Abstract
The aim of this study was to investigate the transfection efficiency of cationic liposomes formulated with phosphatidylcholine (PC) and novel synthesized diethanolamine-based cationic lipids at a molar ratio of 5:1 in comparison with Lipofectamine™ 2000. Factors affecting transfection efficiency and cell viability, including the chemical structure of the cationic lipids, such as different amine head group (diamine and polyamine; and non-spermine and spermine) and acyl chain lengths (C14, C16, and C18) and the weight ratio of liposomes to DNA were evaluated on a human cervical carcinoma cell line (HeLa cells) using the pDNA encoding green fluorescent protein (pEGFP-C2). Characterizations of these lipoplexes in terms of size and charge measurement and agarose gel electrophoresis were performed. The results from this study revealed that almost no transfection was observed in the liposome formulations composed of cationic lipids with a non-spermine head group. In addition, the transfection efficiency of these cationic liposomes was in the following order: spermine-C14 > spermine-C16 > spermine-C18. The highest transfection efficiency was observed in the formulation of spermine-C14 liposomes at a weight ratio of 25; furthermore, this formulation was safe for use in vitro. In conclusion, cationic liposomes containing spermine head groups demonstrated promising potential as gene carriers.
Key words: cationic lipids, cationic liposomes, gene transfection
INTRODUCTION
Gene therapy has become a promising approach for treating incurable diseases such as genetic disorders and nongenetic diseases such as cancer, cardiovascular diseases, and autoimmune diseases (1) by either correcting the genetic defects or overexpressing therapeutically useful proteins. The prerequisites for successful gene therapy include not only therapeutically suitable genes but also the development of safe and effective gene delivery systems (2). Gene vectors can be classified into two major groups: viral vectors and non-viral vectors. Because of the severe side effects caused by viral vectors, such as immunogenicity, mutagenesis, and carcinogenesis, non-viral vectors have become a viable alternative. Among the non-viral vectors, cationic liposomes have been widely investigated (3,4) because of their ability to form complexes with anionic DNA molecules, which are supposed to deliver DNA into the cells via the endosomal pathway (5,6). Furthermore, several studies have reported that cationic liposomes may induce cytosolic DNA release by fusion with the endosomal membrane (6) or endosomal membrane destabilization (5). However, the certain mechanism of gene transfection mediated by cationic liposomes remains unclear.
Cationic liposome-mediated gene delivery is affected by numerous factors, and one of the major factors is the composition of the liposomes. In addition, several studies have revealed that altering the type and amount of cationic lipid in the cationic liposome can enhance the transfection efficiency (3). Most cationic lipids that are used as transfection reagents usually contain three parts: a hydrophobic group, linker group, and a positively charged head group that can interact with DNA, thereby leading to DNA condensation (7). A previous study suggested that the type of cationic lipid head group strongly affected the gene transfection mediated by cationic liposomes prepared from amino acid-based cationic lipids containing a cholesterol tail (8). One of the most successful head groups of cationic lipids is polyamine (7). Among polyamines, spermine, a well-known polyamine that consists of a tetra-amine with two primary and two secondary amino groups, plays an important role as a gene carrier (9). Additionally, grafting galactosylated chitosan with spermine can improve transfection efficiency in vitro (10). Furthermore, spermine derivatives–cationic lipids, such as dioctadecylamidoglycylspermine (DOGS) and dipalmitoylphosphatidylethanolamidospermine (DPPES), are commercially available for gene delivery purposes (11–13).
Therefore, in the present study, cationic liposomes formulated with phosphatidylcholine (PC) and novel synthesized diethanolamine-based cationic lipids, N1,N1-dimyristeroyloxyethyl-1,2-diaminoethane, N1,N1-dimyristeroyloxyethyl-1,3-diaminopropane, N1,N1-dimyristeroyloxyethyl-3-monoazapentane-1,5-diamine, N1,N1-dimyristeroyloxyethyl-spermine, N1,N1-palmitoyloxyethyl-spermine, and N1,N1-steroyloxyethyl-spermine (Fig. 1), at a molar ratio of 5:1 were prepared using the sonication method. The influences of various factors, such as the chemical structure of cationic lipids with different amine head group (diamine and polyamine; non-spermine and spermine), acyl chain lengths (C14, C16, and C18), and weight ratios of liposomes to DNA on the transfection efficiency and cell viability were investigated on a human cervical carcinoma cell line (HeLa cells) using the pDNA encoding green fluorescent protein (pEGFP-C2). Characterizations of these lipoplexes in terms of size and charge measurements and gel retardation assays were performed.
Fig. 1.
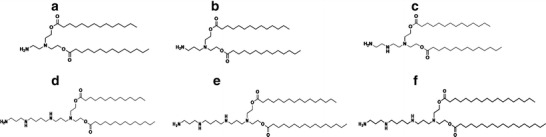
Chemical structure of the cationic lipids: a N 1,N 1-dimyristeroyloxyethyl-1,2-diaminoethane; b N 1,N 1-dimyristeroyloxyethyl-1,3-diaminopropane; c N 1,N 1-dimyristeroyloxyethyl-3-monoazapentane-1,5-diamine; d N 1,N 1-dimyristeroyloxyethyl-spermine; e N 1,N 1-palmitoyloxyethyl-spermine; and f N 1,N 1-steroyloxyethyl-spermine
MATERIALS AND METHODS
Materials
Pure phosphatidylcholine stabilized with 0.1% ascorbyl palmitate (Phospholipon® 90 G; PC) was obtained from Phospholipid GmbH, Nordrhein-Westfalen, Germany. Cationic lipids composed of di(hydroxyethyl)amino core as shown in Fig. 1a–f were synthesized via solid phase synthesis. The synthesis and characterization procedure of these synthetic compounds were described in the previous report (14). HeLa cells—the human cervical cancer cell line—were obtained from American Type Culture Collection (Rockville, Maryland, USA). Modified Eagle's medium (MEM), trypsin-EDTA, penicillin–streptomycin antibiotics, and fetal bovine serum were purchased from Gibco-Invitrogen, New York, USA. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma-Chemical Co., Missouri, USA. The pEGFP-C2 plasmid DNA, encoding green fluorescent protein (GFP), was obtained from Clontech, California, USA. The λHindIII was obtained from Promega, California, USA. Lipofectamine™ 2000 was purchased from Invitrogen, New York, USA. All other reagents employed were of cell culture and molecular biology quality.
Plasmid Preparation
pEGFP-C2 was propagated in Escherichia coli DH5-α and purified by using the Qiagen endotoxin-free plasmid purification kit (Qiagen, Santa Clarita, California, USA). DNA concentration was quantified by measuring UV absorbance at 260 nm using a GeneRay UV Photometer (Biometra®, Goettingen, Germany). The purity of the plasmid was verified by gel electrophoresis (1% agarose gel) in Tris acetate–EDTA buffer, pH 8.0 (TAE buffer), using λDNA/HindIII as a DNA marker.
Preparation of Cationic Liposomes
The cationic liposomes used in this study were prepared from PC and cationic lipids consisting of a di(hydroxyethyl)amino core at a molar ratio of 5:1 using the sonication method. Briefly, the PC and cationic lipids were dissolved in chloroform/methanol (2:1% v/v), and then, the solvents were evaporated under a N2 gas flow to generate a thin lipid film at the bottom of the test tube. The thin film was left in a desiccator overnight to remove the remaining organic solvents, followed by thin film hydration using Tris buffer (20 mM Tris and 150 mM NaCl, pH 7.4). After hydration, the dispersion was sonicated using a bath sonicator for 30 min followed by a probe sonicator (Vibra-Cell™ Ultrasonic Processor, Sonics & Materials, Inc., USA.), each for 30 min in two cycles.
Preparation and Characterization of the Liposomes/DNA Complexes
Various weight ratios of the liposome/DNA complexes were prepared by adding the liposomes solution to the DNA solution (the amount of DNA was fixed at 1 μg). The mixture was gently mixed by pipetting up and down for 3–5 s to initiate the formation of the complex. To completely form the complex, the mixture was held at room temperature for 30 min. Agarose gel electrophoresis was performed to confirm the formation of the complex using a 1% agarose gel (1 g agarose in 100 ml TAE buffer containing ethidium bromide 0.5 μg/ml) and TAE buffer as a running buffer. Electrophoresis was performed for 45 min at 100 V. The volume of sample loaded per well was 10 μl of liposome/DNA complexes containing 1 μg of DNA.
Size and Zeta Potential Measurements
The particle size and surface charge of the liposomes/DNA complexes were examined by photon correlation spectroscopy using a Zeta sizer Nano ZS (Malvern Instruments Ltd, Malvern, UK). The complexes were diluted with distilled water that was passed through a 0.22-μm membrane filter to obtain the volume required for each measurement (approximately 1 ml). All measurements were performed in triplicate at room temperature.
In Vitro Transfection of Liposome/DNA Complexes in HeLa Cells
The day before transfection, HeLa cells were seeded into a 24-well plate at a density of 2 × 104 cells/well in 0.5 ml of complete medium (MEM containing 10% fetal bovine serum, supplemented with 1% l-glutamine, 1% nonessential amino acids solution, 100 U/ml penicillin, and 100 μg/ml streptomycin) and were incubated overnight at 37°C and 5% CO2. Before transfection, the medium was removed, and the cells were incubated with 0.5 ml of the liposome/DNA complexes in serum-free medium at various weight ratios containing 1 μg of pEGFP-C2 for 24 h at 37°C and 5% CO2. Cells transfected with naked pEGFP-C2 were used as a negative control, whereas Lipofectamine™ 2000 complexed with DNA at a weight ratio of 2 was used as a positive control. One day after transfection, the cells were washed with pH 7.4 phosphate-buffered saline (PBS) and replaced with complete medium. After this step, GFP expression was directly observed under a fluorescence microscope (model: GFP-B, wavelengths: excitation filter 480/40, and emission filter 535/50). All transfection experiments were performed in triplicate.
Evaluation of Cell Viability
Cell viability was examined using an MTT assay. HeLa cells were seeded into a 96-well plate at a density of 8 × 103 cells/well in 100 μl of complete medium and were incubated overnight at 37°C and 5% CO2. Before the cytotoxicity study, the medium was removed, and the cells were treated with the liposome/DNA complexes under the same conditions as those used in the transfection experiment. The day after treatment, the solution of the complexes was removed, and the cells were rinsed with PBS followed by incubation with 100 μl of MTT-containing medium (1 mg/ml) for 4 h. The formazan crystals formed in the living cells were dissolved using 100 μl of dimethyl sulfoxide per well. The relative cell viability (in percent) was calculated based on the absorbance observed at 550 nm using a microplate reader (Universal Microplate Analyzer, models AOPUS01 and AI53601, Packard BioScience, Connecticut, USA) and compared to the non-treated cells. The viability of the non-treated cells was defined as 100%.
Statistical Analysis
Significant differences in the transfection efficiency and cell viability were determined using one-way analysis of variance followed by the LSD post hoc test for analyzing transfection efficiency and the Tukey's post hoc test for determining cell viability. The significance level was set at p < 0.05.
RESULTS AND DISCUSSION
Characterization of the Cationic Liposomes/DNA Complexes
The degree of binding between the cationic liposomes and DNA at varying weight ratios of liposomes/DNA was investigated using a gel retardation assay to determine the optimal conditions for the complex formation. The formation of lipoplexes was visualized by agarose gel electrophoresis, as shown in Fig. 2. The results from the agarose gel electrophoresis illustrated that the DNA migration pattern of the cationic liposomes prepared using the cationic lipid with a non-spermine head group at any weight ratio of liposomes/DNA (Fig. 2a–c; lanes 3–8) did not differ from the DNA migration pattern of naked DNA (Fig. 2a–c; lane 2), which indicates that the complex formation could not completely occur at the weight ratios used in this experiment (weight ratios of 5 to 30). In contrast, the complex formation of the liposomes prepared from the cationic lipids with a spermine head group (D, E, and F; spermine-C14, spermine-C16, and spermine-C18) completely formed at weight ratios above 15, 20, and 20, respectively (Fig. 2d–f). If the carrier can form complexes with DNA, the DNA migration will be retarded. In addition, the DNA migration disappears when the complexes completely form (15). Our results showed that the formulations of liposomes composed of spermine–cationic lipids were able to condense with DNA at different binding abilities. The binding ability of these cationic lipids was in the following order: spermine-C14 > spermine-C16 ~ spermine-C18.
Fig. 2.
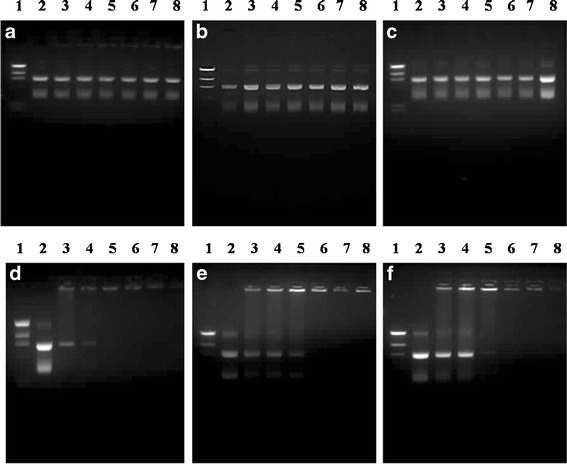
Gel retardation assay of the liposomes/DNA complexes formulated from PC and cationic lipids (a, b, c, d, e, and f, respectively). Lane 1, λHindIII DNA marker; lane 2, pEGFP-C2; lanes 3–8, liposomes/DNA complexes at weight ratios of 5, 10, 15, 20, 25, and 30, respectively
Further investigations of the particle size and zeta potentials of the liposomes/DNA complexes were performed at weight ratios of 5, 15, 25, and 40. The particle size and zeta potentials were plotted against the weight ratios of liposomes/DNA (Fig. 3). The results revealed that the particle size of the lipoplexes were in the range of 72–291 nm. Decreasing particle sizes of the lipoplexes formulated from cationic lipids A, D, E, and F were observed when the weight ratio of liposomes/DNA increased, whereas the particle size of the lipoplexes formulated from cationic lipids B and C slightly decreased with increasing weight ratios. It could be assumed from this finding that the observed size reduction of the lipoplexes when the weight ratios increased was caused by the ability of the liposomes to condense DNA into a compact size (16). Negative values of the zeta potential were observed in the lipoplexes formulated with cationic lipids B and C (lipoplexes B and C) at any weight ratios. In contrast, the lipoplexes formulated with cationic lipids A, D, E, and F (lipoplexes A, D, E, and F) had an initially positive zeta potential value (weight ratio of 5). Furthermore, the zeta potentials of the lipoplexes D, E, and F gradually increased with an increasing weight ratio, whereas the zeta potential of lipoplexes A at weight ratios of 15, 25, and 40 (approximately 8–9 mV) did not differ from its initial value (10 mV). The highest zeta potentials observed at a weight ratio of 25 for the lipoplexes D, E, and F were 67, 69, and 76, respectively. Furthermore, the zeta potentials of lipoplexes D, E, and F at a weight ratio of 25 were not significantly different (p > 0.05). Therefore, lipoplexes D, E, and F, which were prepared from spermine–cationic lipids D, E, and F at a weight ratio of 25, may be utilized as an alternative for gene transfection due to their abilities to form complete complexes with DNA and provide a high net positive surface charge (7).
Fig. 3.
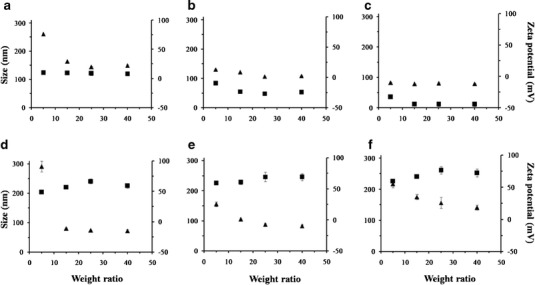
Particle size (filled triangle) and zeta potential (filled square) at varying weight ratios of liposomes/DNA complexes formulated from PC and cationic lipids (a, b, c, d, e, and f). Each value represents the mean ± standard deviation of three measurements
In Vitro Transfection in HeLa Cells
High gene transfection efficiency is a major requirement for developing novel gene delivery systems. The cationic liposome-mediated gene transfection was investigated in a human cervical carcinoma cell line (HeLa cells) using pEGFP-C2. Gene transfection mediated by cationic liposomes was affected by numerous factors, including the compositions of the cationic liposomes. In addition, altering the type and amount of cationic lipids in the cationic liposomes can enhance the transfection efficiency (17,18). In this study, the effect of the chemical structure of the cationic lipids used for preparing liposomes and the weight ratios of liposomes/DNA on the transfection efficiency were evaluated in comparison with Lipofectamine™ 2000 (weight ratio of 2) and naked pDNA transfection (Fig. 4). The cationic lipids used in this study can be classified into three groups based on the differences in the amine head group: (a) diamine head group; cationic lipids A and B that differ in the length of the linker group (C2 and C3) between the primary and tertiary amines, (b) polyamine head group (non-spermine); cationic lipid C, and (c) polyamine head group (spermine); cationic lipids D, E, and F that differ in the acyl chain length (C14, C16, and C18). There were no significant differences in the transfection efficiency between lipoplexes B and C and the naked DNA transfection. However, the transfection efficiency of lipoplex A at weight ratios of 5, 10, and 15 was significantly different from the naked DNA transfection, and the value was very low when compared to the Lipofectamine™ 2000 transfection. In contrast, the transfection efficiency observed from lipoplexes D, E, and F (composed of spermine–cationic lipids C14, C16, and C18) at any weight ratio was significantly different from the naked DNA transfection, and the value was close to the Lipofectamine™ 2000 transfection results. The gene transfection efficiency was significantly affected by the carrier/DNA weight ratios. In addition, as the weight ratio increased, the transfection efficiencies reached the highest levels from 5 to 25 with a decrease by further augmentation of the ratios from 25 to 40 (Fig. 4d–f), which is in agreement with our previous report (19). The highest transfection efficiency yield from lipoplexes D, E, and F was observed at a weight ratio of 25, and the transfection efficiency of these cationic liposomes was in the following order: spermine-C14 (3,047 ± 26 cells/cm2) > spermine-C16 (2,248 ± 32 cells/cm2) > spermine-C18 (1,816 ± 64 cells/cm2). Among the lipoplexes investigated, the highest transfection efficiency was observed in the formulation of spermine-C14 liposomes at the weight ratio of 25. There were no significant differences between the transfection efficiency of lipoplex D and the Lipofectamine™ 2000 transfection (Fig. 5). It could be assumed that lipoplex D (composed of spermine-C14 liposomes) at a weight ratio of 25 provided effective gene carrier ability in vitro. In addition, lipoplexes E and F, which contained liposomes prepared from the spermine–cationic lipid, yielded almost higher transfection efficiency compared to the lipoplexes containing liposomes prepared from the non-spermine cationic lipid. Transfection efficiency was observed only in the complexes between the carrier and DNA that had a positive charge. This result might be due to the polycation/DNA ratio (20). The positive charge increases the binding between the complexes and anionic cell membrane surface, followed by a perturbation of the membrane, which induces endocytosis and uptake of the DNA into the cells (21). The mechanisms of escape from endosomes for lipoplexes and polyplexes are fundamentally different. The release of DNA into the cytoplasm is mediated by the destabilization of the endosome membrane by the cationic lipids (22) due to the trans-bilayer flip–flop of negatively charged phospholipids from the cytoplasmic leaflet to the lumenal leaflet of the endosome (6). Another hypothesis for the escape of lipoplexes from endosomes into cytosol is a detergent-like destabilization of the endosomal membrane (23). Lysosomotropic detergents, such as lipophilic amine, were able to protonate at intralysosomal acidic pH levels, which resulted in enhanced endosomal escape (24). Therefore, the high gene transfection observed from the cationic liposomes containing spermine–cationic lipid in this study might result from the ability of these cationic lipids to become protonated at intralysosomal acidic pH levels, which could result in endosomal membrane destabilization and the cytosolic release of the carried DNA. The exact mechanism of the cationic liposomes containing spermine–cationic lipid mediates efficient gene delivery requires further study.
Fig. 4.
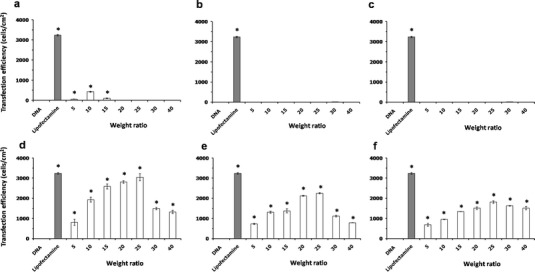
In vitro transfection efficiencies in HeLa cells of liposomes/DNA complexes formulated from PC and cationic lipids (a, b, c, d, e, and f), respectively. Each value represents the mean ± standard deviation of three wells. *Statistically significant (p < 0.05)
Fig. 5.
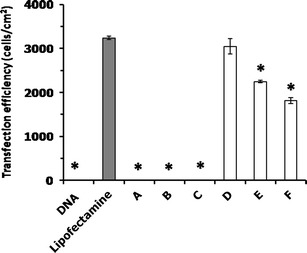
Comparison of the in vitro transfection efficiencies of liposomes/DNA complexes formulated from PC and cationic lipids a, b, c, d, e, and f at a weight ratio of 25 in HeLa cells. Each value represents the mean ± standard deviation of three wells. *Statistically significant (p < 0.05)
The effect of serum on the transfection efficiency of lipoplex D at a weight ratio of 25 was also investigated. Almost no transfection was observed in the presence of serum, and there were no significant differences (p < 0.05) between the transfection efficiency of lipoplex D in the presence of serum and the naked DNA transfection. This finding was in agreement with a previous report (25), which indicated that cationic liposome (lipofectin)-mediated gene transfection was inhibited by serum. Therefore, the resulting inhibition of the lipoplexes transfection by serum is a disadvantage when compared to the polyplex transfection.
Effect of Cationic Liposomes/DNA Complexes on Cell Viability
Suitable gene delivery systems should possess not only high gene transfection efficiency but should also be safe to use (2). To assure the safety of the gene carriers, the cell viability was evaluated on the HeLa cell culture using an MTT assay (Fig. 6). The cell viabilities of lipoplexes D, E, F, at weight ratios of 5 to 25 were greater than 80% and were not significantly different from the control (non-treated cells). Therefore, these liposome formulations presented low toxicity in the concentration that provided the highest transfection efficiency (weight ratio of 25), whereas liposomes A, B, and C had low transfection even though they presented low toxicity (Fig. 6). The cytotoxicity increased with increasing weight ratios of carriers/DNA (16,19). Therefore, it was suggested that lipoplexes prepared form spermine–cationic lipids were safe to be used in vitro.
Fig. 6.
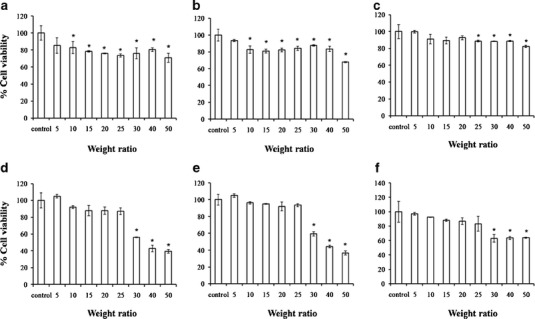
The cell viability in HeLa cells at varying weight ratios of liposomes/DNA complexes formulated from PC and cationic lipids (a, b, c, d, e, and f, respectively). Each value represents the mean ± standard deviation of three wells. *Statistically significant (p < 0.05)
CONCLUSIONS
In the present study, cationic liposomes formulated from PC and novel diethanolamine-based cationic lipids with differences in the amine head group (diamine and polyamine; non-spermine and spermine) and acyl chain lengths (C14, C16, and C18) were investigated in vitro for their potential use as gene carriers. Among these cationic liposomes, the liposomes composed of a cationic lipid with a spermine head group and a C14 acyl chain length demonstrated not only the highest gene transfection but also low cytotoxicity in vitro. Consequently, these cationic liposomes that contained a spermine head group are a promising alternative for further development as a gene carrier.
ACKNOWLEDGMENTS
The authors would like to acknowledge Commission of Higher Education (Thailand) and the Thailand Research Funds through the Golden Jubilee Ph.D. Program (grant nos. PHD/0092/2551 and PHD/0217/2552) and the Center of Excellence for Innovation in Chemistry for the financial support.
REFERENCES
- 1.Mhashilkar A, Chada S, Roth JA, Ramesh R. Gene therapy: therapeutic approaches and implications. Biotechnol Adv. 2001;19:279–297. doi: 10.1016/S0734-9750(01)00063-5. [DOI] [PubMed] [Google Scholar]
- 2.Gabor MR. The future of human gene therapy. Mol Aspects Med. 2001;22:113–142. doi: 10.1016/S0098-2997(01)00004-8. [DOI] [PubMed] [Google Scholar]
- 3.Maitani Y, Igarashi S, Sato M, Hattori Y. Cationic liposome (DC-Chol/DOPE = 1:2) and a modified ethanol injection method to prepare liposomes, increased gene expression. Int J Pharm. 2007;342:33–39. doi: 10.1016/j.ijpharm.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 4.Serikawa T, Suzuki N, Kikuchi H, Tanaka K, Kitagawa T. A new cationic liposome for efficient gene delivery with serum into cultured human cells: a quantitative analysis using two independent fluorescent probes. Biochim Biophys Acta. 2000;1467:419–430. doi: 10.1016/S0005-2736(00)00239-X. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi A, Furuno T, Kawaura C, Nakanishi M. Membrane fusion plays an important role in gene transfection mediated by cationic liposomes. FEBS Lett. 1998;433:169–173. doi: 10.1016/S0014-5793(98)00837-0. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Szoka FC. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 7.Ram IM. Water insoluble and soluble lipids for gene delivery. Adv Drug Deliv Rev. 2005;57:699–712. doi: 10.1016/j.addr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Song H, Luo K, He B, Nie Y, Yang Y, et al. Gene transfer efficacies of serum-resistant amino acids-based cationic lipids: dependence on headgroup, lipoplex stability and cellular uptake. Int J Pharm. 2011;408:183–190. doi: 10.1016/j.ijpharm.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 9.Azzama T, Eliyahua H, Makovitzki A, Linial M, Domb AJ. Hydrophobized dextran-spermine conjugate as potential vector for in vitro gene transfection. J Control Release. 2004;96:309–323. doi: 10.1016/j.jconrel.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Alex SM, Rekha MR, Sharma CP. Spermine grafted galactosylated chitosan for improved nanoparticle mediated gene delivery. Int J Pharm. 2011;410:125–137. doi: 10.1016/j.ijpharm.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 11.Gaucheron J, Santaella C, Vierling P. Transfection with fluorinated lipoplexes based on fluorinated analogues of DOTMA, DMRIE and DPPES. Biochim Biophys Acta. 2002;1564:349–358. doi: 10.1016/S0005-2736(02)00469-8. [DOI] [PubMed] [Google Scholar]
- 12.Jean PB, Barbara D, Jean PL, Jose PM. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci. 1989;86:6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29:3477–3496. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Yingyongnarongkul B, Radchatawedchakoon W, Krajarng A, Watanapokasin R, Suksamrarn A. High transfection efficiency and low toxicity cationic lipids with aminoglycerol-diamine conjugate. Bioorg Med Chem. 2009;17:176–188. doi: 10.1016/j.bmc.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Weecharangsan W, Opanasopit P, Ngawhirunpat T, Apirakaramwong A, Rojanarata T, Ruktanonchai U, et al. Evaluation of chitosan salts as non-viral gene vectors in CHO-K1 cells. Int J Pharm. 2008;348:161–168. doi: 10.1016/j.ijpharm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Chen JL, Wang H, Gao JQ, Chen HL, Liang WQ. Liposomes modified with polycation used for gene delivery: preparation, characterization and transfection in vitro. Int J Pharm. 2007;343:255–261. doi: 10.1016/j.ijpharm.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 17.Ciani L, Casini A, Gabbiani C, Ristori S, Messori L, Martini G. DOTAP/DOPE and DC-Chol/DOPE lipoplexes for gene delivery studied by circular dichroism and other biophysical techniques. Biophys Chem. 2007;127:213–220. doi: 10.1016/j.bpc.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Masotti A, Mossa G, Cametti C, Ortaggi G, Bianco A, Grosso ND, et al. Comparison of different commercially available cationic liposome-DNA lipoplexes: parameters influencing toxicity and transfection efficiency. Colloids Surf B: Biointerfaces. 2009;68:136–144. doi: 10.1016/j.colsurfb.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Opanasopit P, Tragulpakseerojn J, Apirakaramwong A, Ngawhirunpat T, Rojanarata T. Chitosan enhances transfection efficiency of cationic polypeptides/DNA complexes. Int J Pharm. 2011;410:161–168. doi: 10.1016/j.ijpharm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Opanasopit P, Paecharoenchai O, Rojanarata T, Ngawhirunpat T, Ruktanonchai U. Type and composition of surfactants mediating gene transfection of polyethylenimine-coated liposomes. Int J Nanomedicine. 2011;6:975–983. doi: 10.2147/IJN.S18647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith J, Zhang Y, Niven R. Toward development of a non-viral gene therapeutic. Adv Drug Deliv Rev. 1997;26:135–150. doi: 10.1016/S0169-409X(97)00031-8. [DOI] [PubMed] [Google Scholar]
- 22.Tros de Ilarduya C, Sun Y, Düzgünes N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40:159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Elouahabi A, Ruysschaert JM. Formation and intracellular trafficking of lipoplexes and polyplexes. Mol Ther. 2005;11:336–347. doi: 10.1016/j.ymthe.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Liang E, Hughes J. Characterization of a pH-sensitive surfactant, dodecyl-2-(1′-imidazolyl) propionate (DIP), and preliminary studies in liposome mediated gene transfer. Biochim Biophys Acta. 1998;1369:39–50. doi: 10.1016/S0005-2736(97)00172-7. [DOI] [PubMed] [Google Scholar]
- 25.Sato T, Ishii T, Okahata Y. In vitro gene delivery mediated by chitosan. Effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials. 2001;22:2075–2080. doi: 10.1016/S0142-9612(00)00385-9. [DOI] [PubMed] [Google Scholar]


