Summary
Hyperactivity of the hypothalamus–pituitary–adrenal (HPA) axis and increased levels of glucocorticoid hormones in patients with depression have mostly been ascribed to impaired feedback regulation of the HPA axis, possibly caused by altered function of the receptor for glucocorticoid hormones, the glucocorticoid receptor (GR). Antidepressants, in turn, ameliorate many of the neurobiological disturbances in depression, including HPA axis hyperactivity, and thereby alleviate depressive symptoms. There is strong evidence for the notion that antidepressants exert these effects by modulating the GR. Such modulations, however, can be manifold and range from regulation of receptor expression to post-translational modifications, which may result in differences in GR nuclear translocation and GR-dependent gene transcription. The idea that the therapeutic action of antidepressants is mediated, at least in part, by restoring GR function, is consistent with studies showing that decreased GR function contributes to HPA axis hyperactivity and to the development of depressive symptoms. Conversely, excessive glucocorticoid signalling, which requires an active GR, is associated with functional impairments in the depressed brain, especially in the hippocampus, where it results in reduced neurogenesis and impaired neuroplasticity.
In this review, we will focus on the GR as a key player in the precipitation, development and resolution of depression. We will discuss potential explanations for the apparent controversy between glucocorticoid resistance and the detrimental effects of excessive glucocorticoid signalling. We will review some of the evidence for modulation of the GR by antidepressants and we will provide further insight into how antidepressants may regulate the GR to overcome depressive symptoms.
Keywords: Major depressive disorder, Stress, Corticosteroid, Neurogenesis, Neural stem cells, Endocrine system
1. Introduction
According to the World Health Organization, major depression will be the second leading cause of disability by the year 2020 (Blazer, 2000). Diagnosis of depression is mainly based on symptomatic criteria, such as depressed mood, fatigue, low self-esteem and recurrent thoughts of death and suicide, and the heterogeneity of the disease suggests that multiple different biological mechanisms may underlie its aetiology (Nestler et al., 2002; Duman, 2002). Elucidating the neurobiological basis for depression has therefore become one of the most challenging tasks for medical research.
Studies on the molecular basis of depression has so far mainly focused on imbalances of neurotransmitter systems in the brain, especially depletion of the monoamines serotonin, norepinephrine and dopamine (“monoamine hypothesis”). However, depression is precipitated by long-term, chronic exposure to stress, and antidepressant treatment needs to be administered chronically in order to elicit a therapeutic response in depressed patients. These long-term effects of both stress and antidepressants suggest that rather adaptive mechanisms may be involved in the pathogenesis of depression, which cannot be explained by imbalances of fast acting neurotransmitters alone.
Brain regions, such as the hippocampus, undergo structural changes in depressed patients (Sheline et al., 1996) and it has been hypothesized that a loss of hippocampal volume may explain the long lasting mood and memory disturbances in depression (Sahay et al., 2007). However, the cellular and molecular basis for these structural changes is still unclear. Although neuronal cell death, reduced neurogenesis and alterations in neurotrophic proteins, such as brain-derived neurotrophic factor (BDNF), are hypothesized to contribute to hippocampal atrophy and depression (Duman, 2004; Schmidt and Duman, 2007), no causal relationship between hippocampal volume loss, neurogenesis and depressive symptoms has yet been established in patients, and we will discuss the validity of such hypotheses in this review.
A growing body of evidence shows that depressed patients consistently exhibit hyperactivity of the hypothalamus–pituitary–adrenal (HPA) axis, which results in increased levels of the glucocorticoid hormone cortisol in these patients (Pariante, 2009). For example, research from our laboratory has demonstrated that inpatients with chronic, treatment-resistant depression have cortisol outputs throughout the day which are double those of healthy controls (Juruena et al., 2006). Cortisol is known to regulate neuronal survival, neuronal excitability, neurogenesis and memory acquisition, and high levels of cortisol may thus contribute to the manifestation of depressive symptoms by impairing these brain functions. On a molecular level, cortisol exerts its effects in part by activating the glucocorticoid receptor (GR). The GR has been shown to profoundly regulate the expression of neurotrophic factors such as BDNF, to induce neuronal cell death, and to alter adult hippocampal neurogenesis, thus it is conceivable that also abnormalities in GR function, rather than simply in cortisol levels, contribute to the structural changes in the depressed brain (Sousa et al., 2008). Indeed, impaired GR function has been suggested to be causal for HPA axis hyperactivity in depression, as glucocorticoids usually regulate the HPA axis through negative feedback inhibition and thereby reduce the production of glucocorticoids themselves. This effect is thought to be mediated in part by the GR. Therefore, hyperactivity of the HPA axis has been explained by an impaired feedback inhibition of glucocorticoids, possibly due to an impaired or dysfunctional GR (so-called “glucocorticoid resistance”). It thus seems that two opposing mechanisms may operate: on the one hand, depression is characterized by detrimental effects of excessive glucocorticoid signalling, which depend on a functional GR, whereas, on the other hand, GR function may be impaired in depression and thereby causing the high glucocorticoid levels. We will elaborate these two models below and we will discuss how both of them may contribute to depressive symptoms. To do so, we will recapitulate some of the existing data on the role of the GR in major depression and how GR function can be modulated by antidepressants and glucocorticoids. We will conclude by hypothesizing a partial impairment of GR function, which may contribute to depression and represent a future target for antidepressant treatment.
2. Biological correlates of depression: evidence for glucocorticoid hormones
It is a common finding that around 50% of depressed patients (80% if severely depressed) show hyperactivity of the HPA axis (Young et al., 1991; Holsboer, 2000; Pariante and Miller, 2001; Pariante, 2003). The HPA axis is a major part of the neuroendocrine system, which regulates the body’s response to external stressors, e.g., by providing energy and by focusing attention. The HPA axis is governed by the hippocampus, which controls the release of corticotrophin releasing hormone (CRH) and arginine-vasopressin (AVP) from the paraventricular nucleus (PVN) of the hypothalamus (Antoni, 1993). CRH then induces the synthesis of adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland (Holsboer et al., 1987). ACTH in turn stimulates the production of glucocorticoids (cortisol in humans and corticosterone in rodents) in the adrenal cortex and their release into the blood stream (Fig. 1). Glucocorticoids have multiple functions in almost every tissue of the human body, such as regulation of energy metabolism (through increased gluconeogenesis, lipolysis and protein degradation), regulation of immune functions, sexuality and mood. Albeit produced in the periphery, glucocorticoid hormones can act back on the hippocampus, the PVN and the anterior pituitary, to exert GR-mediated negative feedback inhibition on the HPA axis and to inhibit the synthesis and secretion of CRH and ACTH. Ultimately, this regulatory feedback loop maintains low glucocorticoid levels under normal physiological conditions (de Kloet et al., 2007) (Fig. 1). However, in some depressed patients this feedback inhibition is impaired, resulting in constant HPA axis hyperactivity, increased pituitary and adrenal gland volume, and chronic high levels of glucocorticoids in saliva, cerebrospinal fluid, blood plasma and urine (Nemeroff et al., 1992; Pariante, 2009). Impaired negative feedback inhibition of glucocorticoids in depression can be examined indirectly by the dexamethasone suppression test (DST): oral administration of the synthetic glucocorticoid dexamethasone, which specifically binds to the GR, activates HPA axis feedback inhibition in healthy subjects and thereby reduces cortisol secretion. In contrast, cortisol secretion cannot be inhibited by dexamethasone administration to depressed patients, supporting the notion that the GR-mediated negative feedback on the HPA axis is impaired in this condition (“glucocorticoid resistance”). For example, we have recently shown that healthy controls display 85% suppression of cortisol output throughout the day following a small (0.5 mg) dose of dexamethasone, while depressed patients only show approximately 45% suppression (Juruena et al., 2006).
Figure 1.
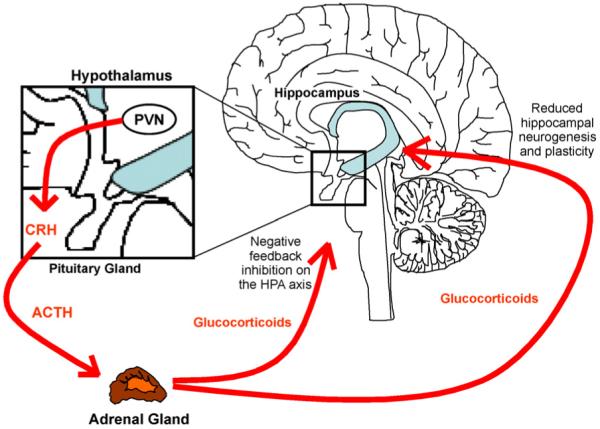
HPA axis.
The correlation between HPA axis hyperactivity, high glucocorticoid levels and depression, has evoked the question whether the high concentrations of glucocorticoids are causal for the development of depression or a mere epiphenomenon of the disease. Interestingly, chronic glucocorticoid treatment induces depression and anxiety-like behaviour in rats (Ardayfio and Kim, 2006; Murray et al., 2008; David et al., 2009), and it has been reported that models of chronic severe stress and depression, as well as direct glucocorticoid administration, induce neural cell death, atrophy of neuronal processes (McEwen and Seeman, 1999; Sapolsky, 2000, 2002) and a reduction of adult hippocampal neurogenesis in rodents (David et al., 2009). For example, stressed rat pups exhibit increased glucocorticoid levels which correlate with a decrease in hippocampal neurogenesis (Tanapat et al., 1998). In turn, removal of the adrenal glands in rats lowers glucocorticoid levels and increases hippocampal neurogenesis, an effect which can be reversed by exogenous glucocorticoid administration (Gould et al., 1992). Therefore, these increased glucocorticoid levels may be causing a reduction in neurogenesis. This is in line with in vitro studies which show that dexamethasone reduces cell proliferation of rodent neural stem cells, supporting a role specifically for the GR in the effects of glucocorticoids on neurogenesis (Kim et al., 2004). Interestingly, mice with a 50% reduction in GR protein in the brain exhibit increased glucocorticoid levels, possibly due to impaired GR-mediated feedback inhibition on the HPA axis, and they also show reduced neurogenesis compared with their control littermates (Kronenberg et al., 2009). It is noteworthy that these data could also be interpreted as showing that reduced GR signalling (again, “GR resistance”) can also impair neurogenesis. Data on neurogenesis in human post-mortem brain tissue is however still controversial, as some studies find no change in neurogenesis in depressed patients (Reif et al., 2006), whereas others find a decrease (Boldrini et al., 2009). Furthermore, chronic treatment with glucocorticoids reduces hippocampal volume in animals (Sapolsky, 1985, 2001) which is in line with the reduced hippocampal gray matter found in depressed patients (Sheline et al., 1996; Videbech and Ravnkilde, 2004; Geuze et al., 2005); however, considering the small number of neurons actually added to the brain by neurogenesis, a reduction in cell birth seems unlikely to account for the macroscopic changes that we see in the depressed brain, such as hippocampal volume loss.
In summary, elevated glucocorticoid levels result in decreased hippocampal neurogenesis (although so may do impaired GR signalling), and this in turn correlates with the induction of depressive symptoms in preclinical studies. However, although degeneration or functional impairment of the hippocampus as a brain region critically involved in mood and memory processing may contribute to the debilitating pathophysiology of depression, no causal relationship between neuronal atrophy, reduced neurogenesis and depression has yet consistently been demonstrated in clinical populations.
But how can we explain the two seemingly opposing concepts that the detrimental effects of excessive glucocorticoid levels on the hippocampus seem to require a functional GR, whereas these same excessive glucocorticoid levels in depression may result from impaired negative feedback inhibition on the HPA axis, caused by a dysfunctional GR? In the following paragraphs, we want to look closer into the molecular mechanisms of GR signalling and how they may help to explain such conflicting findings.
3. The glucocorticoid receptor
Glucocorticoids are steroid hormones which diffuse freely into the cytoplasm of target cells where they bind to two different steroid receptors: the type I or mineralocorticoid receptor (MR) and the type II or glucocorticoid receptor (GR). The MR is expressed mainly in renal tissue, heart and intestine, but also in the limbic brain regions, including the hippocampus (Funder, 1992), where it is involved in blood pressure maintenance and regulation of circadian rhythm (Roberts and Keith, 1994; Odermatt et al., 2008). The MR has a high affinity for glucocorticoids and has thus been suggested to be almost completely occupied during normal physiological conditions with basal endogenous glucocorticoid levels. However, fluctuations in MR expression during the circadian cycle and in response to altered glucocorticoid levels indicate that MR signalling may in fact be a dynamic process with possibly significant changes in MR occupancy during the day (Kalman and Spencer, 2002). The GR is expressed in all tissues of the body and has low affinity for endogenous glucocorticoids. This receptor is therefore only modestly occupied during normal physiological conditions and needs higher glucocorticoid concentrations to be fully activated. For this reason, the GR is considered to be important in depression, in which glucocorticoid concentrations rise to particularly high levels (De Kloet et al., 1998). Understanding GR function has thus become an important line of research on the molecular mechanisms that underlie depression, and we therefore want to focus on the GR in the following sections. However, it is important to bear in mind that the MR may indeed be responsive also to high levels of endogenous glucocorticoids, and it may therefore add to the biological effects of glucocorticoid hormones in depression. Importantly, both MR and GR contribute to negative feedback inhibition of the HPA axis, and thus imbalances in MR/GR expression could help to explain some of the (neuro-) biological disturbances in depression (Juruena et al., 2006).
3.1. The glucocorticoid receptor: functional mechanisms
The mode of action of the GR is very complex: in the absence of glucocorticoids, the GR is packaged into multiprotein-complexes consisting of heat-shock proteins (e.g., Hsp90, Hsp70, Hsp23) and immunophilins (e.g., FKBP5, Cyp44, PP5), which bind to the receptor and keep it sequestered and inactive in the cytoplasm (Pratt and Toft, 1997). Glucocorticoid binding induces a conformational change of the receptor and results in its dissociation from the protein complex, dimerization and translocation into the nucleus (Bledsoe et al., 2002). Although the inactive receptor usually resides in the cytoplasm, constant GR shuttling between the nucleus and the cytoplasm has been reported also in the absence of glucocorticoids (Savory et al., 1999; De Bosscher and Haegeman, 2009). In certain cell types, the GR is even constitutively expressed only in the nucleus, where it is kept in its inactive state by heat-shock proteins (Sanchez et al., 1990). Once activated, the GR homodimer binds to highly conserved glucocorticoid response elements (GREs) on the DNA to activate gene transcription (so-called transactivation). The GR can also translocate as a monomer which results in its binding to other transcription factors such as nuclear factor kappa B (NFkB) (McKay and Cidlowski, 1998), AP-1 (Jonat et al., 1990) or cyclic AMP response element binding protein (CREB) (Focking et al., 2003). Binding to these transcription factors generally results in repression of gene transcription (so-called transrepression). Typical target genes of GR-mediated transrepression include inflammatory cytokines such as interleukin-1β (IL-1β), IL-2, IL-6 and IL-12, interferon-γ (IFN-γ), tumor necrosis factor α (TNFα), cyclooxygenase-2 (COX-2) and inducible NO-synthase (iNOS) (De Bosscher and Haegeman, 2009), and transrepression of these genes accounts for the well known immunosuppressive actions of glucocorticoid hormones. Some studies suggest that repression of gene transcription can also be caused by GR-dimer binding to negative GREs (nGREs), which have been found in the promoter region of CRH (Zhou and Cidlowski, 2005) where they may be of particular relevance for glucocorticoid-mediated negative feedback inhibition on the HPA axis.
To further complicate the already intricate mode of action, GR-dependent gene transcription from the same promoter region can be altered depending on which coregulators are recruited to the promoter-bound receptor. Binding of the GR to the DNA induces a conformational change of the receptor which promotes the recruitment of co-factors such as CBP/p300, P/CAF and p160/SRC to the GR-DNA complex (Duma et al., 2006). These co-factors in turn modulate the interaction of the DNA bound receptor with the basal transcription machinery (Glass and Rosenfeld, 2000). GR activation, nuclear translocation and gene transcription are mainly dependent on the conformation of the receptor and its state of phosphorylation. Indeed, GR needs to be phosphorylated at one of its multiple serine residues in order for the glucocorticoid to bind, and glucocorticoid binding in return induces phosphorylation of the GR (Ismaili and Garabedian, 2004; Rogatsky et al., 1998). Some serine residues, however, are independent of glucocorticoid binding and can be phosphorylated by mitogen protein kinases (MAPK), cyclin-dependent kinase (CDK) (Krstic et al., 1997), glycogen synthase kinase-3 (GSK3) and c-Jun N-terminal kinases (JNK) (Rogatsky et al., 1998). Furthermore, GR phospho-isoforms have been shown to selectively bind to promoters of some GR target genes but not to others, to exhibit different transactivation potential, different subcellular localization of the GR, and differences in protein stability (Blind and Garabedian, 2008; Chen et al., 2008; Davies et al., 2008).
3.2. The glucocorticoid receptor: many of one
To add further to the complexity of the GR system, there is just one gene encoding for the GR, but several different mRNA splice variants are known. Whereas the GRα splice variant is the ligand-dependent nuclear transcription factor, which is abundant in almost every tissue and cell type, the GRβ splice variant does not have transcriptional activity, is expressed in much lower concentrations and inhibits GRα, possibly by competing for the GRE-sequence (Oakley et al., 1996; Fruchter et al., 2005). The GRβ isoform has been found to be involved in several glucocorticoid resistance diseases such as inflammatory bowel disease, asthma and arthritis (Bamberger et al., 1995; Christodoulopoulos et al., 2000). The splice variant GR-γ accounts for 4–8% of total GR, however with only 50% of GRα transactivation activity (Duma et al., 2006; Beger et al., 2003), and splice variants GR-P and GR-A have been described in cells from glucocorticoid-resistant myeloma patients (Krett et al., 1995; Moalli et al., 1993).
Alternative mRNA splicing could represent one of several mechanisms by which responsiveness to glucocorticoids is altered. Now we will discuss such possible mechanisms for the dynamic glucocorticoid responsiveness in more detail.
4. Glucocorticoid responsiveness: possible explanations
As described above, alternative splicing of GR mRNA may result in tissue- and cell-type specific differences in transactivation and transrepression potential. Indeed, it has been shown that the GRα splice variant is decreased in the limbic brain and in peripheral blood mononuclear cells (PBMCs) of depressed patients without changes in GRβ (Alt et al., 2009; Matsubara et al., 2006). Such changes in GRα/GRβ ratio are likely to alter responsiveness to glucocorticoids and may thus contribute to glucocorticoid resistance in depressed patients. Differences in glucocorticoid responsiveness may also be achieved by alterations in nuclear translocation or transactivation ability of the GR. These alterations may be modulated by phosphorylation of the receptor, and differential phosphorylation of the GR by CDK5 and JNK in response to stress has indeed been suggested (Adzic et al., 2009). In addition, cellular membrane pumps such as the multidrug resistant p-glycoprotein (MDR PGP or ABCB1) expel glucocorticoids from the intracellular space and thereby regulate their cellular availability (Karssen et al., 2001; Pariante and Lightman, 2008; Mason et al., 2008). Alterations in MDR PGP activity may thus lead to varying concentrations of available glucocorticoid hormones in different cells and tissues and thereby contribute to glucocorticoid resistance (Carvalho and Pariante, 2008). Moreover, PGP expression in the dentate gyrus of the hippocampus and in neural stem cells has also been described (Karssen et al., 2004; Lin et al., 2006), and regulation of glucocorticoids by PGP specifically in these cells may potentially modulate their effects on hippocampal neurogenesis in depression.
An interesting recent discovery is the involvement of microRNAs (miRNAs) in the regulation of GR expression. miRNAs are single stranded RNA molecules which regulate gene expression by binding to complimentary mRNA molecules and causing their breakdown before the initiation of translation. Rats which are hypersensitive to the effects of stress, have been shown to exhibit reduced GR protein expression in the PVN after repeated restraint stress, which is caused by increased levels of miR-18 (Uchida et al., 2008). miR-18 also decreases GR protein expression and GR-mediated gene transcription in vitro (Vreugdenhil et al., 2009). These data suggest that miRNAs may be yet another potential mechanism by which GR expression, and thus glucocorticoid responsiveness, is altered in different tissues or upon different environmental stimuli. Interestingly, recent studies have shown that mood stabilizers induce changes in hippocampal miRNA levels (Zhou et al., 2009), however, it is yet unknown whether antidepressants can have similar effects.
Finally, epigenetic changes also contribute to GR regulation. For example, maternal licking and grooming in mice changes GR expression by modulating the methylation state of the GR promoter in their offspring, thereby possibly altering HPA axis responsiveness (Weaver et al., 2004). Furthermore, human post-mortem studies have shown that suicide victims with a history of childhood abuse show increased methylation of the GR promoter and decreased GR mRNA expression (McGowan et al., 2009), suggesting that epigenetic changes may influence GR expression and HPA axis responsiveness already early in life, and thereby possibly contribute to the development of depressive symptoms.
Taken together, all these findings display the complexity of GR regulation at several different stages of its function. We will elaborate below how this may be of importance in explaining the role of the GR in depression and antidepressant treatment response.
5. The GR in depression
Considering the critical role of the GR in HPA axis hyperactivity and in mediating the effects of glucocorticoids on brain plasticity and mood, it is not surprising that the GR has been found to be a common mechanism for stress dependent changes in brain function and a potential target of antidepressant drugs. Changes in GR expression, nuclear translocation, co-factor binding and GR-mediated gene transcription may play a fundamental role in altered HPA axis responsiveness to glucocorticoids in the HPA axis tissues, which may contribute to HPA axis hyperactivity. Indeed, studies on immune cells from peripheral blood have shown that the capacity of glucocorticoids to inhibit proliferation of PBMCs in response to polyclonal mitogens is impaired in depressed patients (Lowy et al., 1984; Wodarz et al., 1991; Pariante and Miller, 2001). These findings correlate with the findings in the DST discussed above, in that patients who are non-suppressors of the DST, and thus glucocorticoid resistant, also show reduced dexamethasone-induced inhibition of the lymphocyte proliferative response, and thus impaired GR function (Wodarz et al., 1991). Furthermore, mice with a GR deficiency only in the pituitary show impaired glucocorticoid-mediated negative feedback inhibition and HPA axis hyperactivity without changes in GR expression in the central nervous system (Schmidt et al., 2009). This supports the notion that impaired GR function, specifically in the periphery, may account for glucocorticoid resistance and explain impaired feedback inhibition with resulting HPA axis hyperactivity. However, mice with a deletion of the GR specifically in the hippocampus, but not in peripheral tissues such as the pituitary, also display impaired feedback inhibition, HPA axis hyperactivity and depressive-like behaviour (Boyle et al., 2005). These findings indicate that impaired GR function in both the periphery and also in the central nervous system are relevant for HPA axis hyperactivity and behavioural abnormalities in depression.
It has been proposed that blocking the GR with an antagonist may reduce the effects of high glucocorticoid levels on the brain, and thus represent a potential treatment strategy for depression. Some clinical trials have shown that the GR antagonist RU486 (mifepristone) can overcome neurocognitive impairments and has antidepressant potential in bipolar depressed patients (Young et al., 2004). Other studies however, have only found improvements in psychotic but not in depressive symptoms in patients with psychotic major depression (DeBattista et al., 2006; Flores et al., 2006). Interestingly, chronically stressed rats, as well as rats treated with glucocorticoids, show a decrease in adult hippocampal neurogenesis which can be reversed by treatment with RU486 (Mayer et al., 2006; Oomen et al., 2007). These findings suggest that blocking the GR can counteract the effects of elevated glucocorticoids on neurogenesis, which may possibly underlie some of the neurocognitive improvements upon RU486 treatment as observed in the study mentioned above. However, it has also been reported that chronic injection of RU486 into the dentate gyrus of rats induces, rather than ameliorates, learned helplessness, a behavioural measurement used to model depressive symptoms in animals (Papolos et al., 1993). This finding shows again that not only increased but also impaired GR signalling can induce depressive symptoms. Interestingly, not GR antagonists but GR and MR agonists such as dexamethasone, prednisolone and cortisol, have antidepressant effects in clinical populations, possibly by restoring negative feedback on the HPA axis (Dinan et al., 1997; Bouwer et al., 2000; DeBattista et al., 2000). However, as mentioned above, GR agonists also induce depressive behaviour in preclinical studies in which decreased neurogenesis is concomitantly observed (David et al., 2009). To complicate the matter further, RU486 administration in humans induces elevation of cortisol levels, possibly by blocking GR-mediated negative feedback (Flores et al., 2006), and therefore it is difficult to dissect, once again, whether the clinical effects of RU486 are mediated by blocking or rather by facilitating GR signalling.
So how can we explain that GR activation can, on the one hand, induce depressive symptoms and neurocognitive impairment, and on the other hand, exert antidepressant effects? A possible explanation could be that both GR and MR agonists, as well as GR antagonists, ultimately restore HPA axis negative feedback inhibition and thereby normalize HPA axis hyperactivity. However, it is important to segregate depressive symptoms and mood disturbances from neurocognitive impairment, as different biological mechanisms may be involved, as also shown by the differential effects of RU486 mentioned above. As mood and cognition are two different aspects of depression, their assessment may require distinct psychological tools in clinical studies, or behavioural tests in preclinical experiments, to explain contradictory findings in the literature.
6. Neurobiological mechanisms of antidepressants
Antidepressants not only alleviate depressive symptoms and normalize HPA axis hyperactivity, they also protect from neuronal cell death and from reduction in adult hippocampal neurogenesis. Chronic antidepressant treatment, for example, attenuates dexamethasone-induced neuronal cell death and sublethal neuronal damage in the hippocampus and striatum of rats (Haynes et al., 2004). These neuroprotective effects have been suggested to be mediated, at least in part, by elevated BDNF levels upon antidepressant treatment (Shirayama et al., 2002; Sen et al., 2008).
Data on the effects of antidepressants on neurogenesis are still controversial: whereas some studies show that antidepressants and mood stabilizers increase hippocampal cell proliferation in healthy, non-depressed animals (Malberg et al., 2000; Chen et al., 2000), another study found that oral fluoxetine treatment only increases cell proliferation in depressed, but not in healthy mice (David et al., 2009). Furthermore, co-treatment with fluoxetine counteracts the effects of glucocorticoids to induce depressive behaviour and to reduce adult hippocampal neurogenesis in rodents (David et al., 2009).
Chronic antidepressant treatment has also been shown to counteract hippocampal volume loss (Colla et al., 2007) and to increase cell proliferation and the number of neural precursors in depressed patients but not in healthy controls (Boldrini et al., 2009). As mentioned above, considering that the number of new-born neurons in the hippocampus is only minuscule, it seems improbable to assume that this increase in neurogenesis may explain the gain of hippocampal volume upon antidepressant treatment. It seems more likely that unrelated neurobiological mechanisms may be operating, and that the changes in neurogenesis represent functional adaptations, which aim to counteract depressive symptoms, rather than being a mechanism underlying the macroscopic changes in hippocampal volume upon antidepressant treatment. Interestingly, the effect of antidepressants on neurogenesis can no longer be observed in rats whose glucocorticoid rhythms are flattened (Huang and Herbert, 2006), suggesting that circadian fluctuations in glucocorticoid signalling, most likely via a functional GR, are required for antidepressants to elicit their effects on neurogenesis. Finally, it is important to note that the effects of antidepressants on neurogenesis are independent of the chemical class of antidepressant, indicating that different types of antidepressant drugs may act on a common final target to elicit this effect (Duman, 2004).
7. The GR: a common target of antidepressants?
As outlined above, the GR plays a crucial role in the effects of stress, depression and glucocorticoid hormones on neurogenesis and HPA axis hyperactivity. Targeting the GR at key points of its intricate mode of action may thus conquer the disturbances which underlie depression both in the periphery and in the central nervous system. Indeed, antidepressants of the class of monoamine re-uptake inhibitors regulate GR mRNA expression in primary neuronal cell cultures. More specifically, in neurons of the hypothalamus, the amygdala and the cerebral cortex, antidepressants increase GR mRNA levels after 48 h of treatment, independent of their ability to block monoamine re-uptake (Pepin et al., 1989). These findings are supported by several other studies, which showed that treatment with tricyclic antidepressants increases GR binding affinity and GR mRNA expression in rat hypothalamic and hippocampal neurons, suggesting that antidepressants enhance glucocorticoid sensitivity, specifically in the brain, and thereby may restore GR-mediated feedback inhibition on the HPA axis (Peiffer et al., 1991; Okugawa et al., 1999). However, both increased and decreased GR mRNA expression by antidepressants has been shown in the periphery, dependent on the duration of treatment. Incubation of PBMCs with the antidepressant mirtazapine increased GR mRNA levels in human leukocytes and monocytic cells after 2.5 h and reduced mRNA levels after 4 h, 24 h and 48 h of incubation (Vedder et al., 1999; Heiske et al., 2003). Quantifications of GR protein upon antidepressant treatment is also contradictory, as some studies found an increase in GR protein levels (Pepin et al., 1992; Hery et al., 2000; Lai et al., 2003), whereas others found a decrease (Pariante et al., 1997, 2003a,b; Hery et al., 2000). We will discuss below how such differences in GR expression upon antidepressant treatment may be explained.
Furthermore, studies by us and others have shown that antidepressants induce glucocorticoid-dependent and even glucocorticoid-independent nuclear translocation of the GR, and that they also facilitate glucocorticoid-dependent GR-mediated gene transcription (Pariante et al., 1997, 2003a,b; Herr et al., 2003; Funato et al., 2006). It is important to mention, however, that some studies have reported that antidepressants inhibit GR-mediated gene transcription (Budziszewska et al., 2000; Augustyn et al., 2005). Indeed, in our own work we have shown that antidepressants both increase and decrease GR-mediated gene transcription dependent on the cell type, the function of PGP, and the incubation time (Pariante et al., 1997, 2003a,b; Pariante and Miller, 2001; Carvalho et al., 2008). These differences may underlie different second-messenger signalling mechanisms of antidepressants in different cell types and in different experimental conditions, which brings up the central question of how antidepressants regulate the GR.
8. How do antidepressants regulate the GR?
The alterations in GR function described above may likely represent a point of convergence for several different chemical classes of antidepressants. So far, no direct interaction of the antidepressant compound itself with the GR or any of its interacting proteins has been described, and such a direct interaction may seem unlikely. The pivotal question, however, is how antidepressants actually induce changes in GR function which would then have an effect on the biology of the cell, on changes in neuroplasticity, and ultimately on brain function and mood. Several mechanisms may be involved, including phosphorylation by protein kinases, membrane transporters like the MDR PGP, and miRNAs. In the following section, we will briefly describe each of them.
There is considerable evidence for a cyclic AMP (cAMP)/protein kinase A (PKA) dependent mechanism of GR regulation. In 1992, Rangarajan and colleagues reported that PKA induces GR-transactivation in cells that lack endogenous cAMP response element binding protein (CREB) (Rangarajan et al., 1992). Research by Miller and colleagues showed that the phosphodiesterase type-4 inhibitor rolipram, which prevents the breakdown of cAMP to AMP and therefore enhances cAMP-dependent PKA activation, increases GR-mediated gene transcription in vitro, and also potentiates the effects of antidepressants on increasing GR function (Miller et al., 2002). Accordingly, β2-adrenergic receptor agonists have been shown to cause GR nuclear translocation via a cAMP/PKA-dependent pathway (Eickelberg et al., 1999). These findings are intriguing in view of the fact that antidepressants are suggested to enhance neurogenesis via a cAMP/PKA-dependent effect (Rasenick et al., 1996; Chen et al., 2001). The question remains, however, whether the effects of cAMP and PKA are mediated by direct phosphorylation of the GR protein, or via indirect mechanisms, which may involve multiple regulators of the receptor.
Antidepressants influence several other intracellular protein kinases such as protein kinase C (PKC) and calcium/calmodulin-dependent kinase (CaMK) (Silver et al., 1986; Nalepa and Vetulani, 1991), which seem to be specifically implicated in the antidepressant-induced decrease in GR-mediated gene transcription (Budziszewska et al., 2000; Augustyn et al., 2005). However, an overall reduced GR function may reflect a decrease in GR expression without an actual functional impairment of the GR protein. In particular, reduced GR expression may be a consequence of increased GR translocation, increased GR-mediated gene transcription and subsequent GR downregulation, and thus in fact reflect an increase, rather than a decrease in GR function. Also, activation of different second-messenger pathways may result in differential phosphorylation of the GR, which in turn may cause changes in GR-dependent gene transcription but not completely abolish it. Reporter gene assays as an indicator for GR function may thus deliver conflicting results if only particular genes are being looked at, which may be regulated by one certain GR phosphoisoform, but not by another phospho-isoform.
Research from our laboratory has also shown that antidepressants inhibit membrane transporters, such as the MDR PGP, which, as described above, expels glucocorticoids from the cytoplasm. Again, this effect appears to be independent of the chemical class of the antidepressant. Using both mouse fibroblasts and rat cortical neurons, we have shown that antidepressants increase intracellular glucocorticoid levels by blocking MDR PGP (Pariante et al., 2003a,b) and thus indirectly enhance GR function by increasing the intracellular concentration of glucocorticoids. Specifically, this may explain the time dependent differences in GR expression upon antidepressant treatment described above: inhibiting MDR PGP increases the intracellular concentrations of glucocorticoids and subsequently induces GR nuclear translocation and an initial downregulation of GR expression during the first few hours of treatment. This is followed by GR upregulation after 1 or 2 days, possibly caused by MDR PGP downregulation and/or as a compensatory mechanism following the initial GR downregulation (for a complete review, see Carvalho and Pariante, 2008). Moreover, using an in vivo mouse model, we have shown that the antidepressant desipramine increases GR mRNA expression in the hippocampus, whereas GR expression is downregulated in mice with a MDR PGP knockout, which therefore have more intracellular glucocorticoids and increased GR activation. Interestingly, the opposite effect was observed in the amygdala, where desipramine caused GR downregulation in control mice and upregulation in MDR PGP knockout mice. Furthermore, in MDR PGP knockout mice, desipramine showed a reduced ability to decrease plasma glucocorticoid levels (Yau et al., 2007), which supports the notion that antidepressants regulate HPA axis activity through an effect which may include glucocorticoid transporters such as the MDR PGP.
9. Conclusion
Considering the literature discussed in this review, there is substantial evidence for a crucial involvement of the GR in the development of depression and its related neurobiological disturbances. Furthermore, the effects of antidepressants on several different mechanisms of GR function could be of considerable importance to therapeutic efficacy. These effects of antidepressants on the GR may differ in various cell types and tissues, possibly due to the expression of diverse mRNA splice variants and miRNAs, altered GRα/GRβ ratio, or differential expression of membrane transporters such as the MDR PGP. Also, differential expression of GR-chaperones, transcriptional co-regulators, or differences in phosphorylation status of the receptor, may all contribute significantly to the effects of antidepressants on GR-dependent changes in glucocorticoid resistance, hippocampal volume and neurogenesis.
Glucocorticoid resistance (i.e., impaired GR function) and glucocorticoid-dependent changes in hippocampal plasticity (which requires functional GR signalling) seem to be contradictory at first sight. However, evidence from the literature strongly suggests that glucocorticoid resistance and the resulting HPA axis hyperactivity may represent a potential cause for depression, whereas hippocampal atrophy and decreased hippocampal neurogenesis may be a result of the illness, possibly contributing to neurocognitive impairment. But how is it possible that HPA axis hyperactivity and depression is caused by supposedly impaired GR function, whereas the detrimental effects of glucocorticoids on the hippocampus seem to require the presence of an active GR? Some studies suggest that GR function is different in different tissues. Therefore, impaired GR function may occur in the periphery, specifically in the pituitary, where it contributes to impaired negative feedback inhibition and HPA axis hyperactivity in mice (Schmidt et al., 2009), while allowing for the detrimental effects of high glucocorticoid levels on the hippocampus, which still contains a functional GR. However, the evidence is conflicting, as impaired GR function in the hippocampus also induces HPA axis hyperactivity (Boyle et al., 2004) and reduces neurogenesis (Papolos et al., 1993; Kronenberg et al., 2009). More research will be needed to investigate the differential contribution of specific cell types and tissues to both HPA axis hyperactivity and the effects of glucocorticoids on the brain, especially the hippocampus.
Finally, considering the receptor’s intricate mode of action and its modulation in different tissues, the term “GR function” summarizes a vast number of receptor mechanisms, ranging from nuclear translocation as either monomer or dimer, to transactivation, transrepression, and phosphorylation-dependent differences in co-factor recruitment and gene transcription. It is thus important to delineate these different mechanisms individually in order to explain the “function” of the receptor. Importantly, altered post-translational modifications of the GR in depression may explain partial impairment of GR function, rather than a global dysfunction of the receptor. For example, differential phosphorylation of the GR may result in changes in co-factor recruitment and the subsequent expression of a particular subset of genes. Changes in these post-translational modifications, for example in depression or upon antidepressant treatment, may then result in different GR phospho-isoforms, altered co-factor binding, and hence changes in GR-dependent gene transcription. Such changes may explain how a partial impairment of the GR, or rather an alteration in GR-dependent gene transcription, may lead to decreased HPA axis feedback inhibition and cause HPA axis hyperactivity without preventing the detrimental effects of glucocorticoids on brain plasticity.
So is the GR the pivot of depression and of antidepressant treatment? Indeed, the evidence from the literature strongly suggests an important involvement of the GR in the neurobiological correlates that underlie depression, ranging from glucocorticoid resistance to HPA axis hyperactivity and changes in neural plasticity and neurogenesis. Antidepressants have consistently been shown to impact on all these mechanism in preclinical studies, and they have also been shown to modulate GR function, suggesting that the receptor may play a pivotal role in the onset of depression and in the neurobiological disturbances that contribute to depressive symptoms. If these effects of antidepressants are dependent on the GR directly or on mechanisms downstream of the GR, is yet to be elucidated. However, the receptor’s crucial role in the action of antidepressant drugs suggests that targeting the GR more efficiently may provide potential new ways to overcome depressive symptoms and to alleviate the burden of mental illness.
Acknowledgements
Funded by a studentship to C. Anacker from the NIHR “Biomedical Research Centre for Mental Health”, Institute of Psychiatry and South London and Maudsley NHS Foundation Trust, London, UK, and a Clinician Scientist Fellowship to C.M. Pariante from the Medical Research Council, UK.
References
- Adzic M, et al. Acute or chronic stress induce cell compartment-specific phosphorylation of glucocorticoid receptor and alter its transcriptional activity in Wistar rat brain. J. Endocrinol. 2009;202(1):87–97. doi: 10.1677/JOE-08-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt SR, et al. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front. Neuroendocrinol. 1993;14:6–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Ardayfio P, Kim KS. Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav. Neurosci. 2006;120:249–256. doi: 10.1037/0735-7044.120.2.249. [DOI] [PubMed] [Google Scholar]
- Augustyn M, et al. Effects of some new antidepressant drugs on the glucocorticoid receptor-mediated gene transcription in fibroblast cells. Pharmacol. Rep. 2005;57:766–773. [PubMed] [Google Scholar]
- Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J. Clin. Invest. 1995;95:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger C, et al. Expression and structural analysis of glucocorticoid receptor isoform gamma in human leukaemia cells using an isoform-specific real-time polymerase chain reaction approach. Br. J. Haematol. 2003;122:245–252. doi: 10.1046/j.1365-2141.2003.04426.x. [DOI] [PubMed] [Google Scholar]
- Blazer DG., II Controversies in community-based psychiatric epidemiology: let the data speak for themselves. Arch. Gen. Psychiatry. 2000;57:227–228. doi: 10.1001/archpsyc.57.3.227. [DOI] [PubMed] [Google Scholar]
- Bledsoe RK, et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 2002;110:93–105. doi: 10.1016/s0092-8674(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Blind RD, Garabedian MJ. Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. J. Steroid Biochem. Mol. Biol. 2008;109:150–157. doi: 10.1016/j.jsbmb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer C, Claassen J, Dinan TG, Nemeroff CB. Prednisone augmentation in treatment-resistant depression with fatigue and hypocortisolaemia: a case series. Depress. Anxiety. 2000;12:44–50. doi: 10.1002/1520-6394(2000)12:1<44::AID-DA6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Boyle MP, et al. Genetic dissection of stress response pathways in vivo. Endocr. Res. 2004;30(4):859–863. doi: 10.1081/erc-200044120. [DOI] [PubMed] [Google Scholar]
- Boyle MP, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc. Natl. Acad. Sci. U.S.A. 2005;102(2):473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budziszewska B, Jaworska-Feil L, Kajta M, Lason W. Antidepressant drugs inhibit glucocorticoid receptor-mediated gene transcription—a possible mechanism. Br. J. Pharmacol. 2000;130:1385–1393. doi: 10.1038/sj.bjp.0703445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LA, Pariante CM. In vitro modulation of the glucocorticoid receptor by antidepressants. Stress. 2008;11(6):411–424. doi: 10.1080/10253890701850759. [DOI] [PubMed] [Google Scholar]
- Carvalho LA, et al. Clomipramine in vitro reduces glucocorticoid receptor function in healthy subjects but not in patients with major depression. Neuropsychopharmacology. 2008;33(13):3182–3189. doi: 10.1038/npp.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J. Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol. Psychiatry. 2001;49:753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- Chen W, et al. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol. Endocrinol. 2008;22:1754–1766. doi: 10.1210/me.2007-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colla M, et al. Hippocampal volume reduction and HPA-system activity in major depression. J. Psychiatr. Res. 2007;41:553–560. doi: 10.1016/j.jpsychires.2006.06.011. [DOI] [PubMed] [Google Scholar]
- David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L, et al. Cross talk of signaling pathways in the regulation of the glucocorticoid receptor function. Mol. Endocrinol. 2008;22(6):1331–1344. doi: 10.1210/me.2007-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Haegeman G. Minireview: latest perspectives on antiinflammatory actions of glucocorticoids. Mol. Endocrinol. 2009;23:281–291. doi: 10.1210/me.2008-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Derijk RH, Meijer OC. Therapy Insight: is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nat. Clin. Pract. Endocrinol. Metab. 2007;3:168–179. doi: 10.1038/ncpendmet0403. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Posener JA, Kalehzan BM, Schatzberg AF. Acute antidepressant effects of intravenous hydrocortisone and CRH in depressed patients: a double-blind, placebo-controlled study. Am. J. Psychiatry. 2000;157:1334–1337. doi: 10.1176/appi.ajp.157.8.1334. [DOI] [PubMed] [Google Scholar]
- DeBattista C, et al. Mifepristone versus placebo in the treatment of psychosis in patients with psychotic major depression. Biol. Psychiatry. 2006;60:1343–1349. doi: 10.1016/j.biopsych.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Dinan TG, et al. Dexamethasone augmentation in treatment-resistant depression. Acta Psychiatr. Scand. 1997;95:58–61. doi: 10.1111/j.1600-0447.1997.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Duma D, Jewell CM, Cidlowski JA. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J. Steroid Biochem. Mol. Biol. 2006;102:11–21. doi: 10.1016/j.jsbmb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Duman RS. Structural alterations in depression: cellular mechanisms underlying pathology and treatment of mood disorders. CNS Spectr. 2002;7:140–142. 144–147. doi: 10.1017/s1092852900017454. [DOI] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol. Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, et al. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J. Biol. Chem. 1999;274:1005–1010. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- Flores BH, et al. Clinical and biological effects of mifepristone treatment for psychotic depression. Neuropsychopharmacology. 2006;31(3):628–636. doi: 10.1038/sj.npp.1300884. [DOI] [PubMed] [Google Scholar]
- Focking M, Holker I, Trapp T. Chronic glucocorticoid receptor activation impairs CREB transcriptional activity in clonal neurons. Biochem. Biophys. Res. Commun. 2003;304:720–723. doi: 10.1016/s0006-291x(03)00665-x. [DOI] [PubMed] [Google Scholar]
- Fruchter O, et al. The human glucocorticoid receptor (GR) isoform {beta} differentially suppresses GR{alpha}-induced transactivation stimulated by synthetic glucocorticoids. J. Clin. Endocrinol. Metab. 2005;90(6):3505–3509. doi: 10.1210/jc.2004-1646. [DOI] [PubMed] [Google Scholar]
- Funato H, Kobayashi A, Watanabe Y. Differential effects of antidepressants on dexamethasone-induced nuclear translocation and expression of glucocorticoid receptor. Brain Res. 2006;1117:125–134. doi: 10.1016/j.brainres.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Funder JW. Glucocorticoid receptors. J. Steroid Biochem. Mol. Biol. 1992;43:389–394. doi: 10.1016/0960-0760(92)90074-s. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics. 2. Findings in neuropsychiatric disorders. Mol. Psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LE, Barber D, Mitchell IJ. Chronic antidepressant medication attenuates dexamethasone-induced neuronal death and sublethal neuronal damage in the hippocampus and striatum. Brain Res. 2004;1026:157–167. doi: 10.1016/j.brainres.2004.05.117. [DOI] [PubMed] [Google Scholar]
- Heiske A, Jesberg J, Krieg JC, Vedder H. Differential effects of antidepressants on glucocorticoid receptors in human primary blood cells and human monocytic U-937 cells. Neuropsychopharmacology. 2003;28:807–817. doi: 10.1038/sj.npp.1300056. [DOI] [PubMed] [Google Scholar]
- Herr AS, et al. Antidepressants differentially influence the transcriptional activity of the glucocorticoid receptor in vitro. Neuroendocrinology. 2003;78(1):12–22. doi: 10.1159/000071701. [DOI] [PubMed] [Google Scholar]
- Hery M, Semont A, Fache MP, Faudon M, Hery F. The effects of serotonin on glucocorticoid receptor binding in rat raphe nuclei and hippocampal cells in culture. J. Neurochem. 2000;74:406–413. doi: 10.1046/j.1471-4159.2000.0740406.x. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Holsboer F, von Bardeleben U, Buller R, Heuser I, Steiger A. Stimulation response to corticotropin-releasing hormone (CRH) in patients with depression, alcoholism and panic disorder. Horm. Metab. Res. Suppl. 1987;16:80–88. [PubMed] [Google Scholar]
- Huang GJ, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol. Psychiatry. 2006;59:619–624. doi: 10.1016/j.biopsych.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann. N. Y. Acad. Sci. 2004;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
- Jonat C, et al. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Juruena MF, et al. Different responses to dexamethasone and prednisolone in the same depressed patients. Psychopharmacology (Berl.) 2006;189:225–235. doi: 10.1007/s00213-006-0555-4. [DOI] [PubMed] [Google Scholar]
- Kalman BA, Spencer RL. Rapid corticosteroid-dependent regulation of mineralocorticoid receptor protein expression in rat brain. Endocrinology. 2002;143:4184–4195. doi: 10.1210/en.2002-220375. [DOI] [PubMed] [Google Scholar]
- Karssen AM, et al. Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology. 2001;142:2686–2694. doi: 10.1210/endo.142.6.8213. [DOI] [PubMed] [Google Scholar]
- Karssen AM, Meijer O, Pons D, De Kloet ER. Localization of mRNA expression of P-glycoprotein at the blood—brain barrier and in the hippocampus. Ann. N. Y. Acad. Sci. 2004;1032:308–311. doi: 10.1196/annals.1314.048. [DOI] [PubMed] [Google Scholar]
- Kim JB, et al. Dexamethasone inhibits proliferation of adult hippocampal neurogenesis in vivo and in vitro. Brain Res. 2004;1027:1–10. doi: 10.1016/j.brainres.2004.07.093. [DOI] [PubMed] [Google Scholar]
- Krett NL, Pillay S, Moalli PA, Greipp PR, Rosen ST. A variant glucocorticoid receptor messenger RNA is expressed in multiple myeloma patients. Cancer Res. 1995;55:2727–2729. [PubMed] [Google Scholar]
- Kronenberg G, et al. Reduced hippocampal neurogenesis in the GR(+/−) genetic mouse model of depression. Eur. Arch. Psychiatry Clin. Neurosci. 2009 doi: 10.1007/s00406-009-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic MD, et al. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol. Cell Biol. 1997;17(7):3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, et al. Differential regulation of corticosteroid receptors by monoamine neurotransmitters and antidepressant drugs in primary hippocampal culture. Neuroscience. 2003;118:975–984. doi: 10.1016/s0306-4522(03)00038-1. [DOI] [PubMed] [Google Scholar]
- Lin T, Islam O, Heese K. ABC transporters, neural stem cells and neurogenesis—a different perspective. Cell Res. 2006;16:857–871. doi: 10.1038/sj.cr.7310107. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Reder AT, Antel JP, Meltzer HY. Glucocorticoid resistance in depression: the dexamethasone suppression test and lymphocyte sensitivity to dexamethasone. Am. J. Psychiatry. 1984;141:1365–1370. doi: 10.1176/ajp.141.11.1365. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BL, Pariante CM, Thomas SA. A revised role for P-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wild-type and ABCB1A/B-deficient mice. Endocrinology. 2008;149(10):5244–5253. doi: 10.1210/en.2008-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, et al. Reduced Glucocorticoid Receptor alpha Expression in Mood Disorder Patients and First-Degree Relatives. Biol. Psychiatry. 2006;59(8):689–695. doi: 10.1016/j.biopsych.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Mayer JL, et al. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J. Neuroendocrinol. 2006;18:629–631. doi: 10.1111/j.1365-2826.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol. Endocrinol. 1998;12:45–56. doi: 10.1210/mend.12.1.0044. [DOI] [PubMed] [Google Scholar]
- Miller AH, Vogt GJ, Pearce BD. The phosphodiesterase type 4 inhibitor, rolipram, enhances glucocorticoid receptor function. Neuropsychopharmacology. 2002;27:939–948. doi: 10.1016/S0893-133X(02)00381-0. [DOI] [PubMed] [Google Scholar]
- Moalli PA, Pillay S, Krett NL, Rosen ST. Alternatively spliced glucocorticoid receptor messenger RNAs in glucocorticoid-resistant human multiple myeloma cells. Cancer Res. 1993;53:3877–3879. [PubMed] [Google Scholar]
- Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur. J. Pharmacol. 2008;583:115–127. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Nalepa I, Vetulani J. Involvement of protein kinase C in the mechanism of in vitro effects of imipramine on generation of second messengers by noradrenaline in cerebral cortical slices of the rat. Neuroscience. 1991;44:585–590. doi: 10.1016/0306-4522(91)90079-4. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, et al. Adrenal gland enlargement in major depression. A computed tomographic study. Arch. Gen. Psychiatry. 1992;49(5):384–387. doi: 10.1001/archpsyc.1992.01820050048008. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J. Biol. Chem. 1996;271:9550–9559. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- Odermatt A, Gumy C. mineralocorticoid action: why should we consider influences by environmental chemicals? Biochem. Pharmacol. 2008;76:1184–1193. doi: 10.1016/j.bcp.2008.07.019. Glucocorticoid. [DOI] [PubMed] [Google Scholar]
- Okugawa G, et al. Long-term treatment with antidepressants increases glucocorticoid receptor binding and gene expression in cultured rat hippocampal neurones. J. Neuroendocrinol. 1999;11:887–895. doi: 10.1046/j.1365-2826.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur. J. Neurosci. 2007;26:3395–3401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- Papolos DF, et al. Effects of the antiglucocorticoid RU 38486 on the induction of learned helpless behavior in Sprague-Dawley rats. Brain Res. 1993;615(2):304–309. doi: 10.1016/0006-8993(93)90042-l. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Depression, stress and the adrenal axis. J. Neuroendocrinol. 2003;15:811–812. doi: 10.1046/j.1365-2826.2003.01058.x. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Risk factors for development of depression and psychosis. Ann. N. Y. Acad. Sci. 2009;1179:144–152. doi: 10.1111/j.1749-6632.2009.04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Owens MJ, Miller AH. Steroid-independent translocation of the glucocorticoid receptor by the antidepressant desipramine. Mol. Pharmacol. 1997;52:571–581. doi: 10.1124/mol.52.4.571. [DOI] [PubMed] [Google Scholar]
- Pariante CM, et al. The antidepressant clomipramine regulates cortisol intracellular concentrations and glucocorticoid receptor expression in fibroblasts and rat primary neurones. Neuropsychopharmacology. 2003a;28:1553–1561. doi: 10.1038/sj.npp.1300195. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Kim RB, Makoff A, Kerwin RW. Antidepressant fluoxetine enhances glucocorticoid receptor function in vitro by modulating membrane steroid transporters. Br. J. Pharmacol. 2003b;139:1111–1118. doi: 10.1038/sj.bjp.0705357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer A, Veilleux S, Barden N. Antidepressant and other centrally acting drugs regulate glucocorticoid receptor messenger RNA levels in rat brain. Psychoneuroendocrinology. 1991;16:505–515. doi: 10.1016/0306-4530(91)90034-q. [DOI] [PubMed] [Google Scholar]
- Pepin MC, Beaulieu S, Barden N. Antidepressants regulate glucocorticoid receptor messenger RNA concentrations in primary neuronal cultures. Brain Res. Mol. Brain Res. 1989;6:77–83. doi: 10.1016/0169-328x(89)90031-4. [DOI] [PubMed] [Google Scholar]
- Pepin MC, Govindan MV, Barden N. Increased glucocorticoid receptor gene promoter activity after antidepressant treatment. Mol. Pharmacol. 1992;41:1016–1022. [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A. Mol. Endocrinol. 1992;6:1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- Rasenick MM, Chaney KA, Chen J. G protein-mediated signal transduction as a target of antidepressant and antibipolar drug action: evidence from model systems. J. Clin. Psychiatry. 1996;57(Suppl. 13):49–55. discussion 56—48. [PubMed] [Google Scholar]
- Reif A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry. 2006;11(5):514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Keith LD. Sensitivity of the circadian rhythm of kainic acid-induced convulsion susceptibility to manipulations of corticosterone levels and mineralocorticoid receptor binding. Neuropharmacology. 1994;33:1087–1093. doi: 10.1016/0028-3908(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Rogatsky I, Waase CL, Garabedian MJ. Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3). Species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation. J. Biol. Chem. 1998;273:14315–14321. doi: 10.1074/jbc.273.23.14315. [DOI] [PubMed] [Google Scholar]
- Sahay A, Drew MR, Hen R. Dentate gyrus neurogenesis and depression. Prog. Brain Res. 2007;163:697–722. doi: 10.1016/S0079-6123(07)63038-6. [DOI] [PubMed] [Google Scholar]
- Sanchez ER, et al. Hormone-free mouse glucocorticoid receptors overexpressed in Chinese hamster ovary cells are localized to the nucleus and are associated with both hsp70 and hsp90. J. Biol. Chem. 1990;265:20123–20130. [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoid toxicity in the hippocampus: temporal aspects of neuronal vulnerability. Brain Res. 1985;359:300–305. doi: 10.1016/0006-8993(85)91440-4. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol. Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2001;98(22):12320–12322. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Chickens eggs and hippocampal atrophy. Nat. Neurosci. 2002;5:1111–1113. doi: 10.1038/nn1102-1111. [DOI] [PubMed] [Google Scholar]
- Savory JG, et al. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol. Cell. Biol. 1999;19:1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav. Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, et al. Postnatal glucocorticoid excess due to pituitary glucocorticoid receptor deficiency: differential short- and long-term consequences. Endocrinology. 2009;150(6):2709–2716. doi: 10.1210/en.2008-1211. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol. Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver PJ, Sigg EB, Moyer JA. Antidepressants and protein kinases: inhibition of Ca2+-regulated myosin phosphorylation by fluoxetine and iprindole. Eur. J. Pharmacol. 1986;121:65–71. doi: 10.1016/0014-2999(86)90393-6. [DOI] [PubMed] [Google Scholar]
- Sousa N, Cerqueira JJ, Almeida OF. Corticosteroid receptors and neuroplasticity. Brain Res. Rev. 2008;57:561–570. doi: 10.1016/j.brainresrev.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int. J. Dev. Neurosci. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Uchida S, et al. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur. J. Neurosci. 2008;27:2250–2261. doi: 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- Vedder H, Bening-Abu-Shach U, Lanquillon S, Krieg JC. Regulation of glucocorticoid receptor-mRNA in human blood cells by amitriptyline and dexamethasone. J. Psychiatr. Res. 1999;33:303–308. doi: 10.1016/s0022-3956(99)00006-0. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil E, et al. MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology. 2009;150:2220–2228. doi: 10.1210/en.2008-1335. [DOI] [PubMed] [Google Scholar]
- Weaver IC, et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wodarz N, et al. Normal lymphocyte responsiveness to lectins but impaired sensitivity to in vitro glucocorticoids in major depression. J. Affect Disord. 1991;22(4):241–248. doi: 10.1016/0165-0327(91)90070-9. [DOI] [PubMed] [Google Scholar]
- Yau JL, et al. The antidepressant desipramine requires the ABCB1 (Mdr1)-type p-glycoprotein to upregulate the glucocorticoid receptor in mice. Neuropsychopharmacology. 2007;32:2520–2529. doi: 10.1038/sj.npp.1301389. [DOI] [PubMed] [Google Scholar]
- Young EA, Haskett RF, Murphy-Weinberg V, Watson SJ, Akil H. Loss of glucocorticoid fast feedback in depression. Arch. Gen. Psychiatry. 1991;48:693–699. doi: 10.1001/archpsyc.1991.01810320017003. [DOI] [PubMed] [Google Scholar]
- Young AH, et al. Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology. 2004;29(8):1538–1545. doi: 10.1038/sj.npp.1300471. [DOI] [PubMed] [Google Scholar]
- Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70:407–417. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Zhou R, et al. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34:1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]


