Abstract
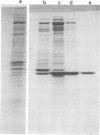
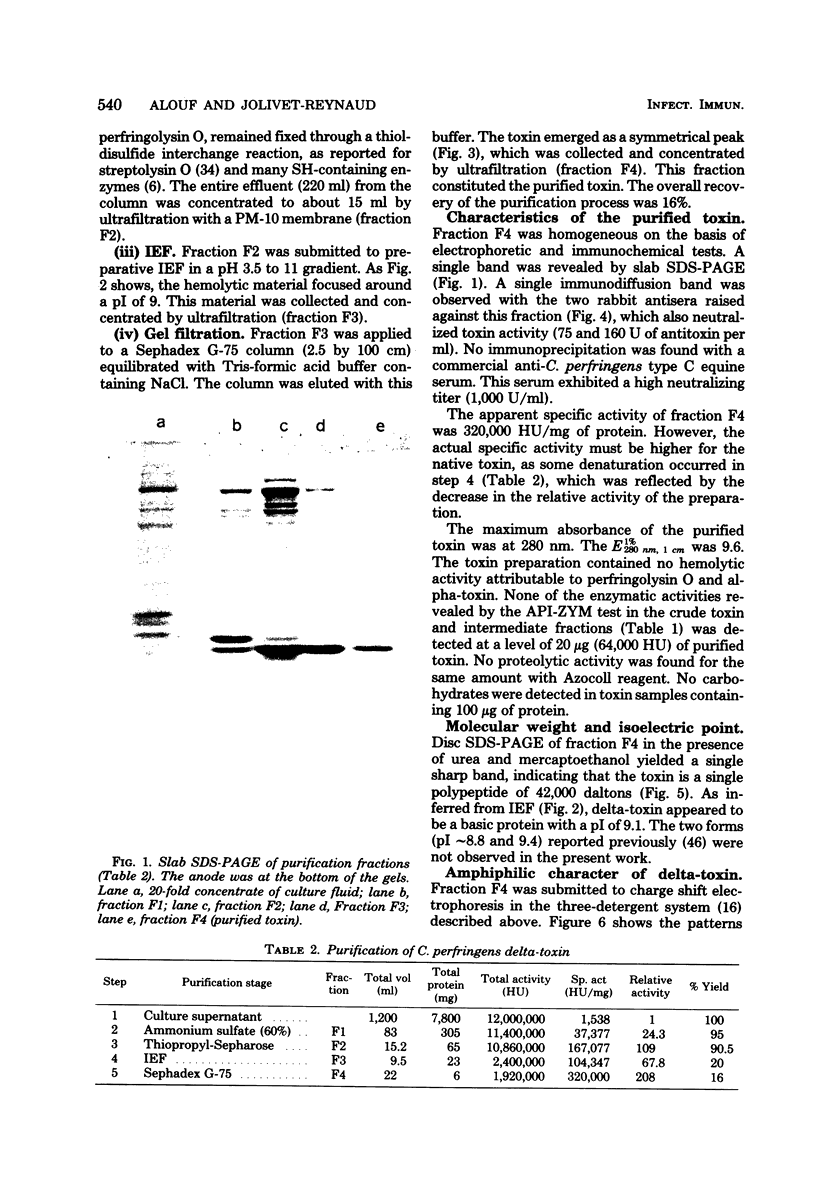
Delta-toxin, an extracellular hemolysin released by Clostridium perfringens type C, was purified from culture supernatant fluid by sequential ammonium sulfate precipitation, thiol-Sepharose gel chromatography, isoelectric focusing, and Sephadex G-75 gel filtration. The purified preparation had a specific activity of 320,000 hemolytic units per mg of protein and was homogeneous, as determined by immunochemical and electrophoretic tests. This toxin was characterized as a single polypeptide chain composed of 391 amino acid residues, 30% of which were hydrophobic. The molecular weight was found to be 42,000, and the isoelectric point was pH 9.1. Delta-toxin appeared to be amphiphilic by charge shift electrophoresis in a three-detergent system. It was immunogenic in rabbits and lethal to mice at a dose of 0.12 micrograms. The lytic activity of delta-toxin was restricted to erythrocytes of even-toed ungulates (sheep, goats, and pigs). This activity was inhibited by GM2 ganglioside but not by other gangliosides, cholesterol, lecithin, or sphingomyelin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
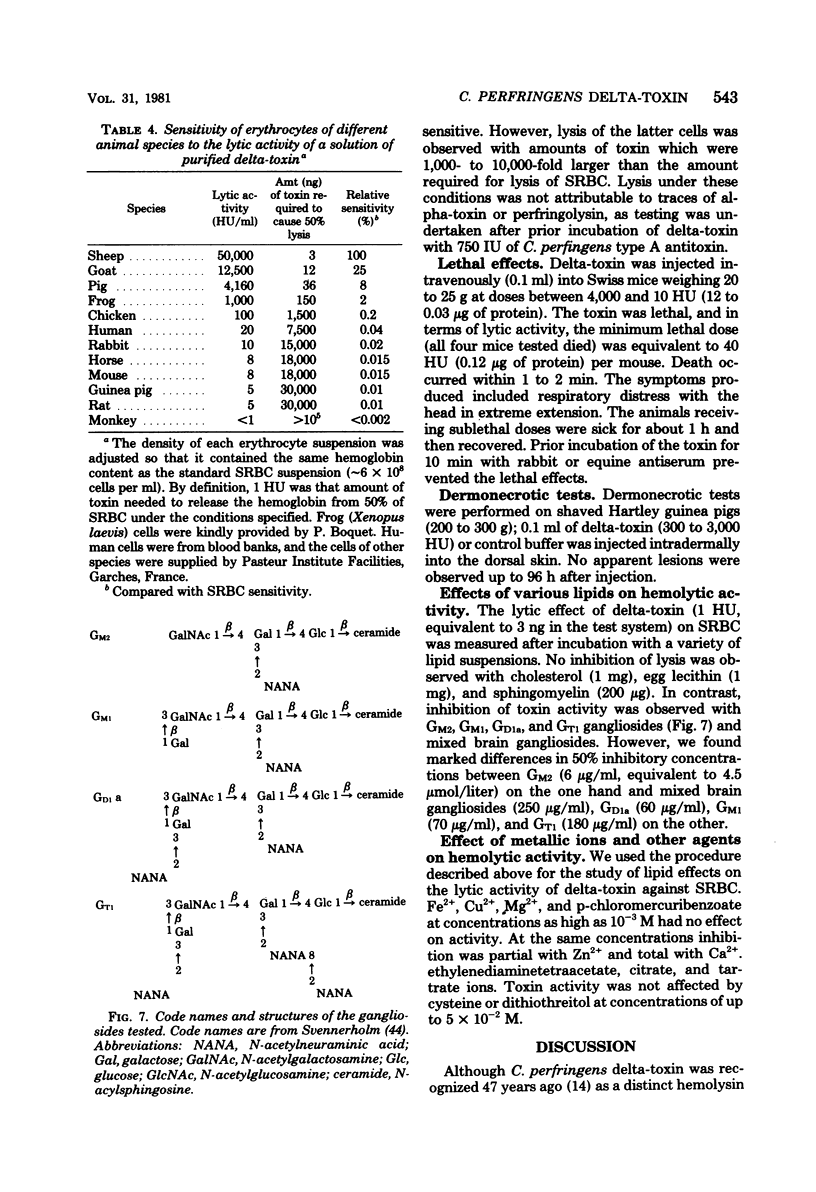
- Alouf J. E., Raynaud M. Action de la streptolysine O sur les membranes cellulaires. I. Fixation sur la membrane érythrocytaire. Ann Inst Pasteur (Paris) 1968 Jun;114(6):812–827. [PubMed] [Google Scholar]
- Barsumian E. L., Cunningham C. M., Schlievert P. M., Watson D. W. Heterogeneity of group A streptococcal pyrogenic exotoxin type B. Infect Immun. 1978 May;20(2):512–518. doi: 10.1128/iai.20.2.512-518.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P. Interaction of diphtheria toxin fragments A, B and protein crm 45 with liposomes. Eur J Biochem. 1979 Oct 15;100(2):483–489. doi: 10.1111/j.1432-1033.1979.tb04192.x. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography by thiol-disulfide interchange. Methods Enzymol. 1974;34:531–544. doi: 10.1016/s0076-6879(74)34069-4. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Bernheimer A. W. Role of cholesterol in the action of cereolysin on membranes. Arch Biochem Biophys. 1978 Oct;190(2):603–610. doi: 10.1016/0003-9861(78)90316-8. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Drazin R. E., Collier R. J. Amino-acid sequence of fragment A, an enzymically active fragment from diphtheria toxin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):69–72. doi: 10.1073/pnas.73.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dévényi T. Modified single-column procedure for the automatic analysis of amino acids. Acta Biochim Biophys Acad Sci Hung. 1969;4(3):297–299. [PubMed] [Google Scholar]
- Formisano S., Johnson M. L., Lee G., Aloj S. M., Edelhoch H. Critical micelle concentrations of gangliosides. Biochemistry. 1979 Mar 20;18(6):1119–1124. doi: 10.1021/bi00573a028. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Charge shift electrophoresis: simple method for distinguishing between amphiphilic and hydrophilic proteins in detergent solution. Proc Natl Acad Sci U S A. 1977 Feb;74(2):529–532. doi: 10.1073/pnas.74.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Purification and characterization of a hemolysin produced by Vibrio cholerae biotype El Tor: another toxic substance produced by cholera vibrios. Infect Immun. 1979 Dec;26(3):1020–1027. doi: 10.1128/iai.26.3.1020-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble M. W., King A., Phillips I. API ZYM: a simple rapid system for the detection of bacterial enzymes. J Clin Pathol. 1977 Mar;30(3):275–277. doi: 10.1136/jcp.30.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Naiki M. Ganglioside and rabbit erythrocyte membrane receptor for staphylococcal alpha-toxin. Infect Immun. 1976 Jan;13(1):289–291. doi: 10.1128/iai.13.1.289-291.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M., Iwamori M., Nagai Y. Interaction between Clostridium botulinum neurotoxin and gangliosides. Biochim Biophys Acta. 1980 Mar 20;628(3):328–335. doi: 10.1016/0304-4165(80)90382-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Möllby R., Wadström T. Purification of phospholipase C (alpha-toxin) from Clostridium perfringens. Biochim Biophys Acta. 1973 Oct 10;321(2):569–584. doi: 10.1016/0005-2744(73)90200-3. [DOI] [PubMed] [Google Scholar]
- OAKLEY C. L., WARRACK G. H. Routine typing of Clostridium welchii. J Hyg (Lond) 1953 Mar;51(1):102–107. doi: 10.1017/s0022172400015527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLANS E. S., JONES V. E. Studies on some soluble antigens of Clostridium welchii types B, C and D. Immunology. 1958 Jul;1(3):268–290. [PMC free article] [PubMed] [Google Scholar]
- Onon E. O. Purification and partial characterization of the exotoxin of Corynebacterium ovis. Biochem J. 1979 Jan 1;177(1):181–186. doi: 10.1042/bj1770181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIVNICK H., HABEEB A. F., GORENSTEIN B., STUART P. F., HAUSCHILD A. H. EFFECT OF PH ON TOXINOGENESIS BY CLOSTRIDIUM PERFRINGENS TYPE C. Can J Microbiol. 1964 Jun;10:329–344. doi: 10.1139/m64-045. [DOI] [PubMed] [Google Scholar]
- Prigent D., Geoffroy C., Alouf J. E. Purification de la streptolysine O par chromatographie covalente sur gel de thiol-agarose. C R Acad Sci Hebd Seances Acad Sci D. 1978 Oct 23;287(10):951–954. [PubMed] [Google Scholar]
- Richards K. L., Douglas S. D. Pathophysiological effects of Vibrio cholerae and enterotoxigenic Escherichia coli and their exotoxins on eucaryotic cells. Microbiol Rev. 1978 Sep;42(3):592–613. doi: 10.1128/mr.42.3.592-613.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogolsky M. Nonenteric toxins of Staphylococcus aureus. Microbiol Rev. 1979 Sep;43(3):320–360. doi: 10.1128/mr.43.3.320-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERNE M., WARRACK G. H. THE TYPES OF CLOSTRIDIUM PERFRINGENS. J Pathol Bacteriol. 1964 Jul;88:279–283. [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Schoettle D. J., Watson D. W. Ganglioside and monosaccharide inhibition of nonspecific lymphocyte mitogenicity by group A streptococcal pyrogenic exotoxins. Infect Immun. 1980 Jan;27(1):276–279. doi: 10.1128/iai.27.1.276-279.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel S. Hydrophobic chromatography. Methods Enzymol. 1974;34:126–140. doi: 10.1016/s0076-6879(74)34012-8. [DOI] [PubMed] [Google Scholar]
- Smith L. D. Virulence factors of Clostridium perfringens. Rev Infect Dis. 1979 Mar-Apr;1(2):254–262. doi: 10.1093/clinids/1.2.254. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tixier G., Alouf J. E. Essai de purification et propriétés de la toxine delta de Clostridium perfringens type C. Ann Microbiol (Paris) 1976 Nov-Dec;127B(4):509–524. [PubMed] [Google Scholar]