Summary
Background
Histones are essential for chromatin packing, yet free histones not incorporated into chromatin are toxic. While in most cells multiple regulatory mechanisms prevent accumulation of excess histones, early Drosophila embryos contain massive extra-nuclear histone stores, thought to be essential for development. Excess histones H2A, H2B, and H2Av are bound to lipid droplets, ubiquitous fat storage organelles especially abundant in embryos. It has been proposed that sequestration on lipid droplets allows safe transient storage of supernumerary histones.
Results
Here we critically test this sequestration hypothesis. We find that histones are anchored to lipid droplets via the previously uncharacterized protein Jabba: Jabba localizes to droplets, co-immunoprecipitates with histones, and is necessary to recruit histones to droplets. Jabba mutants lack the maternal H2A, H2B, and H2Av deposits altogether; presumably, these deposits are eliminated unless sequestered on droplets. Jabba mutant embryos compensate for this histone deficit by translating maternal histone mRNAs. However, when histone expression is mildly compromised, the maternal histone protein deposits are essential for proper early mitoses and for viability.
Conclusions
A growing number of proteins from other cellular compartments have been found to transiently associate with lipid droplets. Our studies provide the first insight into mechanism and functional relevance of this sequestration. We conclude that sequestration on lipid droplets allows embryos to build up extra-nuclear histones stores and provides histones for chromatin assembly during times of high demand. This work reveals a novel aspect of histone metabolism and establishes lipid droplets as functional storage sites for unstable or detrimental proteins.
Introduction
In eukaryotes, histone expression levels need to be carefully balanced. Histones are essential for assembling genomic DNA into chromatin, protecting genome integrity, and regulating transcription [1]. Already mild histone reduction disturbs transcription and increases sensitivity to DNA damage [2]; it may even underlie the increased genomic instability of aging cells [3, 4]. Overabundance of histones is also detrimental; it disrupts gene expression, causes chromosome loss, increases DNA damage sensitivity, and may promote cancer [5-9].
Multiple regulatory mechanisms cooperate to balance the cellular histone supply [7, 10]. Biosynthesis of new histones and their assembly into chromatin are typically tightly coupled. Transcriptional and post-transcriptional regulation limit histone biosynthesis to times of need, and excess histones are proteolytically degraded [7, 11]. Thus, many cells have only few extra-nuclear histones, e.g., just ~0.1% of the entire histone content in human somatic cells [12, 13].
Sometimes, however, significant amounts of extra-nuclear histones accumulate on lipid droplets, the cellular organelles dedicated to fat storage. Proteomic analyses detected histones in lipid-droplet preparations from yeast to insects to humans [14]. For early Drosophila embryos, the presence of histones on lipid droplets has been confirmed in vivo [15]; here, histones equivalent to thousands of diploid nuclei are bound to lipid droplets. We proposed that lipid-droplet binding prevents these supernumerary histones from interfering with cellular processes and protects them from surveillance mechanisms that usually eliminate excess histones [14, 15]. A direct test of these proposals was previously not possible.
The biological role of droplet-bound histones remains unclear. One possibility is that they have novel, droplet-specific functions, e.g. a moonlighting role in regulating lipid metabolism. Alternatively, droplet binding may allow storage of histones for later use in chromatin assembly; droplet-bound histones can indeed be transferred to nuclei [15].
Proteins from diverse cellular compartments have been reported to accumulate on lipid droplets under specific conditions [14, 16]. For example, the cytosolic Hsp70 and inosine monophosphate dehydrogenase relocate to lipid droplets after heat stress or insulin signaling, respectively [17, 18]. Lipid droplets may thus have a general role in sequestering proteins. Droplet binding has been proposed to inactivate harmful proteins, promotes intracellular protein delivery, facilitate assembly of protein complexes, or aid protein degradation [14, 15, 19]. For several viral proteins, transient droplet association is indeed crucial for virus assembly [20, 21]. But it remains unknown if any endogenous proteins are indeed sequestered on lipid droplets to subsequently function elsewhere.
In this manuscript, we investigate the mechanism and significance of histone binding to lipid droplets in Drosophila embryos. We find that the novel protein Jabba physically interacts with histones and recruits them to lipid droplets. Jabba mutant embryos lack the extra-nuclear histone deposits. When mild disruption of histone gene expression is combined with Jabba mutants, embryos die during stages of highest histone demand. Our findings demonstrate that lipid droplets store histones for use in early embryogenesis.
Results
Histones on lipid droplets
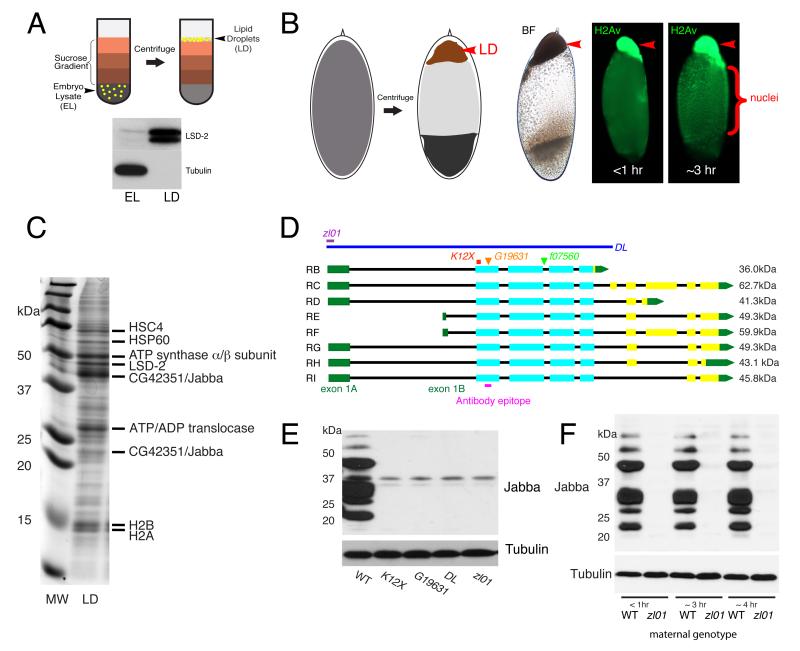
Lipid droplets have a hydrophobic core of neutral lipids surrounded by amphipathic lipids and proteins. Their low buoyant density allows lipid droplets to be separated from other cell components. For example, lipid droplets can be purified from Drosophila embryo lysates by sucrose-gradient centrifugation (Fig. 1A). In the complementary “in-vivo centrifugation” approach [22], intact embryos are centrifuged: the buoyant droplets float up, forming a distinct layer (Fig. 1B).
Figure 1.
Identification and characterization of Jabba. (A) Purification of lipid droplets from embryo lysate (EL) by floatation. During centrifugation, lipid droplets float to the top of the sucrose gradient. The lipid-droplet fraction (LD) is enriched for the droplet protein LSD-2 and depleted for the cytoplasmic protein tubulin. (B) Enrichment of lipid droplets by in-vivo centrifugation. The droplets form a distinct lipid-droplet layer (LD; arrowheads), visible by bright-field (BF) microscopy. Anti-H2Av staining reveals H2Av on the droplet layer of embryos of different ages, as well as within nuclei. (C) Proteins from purified wild-type droplets were separated by SDS PAGE and stained with Coomassie Blue (MW = molecular weight markers, LD = lipid-droplet fraction). Major bands were excised and identified by mass spectrometry. (D) Jabba locus. Exons are shown as boxes: green = UTR, cyan = coding regions shared between all isoforms, yellow = coding regions restricted to some isoforms. Pink bar = peptide used for antibody generation. Extent of deletions in JabbaDL and Jabbazl01 is indicated as well as the location of the non-sense mutation JabbaK12X and of two transposable element insertions. (E) Lysates from ~3 hr old embryos analyzed by anti-Jabba immunoblotting. (F) Embryos from reciprocal crosses between wild-type and Jabbazl01 parents analyzed by anti-Jabba immunoblotting. See also Fig. S1.
Using both approaches, we previously showed that histones H2A and H2B are massively present on lipid droplets of early embryos [15]. Proteomic analysis had also identified histone variant H2Av as candidate droplet protein. Endogenous H2Av is indeed present on biochemically purified lipid droplets (Fig. 4A) and highly enriched in the droplet layer of centrifuged embryos (Fig. 1B): In very young embryos, H2Av resides almost exclusively in the droplet layer; in older embryos, it is present also in nuclei.
Figure 4.
Jabba is necessary for histone recruitment to lipid droplets. (A) Immunoblotting of purified droplets from wild-type and Jabbazl01 embryos. Equal amounts of total protein were loaded. Kinesin heavy chain (KHC) levels are similar. H2A, H2B, and H2Av levels are dramatically reduced in the mutant. (B) Immunostaining of centrifuged embryos (<1 hr). Jabbazl01 embryos lack H2A, H2B, and H2Av signal in the droplet layer (top). (C) Centrifuged embryos from mothers expressing H2Av-GFP, by bright-field and fluorescence microscopy. GFP signal on the droplet layer (arrowhead) is dramatically reduced in the mutant. (D) In uncentrifuged embryos, H2Av-GFP is present in nuclei for both genotypes, but is absent from cytoplasmic puncta (which represent lipid droplets [15]) in Jabbazl01. The exposure for the wild type is double that of the mutant sample; for unknown reasons, H2Av-GFP signal in the nuclei is stronger in Jabbazl01 embryos. Scale bars: 7.5 μm (top), 5 μm (bottom). See also Fig. S4.
Identifying Jabba as candidate histone docking protein
Histones are bound to the droplet surface via electrostatic interactions [15]. If they bind via specific proteins, such histone anchors should be present on lipid droplets in comparable amounts and, just like histones, constitute major droplet proteins. We purified droplets by floatation, separated co-purifying proteins by SDS PAGE, and identified the most prominent bands by mass spectrometry (Fig. 1C). In addition to histones H2A and H2B, we found the cytosolic chaperone HSC70-4 and the mitochondrial proteins HSP60, ATP synthase, and ATP/ADP translocase. Because these proteins are abundant in non-nuclear and non-droplet compartments, they seemed poor candidates for factors that recruit histones specifically to lipid droplets.
We therefore focused on the remaining two candidates, the perilipin LSD-2 [23] and the novel protein CG42351. LSD-2 is not the histone anchor: histones remain bound to purified droplets even if LSD-2 is dislodged with detergents [15]; and, in embryos lacking LSD-2, histones are still present on the droplet layer after centrifugation (Fig. S4A). The prominent band at 42 kDa was identified as CG42351, in the following called Jabba. A minor band at 23 kDa also contained Jabba peptides and likely represents a breakdown product or alternative isoform.
Embryos express multiple forms of Jabba
Jabba is a novel protein, evolutionarily conserved among insects, but not obviously present in other species. Based on FlyBase annotations and our own cloning of Jabba cDNAs, the Jabba locus is predicted to encode eight transcripts (Fig. 1D). These transcripts arise from two different promoters, have coding exons 2 through 5 in common, and then diverge. All peptides recovered by mass spectrometry map to the common region and do not distinguish between isoforms.
Using multiple strategies, we identified eight mutant Jabba alleles, including deletions, non-sense mutations, and transposable-element insertions (Fig. 1D and supplemental experimental procedures). We also generated an antibody against an epitope in the shared exon 2. Immunoblotting (Figs. 1E, S1A, S1B) detected several prominent bands in wild-type embryos. All these proteins are encoded by the Jabba locus, as they are absent in several Jabba mutant alleles. It is unknown which Jabba splice variants give rise to the Jabba proteins present in embryos. Several Jabba bands detected by immunoblotting have a lower molecular weight than the smallest predicted isoform, suggesting post-translational processing.
To test whether the Jabba protein of early embryos is maternally provided or due to new transcription in the embryo, we performed reciprocal crosses between wild-type and Jabba mutant parents (Figs. 1F, S1C). Because the presence of Jabba protein depended strictly on the genotype of the mother, Jabba is maternally provided – like histones and lipid droplets.
Jabba is lipid-droplet associated
We had identified Jabba as 42 and 23 kDa proteins prominent in purified lipid droplets. By immunoblotting, Jabba bands of these sizes were indeed highly enriched on purified droplets (Fig. 2A, Fig. S2B). Other Jabba protein forms were not detected; either these forms localize elsewhere or are lost from droplets during purification.
Figure 2.
Jabba is a lipid-droplet protein. (A) Equal amounts of protein from wild-type lipid-droplet samples (LD) and embryo lysate (EL) analyzed by immunoblotting (see Fig. 1A). Two Jabba bands are highly enriched in the droplet fraction; under these conditions, no signal is detected in the lysate. (B) Embryos expressing the droplet marker GFP-LD stained for GFP (green) and Jabba (red). Jabba is present in rings that colocalize with GFP-LD. Scale bar = 2.5 μm. (C) Anti-Jabba staining of centrifuged wild-type and Jabbazl01 embryos. Jabba is highly enriched in the droplet layer (arrowhead) in the wild type. The middle panel shows a ~10 fold longer exposure of the mutant. (D) Anti-Jabba staining of centrifuged egg chambers. Lipid-droplet layers in the oocyte (arrow) and in nurse cells (arrowhead) are indicated. (E) Kc167 cells transiently expressing Jabba-PB-eGFP (green) stained with LipidTOX (red) to reveal lipid droplets. Scale bar = 5 μm. See also Fig. S2.
Anti-Jabba immunostaining revealed round cytoplasmic structures in the size range of embryonic lipid droplets [24]; these structures showed the ring pattern typical for proteins present on the surface of lipid droplets (Fig. 2B). No such structures were evident in Jabbazl01 embryos. Jabba signal indeed co-localized with GFP-LD (Fig. 2B), a fusion protein present on most embryonic droplets [25]. Jabba signal was also highly enriched in the droplet layer of centrifuged embryos (Fig. 2C). Finally, lipid droplets in early embryos accumulate around the central yolk just before cellularization; this transport requires the Halo protein [26]. Jabba rings also accumulated around the yolk, in a Halo-dependent manner (Fig. S2A). We conclude that Jabba is associated with lipid droplets.
During oogenesis, lipid droplets originate in nurse cells and are transferred through cytoplasmic bridges to the growing oocyte. We employed in-vivo centrifugation to separate the constituents of nurse cells and oocytes by density [15, 22]. Anti-Jabba signal was present exclusively in the droplet layer of nurse cells and oocytes (Fig. 2D). Jabba apparently already localizes to lipid droplets in nurse cells and is deposited with them into the oocyte.
Jabba localizes to lipid droplets also in Drosophila Kc167 cultured cells. Transient expression of GFP-tagged Jabba isoforms yielded the characteristic ring pattern. These rings represent lipid droplets, as shown by co-labeling with a droplet-specific dye or co-expression of Tdt-tagged droplet proteins (Figs. 2E, S2C-G). All three isoforms tested displayed this localization, suggesting that droplet targeting is inherent in the common region.
Jabba is required for histone recruitment to lipid droplets
Embryos lacking Jabba have normal overall triglyceride content and display abundant lipid droplets (Figs. 3A, 3B, S3C). In the mutant embryos, lipid droplets were less evenly distributed than in the wild type and often clustered. The mutant droplets also frequently appeared larger, though currently we cannot resolve whether this is due to fusion of clustered droplets upon fixation.
Figure 3.
Jabba mutants have abundant lipid droplets with altered protein content. (A) Triglyceride levels in 2 hr old wild-type and Jabbazl01 embryos are similar. Error bars represent standard deviations. (B) Lipid droplets in 2 hr old wild-type and Jabbazl01 embryos, revealed by Nile Red staining. Jabbazl01 droplets are unevenly distributed and irregularly shaped, a phenotype that inspired the name Jabba. Scale bar = 5 μm. (C) Equal amounts of proteins of droplets purified from wild-type and Jabbazl01 embryos were separated by SDS PAGE and stained with Coomassie Blue.
Droplets purified from Jabbazl01 and wild-type embryos differed dramatically in their protein composition (Fig. 3C). Several major bands were missing from the mutant sample, including H2A and H2B. Many other bands were still present. In particular, LSD-2 was abundant on the mutant droplets, a fact confirmed by immunoblotting (Fig. S3A). We also found the known droplet proteins kinesin-1 and Klar on Jabbazl01 droplets (Figs. 4A, S3B). Thus, absence of Jabba leads to loss of specific droplet proteins.
We confirmed the absence of histones from Jabba droplets with antibodies. By immunoblotting, H2A and H2B are abundantly present on wild-type, but not Jabba mutant droplets (Fig. 4A). By immunostaining, histone signal was strong in the droplet layer and nuclei of centrifuged wild-type embryos, but absent from the droplet layer of Jabba mutants: Similar results were observed for unfertilized eggs and for embryos of various ages (Figs. S4C, 4B, S4D) as well as for several independently derived Jabba alleles (Figs. 4B, S4B).
Histone variant H2Av displayed the same pattern. In Jabba mutants, H2Av was absent from purified droplets and from the droplet layer of centrifuged embryos (Figs. 4A, 4B, S4C, S4D). We also employed H2Av-GFP transgenes and live imaging of embryos. For centrifuged embryos, presence of H2Av-GFP in the droplet layer requires Jabba (Fig. 4C). In uncentrifuged embryos that express Jabba, H2Av-GFP was present in nuclei and in cytoplasmic rings, previously shown to be lipid droplets [15]. In Jabba mutants, nuclear signal was prominent, but no cytoplasmic rings were detectable (Fig. 4D). We conclude that Jabba is required for H2A, H2B, and H2Av to localize on lipid droplets.
Droplet-bound Jabba and histones form protein complexes
Jabba might act as the histone anchor, physically connecting histones to lipid droplets, or it might act indirectly, e.g., by recruiting or modifying the actual histone anchor. If Jabba recruits histones directly, the amount of Jabba should influence how much histone is present on lipid droplets. In embryos from mothers with a single copy of the Jabba gene (1x Jabba), Jabba levels are roughly halved compared to the wild type (2x Jabba) (Fig. 5A). When centrifuged 3 hr old embryos were stained for H2B, nuclear signal was comparable between embryos from 1x and 2x Jabba mothers, but the droplet-layer signal was greatly reduced for 1x Jabba (Fig. 5B). We observed a similar dependence of histone levels on Jabba dosage using H2Av-GFP (Fig. 5C).
Figure 5.
Jabba is the histone docking protein on droplets. (A) Anti-Jabba immunoblotting in equal numbers of embryos from mothers carrying two, one, or zero copies of a wild-type Jabba gene. Jabba protein levels scale with Jabba gene dosage (2x Jabba = wild type; 1x Jabba = Df(2R)Exel7158/+; 0x Jabba = Jabbazl01). (B) H2B signal on the lipid-droplet layers of centrifuged 2x Jabba embryos (~3 hrs old) is much stronger than that of 1x Jabba, while nuclear signal is similar. (C) Centrifuged H2Av-GFP/+ and Jabbazl01/+, H2Av-GFP/+ embryos. Embryo ages: <1 hr (left panel), ~3 hrs (right panel). Reduced droplet signal with age presumably represents transfer of histones from lipid droplets to nuclei [15]. (D) H2Av-GFP co-immunoprecipitates with Jabba. Lipid droplets purified from H2Av-GFP and wild-type embryos were exposed to anti-GFP antibodies for immunoprecipitation.
To directly test if Jabba physically recruits histones to droplets, we purified lipid droplets from H2Av-GFP expressing embryos and precipitated H2Av-GFP with anti-GFP antibodies. The 42-kDa form of Jabba, but not LSD-2, co-precipitated under these conditions (Fig. 5D). Apparently, Jabba is specifically brought into the pellet, not simply via precipitation of entire droplets. We propose that Jabba acts as histone docking protein and recruits histones to droplets by physical interactions, either directly or via bridging proteins. Since monomeric histones are unstable, it seems likely that the droplet-bound histones are H2A/H2B and H2Av/H2B heterodimers.
Jabba mutants lack maternal H2A/H2B/H2Av deposits
If histones are unable to bind to lipid droplets, they might be present elsewhere in the embryo or they might be eliminated, like excess histones in yeast [7, 11]. Unfertilized Jabbazl01 mutant embryos had massively reduced levels of H2A and H2B, and reproducibly less H2Av (Fig. 6A). Levels of H3, a histone not bound to lipid droplets, were unchanged (Fig. 6B). Embryos from 1x Jabba mothers had intermediate maternal histone deposits (Fig. 6C).
Figure 6.
Jabba mutant embryos lack maternal histone deposits, but can synthesize histones zygotically. (A,B) Compared to wild type, unfertilized Jabbazl01 embryos have barely detectable H2A and H2B, less H2Av, but similar amounts of H3. (C) By immunoblotting, the amount of H2A and H2B in unfertilized 2x Jabba embryos is roughly double that of 1x Jabba embryos. (D) Jabbazl01 embryos can generate H2A and H2B. Levels of both histones go up dramatically as development proceeds (stage 1 ~ 30 min; stage 2 ~ 60 min; stage 4 ~ 2 hrs; stage 5 ~ 3 hrs). (E) By ~3 hrs, Jabbazl01 embryos have reached similar H2B levels as wild type. (F) Unfertilized wild-type and Jabbazl01 embryos have similar levels of H2A mRNA (measured by qRT-PCR, normalized to the wild-type value). Error bars represent standard deviations.
In addition to the maternally supplied histone proteins, wild-type embryos also contain abundant histone messages [10]: maternally deposited histone mRNAs [27, 28] are later supplemented by zygotic transcription [29, 30]. Translation of these messages contributes to the histone protein supply, as overall histone protein levels increase several fold during the first 3 hrs of embryogenesis [15] (Fig. 6E). This source of new histones is apparently unaffected by the absence of Jabba: H2A and H2B levels in Jabbazl01 embryos go up as embryos age (Fig. 6D), such that by stage 5 they have reached close to wild-type levels (Fig. 6E). We also detect similar levels of maternally provided H2A mRNAs in both wild-type and Jabbazl01 embryos (Fig. 6F). Thus, Jabba mutations do not interfere with histone biosynthesis per se. We propose that Jabba mutant embryos lack the maternal histone deposit because extra-nuclear histones are degraded.
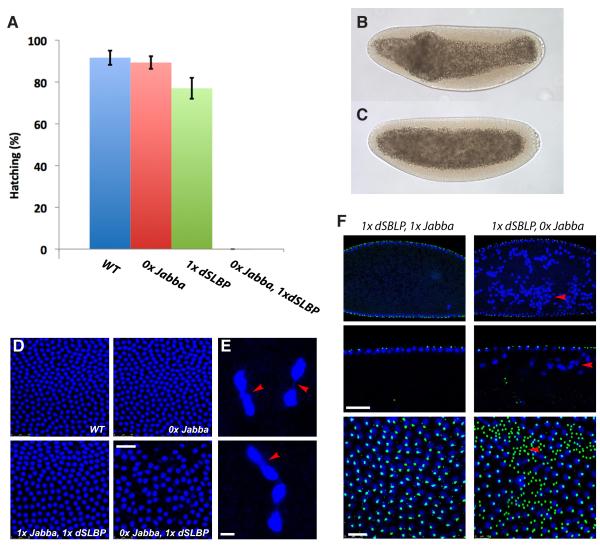
What are the consequences for the embryo? During wild-type development, nuclei initially divide in the center of the embryo (cleavage stages), and most subsequently migrate to the embryo cortex (syncytial blastoderm). Cellularization occurs after four additional cortical mitoses [31]. Severe reduction in maternal histone expression causes aberrant nuclear shapes and distributions, nuclei arrested in mitosis, and ultimately embryonic death before cellularization [28]. Yet DNA staining of Jabba mutant embryos revealed the wild-type pattern of nuclear distributions; in particular, in blastoderm embryos, nuclei at the cortex were evenly spaced and uniform in size (Fig. S5A). Jabba mutant embryos also hatched at wild-type rates (Fig. 7A) and gave rise to fertile adults.
Figure 7.
Reduced histone expression and Jabba mutants are synthetically lethal. (A) Hatching frequency of embryos from mothers of various genotypes. 0x Jabba = Jabbazl01; 1x dSLBP = heterozygous for Df(3R)3450. Error bars represent standard deviations. (B, C) Late syncytial blastoderm embryos (heat-fixed and inspected by DIC microscopy) from 1x dSLBP 0x Jabba mothers, with aberrant yolk distribution (B) or grossly normal morphology (C). By cellularization, most embryos displayed some morphological defects. (D) Surface view of blastoderm embryos; genotype of mothers is indicated (blue = DNA; scale bar = 25 μm) (E) Nuclei of an embryo from a 1x dSLBP 0x Jabba mother showing chromosomes (arrowheads) connecting daughter nuclei late in mitosis. Scale bar = 5 μm. (F) Blastoderm embryos stained for DNA (blue) or centrosomes (green): cross sections at low (top) and high (middle) magnification, plus a surface view (bottom). In the absence of Jabba, nuclei are absent from cortical patches (arrowhead, bottom) and present between the cortex and the central yolk (arrowheads, top and middle). Scale bars = 10 μm. See also Fig. S5 and Movie S6.
Limited histone supply can cause defects during mitosis [28, 32]. In blastoderm embryos, the products of aberrant mitoses are eliminated by nuclear fall-out [33]: Defective nuclei dissociate from their centrosomes and move into the central yolk. Free centrosomes at the cortex are therefore a hallmark of DNA damage and/or defective mitoses. Yet in both wild-type and Jabba mutant embryos, we only rarely detected centrosomes unattached to nuclei (Fig. S5A). Apparently, the lack of the maternal histone protein deposit is neither essential for embryo viability nor does it cause widespread mitotic defects.
Jabba mutations and reduced histone expression are synthetically lethal
Jabba mutants are surprisingly normal, even though a sufficient histone supply is essential for early embryogenesis [28]. We reasoned that biosynthesis of new histones in the zygote (Fig. 6D, E) might generate just enough histones for Jabba embryos to survive. Cultured mammalian cells and yeast, for example, maintain viability even when overall histone incorporation into chromatin is substantially reduced [2].
To test whether Jabba embryos are particularly dependent on new histone synthesis, we employed mutants in the Drosophila stem-loop-binding protein (dSLBP). dSLBP binds the messages of core histones and promotes their correct processing, stability, and translation [28, 34]. Simple reduction in dSLBP dosage caused no overt defects: Embryos from 1x dSLBP mothers were morphologically normal, cellularized, and hatched at near wild-type rates (Fig. 7A). In contrast, embryos from Jabbazl01 1xdSLBP mothers typically reached syncytial stages but then became morphologically abnormal (Fig. 7B). Cellularization, if initiated at all, frequently proceeded abnormally (Fig. S5B). Most embryos failed to hatch (Fig. 7A), and about half ultimately rounded up inside the egg shell (Fig. S5C), reminiscent of the “cannonball” phenotypes observed when pre-cellularization embryos are massively irradiated [35, 36]. The hypomorphic allele dSLBP10 caused similar synthetic lethality (Fig. S5E). Finally, we already observed some reduction in embryo hatching even when we reduced histone expression only zygotically (Fig. S5F).
Two additional strategies to impair histone expression yielded similar results: First, we reduced the dosage of H2Av. Embryos from 1x H2Av mothers developed grossly normally, and hatched at wild-type rates. Embryos from Jabbazl01 1x H2Av mothers displayed severe hatching defects (Fig. S5E); ~10% died as cannonballs. Second, we halved the dosage of core histone genes. Embryo hatching was normal when mothers had one or two copies of Jabba, but was variably reduced when Jabba was absent (data not shown).
DNA staining of embryos from Jabbazl01 1xdSLBP mothers revealed multiple defects. Frequently, nuclei had different sizes, were misshapen and unevenly spaced (Fig. 7D). We also observed trailing chromosomes during anaphase and telophase (Fig. 7E), indicative of defective chromosome segregation. Finally, mutant embryos apparently experienced massive nuclear fall-out because they displayed nuclei in between the cortex and the central yolk, and abundant free centrosomes at the cortex (Fig. 7F). Nuclear fall-out was also evident by bright-field microscopy (Fig. S5D; Movie S6). We conclude that under sensitized conditions the droplet-bound histones are essential for early embryogenesis.
Discussion
Lipid-droplet binding allows accumulation of excess histones
The maternal histone protein deposit is dramatically reduced in Jabba embryos. In principle, lack of these histones could be due to defective histone biosynthesis during oogenesis, lack of transport of histones from nurse cells to oocytes, or degradation of histones if they are not droplet-bound. A defect in histone protein production seems unlikely (Fig. 6): Jabba embryos contain normal levels of maternal histones messages; these messages are abundantly translated in the zygote, and the histone deposit scales with Jabba dosage.
During oogenesis, lipid droplets originate in nurse cells and are then transferred to oocytes. Histones might be absent from oocytes because without droplet-attachment they are left behind in the nurse cells. We disfavor this possibility: first, most nurse-cell contents are transferred to the oocyte later in oogenesis anyway; second, oocytes contain abundant histone mRNAs that could contribute to the intra-oocyte histone pool regardless of transport problems; third, we have failed to detect obvious accumulation of H2B or H2Av-GFP in the nurse-cell cytoplasm of Jabba mutants (not shown).
In yeast, excess histones are destroyed by the proteasome [5, 11]. Indirect evidence suggests that similar pathways are active in animal cells [2]. We propose that in Jabba mutants the maternal histone protein deposit is reduced because histones not bound to lipid droplets are degraded.
Droplet-associated histones support early development
To ensure proper chromatin packaging, the cellular histone supply has to match the available DNA. In early Drosophila embryos, the demand for histones is particularly high: by blastoderm stages, new DNA equivalent to thousands of diploid nuclei needs to be packaged, during S phases that last less than 5 minutes [31]. It had long been thought that this demand was met by the maternal deposits of histone mRNA and protein [10]. Our data now show that embryos in which the maternal histone protein deposit is selectively impaired are viable.
Severe impairment of the embryonic histone supply has been achieved by two distinct strategies: ten-fold reduction of histone mRNA levels via dSLBP mutations [28] or by combining mild disruption of histone expression with a lack of the histone protein deposit via Jabba mutants (this manuscript). In either case, the overall histone supply apparently falls below a critical threshold: many embryos become abnormal by or before blastoderm stages. Under these sensitized conditions, Jabba mutants display massive nuclear fall-out as well as trailing chromosomes in mitosis, consistent with the aberrant mitoses and increased DNA damage sensitivity known to result from reduced histone levels. We conclude that when histone biosynthesis in the embryo is compromised Jabba mutants die because they cannot compensate for the lack of the maternal histone deposit.
This analysis strongly argues that the droplet-bound histones are one of the sources of histones that sustain early development. Indeed, histones can be transferred from droplets to nuclei (as previously shown by droplet transplantation [15]), and levels of droplet-bound histones decrease as embryogenesis proceeds ([15], Fig. 4C). In embryos with impaired histone biosynthesis (1x dSLBP), the histone pool on droplets is markedly reduced by cellularization, compared to wild type (Figs. S5G, S5H), consistent with the notion that these embryos are particularly dependent on the maternally provided histone store.
Microarray and RNA-seq data available on FlyBase [37] suggest that Jabba expression is not restricted to early embryos. What role Jabba may play in other cell types is an exciting challenge for future research. Like in embryos, it may modulate histone availability, though presumably the need for excess histones is reduced later in development. Our analysis (Fig. 3) raises the intriguing possibility that Jabba may also buffer other proteins or may control morphology and spatial distribution of droplets.
Lipid droplets as general protein storage sites
Droplet-associated histones may be widespread: By immunolocalization, histones have been detected on lipid droplets in housefly embryos [15] and mouse oocytes [38] as well as on the lipid-droplet–related microvesicles of mammalian sebocytes [39]. In addition, many droplet proteomes report the presence of specific histones (see, e.g., [14]). In these cases, histones may also be transiently stored on lipid droplets for later use in the nucleus, just like in Drosophila embryos; such a mechanism might, for example, buffer histone availability even if histone synthesis and DNA replication transiently get out of sync or provide a source of histones for DNA repair. This histone buffering capacity is likely enhanced by additional mechanisms, since both classical histone chaperones and certain importins can buffer limited amounts of histones in the cytoplasm [5, 40]. We speculate that the abundant surface of lipid droplets provides high-capacity storage and also keeps the histones from inappropriately entering the nuclei.
The specificity of histone-droplet interactions is remarkable: H2A, H2B, and H2Av are droplet localized, and their stability in the early embryo depends on Jabba. In contrast, H3 is absent from lipid droplets, and its maternal stores are independent of Jabba. It is possible that – like in Xenopus oocytes [41] – histones H3/H4 are stored in the cytoplasm associated with histone chaperones. In contrast, droplet-localized histones are apparently not associated with traditional histone chaperones, since no such proteins have been detected on lipid droplets (Fig. 1C; [15]). We propose that binding to Jabba stabilizes these histones and that they are later handed off to cytoplasmic chaperones that shuttle them to the nuclei for chromatin assembly.
Like histones, many candidate droplet proteins have known functions or localization in other cellular compartments, such as the cytosolic enzyme inosine monophosphate dehydrogenase, the spliceosome activator Prp19p, and the membrane protein caveolin (reviewed in [14]). We previously proposed that some of these proteins are “refugee proteins”, proteins transiently sequestered after or before acting elsewhere in the cell [14, 15]. To our knowledge, our studies on droplet-bound histones provide the strongest evidence yet for droplet sequestration of endogenous proteins and for lipid droplets as a way station for proteins destined elsewhere.
The possibility that lipid droplets modulate protein availability for other cellular compartments suggests that they have novel cellular roles beyond lipid metabolism. If so, altering droplet number or size could affect overall cellular buffering capacity: Too much sequestration surface might reduce available protein below optimal levels, while too little sequestration might allow proteins to build up to toxic levels.
Experimental Procedures
Identification of droplet proteins
Lipid droplets were isolated by floatation as described [25], and normalized by protein content for Western analysis. For mass spectrometry, isolated droplets were separated by SDS PAGE, and proteins were digested in-gel with trypsin. Tryptic peptides were identified by either MALDI or LC-MS/MS.
Microscopic analysis
Living embryos and adult females were centrifuged to separate lipid droplets from other cellular components [15, 22]. Lipid droplets were detected in fixed, dechorionated embryos with Nile Red [25]. To detect GFP, Jabba, histones, or centrosomes by immunostaining, dechorionated embryos were heat-fixed and devitellinized using standard heptane-methanol procedures. Kc167 cells were cultured in Schneider’s medium and induced to accumulate lipid droplets with 400 μM oleic acid. Cells were fixed using 5% paraformaldehyde and counterstained with LipidTOX Deep Red.
Molecular biology
Jabba isoforms RD, RH, and RI were cloned from an adult female cDNA library. Isoforms were expressed in Kc167 cells as N-terminal GFP fusions. For qPCR analysis, cDNA was synthesized using mRNA from embryos less than 2 hours old. Histone message levels were quantified via a Bio-Rad iQ5 detection system.
Supplementary Material
Highlights.
The novel protein Jabba acts as histone anchor on Drosophila lipid droplets.
Jabba mutant embryos lack the extra-nuclear store of histones.
Under sensitized conditions, lipid droplet-bound histones are essential for life.
First confirmed case of lipid droplets as storage sites for endogenous proteins.
Acknowledgements
We thank the Bloomington Drosophila stock center, the Exelixis stock center at Harvard Medical School, and Robert Duronio for fly stocks, Timothy Megraw and Robert Glaser for antibodies, and Alf Herzig for plasmids. We thank Fred Hagen and the University of Rochester Proteomics Center for mass spectrometry analysis, Julia Militar and Scott Hetzel for help with PCR, and Henri Jasper for access to qPCR equipment. We are grateful to Xin Bi, Jeffrey Hayes, Margarete Heck, Steven Gross, and three anonymous reviewers for comments on the manuscript. This work was supported by NIH grants GM64687 and AG031531 (MAW) and by the Max Planck Society (KT, PJT, MB, RPK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.An W, Leuba SH, van Holde K, Zlatanova J. Linker histone protects linker DNA on only one side of the core particle and in a sequence-dependent manner. Proc Natl Acad Sci U S A. 1998;95:3396–3401. doi: 10.1073/pnas.95.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celona B, Weiner A, Di Felice F, Mancuso FM, Cesarini E, Rossi RL, Gregory L, Baban D, Rossetti G, Grianti P, et al. Substantial histone reduction modulates genomewide nucleosomal occupancy and global transcriptional output. PLoS Biol. 2011;9:e1001086. doi: 10.1371/journal.pbio.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat Struct Mol Biol. 2010;17:1218–1225. doi: 10.1038/nsmb.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feser J, Truong D, Das C, Carson JJ, Kieft J, Harkness T, Tyler JK. Elevated histone expression promotes life span extension. Mol Cell. 2010;39:724–735. doi: 10.1016/j.molcel.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunjan A, Verreault A. A Rad53 kinase-dependent surveillance mechanism that regulates histone protein levels in S. cerevisiae. Cell. 2003;115:537–549. doi: 10.1016/s0092-8674(03)00896-1. [DOI] [PubMed] [Google Scholar]
- 6.Singh RK, Liang D, Gajjalaiahvari UR, Kabbaj MH, Paik J, Gunjan A. Excess histone levels mediate cytotoxicity via multiple mechanisms. Cell Cycle. 2010;9:4236–4244. doi: 10.4161/cc.9.20.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunjan A, Paik J, Verreault A. The emergence of regulated histone proteolysis. Curr Opin Genet Dev. 2006;16:112–118. doi: 10.1016/j.gde.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Herrero AB, Moreno S. Lsm1 promotes genomic stability by controlling histone mRNA decay. EMBO J. 2011;30:2008–2018. doi: 10.1038/emboj.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meeks-Wagner D, Hartwell LH. Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission. Cell. 1986;44:43–52. doi: 10.1016/0092-8674(86)90483-6. [DOI] [PubMed] [Google Scholar]
- 10.Marzluff WF, Wagner EJ, Duronio RJ. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat Rev Genet. 2008;9:843–854. doi: 10.1038/nrg2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh RK, Kabbaj MH, Paik J, Gunjan A. Histone levels are regulated by phosphorylation and ubiquitylation-dependent proteolysis. Nat Cell Biol. 2009;11:925–933. doi: 10.1038/ncb1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonner WM, Wu RS, Panusz HT, Muneses C. Kinetics of accumulation and depletion of soluble newly synthesized histone in the reciprocal regulation of histone and DNA synthesis. Biochemistry. 1988;27:6542–6550. doi: 10.1021/bi00417a052. [DOI] [PubMed] [Google Scholar]
- 13.Oliver D, Granner D, Chalkley R. Identification of a distinction between cytoplasmic histone synthesis and subsequent histone deposition within the nucleus. Biochemistry. 1974;13:746–749. doi: 10.1021/bi00701a017. [DOI] [PubMed] [Google Scholar]
- 14.Welte MA. Proteins under new management: lipid droplets deliver. Trends Cell Biol. 2007;17:363–369. doi: 10.1016/j.tcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Cermelli S, Guo Y, Gross SP, Welte MA. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol. 2006;16:1783–1795. doi: 10.1016/j.cub.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 16.Hodges BD, Wu CC. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J Lipid Res. 2010;51:262–273. doi: 10.1194/jlr.R003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, He J, Pu S, Tang C, Xu G. Heat shock protein 70 is translocated to lipid droplets in rat adipocytes upon heat stimulation. Biochim Biophys Acta. 2007;1771:66–74. doi: 10.1016/j.bbalip.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead JP, Simpson F, Hill MM, Thomas EC, Connolly LM, Collart F, Simpson RJ, James DE. Insulin and oleate promote translocation of inosine-5′ monophosphate dehydrogenase to lipid bodies. Traffic. 2004;5:739–749. doi: 10.1111/j.1600-0854.2004.00217.x. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto T, Ohsaki Y, Cheng J, Suzuki M, Shinohara Y. Lipid droplets: a classic organelle with new outfits. Histochem Cell Biol. 2008;130 doi: 10.1007/s00418-008-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 21.Samsa MM, Mondotte JA, Iglesias NG, Assuncao-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009;5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran SL, Welte MA. In-vivo centrifugation of Drosophila embryos. J Vis Exp. 2010 doi: 10.3791/2005. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welte MA, Cermelli S, Griner J, Viera A, Guo Y, Kim DH, Gindhart JG, Gross SP. Regulation of lipid-droplet transport by the Perilipin homologue LSD2. Curr Biol. 2005;15:1266–1275. doi: 10.1016/j.cub.2005.06.062. [DOI] [PubMed] [Google Scholar]
- 24.Welte MA, Gross SP, Postner M, Block SM, Wieschaus EF. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell. 1998;92:547–557. doi: 10.1016/s0092-8674(00)80947-2. [DOI] [PubMed] [Google Scholar]
- 25.Yu YV, Li Z, Rizzo NP, Einstein J, Welte MA. Targeting the motor regulator Klar to lipid droplets. BMC Cell Biol. 2011;12:9. doi: 10.1186/1471-2121-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross SP, Guo Y, Martinez JE, Welte MA. A determinant for directionality of organelle transport in Drosophila embryos. Curr Biol. 2003;13:1660–1668. doi: 10.1016/j.cub.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Anderson KV, Lengyel JA. Changing rates of histone mRNA synthesis and turnover in Drosophila embryos. Cell. 1980;21:717–727. doi: 10.1016/0092-8674(80)90435-3. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan E, Santiago C, Parker ED, Dominski Z, Yang X, Lanzotti DJ, Ingledue TC, Marzluff WF, Duronio RJ. Drosophila stem loop binding protein coordinates accumulation of mature histone mRNA with cell cycle progression. Genes Dev. 2001;15:173–187. doi: 10.1101/gad.862801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanzotti DJ, Kaygun H, Yang X, Duronio RJ, Marzluff WF. Developmental control of histone mRNA and dSLBP synthesis during Drosophila embryogenesis and the role of dSLBP in histone mRNA 3′ end processing in vivo. Mol Cell Biol. 2002;22:2267–2282. doi: 10.1128/MCB.22.7.2267-2282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar BA, Schubiger G. Parameters controlling transcriptional activation during early Drosophila development. Cell. 1986;44:871–877. doi: 10.1016/0092-8674(86)90009-7. [DOI] [PubMed] [Google Scholar]
- 31.Foe VE, Odell GM, Edgar BA. Mitosis and morphogenesis in the Drosophila embryo: point and counterpoint. In: Bate M, Martinez-Arias A, editors. The Development of Drosophuila melanogaster. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. pp. 149–300. [Google Scholar]
- 32.Han M, Chang M, Kim UJ, Grunstein M. Histone H2B repression causes cell-cycle-specific arrest in yeast: effects on chromosomal segregation, replication, and transcription. Cell. 1987;48:589–597. doi: 10.1016/0092-8674(87)90237-6. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan W, Minden JS, Alberts BM. daughterless-abo-like, a Drosophila maternal-effect mutation that exhibits abnormal centrosome separation during the late blastoderm divisions. Development. 1990;110:311–323. doi: 10.1242/dev.110.2.311. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez R, Marzluff WF. The stem-loop binding protein is required for efficient translation of histone mRNA in vivo and in vitro. Mol Cell Biol. 2002;22:7093–7104. doi: 10.1128/MCB.22.20.7093-7104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wieschaus E, Nüsslein-Volhard C. Looking at embryos. In: Roberts DB, editor. Drosophila: A Practical Approach. 2nd edition. Oxford University Press; Oxford: 1998. pp. 179–214. Edition. [Google Scholar]
- 36.Würgler FE, Ulrich H. Radiosensitivity of embryonic stages. In: Ashburner M, Novitski E, editors. The Genetics and Biology of Drosophila. Volume 1c. Academic Press; London: 1976. pp. 1269–1298. [Google Scholar]
- 37.McQuilton P, St Pierre SE, Thurmond J. FlyBase 101 - the basics of navigating FlyBase. Nucleic Acids Res. 2011;40:D706–D714. doi: 10.1093/nar/gkr1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kan R, Jin M, Subramanian V, Causey CP, Thompson PR, Coonrod SA. Potential role for PADI-mediated histone citrullination in preimplantation development. BMC Dev Biol. 2012;12:19. doi: 10.1186/1471-213X-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai A, Sato T, Akimoto N, Ito A, Sumida M. Isolation and identification of histone H3 protein enriched in microvesicles secreted from cultured sebocytes. Endocrinology. 2005;146:2593–2601. doi: 10.1210/en.2004-1478. [DOI] [PubMed] [Google Scholar]
- 40.Jäkel S, Mingot JM, Schwarzmaier P, Hartmann E, Görlich D. Importins fulfil a dual function as nuclear import receptors and cytoplasmic chaperones for exposed basic domains. EMBO J. 2002;21:377–386. doi: 10.1093/emboj/21.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dilworth SM, Black SJ, Laskey RA. Two complexes that contain histones are required for nucleosome assembly in vitro: role of nucleoplasmin and N1 in Xenopus egg extracts. Cell. 1987;51:1009–1018. doi: 10.1016/0092-8674(87)90587-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.