virus M2 protein is restricted to apical surfaces of polarized epithelial cells. J. Virol. 1992;66(9):5542–5552. doi: 10.1128/jvi.66.9.5542-5552.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40(3):627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 60.Zebedee SL, Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J. Virol. 1988;62(8):2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fiers W, De Filette M, Birkett A, Neirynck S, Min Jou W. A ‘universal’ human influenza A vaccine. Virus Res. 2004;103(1–2):173–176. doi: 10.1016/j.virusres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 62.Liu W, Zou P, Ding J, Lu Y, Chen YH. Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect. 2005;7(2):171–177. doi: 10.1016/j.micinf.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Black RA, Rota PA, Gorodkova N, Klenk HD, Kendal AP. Antibody response to the M2 protein of influenza A virus expressed in insect cells. J. Gen. Virol. 1993;74(Pt 1):143–146. doi: 10.1099/0022-1317-74-1-143. [DOI] [PubMed] [Google Scholar]
- 64. Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibodydependent NK cell activity. J. Immunol. 2004;172(9):5598–5605. doi: 10.4049/jimmunol.172.9.5598.. • A possible mechanism by which M2 antibody provides protection.
- 65.Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4(11):3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Treanor JJ, Tierney EL, Zebedee SL, Lamb RA, Murphy BR. Passively transferred monoclonal antibody to the M2 protein inhibits influenza A virus replication in mice. J. Virol. 1990;64(3):1375–1377. doi: 10.1128/jvi.64.3.1375-1377.1990.. • The first demonstration of potential protective effects by M2-specific antibodies in vivo
- 67.Slepushkin VA, Katz JM, Black RA, Gamble WC, Rota PA, Cox NJ. Protection of mice against influenza A virus challenge by vaccination with baculovirus-expressed M2 protein. Vaccine. 1995;13(15):1399–1402. doi: 10.1016/0264-410x(95)92777-y. [DOI] [PubMed] [Google Scholar]
- 68.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 1999;5(10):1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 69.Fan J, Liang X, Horton MS, et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine. 2004;22(23–24):2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 70.De Filette M, Fiers W, Martens W, et al. Improved design and intranasal delivery of an M2e-based human influenza A vaccine. Vaccine. 2006;24(44–46):6597–6601. doi: 10.1016/j.vaccine.2006.05.082. [DOI] [PubMed] [Google Scholar]
- 71.Ionescu RM, Przysiecki CT, Liang X, et al. Pharmaceutical and immunological evaluation of human papillomavirus viruslike particle as an antigen carrier. J. Pharm. Sci. 2006;95(1):70–79. doi: 10.1002/jps.20493. [DOI] [PubMed] [Google Scholar]
- 72.Bessa J, Schmitz N, Hinton HJ, Schwarz K, Jegerlehner A, Bachmann MF. Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: implications for vaccine design. Eur. J. Immunol. 2008;38(1):114–126. doi: 10.1002/eji.200636959. [DOI] [PubMed] [Google Scholar]
- 73.Tompkins SM, Zhao ZS, Lo CY, et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerging Infect. Dis. 2007;13(3):426–435. doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu TM, Grimm KM, Citron MP, et al. Comparative immunogenicity evaluations of influenza A virus M2 peptide as recombinant virus like particle or conjugate vaccines in mice and monkeys. Vaccine. 2009;27(9):1440–1447. doi: 10.1016/j.vaccine.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 75.Ernst WA, Kim HJ, Tumpey TM, et al. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24(24):5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 76. Huleatt JW, Nakaar V, Desai P, et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008;26(2):201–214. doi: 10.1016/j.vaccine.2007.10.062.. • A chimeric M2-vaccine design by fusion to an innate immune-stimulating molecule.
- 77.De Filette M, Min Jou W, Birkett A, et al. Universal influenza A vaccine: optimization of M2-based constructs. Virology. 2005;337(1):149–161. doi: 10.1016/j.virol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Heinen PP, Rijsewijk FA, de Boer-Luijtze EA, Bianchi AT. Vaccination of pigs with a DNA construct expressing an influenza virus M2-nucleoprotein fusion protein exacerbates disease after challenge with influenza A virus. J. Gen. Virol. 2002;83(Pt 8):1851–1859. doi: 10.1099/0022-1317-83-8-1851. [DOI] [PubMed] [Google Scholar]
- 79.Bachmann MF, Rohrer UH, Kündig TM, Bürki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262(5138):1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 80. De Filette M, Martens W, Roose K, et al. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J. Biol. Chem. 2008;283(17):11382–11387. doi: 10.1074/jbc.M800650200.. • Improved immunogenicity by tetrameric M2-vaccine design.
- 81.Song JM, Wang BZ, Park KM, et al. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS ONE. 2011;6(1):e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc. Natl Acad. Sci. USA. 2011;108(2):757–761. doi: 10.1073/pnas.1012199108.. • A new concept to improve cross-protection by supplementing the vaccine with M2 VLPs as a conserved antigenic target.
- 83.Wu F, Yuan XY, Huang WS, Chen YH. Heterosubtypic protection conferred by combined vaccination with M2e peptide and split influenza vaccine. Vaccine. 2009;27(43):6095–6101. doi: 10.1016/j.vaccine.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 84.El Bakkouri K, Descamps F, De Filette M, et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J. Immunol. 2011;186(2):1022–1031. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- 85.Pei S, Xiong N, Zhang Y, Chen S. Increasing M2 epitope density enhances systemic and mucosal immune responses to influenza A virus. Biotechnol. Lett. 2009;31(12):1851–1856. doi: 10.1007/s10529-009-0102-6. [DOI] [PubMed] [Google Scholar]
- 86.Wei G, Meng W, Guo H, et al. Potent neutralization of influenza A virus by a single-domain antibody blocking M2 ion channel protein. PLoS ONE. 2011;6(12):e28309. doi: 10.1371/journal.pone.0028309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turley CB, Rupp RE, Johnson C, et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. 2011;29(32):5145–5152. doi: 10.1016/j.vaccine.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 88.Taylor DN, Treanor JJ, Strout C, et al. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI) Vaccine. 2011;29(31):4897–4902. doi: 10.1016/j.vaccine.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Johansson BE, Brett IC. Changing perspective on immunization against influenza. Vaccine. 2007;25(16):3062–3065. doi: 10.1016/j.vaccine.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 90.Kilbourne ED, Cerini CP, Khan MW, Mitchell JW, Jr, Ogra PL. Immunologic response to the influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. I. Studies in human vaccinees. J. Immunol. 1987;138(9):3010–3013. [PubMed] [Google Scholar]
- 91.Brett IC, Johansson BE. Immunization against influenza A virus: comparison of conventional inactivated, live-attenuated and recombinant baculovirus produced purified hemagglutinin and neuraminidase vaccines in a murine model system. Virology. 2005;339(2):273–280. doi: 10.1016/j.virol.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Kilbourne ED, Johansson BE, Grajower B. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc. Natl Acad. Sci. USA. 1990;87(2):786–790. doi: 10.1073/pnas.87.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sandbulte MR, Westgeest KB, Gao J, et al. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc. Natl Acad. Sci. USA. 2011;108(51):20748–20753. doi: 10.1073/pnas.1113801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Johansson BE, Matthews JT, Kilbourne ED. Supplementation of conventional influenza A vaccine with purified viral neuraminidase results in a balanced and broadened immune response. Vaccine. 1998;16(9–10):1009–1015. doi: 10.1016/s0264-410x(97)00279-x.. • Vaccine supplemented with purified neuraminidase results in improved immunity.
- 95.Martinet W, Saelens X, Deroo T, et al. Protection of mice against a lethal influenza challenge by immunization with yeast-derived recombinant influenza neuraminidase. Eur. J. Biochem. 1997;247(1):332–338. doi: 10.1111/j.1432-1033.1997.00332.x. [DOI] [PubMed] [Google Scholar]
- 96.Deroo T, Jou WM, Fiers W. Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine. 1996;14(6):561–569. doi: 10.1016/0264-410x(95)00157-v. [DOI] [PubMed] [Google Scholar]
- 97.Johansson BE. Immunization with influenza A virus hemagglutinin and neuraminidase produced in recombinant baculovirus results in a balanced and broadened immune response superior to conventional vaccine. Vaccine. 1999;17(15–16):2073–2080. doi: 10.1016/s0264-410x(98)00413-7. [DOI] [PubMed] [Google Scholar]
- 98.Chen J, Fang F, Li X, Chang H, Chen Z. Protection against influenza virus infection in BALB/c mice immunized with a single dose of neuraminidase-expressing DNAs by electroporation. Vaccine. 2005;23(34):4322–4328. doi: 10.1016/j.vaccine.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 99.Chen Z, Kadowaki S, Hagiwara Y, et al. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine. 2000;18(28):3214–3222. doi: 10.1016/s0264-410x(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 100.Li X, Fang F, Song Y, et al. Essential sequence of influenza neuraminidase DNA to provide protection against lethal viral infection. DNA Cell Biol. 2006;25(4):197–205. doi: 10.1089/dna.2006.25.197. [DOI] [PubMed] [Google Scholar]
- 101.Qiu M, Fang F, Chen Y, et al. Protection against avian influenza H9N2 virus challenge by immunization with hemagglutinin- or neuraminidase-expressing DNA in BALB/c mice. Biochem. Biophys. Res. Commun. 2006;343(4):1124–1131. doi: 10.1016/j.bbrc.2006.03.088. [DOI] [PubMed] [Google Scholar]
- 102.Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4(2):e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Webster RG, Reay PA, Laver WG. Protection against lethal influenza with neuraminidase. Virology. 1988;164(1):230–237. doi: 10.1016/0042-6822(88)90640-x. [DOI] [PubMed] [Google Scholar]
- 104.Sylte MJ, Hubby B, Suarez DL. Influenza neuraminidase antibodies provide partial protection for chickens against high pathogenic avian influenza infection. Vaccine. 2007;25(19):3763–3772. doi: 10.1016/j.vaccine.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 105.Gao W, Soloff AC, Lu X, et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 2006;80(4):1959–1964. doi: 10.1128/JVI.80.4.1959-1964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qiao CL, Yu KZ, Jiang YP, et al. Protection of chickens against highly lethal H5N1 and H7N1 avian influenza viruses with a recombinant fowlpox virus co-expressing H5 haemagglutinin and N1 neuraminidase genes. Avian Pathol. 2003;32(1):25–32. doi: 10.1080/0307945021000070688. [DOI] [PubMed] [Google Scholar]
- 107.Pavlova SP, Veits J, Keil GM, Mettenleiter TC, Fuchs W. Protection of chickens against H5N1 highly pathogenic avian influenza virus infection by live vaccination with infectious laryngotracheitis virus recombinants expressing H5 hemagglutinin and N1 neuraminidase. Vaccine. 2009;27(5):773–785. doi: 10.1016/j.vaccine.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 108.Bright RA, Carter DM, Crevar CJ, et al. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS ONE. 2008;3(1):e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pushko P, Tumpey TM, Van Hoeven N, et al. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine. 2007;25(21):4283–4290. doi: 10.1016/j.vaccine.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 110.Bright RA, Carter DM, Daniluk S, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25(19):3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 111. Quan FS, Kim MC, Lee BJ, Song JM, Compans RW, Kang SM. Influenza M1 VLPs containing neuraminidase induce heterosubtypic cross-protection. Virology. 2012;430(2):127–135. doi: 10.1016/j.virol.2012.05.006.. • Neuraminidase VLP vaccines can induce broader cross-protection.
- 112.Compans RW, Dimmock NJ, Meier-Ewert H. Effect of antibody to neuraminidase on the maturation and hemagglutinating activity of an influenza A2 virus. J. Virol. 1969;4(4):528–534. doi: 10.1128/jvi.4.4.528-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schulman JL, Khakpour M, Kilbourne ED. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J. Virol. 1968;2(8):778–786. doi: 10.1128/jvi.2.8.778-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Webster RG, Laver WG, Kilbourne ED. Reactions of antibodies with surface antigens of influenza virus. J. Gen. Virol. 1968;3(3):315–326. doi: 10.1099/0022-1317-3-3-315. [DOI] [PubMed] [Google Scholar]
- 115.Kasel JA, Couch RB, Gerin JL, Schulman JL. Effect of influenza anti-neuraminidase antibody on virus neutralization. Infect. Immun. 1973;8(1):130–131. doi: 10.1128/iai.8.1.130-131.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johansson BE, Kilbourne ED. Immunization with dissociated neuraminidase, matrix, and nucleoproteins from influenza A virus eliminates cognate help and antigenic competition. Virology. 1996;225(1):136–144. doi: 10.1006/viro.1996.0581. [DOI] [PubMed] [Google Scholar]
- 117.Johansson BE, Bucher DJ, Kilbourne ED. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J. Virol. 1989;63(3):1239–1246. doi: 10.1128/jvi.63.3.1239-1246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song JM, Hossain J, Yoo DG, et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405(1):165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 2005;11(Suppl. 4):S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 120.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 121.Kang SM, Compans RW. Host responses from innate to adaptive immunity after vaccination: molecular and cellular events. Mol. Cells. 2009;27(1):5–14. doi: 10.1007/s10059-009-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kool M, Pétrilli V, De Smedt T, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 2008;181(6):3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 123.Sato A, Iwasaki A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc. Natl Acad. Sci. USA. 2004;101(46):16274–16279. doi: 10.1073/pnas.0406268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lambrecht BN, Prins JB, Hoogsteden HC. Lung dendritic cells and host immunity to infection. Eur. Respir. J. 2001;18(4):692–704. [PubMed] [Google Scholar]
- 125.Kwissa M, Kasturi SP, Pulendran B. The science of adjuvants. Expert Rev. Vaccines. 2007;6(5):673–684. doi: 10.1586/14760584.6.5.673. [DOI] [PubMed] [Google Scholar]
- 126.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124(4):849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 127.Zinkernagel RM. Immunology taught by viruses. Science. 1996;271(5246):173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 128.Bachmann MF, Zinkernagel RM, Oxenius A. Immune responses in the absence of costimulation: viruses know the trick. J. Immunol. 1998;161(11):5791–5794. [PubMed] [Google Scholar]
- 129.Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 2007;81(7):3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McBurney SP, Young KR, Ross TM. Membrane embedded HIV-1 envelope on the surface of a virus-like particle elicits broader immune responses than soluble envelopes. Virology. 2007;358(2):334–346. doi: 10.1016/j.virol.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 131.Kang SM, Yoo DG, Lipatov AS, et al. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS ONE. 2009;4(3):e4667. doi: 10.1371/journal.pone.0004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bachmann MF, Lutz MB, Layton GT, et al. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur. J. Immunol. 1996;26(11):2595–2600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 133.Storni T, Lechner F, Erdmann I, et al. Critical role for activation of antigenpresenting cells in priming of cytotoxic T cell responses after vaccination with virus-like particles. J. Immunol. 2002;168(6):2880–2886. doi: 10.4049/jimmunol.168.6.2880. [DOI] [PubMed] [Google Scholar]
- 134.Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J. Immunol. 2004;172(3):1777–1785. doi: 10.4049/jimmunol.172.3.1777. [DOI] [PubMed] [Google Scholar]
- 135.Guo L, Lu X, Kang SM, Chen C, Compans RW, Yao Q. Enhancement of mucosal immune responses by chimeric influenza HA/SHIV virus-like particles. Virology. 2003;313(2):502–513. doi: 10.1016/s0042-6822(03)00372-6. [DOI] [PubMed] [Google Scholar]
- 136.Yao Q, Zhang R, Guo L, Li M, Chen C. Th cell-independent immune responses to chimeric hemagglutinin/simian human immunodeficiency virus-like particles vaccine. J. Immunol. 2004;173(3):1951–1958. doi: 10.4049/jimmunol.173.3.1951. [DOI] [PubMed] [Google Scholar]
- 137.Zhang R, Zhang S, Li M, Chen C, Yao Q. Incorporation of CD40 ligand into SHIV virus-like particles (VLP) enhances SHIV-VLP-induced dendritic cell activation and boosts immune responses against HIV. Vaccine. 2010;28(31):5114–5127. doi: 10.1016/j.vaccine.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pandey JP. Comment on Flagellin as an adjuvant: cellular mechanisms and potential. J. Immunol. 2011;186(3):1299. doi: 10.4049/jimmunol.1090134. author reply 1299. [DOI] [PubMed] [Google Scholar]
- 139.Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J. Immunol. 2010;185(10):5677–5682. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 2008;82(3):1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang BZ, Xu R, Quan FS, Kang SM, Wang L, Compans RW. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS ONE. 2010;5(11):e13972. doi: 10.1371/journal.pone.0013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wei HJ, Chang W, Lin SC, et al. Fabrication of influenza virus-like particles using M2 fusion proteins for imaging single viruses and designing vaccines. Vaccine. 2011;29(41):7163–7172. doi: 10.1016/j.vaccine.2011.05.077. [DOI] [PubMed] [Google Scholar]
- 143.Smith LR, Wloch MK, Ye M, et al. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine. 2010;28(13):2565–2572. doi: 10.1016/j.vaccine.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 144.Lalor PA, Webby RJ, Morrow J, et al. Plasmid DNA-based vaccines protect mice and ferrets against lethal challenge with A/ Vietnam/1203/04 (H5N1) influenza virus. J. Infect. Dis. 2008;197(12):1643–1652. doi: 10.1086/588431. [DOI] [PubMed] [Google Scholar]
- 145.Price GE, Soboleski MR, Lo CY, et al. Vaccination focusing immunity on conserved antigens protects mice and ferrets against virulent H1N1 and H5N1 influenza A viruses. Vaccine. 2009;27(47):6512–6521. doi: 10.1016/j.vaccine.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 146.Jimenez GS, Planchon R, Wei Q, et al. Vaxfectin-formulated influenza DNA vaccines encoding NP and M2 viral proteins protect mice against lethal viral challenge. Hum. Vaccin. 2007;3(5):157–164. doi: 10.4161/hv.3.5.4175. [DOI] [PubMed] [Google Scholar]
- 147.Nayak DP, Balogun RA, Yamada H, Zhou ZH, Barman S. Influenza virus morphogenesis and budding. Virus Res. 2009;143(2):147–161. doi: 10.1016/j.virusres.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 201.Universal influenza vaccine tested successfully in humans. Science Daily. 2008 Jan 24; www.sciencedaily.com/releases/2008/01/080124185522.htm.
- 202.VaxInnate. VaxInnate’s Universal Flu Vaccine Candidate Shown Safe and Immunogenic in Phase I Clinical Study. VaxInnate, NJ, USA: 2008. www.vaxinnate.com/pages/pressreleases/20070925_001.html. [Google Scholar]