Abstract
The main objective of the present study is the physicochemical characterization of naturally available Terminalia catappa gum (Badam gum [BG]) as a novel pharmaceutical excipient and its suitability in the development of gastroretentive floating drug delivery systems (GRFDDS) to retard the drug for 12 h when the dosage form is exposed to gastrointestinal fluids in the gastric environment. As BG was being explored for the first time for its pharmaceutical application, physicochemical, microbiological, rheological, and stability studies were carried out on this gum. In the present investigation, the physicochemical properties, such as micromeritic, rheological, melting point, moisture content, pH, swelling index, water absorption, and volatile acidity, were evaluated. The gum was characterized by scanning electron microscopy, differential scanning calorimetry (DSC), powder X-ray diffraction studies (PXRD), and Fourier transform infrared spectroscopy (FTIR). Gastroretentive floating tablets of BG were prepared with the model drug propranolol HCl by direct compression methods. The prepared tablets were evaluated for all their physicochemical properties, in vitro buoyancy, in vitro drug release, and rate order kinetics. PBG 04 was selected as an optimized formulation based on its 12-h drug release and good buoyancy characteristics. The optimized formulation was characterized with FTIR, DSC, and PXRD studies, and no interaction between the drug and BG was found. Thus, the study confirmed that BG might be used in the gastroretentive drug delivery system as a release-retarding polymer.
KEY WORDS: badam gum, floating, gastroretentive, propranolol HCl, Terminalia catappa
INTRODUCTION
In recent years, there have been important developments in different dosage forms for existing and newly designed drugs and natural products, and semisynthetic as well as synthetic excipients often need to be used for a variety of purposes. Gums and mucilages are widely used natural materials for conventional and novel dosage forms. These natural materials have advantages over synthetic ones since they are chemically inert, nontoxic, less expensive, biodegradable, and widely available (1). They can also be modified in different ways to obtain tailor-made materials for drug delivery systems and thus can compete with the available synthetic excipients.
Lots of investigations were carried out on the suitability of natural excipients as controlled drug carriers like guar gum, gum karaya, xanthan gum, gum olibanum, etc. The main and common problem observed with the majority of these carriers was batch to batch variation in the dosage form due to changes in the rheological and swelling properties. The variation in the source, time of collection, purity, and microbial contamination were also other factors which affect the gum properties. If these problems are properly resolved, then these natural polymers would become suitable alternatives for synthetic polymers. As Badam gum (BG) was being explored for the first time for its pharmaceutical application, physicochemical, microbiological, rheological, and stability studies were carried out on this gum for its suitability in the controlled drug delivery systems.
Terminalia catappa Linn (Combretaceae) is found throughout the warmer parts of India and other countries like Malaysia, Australia, Sri Lanka, Pakistan, and many other South Asian countries. The species is found throughout the South Pacific region, including the Solomon Islands, Vanuatu, and Fiji. In India, it is called as Indian almond, Malabar almond, and Tropical almond. It is a medium-sized tree with leaves clustered towards the ends of the branches.
The various extracts of the leaves and bark of the plant have been reported to have anticancer, antioxidant (2), anti-HIV reverse transcriptase (3), hepatoprotective (4), anti-inflammatory (5,6), and aphrodisiac (7) properties. The fruit of T. catappa contains cyanidin 3-glucoside, corilagin (8,9) (topoisomerase I and II inhibitor), xanthine oxidase inhibitor (10), ellagic acid (11) (anti-HIV), antiasthmatic compound, gallic acid (12), and pentosans (13). They are also reported to contain phytochemicals which are indicative of its potential in the treatment of diabetes, e.g., brevifolin carboxylic acid and ellagic acid, which are aldose reductase inhibitors (14,15). Eugenic acid also showed anticataract activity (14). T. catappa is rich in tannins that are reported to be antidiabetic (16).
The real challenge in the development of an oral controlled release drug delivery system is not only to control the drug release but also to prolong the presence of the dosage form within the gastrointestinal tract (GIT) until complete release of the drug at a desired period of time (17). Indeed, gastric retention has received significant interest in the past few decades. Gastroretentive drug delivery is an approach to prolong gastric residence time, thereby targeting site-specific drug release in the upper GIT for local or systemic effects. Even though lots of technologies are available for gastric retention, the floating approach is most effective due to its additional advantages like random gastric emptying, site-specific drug delivery, better bioavailability, less irritation, fewer side effects (18,19), etc. In the light of these, the present work was carried out to explore the applicability of BG, a natural gum having good swelling property, as an excipient in the design of gastroretentive floating drug delivery systems (GRFDDS) of propranolol HCl.
MATERIALS AND METHODS
Materials
Propranolol HCl was provided by Dr. Reddy’s Laboratories Ltd. (Hyderabad, India). BG samples (grade I) were obtained from the Palaniappa Chettiar Traders, Rasappa Chetty Street, Park Town, Chennai, Tamil Nadu, India. Sodium bicarbonate and magnesium stearate were obtained as gift samples from Unichem Laboratories Ltd. (Goa, India). All other reagents and chemicals were of analytical grade.
Physicochemical Properties of BG
Particle Size Distribution of BG Powder
BG powder was dispersed in liquid paraffin and a smear of the dispersion was examined under microscope. The size of the 500 particles was measured using a calibrated eyepiece micrometer. The size distribution of the BG particles was estimated.
Determination of the Flow Properties of BG Powder (20)
The flow properties of BG were determined by the angle of repose method and compressibility index (CI).
Angle of Repose Method
Angle of repose was determined by fixed funnel and free-standing cone method. A funnel with the end of the stem cut perpendicular to its axis of symmetry was fixed at a given height (h) above the graph paper placed on a flat horizontal surface. The gum powder was carefully poured through the funnel until the apex of the conical pile just touched the tip of the funnel. The radius of the base (r) of the pile was determined and the tangent angle of repose (θ) was calculated by the following equation:
 |
1 |
Compressibility Index
The packing properties were determined by measuring the difference between bulk density (BD) and tapped density (TD) using the standard procedure. In the procedure, a 20-g quantity of BG powder was placed into a 250-mL clean, dry measuring cylinder and the volume, V0, occupied by the sample without tapping was determined. An automated tap density tester (model C-TDA2, Campbell Electronics, Mumbai, India) was used for tapping the granules according to USP Chapter (21) 616 Method I. After 100 taps, the occupied volume, V100, was also noted. The BD and TD were calculated from these volumes (V0 and V100) using the formula: Density = weight/volume occupied by the sample. The Hausner ratio was determined by dividing the TD by the BD, and Carr’s CI (22) was determined using Eq. 2:
 |
2 |
Melting Point of BG
The gum powder was transferred into a capillary tube, and the melting point was determined using the Shiva Scientific Melting Point Apparatus, Mumbai, India.
Determination of Moisture Content of BG
Moisture content was determined using the Karl Fischer auto titrator M/s. MetRohm. The sample was dispersed in methanol, stirred to extract water, and then titrated with standardized Karl Fischer reagent until the end point is reached. Moisture content was determined using the following formula:
 |
3 |
where Vl is the volume of the Karl Fisher reagent, We is the water equivalent, and Sw is the sample weight in milligrams. Fifteen milliliters of the reagent is equivalent to 75 mg of water, and the water equivalent is 5.2.
Determination of the pH Value of BG
The pH of 1% w/v aqueous mucilage of BG was determined using a pH meter (Systronics, Model 361).
Determination of the Swelling Index and Water Retention Capacity of BG
Swelling and water retention capacity of BG were determined using the modified method which was reported by Gauthami and Bhat (23). One gram of BG powder was accurately weighed and transferred to a 100-mL stoppered measuring cylinder. The initial volume occupied by the powder was noted and the volume was made up to 100 mL with distilled water. The cylinder was stoppered, shaken gently, and set aside for 24 h. The volume occupied by the gum sediment was noted after 24 h.
Swelling index (SI) is expressed in percentage and was calculated by the following equation:
 |
4 |
where V0 is the initial volume of the powder in a graduated cylinder and Vt denotes the volume occupied by the swollen gum after 24 h.
The contents of the measuring cylinder from this test were filtered through a muslin cloth and water was allowed to drain completely into a dry 100-mL graduated cylinder. The volume of water collected was noted, and the difference between the original volume of the mucilage and the volume drained was taken as the water retention capacity or water absorption capacity.
Determination of the Volatile Acidity of BG
One gram of the BG powder was accurately weighed and transferred to a 500-mL long-necked flask; 100 mL of water and 5 mL of orthophosphoric acid (85%) were added and allowed to stand until the gum was completely swollen (approximately 24 h). Then, the solution was boiled for 3 h under a reflux condenser and steam was distilled until 30 mL of the distillate was obtained (24).
The distillate was titrated with 0.1 N NaOH using phenolphthalein as indicator. The procedure was repeated for blank. The difference between the two titrations represented the amount of alkali required to neutralize the volatile acid. Each milliliter of 0.1 N NaOH is identical to 0.006005 g of C2H4O2.
Scanning Electron Microscopic Studies of BG
The scanning electron microscope (SEM) photograph of the BG powdered sample was obtained by SEM (Jeol, JSM-840A, Japan) with 20 kV accelerating voltage.
Differential Scanning Calorimetry of BG
The differential scanning calorimetry (DSC) curve of the BG powder was obtained using a differential scanning calorimeter (Mettler Toledo STARe SW 8.10, Model DSC 822e) at a heating rate of 10°C/min from 25°C to 280°C in air (80 mL/min).
Infrared (FTIR) Spectroscopy of BG
The IR spectrum of the BG powder was obtained using the IR spectrophotometer (Perkin-Elmer 841, Model Spectrum One). Powder was analyzed by potassium bromide pellet method in an IR spectrophotometer in the region between 3,500 and 500 cm−1.
Powder X-ray Diffractometry
Powder X-ray diffractometry (PXRD) studies of the BG powder were performed with the Rigaku 30-kV X-ray diffractometer (D/MAX-B) using Ni-filtered Cu-K(α) radiation, a voltage of 35 kV, a current of 20 mA, and receiving slit of 0.2 in. The sample was analyzed over the 2θ range of 2–45°, with scan step size of 0.020° and scan step time of 1 s.
Determination of the Rheological Properties of BG (25–27)
The rheological properties of BG were determined using the Brookfield cone and plate viscometer (Model LV DV-III+ Rheometer). BG concentrations of 0.5%, 1%, 1.5%, 2%, 2.5%, and 3% w/v mucilages were prepared with distilled water and allowed for overnight swelling. The sample (0.5 mL) was placed on the viscometer plate and analyzed for its viscosity, percent torque, shear stress, and shear rate at various speeds. Thixotropic phenomenon was also tested at 25°C using the CP 52 spindle and Rheocalc software of the instrument.
The analysis of rheological data may be enhanced through the use of mathematical models. Non-Newtonian behavior can be simply expressed through an equation, and in some cases, the coefficients of a model can be used to infer the performance of a fluid under conditions of use. Newtonian flow is defined by a proportional response in shear stress for a change in shear rate (a linear relationship). Non-Newtonian fluids will exhibit a nonlinear stress/rate relationship. The Newtonian equation for viscosity has been modified many times to attempt to characterize non-Newtonian behavior. Some of the widely used equations include Bingham, Casson, National Confectioners Association/Chocolate Manufacturers Association (NCA/CMA) Casson, Power law, and IPC Paste:
 |
5 |
 |
6 |
 |
7 |
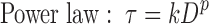 |
8 |
 |
9 |
where τ denotes the shear stress, D denotes the shear rate, η denotes the plastic viscosity, do denotes the yield stress, k denotes the consistency index, p denotes the flow index, n denotes the shear sensitivity, a denotes the gap ratio, and R denotes rotations per minute.
A power law index of p = 1 represents Newtonian flow behavior, p < 1 represents shear thinning (pseudoplastic) behavior, and p > 1 represents shear thickening (dilatant) behavior. Storage period may influence the properties of the natural gum, and hence, rheological properties were also studied for a 1-year-old sample packed in high-density polyethylene (HDPE) bottle and stored under normal conditions (23–25°C) of the laboratory. The same procedure was carried out for the 1-year-old samples.
Microbial Studies on BG
Microbial studies were carried out on the fresh gum powder sample and 1-year-old sample. The samples were analyzed for total viable aerobic microorganism count and the presence of designated microbial species by pour plate method. In this method, 1 μg/mL of the gum solution was inoculated on sterilized molten casein soybean digest agar medium, poured into Petri plates, and allowed to solidify. The plates were kept at 5–10°C for 1 h and were incubated at 37°C for 18 h. The number of colonies formed after the incubation period was counted.
The total fungal count was determined using potato dextrose agar medium and the plates were incubated at room temperature (23–25°C) for 48 h. The presence of designated microbial species in the gum sample was estimated using specific media like mannitol salt agar medium (Staphylococcus aureus), cetrimide agar (Pseudomonas aeruginosa), MacConkey agar medium (Escherichia coli), and deoxycholate citrate agar medium (Salmonella sp.).
Accelerated Stability Studies on BG
BG powder was exposed to accelerated stability conditions as per ICH guidelines (28) to predict the stability of the gum, and the samples were analyzed at regular time intervals.
Preparation of Propranolol HCl Floating Tablets
All the ingredients sufficient for a batch of 100 tablets according to the formulae (shown in Table I) were passed through sieve no. 40 (425 μm). The drug was geometrically mixed with polymer until a homogenous blend was achieved. Sodium bicarbonate was added to the above mixture and mixed for 5 min in a polybag. The blend was lubricated with presifted magnesium stearate through sieve no. 60 (250 μm) for 3 min in polybag. The final blend was then compressed into tablets on a 16-station rotary tablet punching machine (M/s. Cadmach Machinery Co. Pvt. Ltd., India) using 9-mm round plain punches.
Table I.
Formula of BG–Propranolol HCl Floating Tablets
| Ingredients | PBG 01 | PBG 02 | PBG 03 | PBG 04 | PBG 05 | PBG 06 |
|---|---|---|---|---|---|---|
| Propranolol HCl | 80 | 80 | 80 | 80 | 80 | 80 |
| Badam gum | 40 | 80 | 120 | 160 | 200 | 240 |
| Sodium bicarbonate | 13.5 | 18 | 22.5 | 27 | 31.5 | 36 |
| Magnesium stearate | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 |
| Tablet weight (mg) | 135 | 180 | 225 | 270 | 315 | 360 |
Evaluation of Tablets
Floating tablets were evaluated for physicochemical parameters like weight variation, hardness, friability, assay, in vitro buoyancy studies, in vitro dissolution studies, and release rate kinetics.
In Vitro Buoyancy Studies
The floating characteristics of GRFDDS are essential, since they influence the in vivo behavior of the drug delivery system. All the formulated floating tablets were subjected to in vitro buoyancy studies and, for each batch, five tablets were used. The floating lag time was determined in a 1-L glass beaker containing 900 mL of 0.1 N HCl. The time required for the tablet to rise to the surface and float was determined as the floating lag time. The duration of time the dosage form constantly remained on the surface of medium was determined as the total floating time (29).
In Vitro Dissolution Studies
In vitro release of propranolol HCl from the prepared floating tablets was studied using the USP XXIV dissolution test apparatus (Lab India, Disso 2000) employing the paddle stirrer (Apparatus II). Nine hundred milliliters of 0.1 N HCl was used as dissolution medium maintained at a temperature of 37 ± 0.5°C and the paddle was rotated at 50 rpm (29,30). Five milliliters of the sample was withdrawn by means of a syringe fitted with a prefilter at definite time intervals and immediately replaced with 5 mL of fresh medium maintained at 37 ± 0.5°C. The filtered samples were suitably diluted with the dissolution medium wherever necessary and the absorbance of the samples was measured at 289 nm. Dissolution experiments were done in triplicate. The percentage of propranolol HCl released from the floating tablets was calculated and reported.
Release Kinetics
Several mathematical models can be tested to determine which best describes the kinetics and mechanism of drug release from floating tablets (31–36). In the present study, the in vitro drug release data was fitted to the following mathematical models:
| Model | Equation |
| Zero-order |

|
| First-order |

|
| Higuchi |

|
| Hixon–Crowell |
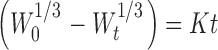
|
| Korsmeyer–Peppas |

|
where Qt is the amount of drug released in time t, Q0 is the initial amount of drug in the tablet, C0 is the initial concentration of drug, Q is the active fraction released per unit of surface, W0 is the initial amount of drug in the pharmaceutical dosage form, Wt is the remaining amount of drug in the pharmaceutical dosage form at time t, Mt is the amount of drug released at time t, M∝ is the amount released at time ∝, Mt/M∝ is the fraction of drug released at time t, and K0, K1, KH, K, and KK are the rate order constants.
RESULTS AND DISCUSSION
The physicochemical properties of BG were evaluated and the results are shown in Table II.
Table II.
Physicochemical Properties of BG Powder
| Property | Mean ± SD |
|---|---|
| Bulk density (g/cc) | 0.721 ± 0.16 |
| Bulkiness (cc/g) | 1.39 ± 0.22 |
| Tapped density (g/cc) | 0.812 ± 0.09 |
| Compressibility index (%) | 11.20 ± 0.08 |
| Hausner ratio | 1.13 ± 0.66 |
| Angle of repose (°) | 31.14 ± 0.91 |
| Melting point (°C) | Charring at 80°C |
| Moisture content (%) | 10.22 ± 0.55 |
| pH | 4.4–4.9 |
| Swelling index (%) | 3,100 ± 0.19 |
| Water retention capacity (mL) | 14.8 ± 0.52 |
| Volatile acidity (%) | 3.6 ± 0.95 |
Determination of the Particle Size Distribution of BG Powder
The particle size distribution of the BG powder is shown in the Fig. 1. When observed under a microscope, BG powder was found to be in the form of irregular granules which were in the range of 25–125 μm, and most of the powder particles were within the range of 75–125 μm.
Fig. 1.
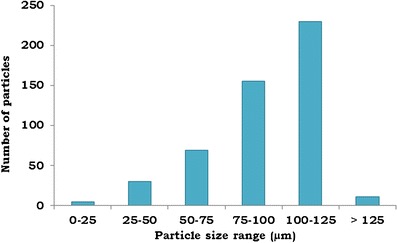
Particle size distribution of BG powder
Determination of the Flow Properties of BG Powder
Powders normally flow under the influence of gravity; dense substances are generally less cohesive than lighter ones, as the weight of the particles for a given volume is increased. Hence, difference in densities of various ingredients of the formulation may lead to improper mixing and filling during manufacturing of formulations, resulting in weight variation and variations in content uniformity of finished products. Hence, determination of the density of any ingredient will be helpful in successful formulation development.
The properties such as BD, TD, true density, CI, and angle of repose are often referred to as the derived properties of the powders, which depend mainly on the particle size distribution, particle shape, and tendency of the particles to adhere together. The results of the flow properties of the BG powder are shown in Table II.
The flow properties of the material can be determined by angle of repose. The angle of repose values in the range of 25–30° and 31–35° indicate excellent and good flow properties for the material, respectively. If the value is >40°, it suggests poor flow of the material. The angle of repose of the BG powder was found to be 31.14°, which indicated good flow properties (21).
CI can also be used as an index of powder flow and is based on experimental research undertaken in which interparticle cohesive properties are investigated. The CI of the BG powder was 11.20% (11–15% for good flow), which indicated good flow.
The Hausner ratio values in between 1.35–1.5 and 1.12–1.18 indicate poor flow and good flow, respectively. The Hausner ratio for BG powder was found to be 1.13, which indicated that the powder demonstrated good flow characteristics.
Melting Point of BG Powder
Normally, natural products often have a range of melting points than pure chemicals. During the melting process of materials containing sugars and polysaccharides, the transition of the solid phase to the liquid phase is followed by charring. The BG powder started charring at a temperature of 80°C.
Determination of the Moisture Content of BG Powder
The moisture content of the excipients can influence the compressing and stability properties of the formulations. The moisture content of BG powder was found to be 10.22%.
Determination of the pH Value of BG Powder
The pH of the 1% w/v BG mucilage was found to be 4.4–4.9, indicating that the gum is acidic in nature.
Determination of the Swelling Index and Water Retention Capacity of BG Powder
The SI of the BG powder was found to be 3,100%. A high value of SI revealed the high swelling ability of the BG powder. The swelling ability of any natural gum depends upon its water retention capacity or water absorption capacity. The water absorption capacity of the BG powder is found to be 14.8 mL.
Determination of Volatile Acidity of BG Powder
The volatile acidity of the BG powder was found to be 3.6%. The viscosity of the gum is directly proportional to the volatile acidity of the gum.
Scanning Electron Microscopic Studies of BG Powder
When examined under a compound microscope, the BG powder was found to be irregular and 0–125 μm in size. The SEM photographs of the BG grains shown in Fig. 2 also revealed that the shape of the BG was irregular with sharp angles.
Fig. 2.
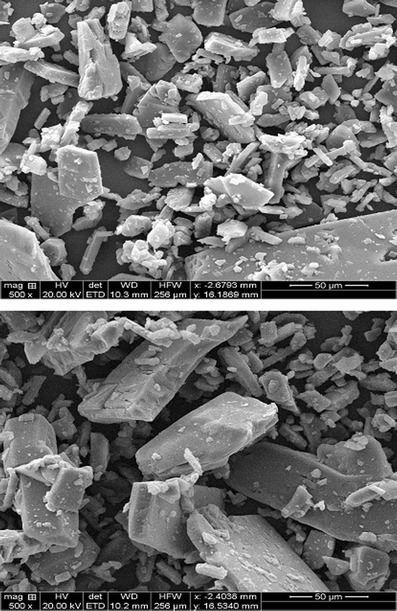
SEM photographs of BG powder
Differential Scanning Calorimetry of BG Powder
DSC measures the heat loss or gain resulting from physical or chemical changes within a sample as a function of temperature. A sharp symmetric melting endotherm can indicate relative purity, whereas a broad asymmetric curve suggests impurities or more than one thermal process (37). The endothermic peak usually indicates the loss of water present in the compound. The DSC thermogram of the BG powder is shown in the Fig. 3. The BG powder exhibited broad endothermic peak at 79.6°C. The produced peak may be due to the loss of free/bound water present in the BG and this also coincided with the charring temperature of the gum.
Fig. 3.

DSC thermogram of BG powder
Infrared (FTIR) Spectroscopy of BG
The Fourier transform infrared spectroscopy (FTIR) spectrum of BG showed the characteristic alcoholic –OH stretch at 3,453 cm−1, C–H stretch at 2,888 cm−1, –C=O stretch at 1,728 cm−1, C=C stretch at 1,628 cm−1, aromatic O–CH2 stretch at 1,272 cm−1, COO– symmetrical stretch at 1,430 cm−1, –CH=CH–O–CH2 stretch at 1,054 cm−1, and –C–O–C asymmetric stretch at 1,370 cm−1. The absorption peaks at 1,728 and 1,272 cm−1 are indicative of acetyl groups and at 1,430 cm−1 is due to the carboxylate groups of uronic acid residues. The FTIR spectrum of the BG powder is shown in Fig. 4.
Fig. 4.

FTIR spectra of BG powder
Powder X-ray Diffractometry
PXRD analysis of the BG sample is shown in Fig. 5. Polymer BG did not show any characteristic peaks in the spectrum when it was exposed to X-ray diffraction. As per the powder crystalline theory, solids are characterized by an extended three-dimensional arrangement of atoms, ions, or molecules in which the components are generally locked into their positions. The components can be arranged in a regular repeating three-dimensional array (a crystal lattice), which results in a crystalline solid, or more or less randomly to produce an amorphous solid (38). Crystalline solids have well-defined edges and faces, diffract X-rays, and tend to have sharp melting points. In contrast, amorphous solids have irregular or curved surfaces and do not give well-resolved X-ray diffraction patterns. By PXRD analysis, it can be concluded that BG is exhibiting an amorphous nature.
Fig. 5.

X-ray diffractogram of BG powder
Determination of the Rheological Properties of BG Powder
To assess the quality of the natural gum rheology is one of the main parameter. The applications of any natural gum depend upon its viscosity. The choice of selecting the natural gum for sustained release depends upon its gelling strength (27). The rheological properties of the BG powder at various concentrations were performed and the results are shown in Fig. 6. The results showed that BG possesses pseudoplasticity. From the results, it was observed that the BG followed a non-Newtonian flow behavior, which was confirmed by the existence of a nonlinear relationship between shear stress and shear rate. Viscosity data were analyzed using the Bingham, Casson, NCA/CMA Casson, Power law, and IPC Paste equations and the results are shown in Table III. The viscosity of 0.5%, 1%, 1.5%, 2%, 2.5%, and 3% w/v BG mucilage at 10 rpm was 188.6, 244.5, 312.8, 428.6, 2,459, and 2,490 cps, respectively. From the results, it was clearly indicated that BG in aqueous media obeys the Power law, with confidence of fit values ranging from 91.7 to 98.6. Systems that exhibit non-Newtonian flow behavior are frequently represented as power law fluids such that the apparent viscosity of the material changes as shear stress is applied to it. The flow index (p) value found to be <1 for all the concentrations of BG indicated that BG has a shear thinning property (pseudoplastic). BG hydrated quickly, swelled rapidly, and formed a thick viscous layer around it. The viscosity and other rheological properties confirmed its suitability for the development of controlled release delivery systems.
Fig. 6.

a Shear stress vs. shear rate and b shear rate vs. viscosity profiles of various concentrations of BG
Table III.
Analysis of Rheological Properties of Various Concentrations of BG
| Concentration of BG (% w/v) | ||||||
|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | |
| Bingham | ||||||
| η (cps) | 30 | 54.5 | 72.9 | 286 | 4,129 | 6,471 |
| τ 0 (dynes/cm2) | 46.2 | 42.9 | 26.5 | 34.9 | 47.9 | 82.4 |
| Cof (%) | 93.1 | 91.1 | 90.3 | 89.7 | 91.3 | 88.6 |
| Casson | ||||||
| η (cps) | 16.2 | 29 | 37 | 121.1 | 2,768 | 3,408 |
| τ 0 (dynes/cm2) | 22.6 | 21.2 | 13.9 | 21 | 24.2 | 41 |
| Cof (%) | 96.6 | 93.3 | 91.4 | 91.1 | 92.3 | 91.9 |
| NCA/CMA Casson | ||||||
| η (cps) | 16.2 | 29 | 37 | 121.1 | 2,768 | 3,408 |
| τ 0 (dynes/cm2) | 21.4 | 20.1 | 13.2 | 19.8 | 22.9 | 38.9 |
| Cof (%) | 96.6 | 93.3 | 91.4 | 91.1 | 92.3 | 91.9 |
| Power law | ||||||
| Consistency index, k (cps) | 911.6 | 1,227 | 1,678 | 2,853 | 12,524 | 15,487 |
| Flow index, n | 0.47 | 0.46 | 0.44 | 0.37 | 0.46 | 0.49 |
| Cof (%) | 98.6 | 94.1 | 93 | 91.7 | 93.8 | 94.8 |
| IPC Paste | ||||||
| 10 rpm viscosity (cps) | 188.6 | 244.5 | 312.8 | 428.6 | 2,459 | 2,490 |
| Shear sensitivity | 0.53 | 0.54 | 0.56 | 0.63 | 0.64 | 0.66 |
| Cof (%) | 98.6 | 94.1 | 93 | 91.7 | 93.8 | 94.8 |
Cof coefficient of fit
The 1-year-old BG sample of 1.5% and 2% w/v concentration stored at normal conditions was evaluated for rheological properties to check its reproducibility, and the comparative results are shown in Fig. 7. The results showed that there was no significant difference between the initial and 1-year-old samples.
Fig. 7.
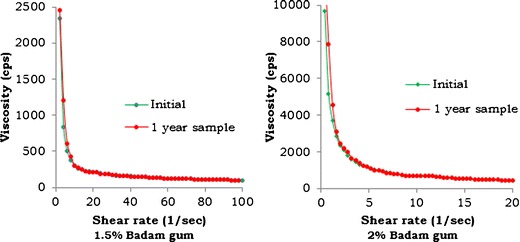
Comparison of shear rate vs. viscosity profiles of 1.5% and 2% w/v BG stored at normal conditions for 1 year
Microbial Studies on BG Powder
Microbial studies were carried out for the immediately collected powder and 1-year-old sample. The samples were analyzed for microbial growth and results showed that BG did not support microbial growth and it is free from all pathogenic organisms. The results are shown in Table IV.
Table IV.
Microbial Load of BG
| Parameter | Result | Specified limit as per I.P. | |
|---|---|---|---|
| Fresh sample | One-year-old sample | ||
| Total aerobic microbial count | 35 CFU/g | 38 CFU/g | NMT 100 CFU/g |
| Total fungal count | 50 CFU/g | 54 CFU/g | NMT 100 CFU/g |
| Microbial species | |||
| S. aureus | Absent/g | Absent/g | Should be absent/g |
| P. aeruginosa | Absent/g | Absent/g | Should be absent/g |
| E. coli | Absent/g | Absent/g | Should be absent/g |
| Salmonella sp. | Absent/g | Absent/g | Should be absent/g |
CFU colony-forming units, NMT not more than
Accelerated Stability Studies on BG Powder
The results of the accelerated stability studies on BG powder are shown in Table V. No significant difference in the physical state of the powder and pH between the initial and final samples. Very slight increase in the moisture content might be due to the absorption of moisture during stability studies. Hence, samples should be stored in airtight HDPE containers. There was no significant change in the viscosity of the BG mucilage (1% w/v), indicating that the gum is stable over a longer period of time.
Table V.
Accelerated Stability Results of BG
| Test condition | Tests carried out | ||||
|---|---|---|---|---|---|
| Date | Period (months) | Physical observation | Moisture content (%) | pH of 1% mucilage | Viscositya (cps) |
| 12-01-2009 | Initial | Off-white crystalline powder | 10.22 | 4.77 | 244.5 |
| 12-02-2009 | 1 | No change | 10.93 | 4.61 | 247.8 |
| 12-03-2009 | 2 | No change | 11.02 | 4.60 | 245.1 |
| 12-04-2009 | 3 | No change | 11.84 | 4.59 | 246.4 |
| 12-07-2009 | 6 | No change | 11.93 | 4.71 | 243.2 |
a1% w/v mucilage at 10 rpm
Tableting Characteristics of the Propranolol HCl Floating Tablets
All the prepared propranolol HCl floating formulations complied with the compendial standards for uniformity of weight (Table VI). The hardness for all the formulations was in the range of 4 to 6 kg/cm2. Assay was found to be in the range of 98.12% to 99.99% of the stated amount of propranolol HCl. The percentage weight loss in the friability test was found to be <0.5% for all the batches. Thus, propranolol HCl gastroretentive floating tablets (GRFT) prepared with the selected polymers were regarded as good quality, fulfilling the entire official and other requirements of tablets.
Table VI.
Tableting Characteristics of BG-Based Propranolol HCl Floating Tablets
| Formulation | Weighta (mg) | Assayb (%) | Hardnessc (kg/cm2) | Friabilityd (%) | FLTc (min) | TFTc (h) |
|---|---|---|---|---|---|---|
| BG | ||||||
| PBG 01 | 135 ± 1.77 | 99.12 ± 0.9 | 4–6 | 0.12 | 2.5–4 | 6 |
| PBG 02 | 180 ± 1.43 | 99.99 ± 0.34 | 4–6 | 0.22 | 1.5–2.5 | 8 |
| PBG 03 | 225 ± 1.09 | 99.92 ± 1.42 | 4–6 | 0.43 | 0.5–1.5 | 10 |
| PBG 04 | 270 ± 1.52 | 99.92 ± 0.99 | 4–6 | 0.21 | 0–0.5 | 12 |
| PBG 05 | 314 ± 0.97 | 98.55 ± 1.21 | 4–6 | 0.27 | 0–0.3 | 14 |
| PBG 06 | 360 ± 0.57 | 99.43 ± 1.11 | 4–6 | 0.23 | 0–0.1 | 16 |
FLT floating lag time, TFT total floating time
aMean ± SD (n = 20)
bMean ± SD (n = 10)
c n = 5
d n = 20
In Vitro Buoyancy Studies
When carbonates and bicarbonates interact with acid, they liberate carbon dioxide. This concept was applied in the preparation of effervescent floating tablets of propranolol HCl. The floating lag time for all the formulations (PBG 01–06) was in the range of 0–4 min, and the total floating time ranged from 6 to 16 h. From the results, it was found that, as the concentration of polymer increased, floating lag time decreased due to the hydrophilic nature of polymer, which allows the penetration of media through the pores formed on the surface of the tablet, and total floating time increased due to the swelling nature of the tablet by forming a swollen mass over the tablet, which keeps the tablet intact for longer duration (39–41). Sodium bicarbonate was necessary in the formulations for floating, which has the property to liberate CO2 in the presence of 0.1 N HCl. The generated gas gets trapped and protected within the gel formed by hydration of the polymer, thus decreasing the density of the tablet. As the density of the tablet falls below 1 g/cm3, the tablet becomes buoyant.
In Vitro Dissolution Studies
Natural polymer BG-based formulations (PBG 01–06) containing drug to polymer ratios 1:0.5, 1:1, 1:1.5, 1:2, 1:2.5, and 1:3 released more than 95% of the drug in 6, 8, 10, 12, 14, and 16 h, respectively. The drug-retarding property was directly proportional to the polymer concentration. PBG 04 retarded the drug release for 12 h with continuous floating on the gastric medium (Fig. 8).
Fig. 8.

Dissolution profiles of BG–propranolol HCl floating tablets
Release Kinetics
The dissolution data were fitted to the kinetic parameters and the correlation coefficient values (r values) of the release kinetics of all the formulations of propranolol HCl are shown in Table VII. All the BG-based formulations followed first-order rate kinetics, except PBG 06 which followed zero-order rate kinetics, which is indicated by higher r value. The relative contributions of drug diffusion and erosion to drug release were further confirmed by subjecting the dissolution data to the Higuchi model and the Hixon–Crowell model. It was found that all the formulations followed non-Fickian diffusion mechanism. From the obtained results, it can be concluded that the drug dissolution property is strongly altered by the quantity of polymer up to 160 mg. When the concentration exceeded that quantity, the drug release rate independent of BG concentration might be because of its high swelling nature, which leads to difficulty for the drug to come out from the swollen mass.
Table VII.
Correlation Coefficient Values and Release Kinetics of BG-Based Propranolol HCl Floating Tablets
| Formulation | Zero order | First order | Higuchi | Hixson–Crowell | Korsmeyer–Peppas | ||||
|---|---|---|---|---|---|---|---|---|---|
| K 0 | r | K 1 | r | r | r | n | r | ||
| PBG 01 | 14.898 | 0.9039 | 0.5513 | 0.9946 | 0.9824 | 0.9588 | 0.3553 | 0.9935 | Non-Fickian |
| PBG 02 | 11.4222 | 0.9261 | 0.3943 | 0.9967 | 0.9898 | 0.9588 | 0.4181 | 0.9904 | Non-Fickian |
| PBG 03 | 8.9086 | 0.9626 | 0.2246 | 0.9946 | 0.9972 | 0.9615 | 0.5021 | 0.9928 | Non-Fickian |
| PBG 04 | 7.8065 | 0.9780 | 0.2008 | 0.9977 | 0.9996 | 0.9805 | 0.6141 | 0.9974 | Non-Fickian |
| PBG 05 | 7.1067 | 0.9832 | 0.1868 | 0.9933 | 0.9988 | 0.9979 | 0.7538 | 0.9965 | Non-Fickian |
| PBG 06 | 6.4899 | 0.9983 | 0.1543 | 0.9624 | 0.9874 | 0.9873 | 0.8613 | 0.9990 | Non-Fickian |
Drug–Polymer Interaction Studies
FTIR Studies
The FTIR spectra of pure drug propranolol HCl, BG, and PBG 04 are shown in Fig. 9. The FTIR spectrum of propranolol HCl showed characteristic secondary amine –N–H stretch at 3,280 cm−1, C–H stretch at 2,964 cm−1, aryl C=C stretch at 1,579 cm−1, aryl O–CH2 asymmetric stretch at 1,240 cm−1, aryl O–CH2 symmetric stretch at 1,030 cm−1, and the peak at 798 cm−1 due to α-substituted naphthalene (42). The FTIR spectrum of BG showed the characteristic alcoholic –OH stretch at 3,453 cm−1, C–H stretch at 2,888 cm−1, –C=O stretch at 1,728 cm−1, and –C–O–C asymmetric stretch at 1,370 cm−1.
Fig. 9.

FTIR spectra of a propranolol HCl, b BG, and c PBG 04
Optimized BG-based formulation (PBG 04) showed all the characteristic peaks of propranolol HCl, with minor shifts in its FTIR spectrum. This spectrum showed secondary amine –N–H stretch at 328 cm−1, C–H stretch at 2,964 cm−1, aryl C=C stretch at 1,579 cm−1, aryl O–CH2 asymmetric stretch at 1,241 cm−1, aryl O–CH2 symmetric stretch at 1,030 cm−1, and the peak at 797 cm−1 due to α-substituted naphthalene. The FTIR data indicated the absence of any chemical interaction between propranolol HCl and the studied polymer BG.
DSC Studies
Any abrupt or drastic change in the thermal behavior of the drug or polymer may indicate a possible drug–polymer interaction. The DSC thermogram of propranolol HCl, BG, and optimized formulation PBG 04 are shown in Fig. 10. Pure drug propranolol HCl showed a sharp endothermic peak at 168.12°C, BG exhibited a broad endothermic peak at 82°C, and the optimized formulation PBG 04 showed peaks at 82.3°C and 166.6°C, representing the polymer and drug’s endothermic peaks, respectively. The DSC thermogram revealed that there is no appreciable change in the nature of the melting endotherms, suggesting that the drug has not lost its characteristic properties even in its formulation form as there is no interaction of the drug with the natural polymer BG used for the study.
Fig. 10.

DSC thermogram of a propranolol HCl, b BG, and c PBG 04
PXRD Studies
The X-ray diffractograms of propranolol HCl, BG, and optimized formulation PBG 04 are shown in Fig. 11. The X-ray diffractograms of propranolol HCl showed sharp peaks at 8.401°, 9.742°, 12.521°, 12.860°, 16.759°, 17.199°, 18.678°, 19.339°, 19.539°, 19.918°, 21.241°, 22.119°, 22.460°, 23.697°, 25.062°, 25.439°, 25.839°, 26.418°, 27.081°, 29.580°, 33.639°, and 34.941° angle (2θ), having the crystalline size of 29.88, 33.63, 34.83, 41.77, 36.99, 45.86, 63.58, 19.02, 17.00, 46.55, 49.07, 22.73, 27.35, 43.28, 46.71, 37.03, 30.90, 58.70, 39.19, 26.55, 36.02, 32.88, and 44.57 nm, respectively, indicated the crystallinity of the drug (Fig. 11a). The average crystal size of the drug was calculated using the Eq. 10 at different peaks obtained in XRD for pure drug, and the average crystal size was found to be 37.57 nm.
Fig. 11.
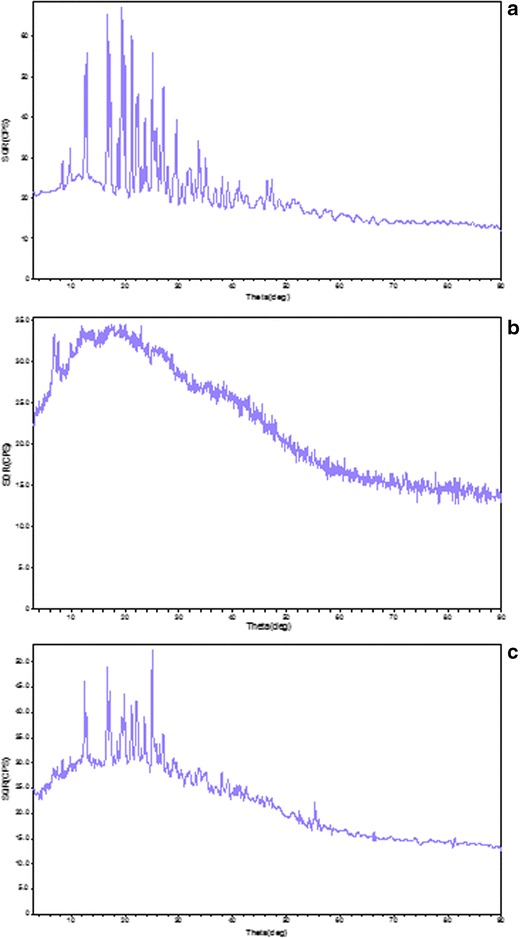
PXRD diffractogram of a propranolol HCl, b BG, and c PBG 04
Crystal size was calculated by the following formula (43,44):
 |
10 |
where k is the Scherrer’s constant (0.89), λ is the X-ray wavelength (0.1549 nm), β is the peak width at half of its height or full width at half maximum (FWHM), and θ is the Bragg angle.
The crystalline size can also cause peak broadening. The Scherrer equation explains the peak broadening in terms of incident beam divergence, which makes it possible to satisfy the Bragg condition for nonadjacent diffraction planes. Once instrument effects have been excluded, the crystallite size is easily calculated as a function of peak width (specified as the FWHM), peak position, and wavelength.
Polymer BG did not show any peaks which indicated its amorphous nature (Fig. 11b). The formulation PBG 04 showed characteristic peaks of pure drug with minor shift and less intensity at 12.498°, 12.839°, 16.720°, 17.159°, 19.400°, 19.878°, 21.201°, 22.080°, 22.339°, 23.638°, 25.061°, 25.420°, and 27.062° angle (2θ), with crystalline size of 32.53, 26.57, 34.01, 39.07, 13.95, 28.33, 36.12, 21.91, 17.15, 27.23, 31.08, 12.03, and 28.32 nm, respectively. In the formulation peaks, it was observed that some drug peaks disappeared at 8.401°, 9.742°, 18.678°, 26.418°, 29.081°, 29.580°, 33.639°, and 34.941° angle (2θ), indicating the reduction of crystallinity (Fig. 11c). The average crystal size of the formulation was found to be 26.79 nm. The reduced crystallinity may be due to the amorphous nature of the polymer and might be due to the fine dispersion of the drug in the polymer during mixing.
The changes observed in the X-ray diffractograms and DSC thermograms and the absence of any changes in the FTIR spectra for the selected formulations indicated that there was no physical or chemical interaction between the drug and polymers used in the study.
CONCLUSION
The results of the physicochemical properties of BG indicated that the gum is acidic in nature, free flowing, and suitable for tablet manufacture. Polymer with high SI/water retention capacity are considered to be very good polymers for the design of controlled/sustained release, especially for diffusion-type drug delivery systems. The high swelling capacity of BG indicated its suitability for the design of diffusion-type controlled drug delivery systems. The results of the present study clearly indicated that the GRFT of propranolol HCl were successfully designed and optimized using the indigenously available natural gum BG.
Based on these findings, it was concluded that natural gum like BG could be explored as a controlled release polymer because of its abundant availability, reliability, efficiency, ecofriendly, and economical features and it is also a good substitute for synthetic polymers in the design of GRFDDS. According to the results obtained, it can also be used as controlled and sustained release polymer. As BG successfully retarded the drug release of highly soluble drug like propranolol HCl, it can be concluded that BG can be used as a retarding polymer for the formulation of other drugs with similar behavior.
ACKNOWLEDGMENTS
The author M.V. Srikanth is thankful to the University Grants Commission, India for awarding a Senior Research Fellowship for carrying out this project. One of the authors, M.V. Srikanth, is thankful to G.V. Murali Mohan Babu, M. Chaitanya Krishna, and B. Sudhakar for providing valuable information to carry out the research work.
Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Girish KJ, Dhiren PS, Vipul DP, Vineet CJ. Gums and mucilages: versatile excipients for pharmaceutical formulations. Asian J Pharm Sci. 2009;4:309–23. [Google Scholar]
- 2.Masuda T, Yonemori S, Oyama Y, Takeda Y, Tanaka T, Andoh T, et al. Evaluation of the antioxidant activity of environmental plants: activity of the leaf extracts from sea shore plants. J Agric Food Chem. 1999;47:1749–54. doi: 10.1021/jf980864s. [DOI] [PubMed] [Google Scholar]
- 3.Tan GT, Pezzuto JM, Kinghorn AD, Hughes SH. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J Nat Prod. 1991;54:143–54. doi: 10.1021/np50073a012. [DOI] [PubMed] [Google Scholar]
- 4.Lin CC, Chen YL, Lin JM, Ujiie T. Evaluation of the antioxidant and hepatoprotective activity of Terminalia catappa. Am J Chin Med. 1997;25:153–61. doi: 10.1142/S0192415X97000172. [DOI] [PubMed] [Google Scholar]
- 5.Lin CC, Hsu YF, Lin TC. Effects of punicalagin and punicalin on carrageenan-induced inflammation in rats. Am J Chin Med. 1999;27:371–6. doi: 10.1142/S0192415X99000422. [DOI] [PubMed] [Google Scholar]
- 6.Chen PS, Li JH, Liu TY, Lin TC. Folk medicine Terminalia catappa and its major tannin component, punicalagin, are effective against bleomycin induced genotoxicity in Chinese hamster ovary cells. Cancer Lett. 2000;152:115–22. doi: 10.1016/S0304-3835(99)00395-X. [DOI] [PubMed] [Google Scholar]
- 7.Ratnasooriya WD, Dharmasiri MG. Effects of Terminalia catappa seeds on sexual behaviour and fertility of male rats. Asian J Androl. 2000;2:213–9. [PubMed] [Google Scholar]
- 8.Hecht SM, Berry DE, MacKenzie LJ, Busby RW, Nasuti CA. A strategy for identifying novel, mechanistically unique inhibitors of topoisomerase I. J Nat Prod. 1992;55:401–13. doi: 10.1021/np50082a001. [DOI] [PubMed] [Google Scholar]
- 9.Kashiwada Y, Nonaka G, Nishioka I, Lee KJ, Bori I, Fukushima Y, et al. Tannins as potent inhibitors of DNA topoisomerase II in vitro. J Pharm Sci. 1993;82:487–92. doi: 10.1002/jps.2600820511. [DOI] [PubMed] [Google Scholar]
- 10.Hatano T, Yasuhara T, Yoshihara R, Agata I, Noro T, Okuda T. Effects of interaction of tannins with co-existing substances VII. Inhibitory effects of tannins and related polyphenols on xanthine oxidase. Chem Pharm Bull (Tokyo) 1990;38:1224–9. doi: 10.1248/cpb.38.1224. [DOI] [PubMed] [Google Scholar]
- 11.Tan GT, Pezzuto JM, Kinghorn AD, Hughes SH. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J Nat Prod. 1991;54:143–54. doi: 10.1021/np50073a012. [DOI] [PubMed] [Google Scholar]
- 12.Dorsch W, Wagner H. New antiasthmatic drugs from traditional medicine. Int Arch Allergy App Immunol. 1991;94:262–5. doi: 10.1159/000235378. [DOI] [PubMed] [Google Scholar]
- 13.Nagappa AN, Thakurdesai PA, Venkat Rao N, Singh J. Antidiabetic activity of Terminalia catappa Linn fruits. J Ethnopharmacol. 2003;88:45–50. doi: 10.1016/S0378-8741(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu M, Horie S, Terashima S, Ueno H, Hayashi T, Arisawa M, et al. Studies on aldose reductase inhibitors from natural products II. Active components of a Paraguayan crude drug ‘Para-paraimi’, Phyllanthus niruri. Chem Pharm Bull (Tokyo) 1989;37:2531–2. doi: 10.1248/cpb.37.2531. [DOI] [PubMed] [Google Scholar]
- 15.Basnet P, Kadota S, Shimizu M, Xu HX, Namba T. Hydroxymatteucinol a new C-methyl flavanone derivative from Matteccia orientalis potent hypoglycemic activity in streptozotocin (STZ)-induced diabetic rat. Chem Pharm Bull (Tokyo) 1993;41:1790–5. doi: 10.1248/cpb.41.1790. [DOI] [PubMed] [Google Scholar]
- 16.Teotia S, Singh M. Hypoglycemic effect of Prunus amygdalus seeds in albino rabbits. Ind J Exp Bio. 1997;35:295–6. [PubMed] [Google Scholar]
- 17.Prajapati ST, Patel LD, Patel DM. Gastric floating matrix tablets: design and optimization using combination of polymers. Acta Pharm. 2008;58:221–9. doi: 10.2478/v10007-008-0006-3. [DOI] [PubMed] [Google Scholar]
- 18.Moes AJ. Gastroretentive dosage forms. Crit Rev Ther Drug Carrier Syst. 1993;10:143. [PubMed] [Google Scholar]
- 19.Fell JT, Whitehead L, Collet H. Prolonged gastric retention using floating dosage forms. Pharm Technol. 2000;24(3):82–90. doi: 10.1016/s0168-3659(97)00266-6. [DOI] [PubMed] [Google Scholar]
- 20.Banker GS, Anderson NR. Tablets. In: Lachman L, Lieberman HA, Kanig JL, editors. The theory and practice of industrial pharmacy. 3. Bombay: Verghese Publishing House; 1991. p. 317. [Google Scholar]
- 21.US Pharmacopeia . USP chapter <616> bulk density and tapped density. Rockville: USP; 2006. p. 2638. [Google Scholar]
- 22.Srikanth MV, Sreenivasa Rao N, Sunil SA, Sharma GS, Uhumwangho MU, Ramana Murthy KV. Dissolution rate enhancement of poorly water soluble bicalutamide by using adsorption process. Int J Pharm Res. 2010;2(4):62–6. [Google Scholar]
- 23.Gauthami S, Bhat VR. A monograph on gum karaya. Hyderabad: National Institute of Nutrition, Indian Council of Medical Research; 1929. p. 29. [Google Scholar]
- 24.Jacob MB. The chemical analysis of foods and food products. 3. New York: Van Nostrand; 1958. p. 476. [Google Scholar]
- 25.Blokshma AH, Bushuk W. Rheology and chemistry of dough. In: Pomarenz Y, editor. Wheat chemistry and technology. St. Paul: American Association of Cereal Chemists, Inc.; 1988. p. 203. [Google Scholar]
- 26.Bushuk W. Rheology: theory and application to wheat flour doughs. In: Faridi H, editor. Rheology of wheat products. St. Paul: American Association of Cereal Chemists, Inc.; 1985. pp. 1–26. [Google Scholar]
- 27.Sadhis VK, Dinakar S, Subodh CP. Rheological characterization and drug release studies of gum exudates of Terminalia catappa Linn. AAPS PharmSciTech. 2008;9(3):885–90. doi: 10.1208/s12249-008-9101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ICH Harmonized Triparatite guideline on “Stability Testing of New Drug Substances and Products Q1A (R2)”, 6 February 2003.
- 29.Srikanth MV, Sreenivasa Rao N, Sunil SA, Janaki Ram B, Ramana Murthy KV. A statistical experimental approach for the development of propranolol HCl gastric floating tablets and its evaluation. Acta Pharma Sin B. 2012;2(1):60–9. doi: 10.1016/j.apsb.2011.12.008. [DOI] [Google Scholar]
- 30.USP 24 NF 19. The official compendia of standards. The United States Pharmacopoeial Convention. Propranolol hydrochloride extended release capsules. 2000; 1429.
- 31.Lazarus J, Cooper J. Absorption, testing, and clinical evaluation of oral prolonged-action drugs. J Pharm Sci. 1961;50:715. doi: 10.1002/jps.2600500902. [DOI] [PubMed] [Google Scholar]
- 32.Wagner JG. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J Pharm Sci. 1969;58:1253. doi: 10.1002/jps.2600581021. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi T. Mechanism of sustained action medication: theoretical analysis of rate release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 34.Korsmeyer R, Gurny R, Peppas N. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [Google Scholar]
- 35.Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1. [PubMed] [Google Scholar]
- 36.Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation: I—theoretical consideration. Ind Eng Chem. 1931;23:923–31. doi: 10.1021/ie50260a018. [DOI] [Google Scholar]
- 37.Prasannaraju Y, Asuntha G, Satyanadam S, Ramana Murthy KV. A study on physical characterization of Hupu gum, a natural polysaccharide for pharmaceutical use. J Innov Trends Pharma Sci. 2011;2(6):174–83. [Google Scholar]
- 38.Averill B, Eldredge P. General chemistry: principles, patterns, and applications, version 1, chapter 12. 2011. eISBN: 978-1-4533-3122-4.
- 39.Kiran C, Umadevi S, Subhash V. Floating matrix dosage form for propranolol hydrochloride based on gas formation technique: development and in vitro evaluation. Sci Pharm. 2010;78:927–39. doi: 10.3797/scipharm.0909-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srikanth MV, Sunil SA, Janaki Ram B, Ramana Murthy KV. Design and in vitro evaluation of effervescent gastric floating drug delivery systems of propranolol HCl. Invest Clin. 2012;53(1):60–70. [PubMed] [Google Scholar]
- 41.Bhosale UV, Kusum Devi V, Nimisha J, Swamy PV. Effect of polymer concentration and viscosity grade on atenolol release from gastric floating drug delivery systems. Indian J Pharm Educ Res. 2010;44(3):267–73. [Google Scholar]
- 42.Srikanth MV, Sunil SA, Sreenivasa Rao N, Latha K, Ramana Murthy KV. Thermal sintering: a novel technique used in the design of gastroretentive floating tablets of propranolol HCl and its statistical optimization using Box–Behnken design. Farmacia. 2012;60(3):411–35. [Google Scholar]
- 43.Khatamian M, Irani M. Preparation and characterization of nanosized ZSM-5 zeolite using kaolin and investigation of kaolin content, crystallization time and temperature changes on the size and crystallinity of products. J Iran Chem Soc. 2009;6:187–94. doi: 10.1007/BF03246519. [DOI] [Google Scholar]
- 44.Weller MT. The application and interpretation of powder X-ray diffraction data, in inorganic materials chemistry. New York: Oxford University Press; 1994. pp. 15–25. [Google Scholar]


