Abstract
The purpose of this research was to investigate the relationship of drug solubility in a complex lipid mixture to that of the individual ingredients with the goal of substantiating a quantitative equation that can be applied in formulation development of lipid dosage forms. To this end, the solubility of four drugs, which span a large range of physicochemical properties, was evaluated in 18 lipid ingredients that cover the major lipid classes. To assess the solubility relation in complex lipid mixtures in an unbiased manner, the experiments were created as an experimental design with the ability to detect cubic curvature in the solubility-lipid composition space. The results demonstrated that for all drugs, irrespective of their significantly distinct physicochemical properties, solubility in lipid mixtures can be readily estimated as a simple weighted average of the drug solubility in the individual ingredients. This result is of great value to formulators who can minimize a large number of solubility experiments once a basis set of solubility is determined in individual lipids.
Key words: drug solubility, lipid dosage form
INTRODUCTION
Many new chemical entities exhibit low solubility in the gastrointestinal tract, leading to poor absorption. Of the many approaches to improve low bioavailability, solubilization with lipids is important for those compounds that display sufficiently high solubility in oil and cosolvent ingredients and is exploited in several commercial products (1). When delivered to the GI tract, these formulations can promote solubility in an aqueous environment and enhance oral absorption (2). A particularly important strategy is to incorporate self emulsifying ingredients in the formulation that spontaneously form oil droplets and/or micelles depending on the type of composition and type of lipids (3). The finely dispersed formulation can significantly improve oral absorption and reduce food effects (4). There are two important objectives with regard to the lipid vehicle from a formulation development perspective. First, it should provide high solubility for the drug, and second, it should emulsify with no precipitation of the drug. To achieve these objectives, formulation development must as a first step encompass solubility screening in a range of lipid materials. This research was dedicated to assessing the solubility of low water solubility drugs in a broad range of lipid materials specifically to determine if there is any global relationship of solubility to composition variables in complex lipid mixtures.
Four model drugs were investigated for their generally low water solubility. To capture a range of compounds as part of the study, the four drugs were chosen from those with low, medium, and high melting points and partition coefficients. Two of the drugs are nonionizable, while one is an acid and one a base. The key properties are summarized in Table I. Particularly notice the extreme contrast between genistein with its very high melting point and moderate log P and probucol with its low melting point and very high log P. Nifedipine and indomethacin are intermediate in terms of melting point and log P but capture the base and acid chemistry of many drugs.
Table I.
Physicochemical Properties of the Drugs
| Drug | Intrinsic S (mg/mL) | log P | Melting point (°C)a | pKa |
|---|---|---|---|---|
| Genistein | ∼0.001b | 3.04c | 299 | NA |
| Probucol | 0.004–0.005 μg/mLd | 10d | 126 | NA |
| Nifedipine | 0.006e | 3.17f | 174 | 2.7g (base) |
| Indomethacin | 0.002–0.007h,i | 4.27j | 155 | 4.50j (acid) |
NA nonionizable over the practical pH range
aManufacturer data
bWu et al. (5)
cRothwell et al. (6)
dChristensen et al. (7)
eCuratolo et al. (8)
fLombardo et al. (9)
gCalculated using Advanced Chemistry Development (ACD/Labs) Software v11.02
hBergstrom et al. (10)
iNokhodchi et al. (11)
jLiu et al. (12)
As a starting point in developing a lipid formulation, it is proposed to assess solubility of a drug in lipids encompassing a broad range of chemistry. Lipids can be classified according to their basic chemical groups and polarity within a chemical class, most commonly using their HLB (hydrophile-lipophile balance) values. The lipid classification and specific lipids chosen in the experiments are provided in Table II.
Table II.
Lipid Classes, Specific Lipids, and HLB Values
| Lipid class | Lipid or cosolvent | HLBa |
|---|---|---|
| Triglyceride | Soybean oil | NA |
| Castor oil | NA | |
| Miglyol 812 | NA | |
| Mono/diglycerides | Capmul MCM | 5 |
| Maisine 35-1 | 4 | |
| Glyceryl monooleate (GMO) | 3 | |
| Propylene glycol esters | Capmul PG-8 | 6 |
| Lauroglycol 90 | 5 | |
| Polyoxylglycerides | Labrasol | 14 |
| Labrafil M 1944 CS | 3–4 | |
| Polyglyceryl esters | Plurol oleique CC497 | 6 |
| Caprol MPGO | 10 | |
| Surfactants | Polysorbate 80 | 15 |
| Cremophor RH40 | 14–16 | |
| Vitamin E TPGS | 13 | |
| Cosolvents | PEG400 | NA |
| Propylene glycol | NA | |
| Ethanol | NA |
NA not applicable
aValues obtained from manufacturer literature
Soybean and castor oil are long chain triglycerides, the former composed mainly of linoleic and oleic acid composition. Castor oil is distinctive in its ricinoleic fatty acid composition that contains an OH group that adds some polarity that may help solubilize certain drugs. Miglyol 812 (glyceryl tricaprylate/caprate) is a fractionated coconut oil representing the medium chain class of triglycerides. Capmul MCM (glyceryl mono and dicaprylate/caprate) and Maisine 35-1 (glyceryl monolinoleate) are mono/diglyceride mixtures, the former in the medium and latter in the long-chain categories. Propylene glycol mono and diesters are represented by Capmul PG-8 (propylene glycol monocaprylate) and Lauroglycol 90 (propylene glycol monolaurate). Polyoxylglycerides encompass a broad range of materials of which Labrasol (PEG-8 glyceryl caprylate/caprate) and Labrafil M 1944 CS (PEG-6 glyceryl oleate) are high and low HLB members. These ingredients are particularly important in their cosurfactant nature to help in self-emulsifying the more oily triglycerides and mono/diglycerides. The polyglyceryl fatty acid esters, Plurol oleique CC497 (polyglyceryl-3 dioleate) and Caprol MPGO (polyglyceryl-3 oleate and polyglyceryl-10 mono-dioleate), also exhibit good self-emulsifying characteristics. Three surfactants, Polysorbate 80 (Polyoxyethylene-20 sorbitan monooleate), Cremophor RH40 (PEG-40 hydrogenated castor oil), and Vitamin E TPGS (d-alpha tocopheryl polyethylene glycol 1000 succinate), were chosen from many due to their extensive use and excellent properties for aiding self-emulsification and forming micelles. The three cosolvents were selected as they are often used for their strong solubilization power of drugs.
MATERIALS AND METHODS
Materials
The drugs were sourced as follows: genistein (DSM, 98%), probucol (MP Biomedical, 99.7%), nifedipine (Tokyo Chemical Industry Co., >98%), and indomethacin (Sigma-Aldrich, 99%).
The lipids were sourced as follows: Soybean oil (Spectrum), Castor oil (Fluka), Miglyol 812 (Sasol), Capmul MCM (Abitec), Maisine 35–1 (Gattefosse), GMO (Abitec), Capmul PG-8 (Abitec), Lauroglycol (Gattefosse), Labrasol (Gattefosse), Labrafil M 1944 CS (Gattefosse), Plurol oleique CC497 (Gattefosse), Caprol MPGO (Abitec), Polysorbate 80 (Spectrum), Cremophor RH40 (BASF), Vitamin E TPGS (Eastman), PEG400 (Fisher Scientific), Propylene glycol (Sigma-Aldrich), and Ethanol (Decon Labs). We are particularly thankful to Abitec, Gattefosse, Sasol, and BASF for supplying gratis samples for this research.
Methods
The solubility procedure involved weighing the drugs to excess in 4-mL clear glass vials and adding 1 mL of vehicle. Exact drug weights and volumes were recorded. Vials were closed with PTFE caps. Samples were sonicated and vortexed for ∼1 min to accelerate wetting and dispersing the powder, and were then added to a rotator (Labquake, Thermo Scientific) in an oven (Precision Thelco Laboratory Oven) set to 40°C, which was monitored with a NIST-traceable thermometer and digital temperature data logger. Care was taken not to add too much excess solid, which can lead to poor liquid movement and slow equilibration. All samples were observed for liquid motion on the rotator. It was generally found that a 3-day equilibration was sufficient to achieve equilibrium. Samples were taken at 3–7 days, with 0.5 mL aliquots of suspensions placed in prewarmed microcentrifuge tubes containing 0.22 μm Nylon filters (Costar® spin-X® centrifuge tube filter, Corning Inc.). The samples were then placed in a prewarmed mini centrifuge (MiniSpin microcentrifuge, Eppendorf) and spun at 10,000 rpm all at 40°C. One sample at a time was removed from the oven/centrifuge and immediately weighed into a volumetric flask. It was generally found that IPA was a good solvent to solubilize the lipids for dilution for assay. All assays were by HPLC, using either an Agilent HP1100 or Dionex Ultimate 3,000 system with UV–VIS detection. The HPLC conditions for the drugs are provided in Table III. Lipid samples were injected separately to ensure no coelution with the drug peak. In general, due to the high solubility of the drug and in some cases sufficiently high wavelength for detection, lipid coelution was not problematic in the method development.
Table III.
Chromatography Conditions
| Parameter | Genistein | Probucol | Nifedipine | Indomethacin |
|---|---|---|---|---|
| Column | Alltech, Alltima HP C18 column, 5 μm (150 × 4.6 mm) | Phenomenex®, Kinetex® C18 column, 2.6 μm (150 × 4.6 mm) | Phenomenex®, Luna® Phenyl-Hexyl, 3 μm (150 × 4.6 mm) | YMC America, YMC-Pack, ODS-A, 5 um (150 × 4.6 mm) |
| Column temperature (°C) | 25 | 25 | 25 | 25 |
| Mobile phase | A: 0.1% Acetic Acid/Water | A: Water | A: Water | A: 0.1% TFA/Water |
| B: ACN | B: Methanol | B: ACN | B: 0.1% TFA/ACN | |
| Gradient | Gradient | Isocratic 95% B for 11 min | Gradient | Isocratic 60% B for 10 min then 95% B rinse for 5 min |
| 5–40% B for 12 min then 40% B for 4 min and 95% B rinse for 8 min | 65–95% B for 10 min then 95% B rinse for 5 min | |||
| Injection volume (μL) | 5 | 5 | 5 | 5 |
| Wavelength (nm) | 259 | 242 | 236 | 240 |
| Run time (min) | 30 | 11 | 20 | 20 |
Experimental Design
The first step in the process was to measure solubility of drugs in pure component lipids. Based on the results, a representative lipid was chosen from its class for which the drug had high solubility. Then lipid mixtures were created using the different lipid classes, considering the functionality of the lipids. For example, lipids in the triglyceride and cosolvent categories serve as a base for achieving high solubility, whereas mono/diglycerides, propylene glycol esters and polyoxylglycerdies also have self-emulsifying properties, and surfactants are required to produce fine microemulsions upon dispersion in water. Four lipids, one of which in some cases was a cosolvent, were chosen to create final mixtures for solubility assessment.
Lipid mixtures were created using experimental design, specifically a simplex lattice mixture design of degree 3 with axial points and a center point repeated 3 times (Minitab 15 statistical software). This design enables exploration of the solubility space with minimal samples to determine cubic curvature in the response, i.e., up to 3-component interactions. Typically, only 2-component interactions are needed to develop a quantitative model, but a degree 3 model was chosen to demonstrate this point. Each lipid level range was the full 0 to 1 (fraction basis). Regression of the solubility data was conducted to determine if quadratic and cubic curvature significantly improved the model over that with linear terms.
RESULTS
Solubility results for the four drugs in pure lipid components are provided in Table IV.
Table IV.
Solubility of Drugs in Pure Lipid Components at 40°C
| Lipid or cosolvent | Genistein (mg/g) | Probucol (mg/g) | Nifedipine (mg/g) | Indomethacin (mg/g) |
|---|---|---|---|---|
| Soybean oil | 0.059 | 86.33 | 1.56 | 2.38 |
| Castor oil | 3.02 | 67.92 | 7.19 | 22.41 |
| Miglyol 812 | 0.34 | 161.79 | 4.78 | 6.04 |
| Capmul MCM | 4.12 | 75.48 | 14.86 | 31.68 |
| Maisine 35-1 | 0.51 | 74.38 | 5.17 | 13.48 |
| Glyceryl monooleate (GMO) | 0.65 | 39.18 | 6.04 | 15.42 |
| Capmul PG-8 | 7.14 | 183.31 | 26.61 | 44.77 |
| Lauroglycol | 3.27 | 157.84 | 12.81 | 26.82 |
| Labrasol | 51.17 | 105.88 | 69.04 | 108.06 |
| Labrafil M 1944 CS | 1.61 | 118.82 | 8.75 | 18.21 |
| Plurol oleique CC497 | 1.31 | 57.78 | 4.83 | 17.14 |
| Caprol MPGO | 3.89 | 61.25 | 5.56 | 47.27 |
| PEG400 | 101.12 | 29.40 | 94.41 | 134.15 |
| Propylene glycol | 12.29 | 2.10 | 10.72 | 21.66 |
| Ethanol | 16.49 | 224.74 | 46.53 | 65.04 |
| Polysorbate 80 | 66.08 | 87.83 | 69.76 | 119.42 |
| Cremophor RH40 | 53.74 | 61.86 | 85.75 | 118.77 |
| Vitamin E TPGS | 33.85 | 88.41 | 65.05 | 86.49 |
Based on the lipids that provided high solubility and considering lipid functionality, four lipids were chosen for each drug. One lipid component was chosen in which the drug demonstrated lower solubility to assess the importance of curvature in the solubility space. Genistein solubility results for mixtures created using the simplex lattice design of degree 3 are provided in Table V. The run sequence was generated randomly.
Table V.
Genistein Solubility in Lipid Mixtures at 40°C
| Run | PEG400 (w/w) | Capmul PG-8 (w/w) | Labrasol (w/w) | Polysorbate 80 (w/w) | Solubility (mg/g) |
|---|---|---|---|---|---|
| 1 | 0.000 | 0.333 | 0.667 | 0.000 | 33.64 |
| 2 | 0.333 | 0.667 | 0.000 | 0.000 | 27.91 |
| 3 | 0.250 | 0.250 | 0.250 | 0.250 | 53.62 |
| 4 | 0.000 | 0.000 | 0.667 | 0.333 | 61.70 |
| 5 | 0.000 | 0.333 | 0.000 | 0.667 | 44.99 |
| 6 | 0.000 | 0.000 | 1.000 | 0.000 | 53.45 |
| 7 | 0.667 | 0.333 | 0.000 | 0.000 | 63.05 |
| 8 | 0.625 | 0.125 | 0.125 | 0.125 | 77.34 |
| 9 | 0.125 | 0.125 | 0.125 | 0.625 | 66.14 |
| 10 | 0.000 | 0.667 | 0.333 | 0.000 | 18.16 |
| 11 | 0.333 | 0.333 | 0.333 | 0.000 | 48.51 |
| 12 | 0.333 | 0.333 | 0.000 | 0.333 | 54.89 |
| 13 | 0.250 | 0.250 | 0.250 | 0.250 | 53.90 |
| 14 | 0.125 | 0.625 | 0.125 | 0.125 | 23.79 |
| 15 | 0.000 | 0.667 | 0.000 | 0.333 | 19.53 |
| 16 | 1.000 | 0.000 | 0.000 | 0.000 | 99.98 |
| 17 | 0.333 | 0.000 | 0.000 | 0.667 | 83.66 |
| 18 | 0.125 | 0.125 | 0.625 | 0.125 | 52.85 |
| 19 | 0.333 | 0.000 | 0.333 | 0.333 | 76.17 |
| 20 | 0.667 | 0.000 | 0.000 | 0.333 | 95.43 |
| 21 | 0.667 | 0.000 | 0.333 | 0.000 | 86.90 |
| 22 | 0.333 | 0.000 | 0.667 | 0.000 | 68.98 |
| 23 | 0.000 | 0.000 | 0.000 | 1.000 | 74.20 |
| 24 | 0.000 | 0.000 | 0.333 | 0.667 | 67.35 |
| 25 | 0.000 | 1.000 | 0.000 | 0.000 | 7.48 |
| 26 | 0.250 | 0.250 | 0.250 | 0.250 | 52.37 |
| 27 | 0.000 | 0.333 | 0.333 | 0.333 | 36.67 |
Additional results for probucol, nifedipine, and indomethacin are provided in the Appendix.
In comparing solubility results from the individual components (Table IV) and the same components tested as part of the mixture design (Tables V, VII, VIII, and IX), it is observed that the variability in the solubility values is generally less than 10%. The largest difference was for probucol in Polysorbate 80, with a difference of ∼20% (Table IV value = 87.83 mg/g and Table VII value = 71.67 mg/g). Another measure of variability is obtained from the replicated center points (0.250 composition) for each drug in Tables V, VII, VIII, and IX. The difference in replicate solubility values is generally far less than 10%.
Table VII.
Probucol Solubility in Lipid Mixtures at 40°C
| Run | Miglyol 812 (w/w) | Capmul PG-8 (w/w) | Labrafil M 1944 CS (w/w) | Polysorbate 80 (w/w) | Solubility (mg/g) |
|---|---|---|---|---|---|
| 1 | 0.000 | 0.000 | 1.000 | 0.000 | 97.56 |
| 2 | 0.250 | 0.250 | 0.250 | 0.250 | 159.48 |
| 3 | 0.333 | 0.000 | 0.333 | 0.333 | 124.45 |
| 4 | 0.667 | 0.000 | 0.333 | 0.000 | 142.19 |
| 5 | 0.000 | 0.000 | 0.000 | 1.000 | 71.67 |
| 6 | 0.125 | 0.125 | 0.625 | 0.125 | 132.23 |
| 7 | 0.625 | 0.125 | 0.125 | 0.125 | 167.46 |
| 8 | 0.333 | 0.667 | 0.000 | 0.000 | 192.66 |
| 9 | 0.000 | 0.000 | 0.333 | 0.667 | 90.99 |
| 10 | 0.000 | 1.000 | 0.000 | 0.000 | 188.65 |
| 11 | 1.000 | 0.000 | 0.000 | 0.000 | 170.37 |
| 12 | 0.000 | 0.667 | 0.333 | 0.000 | 169.97 |
| 13 | 0.125 | 0.125 | 0.125 | 0.625 | 123.61 |
| 14 | 0.333 | 0.000 | 0.000 | 0.667 | 125.66 |
| 15 | 0.250 | 0.250 | 0.250 | 0.250 | 145.90 |
| 16 | 0.333 | 0.333 | 0.000 | 0.333 | 164.87 |
| 17 | 0.125 | 0.625 | 0.125 | 0.125 | 165.44 |
| 18 | 0.667 | 0.000 | 0.000 | 0.333 | 156.09 |
| 19 | 0.000 | 0.000 | 0.667 | 0.333 | 107.41 |
| 20 | 0.000 | 0.333 | 0.333 | 0.333 | 124.11 |
| 21 | 0.000 | 0.333 | 0.667 | 0.000 | 137.77 |
| 22 | 0.000 | 0.333 | 0.000 | 0.667 | 121.87 |
| 23 | 0.333 | 0.000 | 0.667 | 0.000 | 130.72 |
| 24 | 0.000 | 0.667 | 0.000 | 0.333 | 127.87 |
| 25 | 0.250 | 0.250 | 0.250 | 0.250 | 150.78 |
| 26 | 0.667 | 0.333 | 0.000 | 0.000 | 140.36 |
| 27 | 0.333 | 0.333 | 0.333 | 0.000 | 173.39 |
Table VIII.
Nifedipine Solubility in Lipid Mixtures at 40°C
| Run | PEG400 (w/w) | Capmul PG-8 (w/w) | Labrasol (w/w) | Cremophor RH40 (w/w) | Solubility (mg/g) |
|---|---|---|---|---|---|
| 1 | 0.250 | 0.250 | 0.250 | 0.250 | 65.89 |
| 2 | 0.625 | 0.125 | 0.125 | 0.125 | 83.14 |
| 3 | 0.333 | 0.000 | 0.333 | 0.333 | 80.96 |
| 4 | 0.333 | 0.333 | 0.000 | 0.333 | 64.81 |
| 5 | 1.000 | 0.000 | 0.000 | 0.000 | 88.47 |
| 6 | 0.667 | 0.000 | 0.000 | 0.333 | 88.80 |
| 7 | 0.000 | 0.000 | 0.667 | 0.333 | 71.80 |
| 8 | 0.125 | 0.625 | 0.125 | 0.125 | 45.68 |
| 9 | 0.000 | 1.000 | 0.000 | 0.000 | 27.90 |
| 10 | 0.250 | 0.250 | 0.250 | 0.250 | 69.97 |
| 11 | 0.250 | 0.250 | 0.250 | 0.250 | 69.44 |
| 12 | 0.667 | 0.333 | 0.000 | 0.000 | 77.16 |
| 13 | 0.000 | 0.333 | 0.333 | 0.333 | 57.23 |
| 14 | 0.000 | 0.333 | 0.667 | 0.000 | 56.61 |
| 15 | 0.000 | 0.000 | 1.000 | 0.000 | 74.11 |
| 16 | 0.000 | 0.333 | 0.000 | 0.667 | 56.50 |
| 17 | 0.333 | 0.333 | 0.333 | 0.000 | 69.40 |
| 18 | 0.333 | 0.667 | 0.000 | 0.000 | 51.65 |
| 19 | 0.000 | 0.667 | 0.000 | 0.333 | 42.09 |
| 20 | 0.333 | 0.000 | 0.667 | 0.000 | 82.70 |
| 21 | 0.000 | 0.000 | 0.000 | 1.000 | 75.95 |
| 22 | 0.000 | 0.667 | 0.333 | 0.000 | 39.63 |
| 23 | 0.333 | 0.000 | 0.000 | 0.667 | 91.08 |
| 24 | 0.667 | 0.000 | 0.333 | 0.000 | 91.28 |
| 25 | 0.000 | 0.000 | 0.333 | 0.667 | 75.09 |
| 26 | 0.125 | 0.125 | 0.125 | 0.625 | 74.36 |
| 27 | 0.125 | 0.125 | 0.625 | 0.125 | 72.39 |
Table IX.
Indomethacin Solubility in Lipid Mixtures at 40°C
| Run | PEG400 (w/w) | Capmul PG-8 (w/w) | Labrasol (w/w) | Cremophor RH40 (w/w) | Solubility (mg/g) |
|---|---|---|---|---|---|
| 1 | 0.125 | 0.125 | 0.125 | 0.625 | 114.33 |
| 2 | 0.000 | 0.000 | 1.000 | 0.000 | 114.15 |
| 3 | 0.333 | 0.000 | 0.333 | 0.333 | 126.93 |
| 4 | 0.125 | 0.625 | 0.125 | 0.125 | 76.03 |
| 5 | 0.000 | 0.667 | 0.333 | 0.000 | 68.38 |
| 6 | 0.333 | 0.667 | 0.000 | 0.000 | 82.52 |
| 7 | 0.333 | 0.000 | 0.000 | 0.667 | 134.16 |
| 8 | 0.000 | 0.000 | 0.333 | 0.667 | 118.59 |
| 9 | 0.000 | 0.333 | 0.333 | 0.333 | 95.69 |
| 10 | 0.000 | 0.333 | 0.667 | 0.000 | 92.18 |
| 11 | 0.250 | 0.250 | 0.250 | 0.250 | 113.46 |
| 12 | 0.250 | 0.250 | 0.250 | 0.250 | 112.28 |
| 13 | 0.625 | 0.125 | 0.125 | 0.125 | 135.12 |
| 14 | 0.333 | 0.333 | 0.000 | 0.333 | 114.20 |
| 15 | 0.250 | 0.250 | 0.250 | 0.250 | 113.12 |
| 16 | 0.333 | 0.000 | 0.667 | 0.000 | 129.37 |
| 17 | 0.000 | 1.000 | 0.000 | 0.000 | 46.81 |
| 18 | 0.125 | 0.125 | 0.625 | 0.125 | 120.53 |
| 19 | 1.000 | 0.000 | 0.000 | 0.000 | 142.79 |
| 20 | 0.667 | 0.000 | 0.000 | 0.333 | 137.70 |
| 21 | 0.000 | 0.333 | 0.000 | 0.667 | 98.51 |
| 22 | 0.000 | 0.667 | 0.000 | 0.333 | 73.94 |
| 23 | 0.000 | 0.000 | 0.000 | 1.000 | 120.27 |
| 24 | 0.667 | 0.000 | 0.333 | 0.000 | 138.73 |
| 25 | 0.667 | 0.333 | 0.000 | 0.000 | 128.73 |
| 26 | 0.000 | 0.000 | 0.667 | 0.333 | 116.07 |
| 27 | 0.333 | 0.333 | 0.333 | 0.000 | 116.54 |
Regression analysis results are provided in Table VI. All four drugs were also assessed for cubic interactions (analysis not shown), but only quadratic terms had statistically significant p values. To be specific, the full regression model with quadratic terms is given by
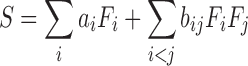 |
1 |
Table VI.
Regression Results for the Four Drugs
| Terms | Coefficients | p value |
|---|---|---|
| Genistein | ||
| PEG400 | 101.35 | a |
| Capmul PG-8 | 6.18 | a |
| Labrasol | 53.56 | a |
| Polysorbate 80 | 74.82 | a |
| PEG400*Capmul PG-8 | −36.00 | 0.000 |
| PEG400*Labrasol | 1.01 | 0.863 |
| PEG400*Polysorbate 80 | 7.91 | 0.188 |
| Capmul PG-8*Labrasol | −20.58 | 0.002 |
| Capmul PG-8*Polysorbate 80 | −37.26 | 0.000 |
| Labrasol*Polysorbate 80 | −1.16 | 0.843 |
| R2 | 0.9973 | |
| Probucol | ||
| Miglyol 812 | 158.20 | a |
| Capmul PG-8 | 184.96 | a |
| Labrafil M1944CS | 98.15 | a |
| Polysorbate 80 | 7.15 | a |
| Miglyol 812*Capmul PG-8 | 14.58 | 0.755 |
| Miglyol 812*Labrafil M1944CS | 52.47 | 0.270 |
| Miglyol 812*Polysorbate 80 | 127.83 | 0.013 |
| Capmul PG-8*Labrafil M1944CS | 69.56 | 0.149 |
| Capmul PG-8*Polysorbate 80 | −5.48 | 0.907 |
| Labrafil M1944CS*Polysorbate 80 | 52.20 | 0.272 |
| R2 | 0.8853 | |
| Nifedipine | ||
| PEG400 | 89.67 | a |
| Capmul PG-8 | 27.04 | a |
| Labrasol | 73.83 | a |
| Cremophor RH40 | 78.11 | a |
| PEG400*Capmul PG-8 | 23.68 | 0.018 |
| PEG400*Labrasol | 22.09 | 0.025 |
| PEG400*Cremophor RH40 | 20.64 | 0.035 |
| Capmul PG-8*Labrasol | −7.83 | 0.397 |
| Capmul PG-8*Cremophor RH40 | −17.28 | 0.072 |
| Labrasol*Cremophor RH40 | −11.80 | 0.208 |
| R 2 | 0.9861 | |
| Indomethacin | ||
| PEG400 | 144.17 | a |
| Capmul PG-8 | 42.79 | a |
| Labrasol | 115.23 | a |
| Cremophor RH40 | 120.76 | a |
| PEG400*Capmul PG-8 | 59.49 | 0.001 |
| PEG400*Labrasol | 25.90 | 0.083 |
| PEG400*Cremophor RH40 | 12.83 | 0.374 |
| Capmul PG-8*Labrasol | 11.37 | 0.430 |
| Capmul PG-8*Cremophor RH40 | 16.59 | 0.254 |
| Labrasol*Cremophor RH40 | −4.94 | 0.730 |
| R 2 | 0.9839 | |
aNot applicable to linear terms, which must be included in the model
The first sum includes the linear terms in the factors and the second sum includes the interaction or quadratic terms (ai and bij are the regression coefficients and Fi are the factors). Once the regression coefficients are determined, solubility can be calculated for any lipid composition.
Although not shown in a table, regression was also conducted using log S, which is a common transformation for solubility data. The narrower range encompassed by log S still resulted in quadratic terms with statistically significant p values for each drug.
DISCUSSION
A major observation in the results is that the regression equations for each drug contained statistically significant quadratic terms. Curvature in the solubility space arises as an interaction for components that are further apart in solubility magnitude. For example, the quadratic interaction terms for genistein all involve Capmul PG-8. This lipid was specifically chosen to produce lower solubility for genistein and as a consequence to assess the importance of curvature in the solubility space. Thus, results with Capmul PG-8 at low and high levels exhibit a larger range of solubility, leading to the statistically significant two-way interaction for this factor with the other ingredients. From a mathematical perspective, it is clear that when solubility data are more widely separated in magnitude, curvature is present that is well modeled by quadratic interaction terms. For probucol, the only significant interaction is for Miglyol 812*Polysorbate 80, which encompasses the two components in which probucol exhibits the largest difference in solubility. Likewise, for nifedipine, the three significant interaction terms all involve PEG400, in which the drug evinces the highest solubility. For indomethacin, the only significant interaction term is for PEG400*Capmul PG-8, which includes the two components for which the solubility difference is largest.
In solubility literature for drugs in aqueous-organic mixtures, there is a significant body of data pointing to a “log-linear” model, as embodied in the relation
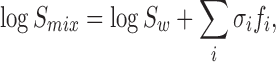 |
2 |
in which Smix is the solubility of the drug in the water-cosolvent mixture, Sw solubility in water, σi the solubilization power, and fi the volume fraction of cosolvent i (13). This equation is simply a statement that the solubility of organic molecules (drugs) in aqueous–organic mixtures is approximated as a geometric average, as expressed by
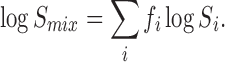 |
3 |
Given the strong solubilizing power of organic solvents for hydrophobic drugs compared to water, it is common for solubility results to span over an order of magnitude in water-cosolvent mixtures (14). The log-linear model captures the significant curvature in the solubility data.
In contrast, in formulation development of drugs in lipid mixtures, formulators will normally select lipids in which the drug is most soluble, and the magnitudes of solubility in different lipids are not as extreme as in water compared to organic solvents. In this work, transforming the solubility results to log S and regressing on log S of the pure components led to slightly better fitting of the data, which is expected given the narrower spread in log S compared to the larger range in S values. However, the regression results still contained the same statistically significant quadratic terms (results not shown).
Although the results clearly illustrate that the solubility space contains curvature as a function of lipid composition, it is worth assessing if the quadratic models provide a practical improvement in solubility estimation from a simple prediction perspective. It is of great value from a formulator’s perspective to be able to estimate a drug’s solubility in a complex lipid mixture from solubility in pure components. The simplest model to use is a weighted average for solubility as given by
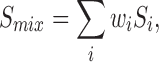 |
4 |
in which Smix is the solubility of the drug in the mixture, Si the solubility in the pure lipid i, and wi the weight fraction of the pure lipid i. This equation requires only solubility data in pure components to predict that in complex mixtures, and requires no regression analysis. There is clear evidence in the regression results that a simple weighted average model works well. Notice that the regression coefficients for the linear terms for all four drugs (Table VI) are very close in magnitude to the pure component solubility values, with one exception for probucol solubility in Polysorbate 80.
As a visual representation for the comparison of models with measured data, the solubility data, weighted average (Calculated S) and quadratic regression (Regression S) results are provided as overlays in Fig. 1 for the four drugs.
Fig. 1.
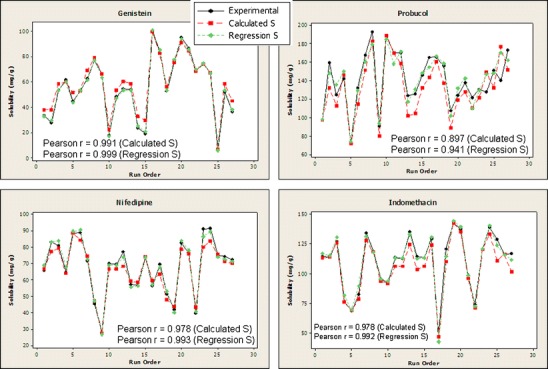
Plot overlays of solubility data (experimental), weighted average prediction (Calculated S), and quadratic regression calculations (Regression S)
It is clearly observed that the improvement in accuracy using the quadratic model, although statistically significant, is practically insignificant in comparison to the simplicity of the weighted average model, especially considering the power of predicting solubility only from pure lipid component results. As a numerical estimation of the model comparisons, the Pearson correlation coefficients are provided in the figures, which demonstrate only a slight improvement of the quadratic (Regression S) over the parameter-less weighted average linear model (Calculated S), which is quite remarkable, considering the latter requires no adjustable parameters.
It was recently noted that the simple weighted average equation worked well in estimating solubility of danazol in two and three component lipid mixtures using Cremophor EL and mono, di and triglyceride mixtures of caprylic/capric acid (15). Our research has demonstrated that this simple relation is broad in its application for acidic, basic and neutral drug molecules covering a wide range in physicochemical properties, and for the major lipids that are used in formulations. In general, the weighted average model can be expected to provide a good solubility estimate in complex lipid mixtures as long as the drug solubility in the individual ingredients is not too different, where second order curvature will become more significant.
CONCLUSION
This research outlines a formulation strategy for lipid materials. Lipids are classified according to their chemistry, functionality and polarity. Solubility is measured in pure components from each class, including lipids with low and high polarity in the class. The solubility screen identifies materials in which the drug is most soluble. Formulators can then combine lipids to produce a formulation with high drug load, considering functional characteristics of the materials. For example, triglycerides and cosolvents are used at higher levels if they are strong solubilizers of a drug to achieve a high dose, even though they generally don’t help solubilization in the GI tract. Coemulsifiers and surfactants are used to promote spontaneous microemulsion/micelle formation and solubilization upon aqueous dispersion in the GI tract. In this formulation process, this article has demonstrated that the solubility of a drug in complex lipid mixtures in general can be modeled with quadratic curvature. However, if the drug solubility values in pure lipid components are close in magnitude (e.g., no more than an order of magnitude), the solubility in a lipid mixture is accurately predicted by a weighted average of the solubility values in the pure components. This result applied to the four drugs examined in this study, and appears to be general for drugs with a wide range of physicochemical properties and for the major lipids used in formulations. The ability to accurately predict solubility of new chemical entity drugs in complex mixtures is of great practical value in formulation development in that formulators only need to measure solubility in individual ingredients, and can then calculate and model solubility in more complex mixtures as a weighted average to aid in formulation design.
Acknowledgments
This research was supported by the George D. Zografi Educational Advancement Fund in the Pharmaceutical Sciences. Jeffrey B. Williams, Research Specialist in the Zeeh Pharmaceutical Experiment Station, is acknowledged for his technical support in developing the HPLC methods.
Appendix
Solubility tables (Tables VII, VIII, and IX) are provided for probucol, nifedipine and indomethacin in lipid mixtures.
Contributor Information
Mark Sacchetti, Phone: +1-608-8901859, FAX: +1-608-2625345, Email: msacchetti@pharmacy.wisc.edu.
Elham Nejati, Email: nejati@wisc.edu, Email: elham.nejati@gilead.com.
References
- 1.Strickley RG. Currently marketed oral lipid-based dosage forms: drug products and excipients. In: Hauss DJ, editor. Oral lipid-based formulations, Drugs and the Pharmaceutical Sciences. New York: Informa Healthcare; 1980. pp. 1–31. [Google Scholar]
- 2.Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev. 2008;60:625–37. doi: 10.1016/j.addr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Grove M, Mullertz A. Liquid self-microemulsifying drug delivery systems. In: Hauss DJ, editor. Oral lipid-based formulations, Drugs and the Pharmaceutical Sciences. New York: Informa Healthcare; 1980. pp. 107–27. [Google Scholar]
- 4.Holt DW, Mueller EA, Kovarik JM, van Bree JB, Kutz K. The pharmacokinetics of Sandimmune Neoral: a new oral formulation of cyclosporine. Transplant Proc. 1994;26:2935–9. [PubMed] [Google Scholar]
- 5.Wu J-G, Ge J, Zhang Y-P, Yu Y, Zhang X-Y. Solubility of genistein in water, methanol, ethanol, propan-2-ol, 1-butanol, and ethyl acetate from 280 to 333K. J Chem Eng Data. 2010;55:5286–8. doi: 10.1021/je100261w. [DOI] [Google Scholar]
- 6.Rothwell JA, Day AJ, Morgan MRA. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J Agric Food Chem. 2005;53:4355–60. doi: 10.1021/jf0483669. [DOI] [PubMed] [Google Scholar]
- 7.Christensen JØ, Schultz K, Mollgaard B, Kristensen HG, Mullertz A. Solubilization of poorly water-soluble drugs during in vitro lipolysis of medium- and long-chain triacylglycerols. Eur J Pharm Sci. 2004;23:287–96. doi: 10.1016/j.ejps.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Curatolo W, Nightingale JA, Herbig SM. Utility of hydroxypropylmethylcellulose acetate succinate (HPMCAS) for initiation and maintenance of drug supersaturation in the GI milieu. Pharm Res. 2009;26:1419–31. doi: 10.1007/s11095-009-9852-z. [DOI] [PubMed] [Google Scholar]
- 9.Lombardo F, Shalaeva MY, Tupper KA, Gao F, Abraham MH. ElogPoct: a tool for lipophilicity determination in drug discovery. J Med Chem. 2000;43:2922–8. doi: 10.1021/jm0000822. [DOI] [PubMed] [Google Scholar]
- 10.Bergström CAS, Strafford M, Lazorova L, Avdeef A, Luthman K, Artursson P. Absorption classification of oral drugs based on molecular surface properties. J Med Chem. 2003;46:558–70. doi: 10.1021/jm020986i. [DOI] [PubMed] [Google Scholar]
- 11.Nokhodchi A, Javadzadeh Y, Siahi-Shadbad MR, Barzegar-Jalali M. The effect of type and concentration of vehicles on the dissolution rate of a poorly soluble drug (indomethacin) from liquisolid compacts. J Pharm Pharm Sci. 2005;8:18–25. [PubMed] [Google Scholar]
- 12.Liu X, Hefesha H, Scriba G, Fahr A. Retention behavior of neutral and positively and negatively charged solutes on an immobilized-artificial-membrane (IAM) stationary phase. Helv Chim Acta. 2008;91:1505–12. doi: 10.1002/hlca.200890164. [DOI] [Google Scholar]
- 13.Yalkowski SH. Solubility and solubilization in aqueous media. New York: Oxford; 1999. pp. 188–91. [Google Scholar]
- 14.Yalkowski SH. Solubility and solubilization in aqueous media. New York: Oxford; 1999. pp. 180–235. [Google Scholar]
- 15.Prajapati HN, Dalrymple DM, Serajuddin ATM. A comparative evaluation of mono-, di- and triglyceride of medium chain fatty acids by lipid/surfactant/water phase diagram, solubility determination and dispersion testing for application in pharmaceutical dosage form development. Pharm Res. 2012;29:285–305. doi: 10.1007/s11095-011-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


