Abstract
First-episode psychosis (FEP) patients show hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis, but the mechanisms leading to this are still unclear. The aim of this study was to investigate the role of stress and antipsychotic treatment on diurnal cortisol levels, and on cortisol awakening response, in FEP. Recent stressful events, perceived stress and childhood trauma were collected in 50 FEP patients and 36 healthy controls using structured instruments. Salivary cortisol was obtained at awakening, at 15, 30, and 60 min after awakening, and at 12 and 8 pm. Patients experienced more recent stressful events, perceived stress and childhood trauma than controls (p < 0.001). Patients had a trend for higher diurnal cortisol levels (p=0.055), with those with less than two weeks of antipsychotics showing significantly higher cortisol levels than both patients with more than two weeks of antipsychotics (p=0.005) and controls (p=0.002). Moreover, patients showed a blunted cortisol awakening response compared with controls, irrespectively of antipsychotic treatment (p=0.049). These abnormalities in patients were not driven by the excess of stressors: diurnal cortisol levels were negatively correlated with the number of recent stressful events (r=−0.36, p=0.014), and cortisol awakening response was positively correlated with a history of sexual childhood abuse (r=0.33, p=0.033). No significant correlations were found between perceived stress or severity of symptoms and cortisol levels, either diurnal or in the awakening response. Our study shows that antipsychotics normalize diurnal cortisol hyper-secretion but not the blunted cortisol awakening response in FEP; factors other than the excess of psychosocial stress explain HPA axis abnormalities in FEP.
Keywords: First-episode psychosis, Cortisol, Stress, Antipsychotic, Childhood trauma, HPA axis
1. Introduction
Previous studies in patients with psychosis have demonstrated that stress is an important factor in the development of psychosis, but the biological mechanisms by which stress affects psychosis remain unclear. One model of psychosis, the vulnerability-stress model, posits that predisposing biological factors increase the sensitivity of some individuals to stress and thus make them more vulnerable to develop psychosis under stressful circumstances (Myin-Germeys and van Os, 2007; Pariante, 2008). Indeed, an excess of stressful life events has been shown to precede the onset of psychosis and psychotic relapse in patients with schizophrenia (Bebbington et al., 1993; Walker et al., 2008). Moreover, childhood adversities have also been linked to an increased risk for development of psychiatric disorders, and have been reported to be more frequent in patients with psychosis than in the general population (Read et al., 2005). Finally, patients with psychosis seem also to perceive daily hassles as more stressful than healthy subjects (Myin-Germeys and van Os, 2007), indicating that they may have a higher sensitivity to stress.
Studies in psychosis have also shown that patients who are in the acute phase of a psychotic disorder, with florid symptoms, newly hospitalized or unmedicated, show hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis, the main biological system involved in mediating the effects of stress (Gallagher et al., 2007; Gunduz-Bruce et al., 2007; Herz et al., 1985; Lammers et al., 1995; Sachar et al., 1970; Tandon et al., 1991). HPA axis activity is activated by the release of the corticotropin releasing hormone (CRH) from the hypothalamus, which in turn activates the secretion of adrenocorticotropic hormone (ACTH) from the pituitary, which finally stimulates the secretion of cortisol from the adrenal gland. Cortisol then interacts with its receptors in multiple target tissues including also the HPA axis, where it is responsible for feedback inhibition of the secretion of ACTH from the pituitary and CRH from the hypothalamus (Pariante and Lightman, 2008). Some authors have suggested that HPA axis hyperactivity could contribute to the pathogenesis of psychotic disorders by increasing brain dopaminergic activity (Walker and Diforio, 1997). Indeed, we have recently described that subjects at high risk of developing psychosis, including healthy first degree relatives of patients with schizophrenia (Mondelli et al., 2008) and subjects with prodromal symptoms (Garner et al., 2005), have a larger pituitary volume, again suggesting that hyperactivity of the HPA axis participates to the predisposition to psychosis.
The hyperactivity of the HPA axis in psychosis is particularly evident at the illness onset, which is often described as the most distressing time. Indeed, studies in subjects experiencing their first psychotic episode have shown increased circulating levels of cortisol and ACTH (Broome et al., 2005; Gunduz-Bruce et al., 2007; Pariante et al., 2004, 2005; Ryan et al., 2003; Ryan et al., 2004; Sachar et al., 1970) and higher rate of dexamethasone non-suppression (Ceskova et al., 2006). Our own work has shown a larger pituitary volume in patients with a first-episode psychosis (Pariante et al., 2004, 2005). This increased volume has been interpreted as indirect evidence of HPA axis hyperactivity as it is present in antipsychotic free patients as well as in patients receiving non-prolactin elevating atypicals, although clearly the volume of the pituitary is even larger in subjects taking prolactin-raising antipsychotics (Pariante et al., 2004, 2005; Pariante, 2008). Moreover, one study has recently described a blunted cortisol awakening response – a marker of dynamic HPA responsivity to a naturalistic stressor (Clow et al., 2004; Roberts et al., 2004) – in a small group of patients with recent onset of psychosis (Pruessner et al., 2008). In contrast, data in patients with established psychosis show that long-term antipsychotic treatment normalizes HPA axis hyperactivity (Tandon et al., 1991). Indeed, previous studies have shown that treatment with atypical antipsychotics, such as olanzapine, clozapine or risperidone, causes reduction in cortisol levels in patients with schizophrenia as well as in healthy controls (Cohrs et al., 2006; Hatzimanolis et al., 1998; Mann et al., 2006; Markianos et al., 1999; Meltzer, 1989; Scheepers et al., 2001; Zhang et al., 2005); interestingly studies investigating typical antipsychotics like haloperidol, pimozide or sulpiride, reported that cortisol levels are unaffected by these drugs in healthy volunteers (Cohrs et al., 2006). Therefore, studies in first episode are particularly relevant to understand the role of stress and HPA axis hyperactivity in psychosis, while avoiding confounders such as the long-term antipsychotic treatment and chronicity of the illness.
Several factors have been hypothesized to explain the HPA axis hyperactivity at the onset of psychosis: an increased level of stressful life events preceding the onset (Bebbington et al., 1993; Garner et al., 2005); an increased sensitivity to stress (Myin-Germeys and van Os, 2007); an increased rate of childhood trauma (Fisher et al., 2009; Nemeroff, 2004; Read et al., 2005); the distress and severity of the psychotic experience (Dinan, 2005); heavy tobacco smoking (De Leon and Diaz, 2005); and an increased use of cannabis (Di Forti et al., 2007), which in turn increases cortisol levels in humans (D’Souza et al., 2004, 2005). Surprisingly, however, these factors have not been yet studied together with HPA axis activity in first-episode psychosis. Therefore, in our study we investigate HPA axis activity (salivary cortisol during the day and in response to awakening) together with all of the putative mechanisms described above as well as the effects of antipsychotic treatment, in a sample of patients at their first-episode psychosis, and in healthy controls from the same geographical area.
2. Methods
2.1. Subjects
First-episode psychosis patients were recruited in London (UK) from the Lambeth, Southwark and Croydon inpatient and outpatient units, part of the South London and Maudsley (SLAM) NHS Foundation Trust, as part of the Genetic and Psychosis (GAP) study. The recruitment strategy was based on contacting inpatients and outpatients services regularly, interviewing staff and reviewing clinical notes, and approaching all subjects aged 18–65 who presented for the first time to these services for a functional psychotic illness (ICD10 F10–19, excluding coding F1x.0 for Acute intoxication; F20–29 and F30–39, psychotic codings) (World Health Organisation, 1992). Patients with organic psychosis, learning disabilities or requiring a translator because of lack of English fluency were excluded from the study. Controls were recruited from the same catchment’s area as the patients through advertisement in local newspapers, hospitals and job centers, as well as from existing volunteer databases. Controls were screened using the Psychosis Screening Questionnaire (PSQ) (Bebbington and Nayani, 1995), and excluded if they met criteria for a present or past psychotic disorder. Both patients and controls were excluded if taking any kind of hormonal treatment. The study was approved by the local Ethical Committee, in accordance with the code of ethics of the World Medical Association, and written informed consent was obtained by all participants.
We recruited and assessed 50 patients with first-episode psychosis and 36 healthy controls. None of the controls was clinically depressed or treated with antidepressants at the time of the study. Seventeen patients received a DSM-IV diagnosis of schizophrenia, nine of schizophreniform disorder, thirteen of psychotic disorder not otherwise specified, nine of delusional disorder, one of schizoaffective depressive disorder, and one of schizoaffective bipolar disorder. Seven patients were drug naïve, ten had less than two weeks of treatment (3 were on quetiapine, 3 on risperidone, 2 on aripriprazole, 1 on haloperidol, and 1 on olanzapine) and 33 were treated with antipsychotic drugs for more than two weeks (16 were on olanzapine, 12 on risperidone, 2 on aripriprazole, 2 on haloperidol, and 1 on amisulpiride). Patients were further divided into those with less than two weeks (n=17) and those with more than two weeks (n=33) of antipsychotic treatment, based on recent studies providing evidence that the greater “psychotic” improvement is seen within the first two weeks of treatment (Agid et al., 2006).
2.2. Questionnaires and clinical assessment
Socio-demographic data were collected using a modified version of the MRC Sociodemographic Schedule (Mallett et al., 2002). Validation of clinical diagnosis was obtained using the Operational Criteria (OPCRIT) (McGuffin et al., 1991), reviewing the case notes in the first month following first contact with services. The presence or absence of symptoms was measured by the OPCRIT checklist using the strict OPCRIT definitions. Inter-rater reliability between researchers for the OPCRIT was very good (Cronbach’s alpha=0.91). The case notes of the first month following first contact with psychiatric services were also used to obtain a total symptom score by summing the individual symptom item scores according to Wing and Sturt’s (1978) procedure for the Present State Examination.
We collected information about stressful life events, in the previous six months, using the Brief Life Events questionnaire (Brugha and Cragg, 1990). This questionnaire assesses both the number and the emotional impact of life stressors involving moderate or long-term threat, such as illness or injury, death of a close friend or relative, unemployment, financial loss and loss of important relationships. We also measured the perceived stress, in the previous month, using the Perceived Stress Scale (Cohen and Williamson, 1988). This is a 10-item scale measuring the degree to which situations in one’s life are appraised as stressful; the items are designed to tap how unpredictable, uncontrollable, and overloaded respondents find their lives in the last month. Information about childhood trauma were also collected, using a modified version of the Childhood Experience of Care and Abuse (CECA) Questionnaire (Bifulco et al., 2005), including information about loss of parents, separation from parents for more than 6 months, and physical and sexual abuse before the age of 17 years. Cut-points published by Bifulco et al. (2005) were used to dichotomize responses on the physical abuse variable into severe and nonsevere categories. A composite variable was created using the four dichotomized variables considered (loss of parents, separation from parents for more than 6 months, severe physical abuse and presence of sexual abuse): the score of this variable ranged from 0 (absence of any childhood trauma) to 4 (presence of all the four childhood traumas investigated). This variable was then further dichotomized as 0 if no childhood trauma was present (‘absence of childhood trauma’) and 1 if one or more types of maltreatment experienced was present (‘presence of childhood trauma’). Tobacco smoking was evaluated by direct questions about current and past history of tobacco smoking. Finally, current and lifetime cannabis use was evaluated with the Cannabis Experience Questionnaire (Barkus et al., 2006).
2.3. Salivary cortisol assessment
Saliva samples were collected to measure salivary cortisol using Salivettes (Sarstedt, Leicester, UK) in which saliva is absorbed in a cotton roll. Subjects were instructed to collect saliva samples by chewing the cotton roll for 2 min, immediately after awakening (0 min) and 15, 30 and 60 min after awakening, and again at 12 pm and at 8 pm. In 11 patients and 24 controls, we also completed a second-day collection to confirm reliability on the collection of the 4 samples during the first hour from awakening (Pearson’s correlation between the first and second days ranged 0.62–0.71, p < 0.001, for each time point). Subjects were instructed to wake up before 10 am, to take the first sample while still in bed, and then not to have breakfast or brush their teeth during the first hour of awakening, and in the 30 min before taking the sample at 12 pm and 8 pm. This was in order to avoid falsely high cortisol values due to plasma exudates from minor bleeding in the oral cavity, or from meal-stimulated rises in cortisol. On the arrival to the laboratory the salivettes were frozen at −20 ° C. After thawing, they were centrifuged at 3500 rev/min for 10 min, which resulted in a clear supernatant of low viscosity. Saliva cortisol concentrations were determined using the “Immulite” — DPC’s Immunoassay analyzer (www.diagnostics.siemens.com). The plasma cortisol assay of the analyzer was suitably modified and then validated for these measurements. A set of 10 cortisol standards in saline were used in each assay to plot a calibration graph. This was highly reproducible with slope of (mean± SEM) 0.197 ± 0.004. The method correlated well with a previously published TR-FIA (Juruena et al., 2006). It had analytical sensitivity of 0.2 nmol/l and inter/intra assay precision (% CV) of less than 10% (cortisol concentration range 5 to 25 nmol/l). All samples from the same subject were analyzed in the same run.
2.4. Data analyses
To limit the number of statistical comparisons, all HPA axis analyses were conducted using two summary measures: the Area Under the Curve (AUC) of cortisol levels during the day (0 min, noon and 8 pm) and the AUC of the increase (AUCi) of cortisol levels after awakening (from 0 min to 15, 30, and 60 min after awakening). Both formulas for the calculation of the AUCs were derived from the trapezoid formula (Pruessner et al., 2003).
Data were analyzed using the Statistical Package for Social Sciences, Version 15.0 (SPSS Inc.). Continuous variables are presented as mean ± standard error mean. The independent sample t-test was used to compare means of continuous variables between patients and controls (e.g., number of stressful events, perceived stress score, cortisol AUC during the day and cortisol AUCi in response to awakening). Chi-square test was used to compare categorical variables (e.g., gender, presence of childhood trauma) between patients and controls. A one-way ANOVA, followed by Bonferroni post-hoc analyses, was used to investigate differences in continuous variables between patients taking antipsychotic treatment for less than two weeks, patients taking antipsychotic treatment for more than two weeks, and controls.
Finally, to examine hypothesized predictors of HPA axis activity, we correlated the two HPA axis summary measures (AUC during the day and cortisol AUCi in response to awakening) with the hypothesized factors described above, using Pearson’s correlation (for parametric measures such as Perceived Stress Scale score, severity of symptoms, and frequency of cannabis use), Spearman’s correlation (for non-parametric measures such as number of recent stressful life events and number of significant childhood trauma), and partial correlation, as appropriate.
3. Results
First-episode psychosis patients did not differ in age, gender, and relationship status from healthy controls; similarly, there were no differences in these variables between patients with less than two weeks of antipsychotic treatment, more than two weeks of antipsychotic treatment, and controls (see Table 1). However, patients and controls differed significantly for ethnicity, as the group of patients included a smaller number of white subjects when compared with the group of controls (p=0.001, see Table 1). This represents the ethnic distribution of patients with psychosis in this geographical area and has been described extensively before (Fearon et al., 2006; Kirkbride et al., 2006; Morgan et al., 2006). Ethnicity was therefore included as a covariate in confirmatory analyses. The rate of current tobacco smokers was significantly higher in the patients than in controls (see Table 1). The mean duration of antipsychotic treatment in our patients was 31.6 ± 4.3 days (range 0–119 days). As expected (Di Forti et al., 2007), patients tended to smoke more cannabis; the rate of current cannabis users in the patients’ group was double than the one in controls, however this difference did not reach statistical significance (see Table 1).
Table 1.
Socio-demographic characteristics of first-episode psychosis patients and healthy controls
| Patients |
Controls |
Test and significance | |||
|---|---|---|---|---|---|
| All (n=50) | <2 weeks of treatment (n=17) |
>2 weeks of treatment (n=33) |
(n=36) | ||
| Age (years) | 29.2±1.1 | - | - | 27.3±0.8 | t=−1.3, df=1, 84, p=0.2 |
| - | 26.5±1.2 | 30.6±1.5 | 27.3±0.8 | F=3.1, df=2, 85, p=0.05 | |
| Gender M/F (% of males) | 32/18 (64%) | - | - | 26/10 (72.2%) | χ2=0.6, p=0.5 |
| - | 13/4 (76.5%) | 19/14 (57.6%) | 26/10 (72.2%) | χ2=2.5, p=0.3 | |
| Ethnicity (% of white) | 26% | - | - | 63.9% | χ2=12.4, p=0.01 |
| - | 23.5% * | 27.3% * | 63.9% | χ2=12.4, p=0.02 | |
| Relationship status (% single) | 68.8% | - | - | 58.3% | χ2=1.0, p=0.4 |
| - | 66.7% | 69.7% | 58.3% | χ2=1.0, p=0.6 | |
| Current tobacco smokers % | 51.2% | - | - | 19.4% | χ2=8.5, p=0.003 |
| - | 42.9% | 55.2% | - | χ2=9.1, p=0.01 | |
| Cannabis, current users % | 25.5% | - | - | 11.4% | χ2=2.5, p=0.2 |
| - | 28.6% | 24.2% | 11.4% | χ2=2.7, p=0.3 | |
Bonferroni post-hoc analyses
p<0.05 vs controls
p<0.05 vs patients with less or more than 2 weeks of antipsychotic treatment.
3.1. Stress variables
First-episode psychosis patients reported approximately three-fold more recent stressful life events, two-fold higher perceived stress levels, and two-fold higher rates of childhood trauma than healthy controls (p < 0.001 for all variables, see Table 2).
Table 2.
Stress measurements, Area Under the Curve (AUC) of cortisol during the day, and AUC with respect to increase (AUCi) of cortisol awakening response in first-episode psychosis patients and controls
| Patients |
Controls |
Test and significance | |||
|---|---|---|---|---|---|
| All (n=50) | <2 weeks of treatment (n=17) |
>2 weeks of treatment (n=33) |
(n=36) | ||
| Number of stressful life events | 2.3±0.3 | - | - | 0.7±0.2 | t=−4.8, df=1, 81, p<0.001 |
| - | 2.1±0.5 * | 2.4±0.3 * | 0.7±0.2 | F=10.3, df=2, 82, p<0.001 | |
| Perceived Stress Scale score | 19.8±1.0 | - | - | 11.9±0.9 | t=−5.4, df=1, 80, p<0.001 |
| - | 20.2±2.0 * | 19.6±1.2 * | 11.9±0.9 | F=14.4, df=2, 81, p<0.001 | |
| Childhood trauma (% with at least one trauma) | 85.7% | - | - | 38.7% | χ2=17.5, p<0.001 |
| - | 92.3% * | 82.8% * | 38.7% | χ2=17.9, p<0.001 | |
| AUC (nmol h/l) cortisol during the day | 90.6±7.2 | - | - | 74.5±4.0 | t=−2.0, df=1, 82, p=0.055 |
| - | 115.2±17.8 *, ** | 77.1±4.1 | 74.5±4.0 | F=7.1 df=2, 83, p=0.001 | |
| AUCi (nmol min/l) cortisol awakening response | 134.8±36.9 | - | - | 245.7±40.5 | t=2.0, df=1, 84, p=0.049 |
| - | 119.8±93.1 | 142.6±30.4 | 245.7±40.5 | F=2.0, df=2, 85, p=0.1 | |
Bonferroni post-hoc analyses
p<0.05 vs controls
p<0.05 vs patients with less or more than 2 weeks of antipsychotic treatment.
3.2. Cortisol levels
The mean awakening time (on the day of cortisol collection) was 8 am for both patients and controls. Salivary cortisol levels during the day are shown in Figs. 1 and 2, and the AUCs are presented in Table 2. Patients showed a trend for higher levels of cortisol levels during the day compared with healthy controls, but this did not reach the statistical significance (AUC, t=−2.0, df=1, 82, p=0.055, see Table 2 and Fig. 1). However, cortisol levels during the day were significantly different between patients with less than two weeks of antipsychotic treatment, patients with more than two weeks of antipsychotic treatment and controls (F=7.1, df=2, 81, p=0.001; see Table 2 and Fig. 2). The post-hoc analyses showed that patients with less than two weeks of treatment had higher cortisol levels during the day than both patients with more than two weeks of treatment (p=0.005), and healthy controls (p=0.002). In contrast, patients with more than two weeks of treatment did not differ from healthy controls in cortisol levels during the day (p=1.0, see Table 2 and Fig. 2). Finally, we run exploratory analyses on the small group of drug naïve patients (n=7). We did not find any difference in cortisol levels during the day between drug naïve (107.4 ± 15.5 nmol h/l) and patients who were treated but for less than 2 weeks of antipsychotic treatment (n=10; 120.7 ± 28.9 nmol h/l; p=0.7). Indeed, similar to the overall group of patients with less than two weeks of treatment, drug naïve patients also had higher cortisol levels than controls, although this only reached trend statistical significance (p=0.08).
Fig. 1.
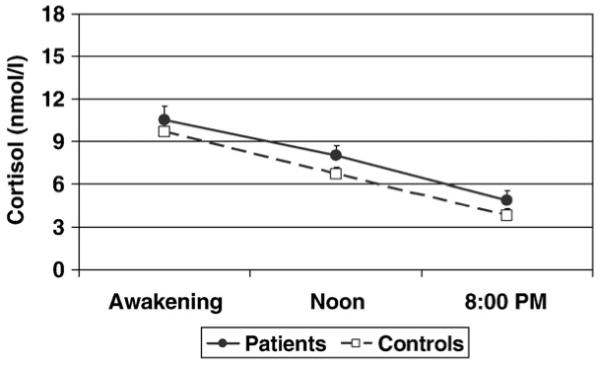
Cortisol levels during the day infirst-episode psychosis patients (closed symbol) and in controls (open symbol).
Fig. 2.
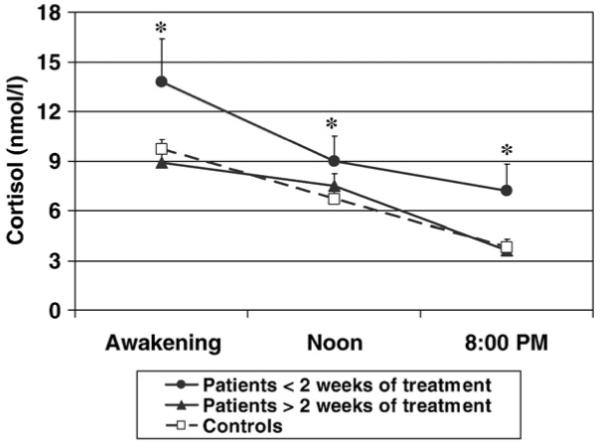
Cortisol levels during the day in patients with less than two weeks of antipsychotic treatment, patients with more than two weeks of antipsychotic treatment, and controls. * indicates a statistically significant difference in the Area Under the Curve (AUC) of cortisol levels during the day between patients with less than two weeks of antipsychotic treatment and both controls and patients with more than two weeks of antipsychotic treatment.
Cortisol awakening response is presented in Fig. 3, and Table 2. First-episode psychosis patients showed a significantly lower cortisol awakening response than healthy controls (AUCi of changes; t=2.0, df=1, 84, p=0.049, see Table 2). In contrast with the AUC during the day, there was no difference in the cortisol awakening response between patients with less than two weeks of treatment and patients with more than 2 weeks of treatment (p=1.0); moreover, both groups of patients had the same degree of blunted cortisol awakening response compared with controls, although this only reached trend statistical significance in the unadjusted comparison (p=0.097 for both; see Table 2 and Fig. 3).
Fig. 3.
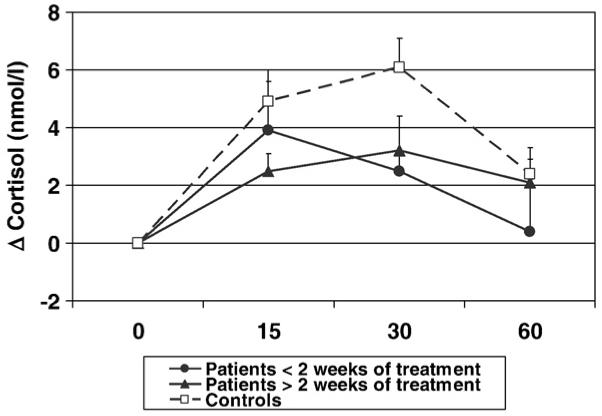
Cortisol awakening response in patients with less than two weeks of antipsychotic treatment, patients with more than two weeks of antipsychotic treatment, and controls. Cortisol levels are presented as delta changes from time 0 of the awakening.
In order to confirm that the differences found between patients and controls were not due to the difference in ethnicity, we repeated the analyses described above using ethnicity as a covariate, and found the same results (data not shown).
3.3. Correlation analyses
We ran correlation analyses to look at hypothesized predictors of cortisol levels during the day and in response to awakening. Because, as shown above, cortisol levels during the day (but not the cortisol awakening response) were influenced by the antipsychotic treatment, the number of days of antipsychotic treatment was included as covariate in all the analyses looking at cortisol during the day. The results of correlation analyses are presented in Table 3.
Table 3.
Correlation analyses of stress measures, frequency of current cannabis use and severity of symptoms with Area Under the Curve (AUC) of cortisol secretion during the day and the AUC with respect to increase (AUCi) of the cortisol awakening response in first-episode psychosis patients and healthy controls
| Patients |
Controls |
|||
|---|---|---|---|---|
| AUC cortisol During day |
AUCi cortisol Awakening |
AUC cortisol During day |
AUCi cortisol Awakening |
|
| Number of stressful life events |
r=−0.36 | r=0.15 | r=0.42 | r=0.14 |
| p=0.014 | p=0.3 | p=0.013 | p=0.4 | |
| Perceived stress scale |
r=−0.25 | r=0.18 | r=0.11 | r=−0.12 |
| p=0.1 | p=0.2 | p=0.5 | p=0.5 | |
| Childhood trauma |
r=−0.23 | r=0.28 | r=0.12 | r=−0.26 |
| p=0.2 | p=0.077 | p=0.5 | p=0.2 | |
| Tobacco smoking |
r=0.012 | r=−0.022 | r=−0.111 | r=0.206 |
| p=0.9 | p=0.9 | p=0.5 | p=0.2 | |
| Frequency of current cannabis use |
r=−0.27 | r=0.32 | r=−0.06 | r=0.02 |
| p=0.074 | p=0.029 | p=0.7 | p=0.9 | |
| Severity of symptoms |
r=−0.04 | r=0.01 | - | - |
| p=0.8 | p=1.0 | - | - | |
Significant correlations indicated that, in healthy controls, the number of stressful life events in the last 6 months correlated positively with the cortisol levels during the day; in contrast, in patients with first-episode psychosis, the number of stressful life events in the last 6 months correlated negatively with the cortisol levels during the day. Also, frequency of current cannabis use was positively correlated with the cortisol awakening response in patients, but not in controls.
Interestingly, previous studies have shown that cannabis use in patients with first-episode psychosis is associated with a history of a specific form of childhood trauma, sexual abuse (Compton et al., 2004); in turn, sexual abuse has been shown more consistently to predispose to HPA axis hyperactivity (Heim et al., 2000). Therefore, we explored the association between cannabis use, childhood sexual abuse and cortisol levels in these patients. Current frequency of cannabis use was positively correlated with a history of childhood sexual abuse (r=0.33, p=0.041), and in turn childhood sexual abuse was correlated with the cortisol awakening response (r=0.33, p=0.033). Indeed, the correlation between frequency of cannabis use and cortisol awakening response was not significant after covarying for childhood sexual abuse (r=0.27, p=0.1).
4. Discussion
In our study, patients with first-episode psychosis have a trend for higher cortisol levels during the day, but this difference is entirely driven by those with less than two weeks of antipsychotic treatment. These patients also have significantly blunted cortisol awakening response, irrespectively of antipsychotic treatment. Finally, patients have more recent stressful events, higher levels of perceived stress, and higher rates of childhood trauma, but these do not seem to explain the HPA axis abnormalities.
The increased cortisol levels during the day in patients with less than two weeks of antipsychotic treatment are consistent with previous studies in drug-free patients with first-episode psychosis, including the work by Ryan et al. showing higher afternoon ACTH and cortisol levels (Ryan et al., 2004), and our own work showing an increased pituitary volume (Pariante et al., 2005). The normalizing effect of antipsychotic treatment on cortisol levels is also in agreement with previous studies in patients with chronic schizophrenia, showing a reduction of cortisol levels following treatment with atypical antipsychotics (Ismail et al., 1998; Jakovljevic et al., 2007; Mann et al., 2006; Popovic et al., 2007; Zhang et al., 2005). Moreover, the only study published to date on the effect of antipsychotic treatment on HPA axis activity in first-episode psychosis has also shown normalization of HPA axis activity with antipsychotic treatment, as indicated by a reduction in the rate of dexamethasone non-suppression from 18% to 5% after 4–6 weeks of antipsychotic treatment (Ceskova et al., 2006). Of note, atypical antipsychotics decrease cortisol levels also in healthy controls (Cohrs et al., 2006). This suggests that our results may be related not only to the reduction of HPA axis hyperactivity as a reflection of the improvement of psychotic symptoms (Cohrs et al., 2006; Meltzer, 1989), but also to the direct antipsychotic pharmacodynamic action. Interestingly, recent studies have also shown that drugs reducing the effects of glucocorticoid excess, such as glucocorticoid antagonists, are effective in alleviating psychotic symptoms in patients with psychotic depression (Debattista and Belanoff, 2006; Schatzberg and Lindley, 2008). Therefore, it is possible that the capability of antipsychotics to decrease diurnal cortisol secretion plays a role in their therapeutic action (Flores et al., 2006).
The patients with first-episode psychosis also showed more stressful events in the last 6 months, higher levels of perceived stress, and higher rates of childhood trauma, compared with controls. These results are consistent with a number of previous studies examining these variables in patients with psychosis (Bebbington et al., 1993; Myin-Germeys et al., 2001; Read et al., 2005). Of note, however, to our knowledge this is the first study demonstrating more recent stressful events and more perceived stress in first-episode psychosis. Interestingly, and in contrast with what we hypothesized, diurnal cortisol hyper-secretion in our patients was not explained by these stress measures. Indeed, levels of cortisol during the day correlated negatively with the number of stressful events, and showed non-significant negative correlations with perceived stress and childhood trauma. Of note also is that the relationship between cortisol levels during the day and stress measures was different in patients and controls, since cortisol levels in controls correlated positively with the number of stressful events, and showed not-significant positive correlations with perceived stress and childhood trauma. A possible hypothesis to explain the negative correlations between stress measures and cortisol levels in patients could be the excessive load of stressful events in this sample, with some patients showing as much as five-fold the number of stressful events of controls. Indeed, post-traumatic stress disorder (PTSD), a psychiatric condition caused by highly stressful and life-threatening situations, has also been associated with cortisol hyposecretion (Yehuda, 2001), suggesting that an extreme excess of stressful situations could determine a decrease in the activation of the HPA axis. Accordingly, in our study, patients in the top 20th percentile for number of stressful life events had similar cortisol levels during the day of healthy controls (data not shown). Interestingly, these results are consistent with a previous study by Oquendo et al. (2003) reporting that depressed patients with PTSD show lower cortisol levels than depressed patients without PTSD, again suggesting that the presence of extremely stressful and traumatic experience can determine a decrease in the activation of the HPA axis also in depression, another condition characterized by HPA axis hyperactivity.
The high cortisol levels during the day in our patients were also not explained by the high rate of tobacco smoking in these subjects. Indeed, the association between schizophrenia and heavy smoking or nicotine dependence is widely recognized (De Leon and Diaz, 2005) and previous studies have reported nicotine as a strong activator of the HPA axis (Rohleder and Kirschbaum, 2006). The lack of association between tobacco smoking and cortisol levels in our first-episode psychosis patients is however in agreement with a previous study in patients with chronic schizophrenia where no difference in cortisol levels was found between smokers and non-smokers (Iancu et al., 2007).
We also failed to find any correlation between cortisol levels and severity of symptoms. However, we measured the severity of symptoms in the first month after contact with psychiatric services, and this time-frame did not always correspond to the time when the cortisol was collected and thus reflect the severity of psychotic symptoms at the time of cortisol collection. A further possible explanation for the lack of correlation between cortisol levels and severity of symptoms in our subjects could be related to other hormones counteracting the effects of cortisol. Interestingly, one previous study has shown high serum levels of dehydroepiandrosterone-sulphate (DHEA-S), a hormone with anti-glucocorticoid action, in patients with first-episode psychosis (Strous et al., 2004), while lower levels of DHEA have been described late in the course of chronic illness (Maninger et al., 2009). The DHEA-to-cortisol ratio has been described as more informative than either DHEA or cortisol absolute concentrations in particular regarding the possible effect of these hormones on cognitive function (Maninger et al., 2009). Unfortunately, DHEA measurement would have required a different saliva collection protocol, and given the already large number of assessments, we did not measure DHEA levels in our subjects. Future studies would need to address this hypothesis.
Other factors not examined in our paper might play a role in the HPA axis hyperactivity found in our patients. Indeed, the severity of the non-psychotic, prodromal symptoms preceding the onset may be important, as suggested by our work demonstrating a larger pituitary volume weeks before the clinical onset of the psychosis (Garner et al., 2005) — but unfortunately this measure was not available. Another possible factor, explaining the high cortisol levels in first-episode psychosis could be the anxiety associated with experiencing psychotic symptoms for the first time; indeed, Ceskova et al. (2006) found an association between anxiety and predexamethasone cortisolemia before starting antipsychotic treatment in patients with first-episode psychosis. Furthermore, we have also recently described a larger pituitary volume in healthy first degree relatives of patients with schizophrenia (Mondelli et al., 2008), and proposed that the HPA axis hyperactivity may be related to a genetic predisposition to over-activate the axis. Family history for psychosis was recorded in this sample, but unfortunately only four patients had family history of psychosis, not allowing for any meaningful comparisons.
We also found a blunted cortisol awakening response in our patients, which was not influenced by antipsychotic treatment. It is important to stress that this is the first time that a blunted cortisol awakening response has been described in the context of higher diurnal cortisol levels. Euthymic or acutely ill patients with major depression, a condition usually characterized by elevated cortisol levels during the day (Pariante and Lightman, 2008), tend to show increased cortisol awakening response (Bhagwagar et al., 2003; Bhagwagar et al., 2005). In contrast, subjects with chronic fatigue syndrome (Roberts et al., 2004), and post-traumatic stress disorder (Rohleder et al., 2004; Wessa et al., 2006), conditions usually characterized by lower cortisol levels during the day (Cleare, 2003; Yehuda, 2001), also tend to show decreased cortisol awakening responses (Roberts et al., 2004). This suggests that HPA axis dysfunction in psychosis is not simply a correlate of depression or other general psychopathological symptoms but has a specific profile, perhaps linked to a different genetic background or a different developmental trajectory of the stress abnormalities. Awakening acts as a mild stressor (Roberts et al., 2004), and thus the blunted cortisol awakening response is consistent with previous studies in patients with chronic schizophrenia, also finding a blunted cortisol response to psychological and psychosocial stressors (Breier et al., 1988; Jansen et al., 1998, 2000), even in the presence of normal baseline cortisol levels (Breier et al., 1988; Jansen et al., 2000).
Our data are also consistent with a very recent study reporting a blunted cortisol awakening response in men, but not in women, with a recent onset of psychosis (Pruessner et al., 2008). These authors did not explain this gender difference, but it is of note that women with first-episode psychosis have been described to have a higher rate of childhood sexual abuse than men (Fisher et al., 2009), and that our data show that childhood sexual abuse increases cortisol awakening response, thus potentially normalizing the blunted response in women. This could explain the results by Pruessner et al. (2008), although these authors did not assess childhood trauma and therefore this hypothesis cannot be confirmed. Interestingly, we did not detect any difference in cortisol response to awakening between men and women with first episode of psychosis (data not shown), and also did not find a difference in the rate of childhood sexual abuse between these two groups (approximately 20% in both groups) — which is consistent with our hypothesized explanation for the results by Pruessner et al. (2008).
A few limitations need to be acknowledged. First, this is not a longitudinal study, and therefore we can only speculate that the differences between patients with less and patients with more than two weeks of antipsychotic treatment are due to progressive changes in HPA axis activity related to the length of antipsychotic treatment. Theoretically, patients that could be assessed for this study already with no or little antipsychotic treatment could have different psychopathological, clinical or biological profiles compared with those that could not be assessed until after two or more weeks of antipsychotics. Second, patients and controls differed in ethnicity; however, all the statistical analyses were repeated using ethnicity as a covariate and confirmed the significance of the results obtained. Third, although the subjects completed information sheets during the saliva collection, we did not supervise directly the saliva collection in our subjects. Fourth, our measures of stress were derived from self-reported questionnaires and not corroborated by external evidence, and may represent a less reliable measure of stress. Last, depression is widely recognized to affect HPA axis activity, but we did not perform specific mood evaluations in our sample. Although only 2 of our patients (and none of our controls) had diagnosis of affective psychosis, a specific assessment of depressive symptoms would have given the opportunity to clarify whether high cortisol levels in our patients could be driven by depressive symptoms.
In conclusion, this study shows, in patients with first-episode psychosis, a diurnal cortisol hyper-secretion (in less treated patients) and a blunted cortisol response to the stress of awakening. However, these abnormalities do not seem to be explained by the excess of subjective and objective stress measures. Our findings confirm the strong association between stress and psychosis, but that the bio-psycho-social mechanisms underlying this association are complex and require further study.
Acknowledgements
This project is supported by a King’s College Development Trust (UK) and a NARSAD Mental Health Research Association. Young Investigator Award to Valeria Mondelli, by the South London and Maudsley NHS Foundation Trust & Institute of Psychiatry NIHR Biomedical Research Centre for Mental Health, and by the British Academy. Carmine M. Pariante’s research is also supported by the UK Medical Research Council, the NARSAD, and the Commission of European Communities 7th Framework Programme Collaborative Project Grant Agreement no. 22963 (Mood Inflame).
Role of funding source
The funding sources had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
All other authors declare that they have no conflicts of interest.
References
- Agid O, Seeman P, Kapur S. The “delayed onset” of antipsychotic action—an idea whose time has come and gone. J. Psychiatry Neurosci. 2006;31:93–100. [PMC free article] [PubMed] [Google Scholar]
- Barkus EJ, Stirling J, Hopkins RS, Lewis S. Cannabis-induced psychosis-like experiences are associated with high schizotypy. Psychopathology. 2006;39:175–178. doi: 10.1159/000092678. [DOI] [PubMed] [Google Scholar]
- Bebbington P, Nayani T. The psychosis screening questionnaire. Int. J. Methods Psychiatr. Res. 1995;5:11–19. [Google Scholar]
- Bebbington P, Wilkins S, Jones P, Foerster A, Murray R, Toone B, Lewis S. Life events and psychosis. Initial results from the Camberwell Collaborative Psychosis Study. Br. J. Psychiatry. 1993;162:72–79. doi: 10.1192/bjp.162.1.72. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Increase in concentration of waking salivary cortisol in recovered patients with depression. Am. J. Psychiatry. 2003;160:1890–1891. doi: 10.1176/appi.ajp.160.10.1890. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen P. Increased salivary cortisol after waking in depression. Psychopharmacology (Berl) 2005:1–4. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Bernazzani O, Moran PM, Jacobs C. The childhood experience of care and abuse questionnaire (CECA.Q): validation in a community series. Br. J. Clin. Psychol. 2005;44:563–581. doi: 10.1348/014466505X35344. [DOI] [PubMed] [Google Scholar]
- Breier A, Wolkowitz OM, Doran AR, Bellar S, Pickar D. Neurobiological effects of lumbar puncture stress in psychiatric patients and healthy volunteers. Psychiatry Res. 1988;25:187–194. doi: 10.1016/0165-1781(88)90050-9. [DOI] [PubMed] [Google Scholar]
- Broome MR, Woolley JB, Tabraham P, Johns LC, Bramon E, Murray GK, Pariante C, McGuire PK, Murray RM. What causes the onset of psychosis? Schizophr. Res. 2005;79:23–34. doi: 10.1016/j.schres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Brugha TS, Cragg D. The List of Threatening Experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatr. Scand. 1990;82:77–81. doi: 10.1111/j.1600-0447.1990.tb01360.x. [DOI] [PubMed] [Google Scholar]
- Ceskova E, Kasparek T, Zourkova A, Prikryl R. Dexamethasone suppression test in first-episode schizophrenia. Neuro. Endocrinol. Lett. 2006;27:433–437. [PubMed] [Google Scholar]
- Cleare AJ. The neuroendocrinology of chronic fatigue syndrome. Endocr. Rev. 2003;24:236–252. doi: 10.1210/er.2002-0014. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the United Stated. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: 1988. [Google Scholar]
- Cohrs S, Roher C, Jordan W, Meier A, Huether G, Wuttke W, Ruther E, Rodenbeck A. The atypical antipsychotics olanzapine and quetiapine, but not haloperidol, reduce ACTH and cortisol secretion in healthy subjects. Psychopharmacology (Berl) 2006;185:11–18. doi: 10.1007/s00213-005-0279-x. [DOI] [PubMed] [Google Scholar]
- Compton MT, Furman AC, Kaslow NJ. Preliminary evidence of an association between childhood abuse and cannabis dependence among African American first-episode schizophrenia-spectrum disorder patients. Drug Alcohol Depend. 2004;76:311–316. doi: 10.1016/j.drugalcdep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- De Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Debattista C, Belanoff J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends Endocrinol. Metab. 2006;17:117–121. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Di Forti M, Morrison PD, Butt A, Murray RM. Cannabis use and psychiatric and cogitive disorders: the chicken or the egg? Curr. Opin. Psychiatry. 2007;20:228–234. doi: 10.1097/YCO.0b013e3280fa838e. [DOI] [PubMed] [Google Scholar]
- Dinan TG. Stress: the shared common component in major mental illnesses. Eur. Psychiatry. 2005;20(Suppl 3):S326–S328. doi: 10.1016/s0924-9338(05)80184-1. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, bi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol. Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Fearon P, Kirkbride JB, Morgan C, Dazzan P, Morgan K, Lloyd T, Hutchinson G, Tarrant J, Fung WL, Holloway J, Mallett R, Harrison G, Leff J, Jones PB, Murray RM. Incidence of schizophrenia and other psychoses in ethnic minority groups: results from the MRC AESOP Study. Psychol. Med. 2006;36:1541–1550. doi: 10.1017/S0033291706008774. [DOI] [PubMed] [Google Scholar]
- Fisher H, Morgan C, Dazzan P, Craig TK, Morgan K, Hutchinson G, Jones PB, Doody GA, Pariante C, McGuffin P, Murray RM, Leff J, Fearon P. Gender differences in the association between childhood abuse and psychosis. Br. J. Psychiatry. 2009;194:319–325. doi: 10.1192/bjp.bp.107.047985. [DOI] [PubMed] [Google Scholar]
- Flores BH, Kenna H, Keller J, Solvason HB, Schatzberg AF. Clinical and biological effects of mifepristone treatment for psychotic depression. Neuropsychopharmacology. 2006;31:628–636. doi: 10.1038/sj.npp.1300884. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Watson S, Smith MS, Young AH, Ferrier IN. Plasma cortisol-dehydroepiandrosterone (DHEA) ratios in schizophrenia and bipolar disorder. Schizophr. Res. 2007;90:258–265. doi: 10.1016/j.schres.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, Brewer WJ, Smith DJ, Dazzan P, Berger GE, Yung AR, van den BM, Murray R, McGorry PD, Pantelis C. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol. Psychiatry. 2005;58:417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Szeszko PR, Gueorguieva R, Ashtari M, Robinson DG, Kane JM, Bilder RM. Cortisol levels in relation to hippocampal sub-regions in subjects with first episode schizophrenia. Schizophr. Res. 2007;94:281–287. doi: 10.1016/j.schres.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Hatzimanolis J, Lykouras L, Markianos M, Oulis P. Neurochemical variables in schizophrenic patients during switching from neuroleptics to clozapine. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22:1077–1085. doi: 10.1016/s0278-5846(98)00064-5. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary–adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Herz MI, Fava GA, Molnar G, Edwards L. The dexamethasone suppression test in newly hospitalized schizophrenic patients. Am. J. Psychiatry. 1985;142:127–129. doi: 10.1176/ajp.142.1.127. [DOI] [PubMed] [Google Scholar]
- Iancu I, Tchernihovsky E, Maayan R, Poreh A, Dannon P, Kotler M, Weizman A, Strous RD. Circulatory neurosteroid levels in smoking and non-smoking chronic schizophrenia patients. Eur. Neuropsychopharmacol. 2007;17:541–545. doi: 10.1016/j.euroneuro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Ismail K, Murray RM, Wheeler MJ, O’Keane V. The dexamethasone suppression test in schizophrenia. Psychol. Med. 1998;28:311–317. doi: 10.1017/s0033291797006521. [DOI] [PubMed] [Google Scholar]
- Jakovljevic M, Pivac N, Mihaljevic-Peles A, Mustapic M, Relja M, Ljubicic D, Marcinko D, Muck-Seler D. The effects of olanzapine and fluphenazine on plasma cortisol, prolactin and muscle rigidity in schizophrenic patients: a double blind study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:399–402. doi: 10.1016/j.pnpbp.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Gademan PJ, De Jonge RC, van der Linden JA, Kahn RS. Blunted cortisol response to a psychosocial stressor in schizophrenia. Schizophr. Res. 1998;33:87–94. doi: 10.1016/s0920-9964(98)00066-8. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Kahn RS. Selective impairments in the stress response in schizophrenic patients. Psychopharmacology (Berl) 2000;149:319–325. doi: 10.1007/s002130000381. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Cleare AJ, Papadopoulos AS, Poon L, Lightman S, Pariante CM. Different responses to dexamethasone and prednisolone in the same depressed patients. Psychopharmacology (Berl) 2006;189:225–235. doi: 10.1007/s00213-006-0555-4. [DOI] [PubMed] [Google Scholar]
- Kirkbride JB, Fearon P, Morgan C, Dazzan P, Morgan K, Tarrant J, Lloyd T, Holloway J, Hutchinson G, Leff JP, Mallett RM, Harrison GL, Murray RM, Jones PB. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AeSOP study. Arch. Gen. Psychiatry. 2006;63:250–258. doi: 10.1001/archpsyc.63.3.250. [DOI] [PubMed] [Google Scholar]
- Lammers CH, Garcia-Borreguero D, Schmider J, Gotthardt U, Dettling M, Holsboer F, Heuser IJ. Combined dexamethasone/corticotropin-releasing hormone test in patients with schizophrenia and in normal controls: II. Biol. Psychiatry. 1995;38:803–807. doi: 10.1016/0006-3223(95)00065-8. [DOI] [PubMed] [Google Scholar]
- Mallett R, Leff J, Bhugra D, Pang D, Zhao JH. Social environment, ethnicity and schizophrenia. A case–control study. Soc. Psychiatry Psychiatr. Epidemiol. 2002;37:329–335. doi: 10.1007/s00127-002-0557-4. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Rossbach W, Muller MJ, Muller-Siecheneder F, Pott T, Linde I, Dittmann RW, Hiemke C. Nocturnal hormone profiles in patients with schizophrenia treated with olanzapine. Psychoneuroendocrinology. 2006;31:256–264. doi: 10.1016/j.psyneuen.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Markianos M, Hatzimanolis J, Lykouras L. Switch from neuroleptics to clozapine does not influence pituitary–gonadal axis hormone levels in male schizophrenic patients. Eur. Neuropsychopharmacol. 1999;9:533–536. doi: 10.1016/s0924-977x(99)00040-1. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch. Gen. Psychiatry. 1991;48:764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Clinical studies on the mechanism of action of clozapine: the dopamine–serotonin hypothesis of schizophrenia. Psychopharmacology (Berl) 1989;99(Suppl):S18–S27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Dazzan P, Gabilondo A, Tournikioti K, Walshe M, Marshall N, Schulze KK, Murray RM, McDonald C, Pariante CM. Pituitary volume in unaffected relatives of patients with schizophrenia and bipolar disorder. Psychoneuroendocrinology. 2008;33:1004–1012. doi: 10.1016/j.psyneuen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Morgan C, Craig T, Leff J, Murray R, Abdul-Al R, Lappin JM, Jones P, Dazzan P. Clinical and social determinants of duration of untreated psychosis in the AESOP first-episode psychosis study. Br. J. Psychiatry. 2006;189:446–452. doi: 10.1192/bjp.bp.106.021303. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin. Psychol. Rev. 2007;27:409–424. doi: 10.1016/j.cpr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Arch. Gen. Psychiatry. 2001;58:1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J. Clin. Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- Oquendo MA, Echavarria G, Galfalvy HC, Grunebaum MF, Burke A, Barrera A, Cooper TB, Malone KM, John MJ. Lower cortisol levels in depressed patients with comorbid post-traumatic stress disorder. Neuropsychopharmacology. 2003;28:591–598. doi: 10.1038/sj.npp.1300050. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Pituitary volume in psychosis: the first review of the evidence. J. Psychopharmacol. 2008;22:76–81. doi: 10.1177/0269881107084020. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Vassilopoulou K, Velakoulis D, Phillips L, Soulsby B, Wood SJ, Brewer W, Smith DJ, Dazzan P, Yung AR, Zervas IM, Christodoulou GN, Murray R, McGorry PD, Pantelis C. Pituitary volume in psychosis. Br. J. Psychiatry. 2004;185:5–10. doi: 10.1192/bjp.185.1.5. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Dazzan P, Danese A, Morgan KD, Brudaglio F, Morgan C, Fearon P, Orr K, Hutchinson G, Pantelis C, Velakoulis D, Jones PB, Leff J, Murray RM. Increased pituitary volume in antipsychotic-free and antipsychotic-treated patients of the AEsop first-onset psychosis study. Neuropsychopharmacology. 2005;30:1923–1931. doi: 10.1038/sj.npp.1300766. [DOI] [PubMed] [Google Scholar]
- Popovic V, Doknic M, Maric N, Pekic S, Damjanovic A, Miljic D, Popovic S, Miljic N, Djurovic M, Jasovic-Gasic M, Dieguez C, Casanueva FF. Changes in neuroendocrine and metabolic hormones induced by atypical antipsychotics in normal-weight patients with schizophrenia. Neuroendocrinology. 2007;85:249–256. doi: 10.1159/000103868. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Boekestyn L, Bechard-Evans L, Abadi S, Vracotas N, Joober R, Pruessner JC, Malla AK. Sex differences in the cortisol response to awakening in recent onset psychosis. Psychoneuroendocrinology. 2008;33(8):1151–1154. doi: 10.1016/j.psyneuen.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Read J, van Os J, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr. Scand. 2005;112:330–350. doi: 10.1111/j.1600-0447.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Wessely S, Chalder T, Papadopoulos A, Cleare AJ. Salivary cortisol response to awakening in chronic fatigue syndrome. Br. J. Psychiatry. 2004;184:136–141. doi: 10.1192/bjp.184.2.136. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Kirschbaum C. The hypothalamic–pituitary–adrenal (HPA) axis in habitual smokers. Int. J. Psychophysiol. 2006;59:236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol. Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am. J. Psychiatry. 2003;160:284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary–adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29:1065–1070. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Sachar EJ, Kanter SS, Buie D, Engle R, Mehlman R. Psychoendocrinology of ego disintegration. Am. J. Psychiatry. 1970;126:1067–1078. doi: 10.1176/ajp.126.8.1067. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Lindley S. Glucocorticoid antagonists in neuropsychotic disorders. Eur. J. Pharmacol. 2008;583:358–364. doi: 10.1016/j.ejphar.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Scheepers FE, Gespen de Wied CC, Kahn RS. The effect of olanzapine treatment on m-chlorophenylpiperazine-induced hormone release in schizophrenia. J. Clin. Psychopharmacol. 2001;21:575–582. doi: 10.1097/00004714-200112000-00006. [DOI] [PubMed] [Google Scholar]
- Strous RD, Maayan R, Lapidus R, Goredetsky L, Zeldich E, Kotler M, Weizman A. Increased circulatory dehydroepiandrosterone and dehydroepiandrosterone-sulphate in first-episode schizophrenia: relationship to gender, aggression and symptomatology. Schizophr. Res. 2004;71:427–434. doi: 10.1016/j.schres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Tandon R, Mazzara C, DeQuardo J, Craig KA, Meador-Woodruff JH, Goldman R, Greden JF. Dexamethasone suppression test in schizophrenia: relationship to symptomatology, ventricular enlargement, and outcome. Biol. Psychiatry. 1991;29:953–964. doi: 10.1016/0006-3223(91)90353-n. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol. Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu. Rev. Clin. Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Wing JK, Sturt E. The PSE-ID-CATEGO System: Supplementary Manual Medical Research Council. 1978 [Google Scholar]
- World Health Organisation The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines Geneva. 1992 [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J. Clin. Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Cao LY, Wu GY, Shen YC. Cortisol and cytokines in chronic and treatment-resistant patients with schizophrenia: association with psychopathology and response to antipsychotics. Neuropsychopharmacology. 2005;30:1532–1538. doi: 10.1038/sj.npp.1300756. [DOI] [PubMed] [Google Scholar]


