Abstract
Depression and fatigue are frequent side effects of interferon-α (IFN-α) treatment, and there is compelling evidence that the inflammatory response system (including interleukin-6, IL-6) and the serotonergic system is important in the pathophysiology of such symptoms. Functional polymorphisms in the promoter region of the IL-6 gene (rs1800795) and serotonin transporter gene (5-HTTLPR) have been identified as regulating these systems. The present study aimed to determine if these polymorphisms were associated with the development of depression and fatigue during IFN-α and ribavirin treatment. Ninety-eight Caucasian patients receiving pegylated IFN-α and ribavirin treatment for chronic hepatitis C virus at King’s College Hospital, London, and Emory University Hospital, Atlanta, participated in this prospective cohort study. Symptoms of depression and fatigue were measured before treatment and at weeks 4, 8, 12 and 24 during treatment. The ‘low IL-6’ synthesizing genotype (CC) was associated with significantly fewer symptoms of depression (effect size = 0.7 at week 24; F = 9.4, d.f. = 436, P = 0.002). The ‘high transcription’ serotonin transporter (5-HTT) genotype (LL) was also associated with significantly fewer symptoms of depression, but with a much smaller effect (effect size = 0.2 at week 24; F = 4.5, d.f. = 436, P = 0.03). Neither polymorphisms were associated with symptoms of fatigue (IL-6: F = 1.2, d.f. = 430, P = 0.2; 5-HTT: F = 0.5, d.f. = 430, P = 0.5). The smaller effects of the 5-HTT polymorphism on depression may be explained by an interaction between the genes (F = 5.0, d.f. = 434, P = 0.02): the ‘protective’ effect of the 5-HTTLPR polymorphism was evident only in the presence of the ‘low IL-6’ genotype (F = 5.4, d.f. = 64, P = 0.02), not in the presence of the ‘high IL-6’ genotype (F = 2.2, d.f. = 369, P = 0.1). The association between the IL-6 polymorphism and reduced risk of depressive symptoms confirms the role of the inflammatory response system in the pathophysiology of IFN-α-induced depression; in contrast, the effect of the 5-HTT gene was small and perhaps dependent on the status of the inflammatory response.
Keywords: interleukin-6, serotonin transporter, depressive symptoms, fatigue, cytokine, immunotherapy
Introduction
There is compelling evidence that the inflammatory response system and the serotonergic system are important in the pathophysiology of depression and fatigue induced by interferon-α (IFN-α). Nevertheless, it is yet to be clarified whether genetic polymorphisms regulating these systems can predict the development of these psychopathological symptoms. The innate immune system cytokine, IFN-α, is the cornerstone of all pharmacological treatment for chronic hepatitis C virus (HCV) infection; the most effective treatment for this disabling and potentially life-threatening condition is a combination of pegylated (PEG) IFN-α and the antiviral agent ribavirin.1 IFN-α frequently induces a number of psychopathological side effects, including depression and fatigue, which impair patients’ quality of life. Moreover, depression and fatigue have been associated with lower viral clearance rates in some2,3 but not all studies;4,5 inconsistencies in the literature may be due to the confounding effects of antidepressant treatment.6 Identifying whether genetic factors predict IFN-α-induced psychopathological side effects will help clarify the molecular mechanisms underlying these symptoms, and could potentially assist in recognizing patients who will benefit from targeted prophylactic treatment. In turn, understanding the molecular mechanisms underlying IFN-α-induced psychopathological symptoms may also help clarify the mechanisms by which the innate immune system contributes to the development of depression and fatigue in patients with psychiatric disorders.
Innate immune system cytokines appear to be important in the pathophysiology of the psychopathological symptoms induced by IFN-α. IFN-α is used to treat HCV because of its potent antiviral, antiproliferation and immunomodulatory properties.7 Specifically, IFN-α modulates the cytokine network, stimulating the synthesis and secretion of proinflammatory cytokines.8,9 In the context of viral clearance these cytokines are useful, but unfortunately the association between proinflammatory cytokines and unpleasant behavioural changes is also well established. For example, during infection or inflammation, people and animals exhibit ‘sickness behaviours’ such as malaise, anorexia, sleep disturbances, fatigue and depression.10 Interleukin-6 (IL-6) appears to be important in IFN-α-induced symptoms, as demonstrated by studies showing increased plasma IL-6 concentrations during IFN-α treatment which correlate with the severity of psychopathological symptoms, including depression.11 Moreover, higher plasma IL-6 concentrations prior to starting IFN-α treatment have been found to predict depressive symptoms during treatment.12 These findings are also consistent with studies showing elevated concentrations of IL-6 in patients experiencing depression and, albeit less consistently, fatigue, as part of infection-induced sickness behaviour,13 major depressive disorders14-16 and chronic fatigue syndrome.17
A functional G > C single nucleotide polymorphism (SNP, rs1800795) has been identified in the promoter region of the IL-6 gene, which is associated with differential IL-6 expression and ultimately IL-6 plasma concentrations. Specifically, the C allele is associated with lower plasma concentrations of IL-6 during immune activation than the G allele.18 Moreover, the C allele is also associated with lower risk of autoimmune disorders,18,19 as well as less severe symptoms and lower mortality rates during cancer and cardiovascular diseases.20,21
Innate immune system cytokines have been suggested to induce depressive symptoms not only through direct effects on the brain, but also through their modulation of the serotonergic system. Specifically, these cytokines upregulate the expression of the first enzyme in the kynurenine pathway, indoleamine 2,3-dioxygenase (IDO).22 IDO is present in non-hepatic organs throughout the body and can be induced by inflammatory cytokines in a wide range of cells including myeloid-lineage cells, epithelial cells and fibroblasts.23 IDO metabolizes tryptophan, therefore when IDO is over-stimulated it may lead to a reduction of plasma tryptophan24,25 and consequently to lower concentrations of serotonin in the brain.26 The evidence that this pathway is activated in patients receiving IFN-α,27 along with the well-documented relationship between altered serotonergic neurotransmission and depression,28 implicates the serotonergic pathway in the pathophysiology of IFN-α-induced depressive symptoms. Of course, tryptophan availability is not the only factor influencing serotonergic neurotransmission. In particular, the serotonin transporter (5-HTT) affects the reuptake of serotonin into the presynaptic terminal and therefore ceases serotonergic neurotransmission; it is by blocking this mechanism that antidepressants, and in particular selective serotonin reuptake inhibitors (SSRIs), are argued to initiate their therapeutic effect.29 Indeed, because of the role of the 5-HTT in antidepressant action, it has been suggested that this transporter may be involved in the pathophysiology of depressive disorders.30
A common functional insertion/deletion polymorphism (5-HTTLPR) in the promoter region of the 5-HTT gene has been identified as altering promoter activity. It is widely hypothesized that the long (L) allele (16 repeats) is associated with higher transcription of 5-HTT compared to the short (S) allele (14 repeats), and therefore with increased serotonin binding and reuptake.31-34 The presence of the LL genotype has been found to be ‘protective’ against depression following stressful events30 and against suicide,35 as well as to predict a better response to SSRI antidepressants,36,37 although a recent study found no effects of this genotype on the occurrence of IFN-α-induced depression.5
In order to investigate the potential effects of these genetic polymorphisms on IFN-α-induced psychopathological symptoms, we conducted a prospective study in patients receiving PEG IFN-α and ribavirin treatment for HCV. The study was conducted in parallel in London, UK, and in Atlanta, GA, USA. Based on the previously mentioned evidence linking high concentrations of IL-6 and low availability of serotonin with psychopathological symptoms, we hypothesized that patients who carry two copies of the C ‘low IL-6’ allele, or of the L ‘high transcription’ 5-HTT allele, would develop significantly less depression and fatigue during PEG IFN-α and ribavirin treatment. Moreover, based on the previously mentioned evidence linking immune activation with lower tryptophan availability, we hypothesized that there would be an interaction between the IL-6 and the 5-HTT gene, so that the effects of the 5-HTT polymorphism would be evident only in the presence of normal tryptophan availability, that is, in the presence of the ‘low IL-6’ synthesis polymorphism. At the time when the study was conducted, there was no information on the putative role of serotonin-related genes in the development of IFN-α-induced depression; however, a recent paper, mentioned above, has found, in a large sample of German patients, that this 5-HTT polymorphism as well as a variation in the tryptophan hydroxylase-2 (TPH2) have no effect on the development of IFN-α-induced depression, whereas a polymorphism in the serotonin 5HT1a receptor gene has a significant effect on IFN-α-induced depression.5 The TPH2 and the 5HT1a receptor genes have not been analysed in our sample.
Materials and methods
Subjects
All 98 subjects in this study had chronic HCV infection with compensated liver disease and were scheduled to receive PEG IFN-α-2b (1.5 μg kg−1, weekly, via subcutaneously injection) and daily ribavirin (800–1200 mg) for at least 24 weeks. Subjects were recruited between January 2001 and July 2003 from the Institute of Liver Studies, King’s College Hospital, London, UK and Emory University Hospital, Atlanta, GA, USA; the two groups included 55 Caucasian individuals (42 Men, 13 women; mean age ± s.d. = 45.5 ± 7 years) and 43 Caucasian individuals (21 Men, 22 women; mean age ± s.d. = 45.6 ± 7 years), respectively. The two groups were recruited and studied independently, but with extensive liaison between the principal investigators at the planning stage to facilitate pooling of the data. This paper presents the only genetic study conducted on this pooled sample, and only these two specific hypothesis-driven candidate genes have been tested. Because of differences in frequency of the IL-6 and 5-HTT polymorphisms between ethnic groups,18,38 only Caucasian subjects were included in the genetics study presented here. Further information on the psychopathological symptoms experienced by the complete sample is described elsewhere.2,39
Subjects had a baseline psychiatric evaluation of current mental state and previous psychiatric history, using the Clinical Interview Schedule—Revised40 in the UK sample and the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th edn (SCID)41 in the USA sample. Subjects with a current severe depression or psychotic illness were excluded, as were those with a history of substance abuse who had not been abstinent for at least 6 months. Other exclusion criteria included prior treatment with IFN-α therapy, pregnancy, autoimmune disorder and any cause for liver disease other than HCV. The study was approved by King’s College Hospital Research Ethics committee and by the Emory University School of Medicine Institutional Review Board, and all subjects provided written informed consent.
Study design
The study utilized a prospective cohort design. Subjects were evaluated at baseline (immediately before treatment began) and at weeks 4, 8, 12 and 24 during PEG IFN-α and ribavirin treatment. Psychopathological symptoms at both sites were evaluated at each visit through self-report questionnaires previously used by the authors in similar populations.42-44 For symptoms of depression, the UK site used the Beck Depression Inventory,45 whereas the USA site used the Zung Self-Rating Depression Scale (SDS) expressed as 100 point (SDS Index).46 Fatigue was assessed at both sites using the Chalder Fatigue Questionnaire.47 The Chalder Fatigue Questionnaire has 14 items that measure mental and physical fatigue. Subjects select the most appropriate answer to questions such as ‘Do you have problems with tiredness?’ from four responses that lie along a continuum: ‘Less than usual’, ‘no more than usual’, ‘worse than usual’ and ‘much worse than usual’.
Antidepressant administration was dictated by the clinical judgement of treating physicians and was not controlled by study protocol. Nevertheless, concomitant medications including antidepressants were recorded by study personnel at each assessment.
DNA extraction
Genomic DNA was extracted from cheek swabs using the Freeman method48 and from buffy coats using the Qiagen M48 robotic DNA extraction protocol (Qiagen Inc., Valencia, CA, USA) from patients from both the United Kingdom and the United States, respectively.
Genotyping the IL-6 polymorphism
The SNP in the promoter region of the IL-6 gene (rs1800795) was genotyped using a PCR-restriction fragment length polymorphism assay as described in Fishman et al.18 The region including the polymorphism was amplified using the following primers: forward: 5′-CAGGAAGAACTCAGATGACTGG-3′; reverse: 5′-GCTGGGCTCCTGGAGGGG-3′. PCR conditions were 20 μM of each primer, 2.5 μM of each dNTP, 1 U Taq, 10 × PCR buffer, 25 mM MgCl2 and 10 ng μl−1 genomic DNA. An initial denaturation step at 95 °C for 10 min was followed by 30 cycles of the following conditions, 95 °C for 15 s, then 55 °C for 15 s and 72 °C for 1 min, with a final extension at 72 °C for 10 min. The PCR product (612 bp) was then digested with 1 U SfaNI restriction enzyme at 37 °C for 90 min and the digested products were visualized by electrophoresis on a 3% agarose gel. The C/C homozygote samples produced an undigested product of 612 bp. The G/G homozygote samples produced two visible products of 377 and 235 bp. The G/C heterozygote samples produced three visible products of 612, 377 and 235 bp.
Genotyping the 5-HTT Polymorphism
The polymorphism in the promoter region of the 5-HTT gene (5-HTTLPR) was genotyped applying PCR amplification using the following primers: forward: 5′-GAATGCCAGCACCTAACC-3′; reverse: 5-ATACTGCGAGGGGTGCAG-3. PCR was carried out in 384-well plates in a 10 μl volume with 10 ng DNA. Each PCR reaction contained 0.5 μm of each primer, 0.08 μm of dATP, dCTP and dTTP and 0.04 μm of dGTP, 0.2 μM of 7-deazaGTP (Amersham Biosciences, Piscataway, NJ, USA), 5% dimethyl sulphoxide and 1.25 U of AmpliTaq Gold (Applied Biosystems, Foster City, CA, USA). The cycling parameters were as follows 95 °C for 5 min; then 94 °C for 30 s, 63 °C for 30 s and 72 °C for 1 min for one cycle; then the annealing temperature was reduced to 62 °C for one more cycle and then to 59.5 °C for 38 cycles. The PCR products were then separated using an Applied Biosystems 3730 genetic analyzer and analysed with Applied Biosystems Genemapper 4.0 software. Fragment lengths for the L allele were 291 and 247 bp for the S allele.
Statistical analyses
SPSS was used to conduct the main statistical analyses on the combined sample; the scores from the self-report scales were standardized as the effect size of the difference between baseline and each subsequent time point. Specifically, the difference between each subject’s score at each time point and the mean sample score at the baseline was calculated, and then divided by the standard deviation of the sample score at the baseline. A Mixed Linear Model approach for longitudinal data was used to investigate the between-groups effects of the IL-6 and 5-HTT polymorphisms, whereas Dunnett’s post-hoc test was used to detect within-group changes between baseline and successive time points. Baseline (pre-treatment) measures of psychopathology were used as co-variates in the analysis. T-tests and χ2-analyses were used to test for differences between groups in sociodemographic and clinical features. Data are presented as mean ± s.e.m. Two-tailed hypotheses were used with a statistical significance level set at P < 0.05.
For statistical analyses, GG and GC (IL-6 gene) individuals, as well as SS and SL (5-HTT gene) individuals, were grouped together because in most functional analyses the G and the S alleles appear to have a dominant mode of action in their respective genes.18,31,32,49
Results
Distribution of genotypes
The IL-6 and 5-HTT polymorphism frequencies are presented in Table 1, for the sample as a whole and by country of recruitment; the genotypic distribution of the whole sample was in Hardy–Weinberg equilibrium for the IL-6 (χ2 = 0.01, d.f. = 1, P = 0.9) and 5-HTT (χ2 = 0.8, d.f. = 1, P = 0.9) polymorphisms. There was no difference between the two IL-6 genotype groups, or the two 5-HTT genotype groups, in age, gender distribution, history (including baseline) of psychiatric diagnoses, use of antidepressant medications at baseline or at any time during treatment and use of other concomitant medications (sedatives in eight subjects, other medications in three subjects) (see Table 2).
Table 1.
Genotype frequencies (number (percentage)) in the HCV clinical cohort
| IL-6 genotype |
Total cohort (n = 98) |
UK (n = 55) |
USA (n = 43) |
|---|---|---|---|
|
| |||
| CC | 15 (15) | 10 (18) | 5 (11) |
| GC | 42 (43) | 21 (38) | 21 (49) |
| GG | 41 (42) | 24 (44) | 17 (40) |
| C allele | 0.367 | 0.373 | 0.360 |
|
5-HTT
genotype |
Total cohort (n = 98) |
UK (n = 55) |
USA (n = 43) |
|---|---|---|---|
| LL | 44 (45) | 25 (45) | 19 (44) |
| SL | 43 (44) | 23 (42) | 20 (47) |
| SS | 11 (11) | 7 (13) | 4 (9) |
| L allele | 0.668 | 0.664 | 0.674 |
Abbreviations: 5-HTT, serotonin transporter; IL, interleukin.
Table 2.
Characteristics of the cohort (frequency (percentage)) according to genotype
|
IL-6 polymorphism
groups |
CC (n = 15) |
GC and GG (n = 83) |
P-valuea |
|---|---|---|---|
| Age, year (mean ± s.d.) | 44.80 ± 5.7 | 45.72 ± 7.2 | 0.6 |
| Gender (%) | |||
| Woman | 3 (20) | 32 (38.6) | 0.2 |
| Men | 12 (80) | 51 (61.4) | |
| History of drug addiction | |||
| Yes | 5 (33.3) | 34 (41.0) | 0.8 |
| No | 10 (66.7) | 49 (59.0) | |
| History of depression | |||
| Yes | 2 (13.3) | 26 (31.3) | 0.2 |
| No | 13 (86.7) | 57 (68.7) | |
| Use of antidepressants any time during IFN-α treatment | |||
| Yes | 4 (26.7) | 26 (31.3) | 1.00 |
| No | 11 (73.3) | 57 (68.7) | |
| Use of antidepressants prophylactically | |||
| Yes | 4 (26.7) | 17 (20.5) | 0.7 |
| No | 11 (73.3) | 66 (79.5) | |
| Use of concomitant medication | |||
| Yes | 0 (0.0) | 11 (13.3) | 0.2 |
| No | 15 (100.0) | 72 (86.7) | |
|
5-HTT polymorphism
groups |
LL (n = 44) |
LS and SS (n = 54) |
P-valuea |
|---|---|---|---|
| Age, year (mean ± s.d.) | 45.18 ± 7.71 | 45.91 ± 6.47 | 0.6 |
| Gender (%) | |||
| Woman | 18 (40.9) | 17 (31.5) | 0.4 |
| Men | 26 (59.1) | 37 (68.5) | |
| History of drug addiction | |||
| Yes | 18 (41.0) | 21 (38.9) | 1.00 |
| No | 26 (59.0) | 33 (61.1) | |
| History of depression | |||
| Yes | 13 (29.5) | 15 (27.8) | 1.00 |
| No | 31 (70.5) | 39 (71.2) | |
| Use of antidepressants any time during IFN-α treatment | |||
| Yes | 10 (22.7) | 20 (37.0) | 0.2 |
| No | 34 (77.3) | 34 (63.0) | |
| Use of antidepressants prophylactically | |||
| Yes | 9 (20.5) | 12 (22.2) | 1.00 |
| No | 35 (79.5) | 42 (77.8) | |
| Use of concomitant medication | |||
| Yes | 5 (11.4) | 6 (11.1) | 1.00 |
| No | 39 (88.6) | 48 (88.9) | |
Abbreviation: IL, interleukin.
χ2-test, except for the age differences which was analysed using Student’s t-test.
Effects of IL-6 genotype on psychopathological symptoms
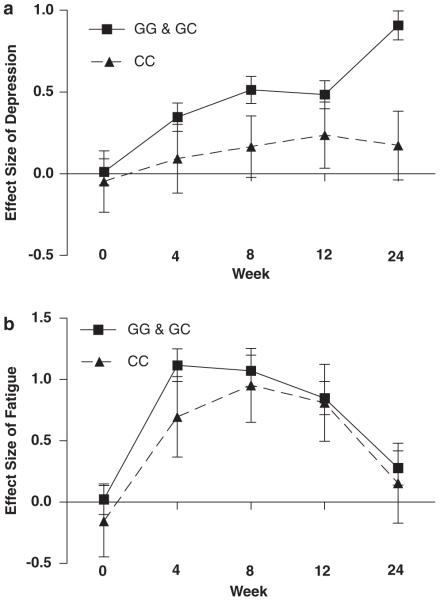
We found a main effect of IL-6 genotype on depressive symptoms during IFN-α and ribavirin treatment (Figure 1a), but not on fatigue (Mixed Linear Model, F = 1.2, d.f. = 430, P = 0.3; Figure 1b). Specifically, and consistent with our hypothesis, CC subjects developed fewer depressive symptoms compared to the grouped GG/GC genotypes (F = 9.4, d.f. = 436, P = 0.002). This difference was large: at week 24, there was a 0.7 effect size difference between the genotype groups. Moreover, subjects with the CC genotype did not show a statistically significant increase in depressive symptoms at any time points when compared with baseline (Dunnett’s test, P-value 0.8–1.0), whereas subjects with the GG/GC genotypes showed a statistically significant increase in depressive symptoms at weeks 8, 12 and 24 (Dunnett’s test, P-value < 0.001–0.03).
Figure 1.
Depressive and fatigue symptom scores in the two interleukin-6 (IL-6) genotype groups. Symptoms are expressed as effect size (mean change from baseline ± s.e.m.) during 24 weeks of interferon-α (IFN-α) and ribavirin treatment. (a) Subjects with the CC genotype (n = 15) experienced significantly fewer depressive symptoms than the combined GG and GC genotype group (n = 83) (P = 0.002). (b) There was no difference in fatigue symptoms experienced by the CC genotype (n = 15) and the combined GG and GC genotype group (n = 83) (P = 0.3).
Similar results (but with less statistical power) were obtained when the analyses were conducted separately according to the country of recruitment. In the English sample, CC subjects developed fewer depressive symptoms compared to the grouped GG/GC genotypes (F = 4.3, d.f. = 222, P = 0.04). Moreover, at week 24, there was a 0.85 effect size difference between the genotype groups. Finally, the subjects with the CC genotype did not show a statistically significant increase in depressive symptoms at any time points when compared with baseline (Dunnett’s test, P ranging 0.9–1.0), whereas subjects with the GG/GC genotypes showed a statistically significant increase in depressive symptoms at week 24 (Dunnett’s test, P = 0.05). In the American sample, CC subjects also developed fewer depressive symptoms compared to the grouped GG/GC genotypes, but this did not reach statistical significance (F = 3.1, d.f. = 211, P = 0.078). Moreover, at week 24 there was a 0.4 effect size difference between the genotype groups. Finally, subjects with the CC genotype did not show a statistically significant increase in depressive symptoms at any time points when compared with baseline (Dunnett’s test, P ranging 0.3–1.0), whereas subjects with the GG/GC genotypes showed a statistically significant increase in depressive symptoms at weeks 8, 12 and 24 (Dunnett’s test, P ranging < 0.001–0.03).
Effects of 5-HTT genotype on psychopathological symptoms
We found a main effect of 5-HTT genotype on depressive symptoms during IFN-α and ribavirin treatment (Figure 2a), but not on fatigue (F = 0.5, d.f. = 430, P = 0.5; Figure 2b). Specifically, and consistent with our hypothesis, LL subjects developed significantly fewer depressive symptoms during treatment than the grouped SS/SL genotypes (F = 4.5, d.f. = 436, P = 0.03). However, although statistically significant, this difference was smaller than the difference (described above) associated with the IL-6 genotype: at week 24, there was a 0.2 effect size difference between the genotype groups. Comparisons between baseline and subsequent time points also indicate an effect of 5-HTT genotype, although not as strong as that of the IL-6 genotype: subjects with the LL genotype showed a statistically significant increase in depressive symptoms but only at week 24 compared with baseline (Dunnett’s test, P = 0.003), whereas subjects with the SS/SL genotypes showed a statistically significant increase in depressive symptoms at both weeks 8 and 24, and a statistical trend at week 4 (Dunnett’s test, P ranging < 0.001–0.06).
Figure 2.
Depressive and fatigue symptom scores in the two 5-HTT genotype groups. Symptoms are expressed as effect size (mean change from baseline ± s.e.m.) during 24 weeks of interferon-α (IFN-α) and ribavirin treatment. (a) Subjects with the LL genotype (n = 45) experienced significantly fewer depressive symptoms than the combined SS and SL genotype group (n = 54) (P = 0.03). (b) There was no difference in fatigue symptoms experienced by the LL genotype (n = 45) and the combined SS and SL genotype group (n = 543) (P = 0.5).
Again, similar results (but with less statistical power) were obtained when the analyses were conducted separately according to the country of recruitment. In the English sample, LL subjects developed fewer depressive symptoms compared to the grouped SS/SL genotypes, but this was not statistically significant (F = 0.4, d.f. = 222, P = 0.5); the largest difference between the genotype groups was at week 8 (0.3 effect size); and neither genotype groups show a statistically significant increase in depressive symptoms at any time points when compared with baseline (Dunnett’s test, P ranging 0.2–1.0). In the American sample, LL subjects also developed fewer depressive symptoms compared to the grouped SL/SS genotypes (F = 4.0, d.f. = 211, P = 0.047); at week 24 there was a 0.4 effect size difference between the genotype groups; and subjects with the LL genotype showed a statistically significant increase in depressive symptoms only at week 24 (Dunnett’s test, P = 0.03), whereas subjects with the SL/SS genotypes showed a statistically significant increase in depressive symptoms at week 24 (P < 0.001) and statistical trends for an increase at weeks 4, 8 and 12 (Dunnett’s test, P ranging 0.068–0.099).
Interaction between genes
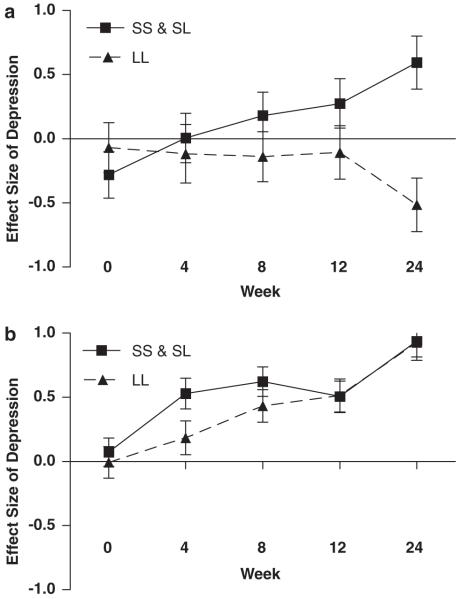
In addition to the main effect of both polymorphisms on depressive symptoms during treatment, there was also a gene–gene interaction (F = 5.0, d.f. = 434, P = 0.02). We investigated this interaction by stratified analysis based on our a priori hypothesis. Specifically, we tested the effects of the 5-HTT polymorphism within each of the two IL-6 genotype groups separately. Within the group of subjects who had the ‘low IL-6’ genotype, the LL 5-HTT genotype was associated with significantly fewer depressive symptoms (F = 5.4, d.f. = 64, P = 0.02; Figure 3a): at week 24, there was a 1.1 effect size difference between the genotype groups. However, the LL polymorphism was not associated with a protective effect in patients who had the ‘high IL-6’ genotypes (F = 2.2, d.f. = 369, P = 0.1; Figure 3b): at week 24, there was virtually no difference (0.01 effects size) between the genotype groups.
Figure 3.
Interaction between interleukin-6 (IL-6) and 5-HTT genotype on depressive symptom scores. Symptoms are expressed as effect size (mean change from baseline ± s.e.m.) during 24 weeks of interferon-α (IFN-α) and ribavirin treatment. There was a significant gene–gene interaction (P = 0.02). (a) Within the CC genotype group, LL genotype subjects (n = 5) experienced significantly fewer depressive symptoms than the SS/SL genotype subjects (n =7) (P = 0.02). (b) Within the GG/GC genotype group, there was no difference in depressive symptoms experienced by the LL genotype subjects (n = 33) and the SS/SL genotype subjects (n = 39) (P = 0.1).
Discussion
This study is the first to report an association between the G > C polymorphism in the IL-6 gene and the 5-HTTLPR insertion/deletion polymorphism in the 5-HTT gene, and protection against the development of depressive symptoms induced by IFN-α treatment. Specifically, carrying two copies of the ‘low IL-6’ C allele or of the ‘high transcription’ L polymorphism was associated with a less marked increase in depressive symptoms. The protective effect of ‘low IL-6’ genotype was large and present irrespective of the 5-HTT polymorphism. In contrast, the effect of the 5-HTT polymorphism (in the whole sample) was small, and subsequent subgroup analyses show that this effect was only evident in patients who also carry the ‘low IL-6’ CC genotype. Neither polymorphism offered any protection against the fatigue developed during treatment.
The finding that the ‘low IL-6’ genotype is associated with fewer depressive symptoms during IFN-α treatment is consistent with evidence that plasma IL-6 positively correlates with depressive symptoms during IFN-α treatment,11 infections13 and major depression.14-16 Our data also supports previously published preliminary finding from the American sample, examining the role of the IFN-α receptor gene in the development of depression.50 The authors found that the 5/5 or 5/14 genotype of a GT repeat dinucleotide microsatellite polymorphism was associated with a larger increase in depressive scores during IFN-α treatment. Because this genotype has also been associated with a superior antiviral activity,51 these data further support the notion that higher immunological activity is associated with the development of IFN-α-induced depression.
We found that the LL 5-HTT genotype was associated with a much smaller protective effect than the CC IL-6 genotype; interestingly Kraus and co-workers5 found no effect of this polymorphism on IFN-α-induced depression. Of course, previous studies have demonstrated, in psychiatric populations, that the LL 5-HTT genotype is protective against depression30 and suicide,35 and is associated with a better clinical response to antidepressants.36,37 Therefore, it is interesting to speculate that the protective effects of the LL genotype can only be evident under specific biological circumstances—for example, in the presence of low inflammation as discussed below. In contrast with the 5-HTT genotype, a 5HT1A receptor gene polymorphism (C-1019G) has been shown by Kraus and co-workers5 to have a profound effect on IFN-α-induced depression, measured as both occurrence of clinically significant symptoms and as changes in depressive symptoms from baseline.
It is somewhat surprising that in our study neither the IL-6 nor the 5-HTT polymorphism was associated with fatigue. Plasma concentrations of IL-6 have previously been found to correlate with fatigue;13 but our findings do not necessarily contradict such evidence. It is important to note that the absence of an association between this polymorphism and fatigue does not exclude IL-6 from the pathophysiology of fatigue, because factors other than the polymorphism may have induced elevated IL-6. However, the role of IL-6 in fatigue is not certain because it can be difficult to differentiate the role of IL-6 and IL-1 in inducing behavioural changes as they are both typically elevated during immune activation. Our study is consistent with a recent paper that found no association between this IL-6 polymorphism and chronic fatigue syndrome.52 There is a paucity of data regarding the relationship between serotonin transporter and fatigue, and the results have thus far been inconsistent. In one small association study, patients with chronic fatigue syndrome had higher prevalence of the L allele compared to controls,53 but a recent neuroimaging study has shown reduced concentrations of 5-HTT in the brain of patients with chronic fatigue syndrome.54
Even if depression and fatigue are both cytokine-induced ‘sickness behaviours’ and typically occur together, do the differences in their genetic regulation indicate a different pathophysiology? Dimensional analysis of symptoms experienced by patients taking IFN-α for malignant melanoma reveals that fatigue (together with psychomotor slowing and anorexia) is distinct from depressive symptoms (depressed mood, anhedonia and anxiety): fatigue occurs earlier in treatment and responds less to antidepressant treatment, whereas depressive symptoms develop later in the treatment and respond more to antidepressants.55 In our previous prospective cohort studies of patients taking IFN-α for HCV (both in the United States and the United Kingdom), the symptoms follow this same pattern, with increases in fatigue evident earlier than depressive symptoms.2,39 However, not all studies have shown depressive symptoms peaking at the end of the treatment, as in this paper and our previous reports;2,39 indeed, samples where depressive symptoms reach maximum scores already at weeks 12–16 have also been described.56,57 Two biological mechanisms also appear to be associated with developing depression but not fatigue during IFN-α treatment. First, patients who have a larger response of the corticotropin-releasing hormone-mediated stress pathway to an initial dose of IFN-α are significantly more likely to develop major depression during treatment, but not fatigue.58 Second, the decrease of plasma tryptophan and the increase of kynurenine and neopterin during IFN-α treatment (indicating increased IDO activity) correlate with the development of depression, but not fatigue.59 Taken together with the present study, these differences between depression and fatigue in time course, response to treatment and correlations with biological variables, suggest that IFN-α-induced depression and fatigue are distinct and reflect different pathophysiological processes.
Our data also indicate the presence of an interaction between the IL-6 and the 5-HTT genes in the development of depressive symptoms. Although the ‘high IL-6’ carriers were consistently more depressed than ‘low IL-6’ carriers, within the ‘high IL-6’ group there was no protective effect associated with the 5-HTT LL genotype: the protective effect of the LL genotype was only evident within the ‘low IL-6’ CC group. Although we did not measure tryptophan availability, these findings indirectly support previous studies indicating that the effects of this 5-HTT polymorphism are influenced by the availability of tryptophan.60,61 Theoretically, subjects with ‘high IL-6’ genotype could have hyperactive IDO and therefore reduced plasma tryptophan, whereas subjects with the ‘low IL-6’ genotype would have normal plasma tryptophan. As suggested above, the fact that putative protective effect of 5-HTT LL genotype is dependent on other conditions (genetic and immunological) could explain the overall small protective effect in our study as well as the lack of any protective effect in the study by Kraus and co-workers.5
One of our aims in conducting this study was to predict which patients were at an increased risk of developing psychopathological symptoms, with the aspiration that genotyping could be used in the future as a clinical screening tool, to offer targeted prophylactic treatment. Although plasma IL-6 concentrations before starting IFN-α treatment have been found to predict the development of symptoms,12 measuring plasma cytokines at one time point does not provide a reliable cytokine secretion profile62 and is therefore not an ideal screening tool to identify trait vulnerability within a clinical setting. Our findings indicate that those patients with both the CC (IL-6) and LL (5-HTT) polymorphisms are less likely to develop severe symptoms, but this identifies a relative small group of resilient individuals, rather than those at greatest risk who would most benefit from prophylactic treatment. Nonetheless, considering the debate around the use of prophylactic treatment with antidepressants,63,64 and the administration of IFN-α in patients with mental disorders,42,43,65 even identifying a ‘very low risk’ group is important.
A few limitations must be mentioned. This study was comprised of subjects recruited in both the United Kingdom and the United States, and depression was measured using two different scales in the two sites. However, in our analysis, we minimized the difference between sites, by standardizing the scores to effect size and adjusting for baseline measures. Moreover, when the data were analyzed in accordance with country of recruitment, the direction of the findings was similar across the two centres (albeit with lower statistical power, and more consistently for the IL-6 genotype then for the 5-HTT genotype). Of course, the findings might be confounded by the use of antidepressant or other psychotropic medications; reassuringly, there were no differences between the genotype groups in these variables. However, the small number of subjects precluded us from conducting further subgroup analyses, and therefore we cannot exclude differences in the pattern of use of psychotropic medications; for example, the protective genotypes might be associated with early use of antidepressants and hence with lower depression scores as a result of antidepressant treatment. Finally, our findings of an interaction between the IL-6 and the 5-HTT genes should be approached with caution, given the relatively small sample size and the lack of concomitant biological measures, such as plasma and cerebrospinal fluid concentrations of IL-6 or tryptophan. A strength of this study is that both candidate genes were hypothesis driven, and based on previous research that has identified these SNPs as being functional. Moreover, the allelic frequencies in our sample were similar to those in the general population, as previously reported in the literature: IL-6 C allele = 37% in our sample vs 40% in the study of Fishman et al.18 and 5-HTT L allele = 66% in our sample vs 65% in Hu et al.66 Therefore, we would suggest that data from this sample may be extrapolated to the general Caucasian population.
In conclusion, our study shows that functional polymorphisms in the IL-6 gene (rs1800795) and, with a smaller effect, the serotonin transporter gene (5-HTTLPR) are protective factors against the development of depressive (but not fatigue) symptoms during IFN-α treatment, confirming the role of the inflammatory response and serotonergic systems in the pathophysiology of IFN-α-induced depression and possibly in the pathophysiology of major depression.
Acknowledgments
Dr CM Pariante and the UK arm of this study were funded by grants from the Guy’s and St Thomas’ Charitable Foundation, the NIHR Specialist Biomedical Research Centre for Mental Health at South London and Maudsley NHS Trust and Institute of Psychiatry (Kings’ College London), the UK Medical Research Council and the NARSAD. The USA arm of the study was funded by grant numbers K05 MH069124 and R01 MH60723 (National Institute of Mental Health), a NARSAD Independent Investigator Award to J Cubells, and by support from the Schering-Plough corporation.
Footnotes
Disclosure/Conflict of Interest
Professor AH Miller has served as a consultant or an advisory board member for Schering-Plough and Centocor, and has received research funding from Janssen/Johnson and Johnson, GlaxoSmithKline and Schering-Plough.
References
- 1.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 2.Maddock C, Landau S, Barry K, Maulayah P, Hotopf M, Cleare AJ, et al. Psychopathological symptoms during interferon-alpha and ribavirin treatment: effects on virologic response. Mol Psychiatry. 2005;10:332–333. doi: 10.1038/sj.mp.4001634. [DOI] [PubMed] [Google Scholar]
- 3.Raison CL, Broadwell SD, Borisov AS, Manatunga AK, Capuron L, Woolwine BJ, et al. Depressive symptoms and viral clearance in patients receiving interferon-alpha and ribavirin for hepatitis C. Brain Behav Immun. 2005;19:23–27. doi: 10.1016/j.bbi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Loftis JM, Hauser P, Rifai MA. The association between viral clearance and depression in patients with hepatitis C receiving interferon-alpha and ribavirin. Brain Behav Immun. 2005;19:271–272. doi: 10.1016/j.bbi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Schafer A, Scheurlen M, Weissbrich B, Schottker K, Kraus MR. Sustained virological response in the antiviral therapy of chronic hepatitis C: is there a predictive value of interferon-induced depression? Chemotherapy. 2007;53:292–299. doi: 10.1159/000102584. [DOI] [PubMed] [Google Scholar]
- 6.Raison CL, Capuron L, Miller AH. Depressive symptoms and viral clearance: in response. Brain Behav Immun. 2005;19:273–274. doi: 10.1016/j.bbi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, Bordens R, et al. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998;58:2489–2499. [PubMed] [Google Scholar]
- 8.Biro L, Varga L, Par A, Nemesanszky E, Telegdy L, Ibranyi E, et al. C5b-9 and interleukin-6 in chronic hepatitis C. Surrogate markers predicting short-term response to interferon alpha-2b. Scand J Gastroenterol. 2000;35:1092–1096. doi: 10.1080/003655200451234. [DOI] [PubMed] [Google Scholar]
- 9.Kido M, Kumagai N, Toda K, Tsuchimoto K, Komiyama T. Differential induction of serum interleukin-6 and -12 by interferon-alpha and -beta administration in chronic hepatitis C patients. Hepatol Res. 2003;27:101–108. doi: 10.1016/s1386-6346(03)00200-6. [DOI] [PubMed] [Google Scholar]
- 10.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 11.Bonaccorso S, Puzella A, Marino V, Pasquini M, Biondi M, Artini M, et al. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res. 2001;105:45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- 12.Wichers MC, Kenis G, Leue C, Koek G, Robaeys G, Maes M. Baseline immune activation as a risk factor for the onset of depression during interferon-alpha treatment. Biol Psychiatry. 2006;60:77–79. doi: 10.1016/j.biopsych.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Vollmer-Conna U, Fazou C, Cameron B, Li H, Brennan C, Luck L, et al. Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans. Psychol Med. 2004;34:1289–1297. doi: 10.1017/s0033291704001953. [DOI] [PubMed] [Google Scholar]
- 14.Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- 15.Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, Maes M, et al. Indicators of immune activation in major depression. Psychiatry Res. 1996;64:161–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- 16.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 17.Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schurmeyer TH, et al. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fife BL. The role of constructed meaning in adaptation to the onset of life-threatening illness. Soc Sci Med. 2005;61:2132–2143. doi: 10.1016/j.socscimed.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Antonicelli R, Olivieri F, Bonafe M, Cavallone L, Spazzafumo L, Marchegiani F, et al. The interleukin-6-174 G > C promoter polymorphism is associated with a higher risk of death after an acute coronary syndrome in male elderly patients. Int J Cardiol. 2005;103:266–271. doi: 10.1016/j.ijcard.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 21.DeMichele A, Martin AM, Mick R, Gor P, Wray L, Klein-Cabral M, et al. Interleukin-6-174G→C polymorphism is associated with improved outcome in high-risk breast cancer. Cancer Res. 2003;63:8051–8056. [PubMed] [Google Scholar]
- 22.Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J Psychiatry Neurosci. 2004;29:11–17. [PMC free article] [PubMed] [Google Scholar]
- 23.King NJ, Thomas SR. Molecules in focus: indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol. 2007;39:2167–2172. doi: 10.1016/j.biocel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Wirleitner B, Neurauter G, Schrocksnadel K, Frick B, Fuchs D. Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10:1581–1591. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- 25.Russo S, Kema IP, Fokkema MR, Boon JC, Willemse PH, de Vries EG, et al. Tryptophan as a link between psychopathology and somatic states. Psychosom Med. 2003;65:665–671. doi: 10.1097/01.psy.0000078188.74020.cc. [DOI] [PubMed] [Google Scholar]
- 26.Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115(Part 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 27.Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 28.Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 29.Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51:215–235. doi: 10.1016/s0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- 30.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 31.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 32.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 33.Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, et al. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- 34.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 35.Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: II. Suicidal behavior. Mol Psychiatry. 2003;8:646–653. doi: 10.1038/sj.mp.4001336. [DOI] [PubMed] [Google Scholar]
- 36.Lerer B, Macciardi F. Pharmacogenetics of antidepressant and mood-stabilizing drugs: a review of candidate-gene studies and future research directions. Int J Neuropsychopharmacol. 2002;5:255–275. doi: 10.1017/S1461145702002936. [DOI] [PubMed] [Google Scholar]
- 37.Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5:32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- 39.Raison CL, Broadwell SD, Borisov AS, Manatunga AK, Capuron L, Woolwine BJ, et al. Depressive symptoms and viral clearance in patients receiving interferon-alpha and ribavirin for hepatitis C. Brain Behav Immun. 2005;19:23–27. doi: 10.1016/j.bbi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med. 1992;22:465–486. doi: 10.1017/s0033291700030415. [DOI] [PubMed] [Google Scholar]
- 41.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Association; Washington, DC: 1997. [Google Scholar]
- 42.Maddock C, Baita A, Orru MG, Sitzia R, Costa A, Muntoni E, et al. Psychopharmacological treatment of depression, anxiety, irritability and insomnia in patients receiving interferon-alpha: a prospective case series and a discussion of biological mechanisms. J Psychopharmacol. 2004;18:41–46. doi: 10.1177/0269881104040230. [DOI] [PubMed] [Google Scholar]
- 43.Pariante CM, Orru MG, Baita A, Farci MG, Carpiniello B. Treatment with interferon-alpha in patients with chronic hepatitis and mood or anxiety disorders. Lancet. 1999;354:131–132. doi: 10.1016/S0140-6736(98)04793-X. [DOI] [PubMed] [Google Scholar]
- 44.Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 46.Zung WW. A Self-rating Depression Scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 47.Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. 1993;37:147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 48.Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav Genet. 2003;33:67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- 49.Beitchman JH, Baldassarra L, Mik H, De Luca V, King N, Bender D, et al. Serotonin transporter polymorphisms and persistent, pervasive childhood aggression. Am J Psychiatry. 2006;163:1103–1105. doi: 10.1176/ajp.2006.163.6.1103. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida K, Alagbe O, Wang X, Woolwine B, Thornbury M, Raison CL, et al. Promoter polymorphisms of the interferon-alpha receptor gene and development of interferon-induced depressive symptoms in patients with chronic hepatitis C: preliminary findings. Neuropsychobiology. 2005;52:55–61. doi: 10.1159/000086605. [DOI] [PubMed] [Google Scholar]
- 51.Matsuyama N, Mishiro S, Sugimoto M, Furuichi Y, Hashimoto M, Hijikata M, et al. The dinucleotide microsatellite polymorphism of the IFNAR1 gene promoter correlates with responsiveness of hepatitis C patients to interferon. Hepatol Res. 2003;25:221–225. doi: 10.1016/s1386-6346(02)00269-3. [DOI] [PubMed] [Google Scholar]
- 52.Carlo-Stella N, Badulli C, De Silvestri A, Bazzichi L, Martinetti M, Lorusso L, et al. A first study of cytokine genomic polymorphisms in CFS: positive association of TNF-857 and IFNgamma 874 rare alleles. Clin Exp Rheumatol. 2006;24:179–182. [PubMed] [Google Scholar]
- 53.Narita M, Nishigami N, Narita N, Yamaguti K, Okado N, Watanabe Y, et al. Association between serotonin transporter gene polymorphism and chronic fatigue syndrome. Biochem Biophys Res Commun. 2003;311:264–266. doi: 10.1016/j.bbrc.2003.09.207. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto S, Ouchi Y, Onoe H, Yoshikawa E, Tsukada H, Takahashi H, et al. Reduction of serotonin transporters of patients with chronic fatigue syndrome. Neuroreport. 2004;15:2571–2574. doi: 10.1097/00001756-200412030-00002. [DOI] [PubMed] [Google Scholar]
- 55.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 56.Schaefer M, Hinzpeter A, Mohmand A, Janssen G, Pich M, Schwaiger M, et al. Hepatitis C treatment in ‘difficult-to-treat’ psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46:991–998. doi: 10.1002/hep.21791. [DOI] [PubMed] [Google Scholar]
- 57.Kraus MR, Schafer A, Al Taie O, Scheurlen M. Prophylactic SSRI during interferon alpha re-therapy in patients with chronic hepatitis C and a history of interferon-induced depression. J Viral Hepat. 2005;12:96–100. doi: 10.1111/j.1365-2893.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 58.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 59.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 60.Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, et al. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry. 2006;63:978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- 61.Moreno FA, Rowe DC, Kaiser B, Chase D, Michaels T, Gelernter J, et al. Association between a serotonin transporter promoter region polymorphism and mood response during tryptophan depletion. Mol Psychiatry. 2002;7:213–216. doi: 10.1038/sj.mp.4000962. [DOI] [PubMed] [Google Scholar]
- 62.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient’s initial affective state. N Engl J Med. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- 64.Rosenstein DL, Lerner D, Cai J, Capuron L, Ravaud A. More on the depressive effects of interferon Alfa. N Engl J Med. 1999;341:849–850. doi: 10.1056/NEJM199909093411116. [DOI] [PubMed] [Google Scholar]
- 65.Pariante CM, Landau S, Carpiniello B. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis. NEngl J Med. 2002;347:148–149. doi: 10.1056/NEJM200207113470221. [DOI] [PubMed] [Google Scholar]
- 66.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]