Abstract
Usual treatment for Helicobacter pylori-induced peptic ulcer includes a ‘triple therapy’ consisting of two antibiotics (amoxicillin and clarithromycin) and a proton pump inhibitor (omeprazole). The objective of this project work was defined with a view to retain the drug in stomach for better antiulcer activity and substituting one of the synthetic drugs in this therapy with a herbal alternative. Hence, aim of the present work was to design and develop a bilayer floating tablet of amoxicillin and Aloe vera gel powder for the treatment of peptic ulcer. A. vera gel powder is used for its cytoprotective action. Bilayer floating tablets were prepared by applying direct compression technique. The proportion of sodium bicarbonate and citric acid was adjusted to get the least possible lag time with good matrix integrity and total floating time. Polymer concentration was adjusted to get the maximum release in 8 h. The formulation was developed using hydroxypropyl methyl cellulose (HPMC) K4M and HPMC K100M in a ratio of 85:15 along with 1:4 ratio of effervescent agents was found to give floating lag time of less than 1 min with total floating time of more than 8 h and 97.0% drug release in 8 h. In vivo study in rats meets the requirement of antiulcer activity for bilayer tablet in comparison to single amoxicillin as standard.
KEY WORDS: Aloe vera, amoxicillin, bilayer, floating, peptic ulcer
INTRODUCTION
A National Institute of Health Consensus Panel (1994) suggested that all patients with Helicobacter pylori infection need eradication therapy with antimicrobial agents in addition to anti-secretory drugs. Triple therapy includes proton pump inhibitor bid, amoxicillin 1 g bid and clarithromycin 500 mg bid (1).
In context to the above literature, an attempt is made of combining amoxicillin and Aloe vera gel powder in the form of a bilayer tablet; which can work as an alternative to triple therapy where proton pump inhibitor is replaced by a herbal component which can help in showing better response of amoxicillin against H. pylori by acting as a cytoprotective agent. Reduction in the number of synthetic drug molecules in the therapy can reduce the chances of side effects associated with synthetic drug molecules.
Amoxicillin (α-aminohydroxybenzylpenicillin) is a semisynthetic antibiotic, belonging to the β-lactam family (penicillin). Elevation of beta-lactam concentration demonstrates increased bacterial killing, only until a finite point which tends to be about four times the minimal inhibitory concentration. Further elevation is not associated with increased bactericidal potency (2). The physicochemical properties of mucus vary considerably at different pH values and acid inhibition (for example by omeprazole) may increase the permeability of mucus to antibiotics (3). However, long-term monotherapy of gastric ulcer patients with amoxicillin is ineffective even at high daily doses, apparently due to limited contact time with the target site when administered in a conventional oral dosage form (4).
A. vera gel exhibited concentration-dependent inhibition of gastric acid secretions by direct interaction with the acid-producing cells or possible interaction with H2 receptors on the parietal cells. A. vera gel has been demonstrated to protect human beings and rats against gastric ulceration. This antiulcer activity is due to its anti-inflammatory, cytoprotective, healing and mucus stimulatory effects (5). A. vera has gastroprotective activity at lower concentrations due to the presence of lectins. Lectins are proteins/glycoproteins which are capable of recognizing and binding to carbohydrate moieties. It has been shown that lectins inhibit aminopyrine uptake by parietal cells. Thus, the ability of gel powder to inhibit gastric acid output maybe as a result of direct action on the acid-producing cells (6). Retention of amoxicillin and A. vera gel in the stomach would result in better action against peptic ulcer by maintaining the effective drug concentration. Thus, the objective of the present investigation involves development and evaluation of a bilayer gastro-retentive floating tablet of amoxicillin and A. vera gel powder for treatment of peptic ulcers. Here, amoxicillin will be systemically absorbed and A. vera gel powder will show local effect.
MATERIALS AND METHODS
Materials
Amoxicillin trihydrate Indian Pharmacopoeia (IP), A. vera gel powder of pharmaceutical grade and all grades of hydroxypropyl methyl cellulose (HPMC) were obtained as a gift sample from FDC Ltd. Mumbai, Maple Biotech Pune and Colorcon Asia Pvt Ltd. Goa, respectively. All other chemicals and reagents used were of analytical grade.
Animals
Healthy Wistar albino male rats weighing approximately 200–250 g were used for the study. All the experimental procedures used in this study were reviewed and followed by Institutional Animal Ethics Committee of STES’s Sinhgad College of Pharmacy, Pune, constituted under Committee for Purpose of Control and Supervision of Experiments on Animals.
Method
Preparation of Bilayer Floating Tablets
Amoxicillin trihydrate, sodium bicarbonate (NaHCO3), citric acid, Avicel PH 102 and HPMC were passed from sieve of # 40 and mixed in a planetary mixer (Model 026-03, Gem Machinery Company, Mumbai) for 5 min. Magnesium stearate and talc were added to the above mixture. The whole bulk of powder was then mixed thoroughly for 10 min. The powder was then compressed on multi-station rotary tabletting machine (Model JM-6, Gem Machinery Company, Mumbai) at low pressure using 16.5 × 8 mm caplet punches to form a first layer. A. vera gel powder was added on this first layer and compressed at high pressure to obtain a bilayer tablet. The formulations of the all batches are shown in Table I.
Table I.
Composition of Bilayer Tablets
| Ingredient (mg per tablet) | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|
| First layer | ||||||
| Amoxicillin trihydrate | 500 | 500 | 500 | 500 | 500 | 500 |
| HPMC K4M | 80 | 90 | 70 | 85 | 80 | 85 |
| HPMC K100M | 20 | 10 | 30 | 15 | 20 | 15 |
| NaHCO3 | 120 | 120 | 120 | 120 | 100 | 100 |
| Citric acid | 30 | 30 | 30 | 30 | 25 | 25 |
| Avicel pH (102) | 50 | 50 | 50 | 50 | 50 | 50 |
| Magnesium stearate | 15 | 15 | 15 | 15 | 15 | 15 |
| Talc | 10 | 10 | 10 | 10 | 10 | 10 |
| Second layer | ||||||
| Aloe vera | 32 | 32 | 32 | 32 | 32 | 32 |
| Avicel pH (102) | 18 | 18 | 18 | 18 | 18 | 18 |
| Colour | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
| Total (mg) | 875 | 875 | 875 | 875 | 850 | 850 |
q.s Quantity sufficient
Evaluation of Floating Tablets
The prepared tablets were evaluated for hardness, thickness, weight variation, friability, content uniformity, floating lag time, total floating time and drug release studies.
Content Uniformity
Twenty tablets were crushed and powder equivalent to the weight of one tablet was dissolved in buffer pH 1.2. Further, suitable dilutions were made and absorbance was measured at 272 nm wavelength using a UV spectrophotometer. The results obtained were compared with acceptance criteria of I.P. (7).
Floating Lag Time, Total Floating Time Determination and Drug Release Studies
Floating lag time and total floating time were determined in USP dissolution apparatus II (Electrolab, TDT-08L, USA) simultaneously with drug release studies using 900 mL buffer pH 1.2 maintained at 37 ± 0.5°C and at 50 rpm. The time required for the tablet to rise to the surface and float was determined as floating lag time. Total floating time is the total time for which the tablet floats in dissolution medium including floating lag time (8). The tablet did not stick to the dissolution vessel or the shaft. It was found to float freely in the vessel without being hindered by the shaft movement. For drug release studies, 10 mL of sample was withdrawn at 1, 2, 3, 4, 5, 6, 7 and 8 h and was replaced with equal volume of dissolution medium. After suitable dilution, amount of drug released was estimated by UV spectrophotometric method at 272 nm (9).
Weight Gain and Water Uptake
The study is done by immersing the dosage form in buffer pH 1.2 and the change in tablet weight at regular 1 h interval until 8 h is recorded. In this study, initial weight (Wo) of the tablet was noted and tablets are immersed in buffer pH 1.2. At time t tablet was removed from beaker and the excess surface liquid was removed carefully using the paper. The swollen tablets were then reweighed (Wt) and water uptake (WU) is measured in the terms of percent weight gain (8), as given by equation.
 |
1 |
Wt is weight of the dosage form at time t and Wo is initial weight. The test was continued for a total period of 8 h.
Dissolution Kinetic Study
The optimized formulation was subjected to various mathematical models to understand the release pattern. The study was carried out using PCP-Disso-V3 software. The value of the coefficient of regression suggests the best fit kinetic model.
In Vivo Studies
In vivo study was carried out for optimized formulation by pylorus ligation-induced ulcer model. Five groups of Wistar rats (six in each group) were used for the study. Animals were fasted for 24 h before pylorus ligation but allowed to have free access to water. Suspensions of omeprazole, amoxicillin and bilayer tablet were prepared by mixing with gum acacia. Following groups of animals were considered for the study:
-
Group I
Normal control
-
Group II
Gastric ulcer control (vehicle solution without drug)
-
Group III
Standard omeprazole (20 mg/kg)
-
Group IV
Marketed amoxicillin (50 mg/kg)
-
Group V
Bilayer tablet suspension of amoxicillin trihydrate and A. vera (50 and 3.2 mg/kg, respectively)
Thirty minutes after oral dosing, the Wistar rats were anaesthetized with ketamine (45 mg/kg) and pylorus was ligated. During this process, care was taken to avoid the traction to the pylorus or damage to its blood supply. Stomach was then closed by interrupted sutures. After 5 h of ligation, all animals were sacrificed and stomachs were removed, cut along the greater curvature and observed for ulcer. The numbers of ulcers were counted using a magnifying glass. The following arbitrary scoring system was used to grade the incidence and severity of lesions: (a) score 0 = no ulcer (b) score 10 = denuded epithelium; (c) score 20 = petechial and flank haemorrhages; (d) score 30 = one or two ulcers; and (e) score 40 = multiple ulcers; the ulcer index (UI) (10) and percent inhibition of ulceration were determined using following formulae:
 |
2 |
where UN is the average of number of ulcers per animal, Us is the mean severity of ulcer score and Up is the percentage of animals with ulcer incidence.
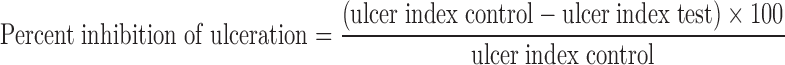 |
3 |
Histopathological Evaluation
The stomach samples of all groups were preserved in 10% buffered formalin and processed for routine paraffin block preparation and stained with haematoxylin and eosin. Samples were examined under the microscope for histopathological changes such as degeneration, haemorrhage, edematous appearance, erosion and necrosis and compared with normal histopathology of rat stomach of normal control.
Statistical Analysis
In vivo experimental results were expressed as mean ± S.E.M. Data were analysed by analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, with the level of significance set at P < 0.05. All the in vivo experimental results were expressed as mean ± S.E.M. of three parallel measurements. P values < 0.05 were considered significant (11).
RESULTS
Results for weight variation were found to be within the limits prescribed by IP. Friability test of all six formulations was also found to be within USP limit, indicating enough resistance to the mechanical shock and abrasion. Hardness of the tablet was found to between 9 and 10 kg/cm2. Drug content uniformity for amoxicillin was also within the acceptance criteria of the IP (Table II).
Table II.
Physical Parameters of the Formulation
| Batch code | Thickness (mm) | Hardness (kg/cm2) | Friability (%) | Uniformity of weight (mg) | Uniformity of content (%) |
|---|---|---|---|---|---|
| T1 | 5.5 ± 0.04 | 9 ± 0.03 | 0.79 ± 0.03 | 875 ± 10 | 98.5 ± 1.2 |
| T2 | 5.4 ± 0.02 | 10 ± 0.02 | 0.82 ± 0.05 | 875 ± 8 | 99.0 ± 0.9 |
| T3 | 5.8 ± 0.01 | 9.5 ± 0.04 | 0.85 ± 0.07 | 875 ± 8 | 98.5 ± 0.9 |
| T4 | 5.6 ± 0.03 | 9.8 ± 0.03 | 0.81 ± 0.04 | 875 ± 9 | 98.7 ± 1.1 |
| T5 | 5.9 ±0.02 | 9.6 ± 0.04 | 0.80 ± 0.04 | 850 ± 10 | 98.4 ± 1.3 |
| T6 | 5.1 ± 0.03 | 9.9 ± 0.05 | 0.87 ± 0.09 | 850 ± 7 | 99.0 ± 0.8 |
Mean ± SD (n = 6)
Floating Lag Time and Total Floating Time Determination
Bilayer floating tablets were prepared with the aim of having maximum drug release in 8 h and with minimum floating lag time and good matrix integrity. Table III shows results for floating lag time, total floating time and matrix integrity. Formulations T1 to T4 containing 30 mg of citric acid and 120 mg of sodium bicarbonate (1:4) met the requirements for floating lag time and total floating time, but matrix integrity was lost in less than 8 h. To improve upon the matrix integrity, formulations F5 and F6 were developed. Formulations T5 and T6 containing lesser amount of effervescent agents, i.e. 25 mg of citric acid and 100 mg of sodium bicarbonate (1:4) showed excellent results for matrix integrity in addition to good floating lag time and total floating time.
Table III.
Floating Lag Time, Total Floating Time and Matrix Integrity of the Formulations
| Formulation code | Floating lag time (s) | Total floating time (h) | Matrix integrity |
|---|---|---|---|
| T1 | 28 ± 3 | >8 | ++ |
| T2 | 36 ± 2 | >8 | ++ |
| T3 | 31 ± 2 | >8 | ++ |
| T4 | 24 ± 2 | >8 | ++ |
| T5 | 27 ± 3 | >8 | +++ |
| T6 | 25 ± 2 | >8 | +++ |
Mean ± SD (n = 6)
++ meets the requirement, +++ exceeds the requirement
Weight Gain and WU
In weight gain study, it was observed that swelling increased up to 40% of tablet weight in 5–6 h but after that it decreased.
Drug Release Studies
The formulations T1 to T6 were subjected to 8 h of dissolution study. T1, T2, T3 and T4 showed percent cumulative release of 90.4%, 93.4%, 94.8% and 98.8%, respectively. The formulation T5 containing 25 mg of citric acid and 100 mg of sodium bicarbonate showed 92.4% drug release and T6 showed 97.0% drug release at the end of 8 h (Fig. 1).
Fig. 1.
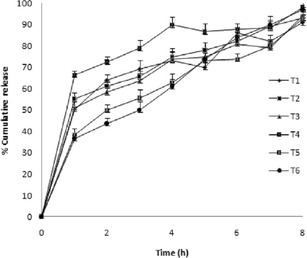
In vitro release profile of formulations T1–T6 (mean ± SD, n = 6)
In Vivo Studies
In vivo study for comparing the effect of amoxicillin trihydrate and aloe in combination for ulcer therapy was carried out and parameters estimated were ulcer index and percent inhibition of ulceration. Results of group II show ulcers while results of groups III, IV and V show reduction in ulceration after 5 h of the treatment (Fig. 2). The values of ulcer index and percent inhibition of ulceration are given in Table IV. The ulcer index of groups II, III, IV and V were 14.6 ± 0.33, 5.0 ± 0.16, 10.1 ± 0.36 and 3.6 ± 0.19, respectively (Fig. 3). The percent inhibition of ulceration of groups III, IV and V is 65.4 ± 0.76, 30.6 ± 0.75 and 74.8 ± 0.85%, respectively (Fig. 4). Reduction in ulceration for pure amoxicillin trihydrate is 30.6 ± 0.75% but in case of the floating tablet of amoxicillin trihydrate along with A. vera gel powder shows 74.8 ± 0.85% of ulcer inhibition. A. vera gel powder inhibits acid secretion due to the presence of lectins in the plant. Lectins inhibit aminopyrine uptake by parietal cells (6). Thus, it can be concluded that amoxicillin and A. vera in combination has both inhibitory and gastroprotective effect. This suggests use of amoxicillin and A. vera gel powder in combination to treat peptic ulcer treatment.
Fig. 2.
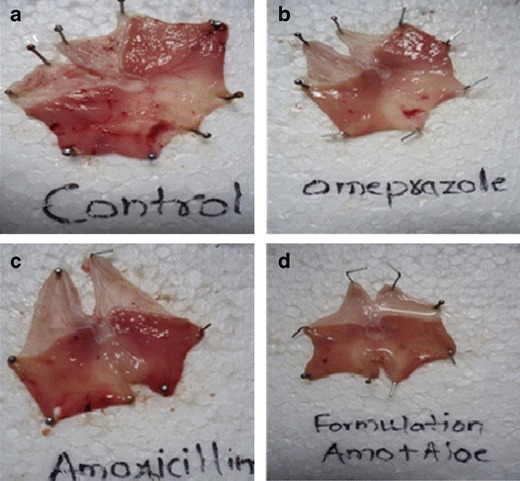
Photographs of rat stomach showing ulcerated area. a Ulcer control. b Treated with standard omeprazole. c Treated with amoxicillin. d Treated with optimized formulation
Table IV.
Effect of Treatment on Pylorus Ligation-Induced Gastric Ulcer in Rats
| Treatment | Dose (mg/kg) | Ulcer index | % inhibition of ulceration |
|---|---|---|---|
| Ulcer control | – | 14.6 ± 0.34*** | – |
| Standard omeprazole | 20 (p.o.) | 5.1 ± 0.17*** | 65.4 ± 0.76*** |
| Amoxicillin | 50 (p.o.) | 10.2 ± 0.37 | 30.6 ± 0.75* |
| Amoxicillin + Aloe vera | 3.2 + 50 (p.o.) | 3.7 ± 0.19*** | 74.8 ± 0.86*** |
Values are represented as mean ± S.E.M.; n = 6 in each group
**p < 0.05, ***p < 0.001, for ulcer control (one-way ANOVA followed by Tukey’s multiple comparison test)
Fig. 3.
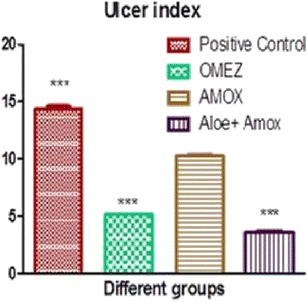
Graph of ulcer index in different groups
Fig. 4.

Graph of percent inhibition of ulceration in different treatment groups
Histopathological Evaluation
Histopathological studies for the ulcer control group show disruption in intact mucosa with inflammatory cells when compared to normal rat stomach (Fig. 5a–d). However, animals treated with omeprazole showed almost normal pattern (Fig. 5e, f). In case of amoxicillin-treated animals, the microphotographs show superficial ulcer with inflammatory cells at the base of ulcerated area indicated by arrows in Fig. 5g, h. Optimized formulation showed no ulcer and retains normal stomach physiology (Fig. 5i, j).
Fig. 5.

Histopathological studies of rat stomach: Normal stomach (a and b), ulcer control group (c and d), omeprazole-treated group showing almost normal pattern (e and f) and amoxicillin-treated group showed superficial ulcer (g and h); arrows indicating inflammatory cells, animals treated with optimized formulation showed no ulcer (i and j)
DISCUSSION
Amoxicillin has a short half life of 1–2 h. Retaining amoxicillin in the stomach region of GIT will lead to better absorption of amoxicillin. A. vera gel has mucoprotective effect and is also reported to have antiulcer activity. Considering these facts, for amoxicillin and A. vera, the objective of this project work was to design and develop a bilayer floating tablet of amoxicillin and A. vera gel powder for the treatment of peptic ulcer.
Food and Drug Administration has suggested that the extrapolation of animal dose to human dose is correctly performed only through normalization to BSA, which often is represented in milligrams per square meter. Based on literature survey, dose used into rats was calculated based on surface area for humans, 3.3 mg/kg (Wistar albino rat dose) multiplied by the Km factor 6 for rat and then divided by the Km factor 37 for a human (12). This calculation resulted in the determination of human equivalent dose for A. vera gel of 0.53 mg/kg, which equates to a 32 mg dose of A. vera for a 60-kg person.
Floating Lag Time and Total Floating Time Determination
If the sodium bicarbonate concentration is much higher than polymer concentration in a formulation, rapid erosion of tablet occurs. This may be due to the release of carbon dioxide and its escape at a faster rate. If the polymer is in sufficient concentration, the evolved gas will get entrapped in the polymer network leading to the floating of the tablet; thus, it indicates that lower polymer levels with higher levels of sodium bicarbonate in a formulation result in erosion rather than floating. Similarly, if the polymer concentration is higher than that of sodium bicarbonate, the desired drug release profile may not be achieved as the higher polymer portions can delay the drug release. Thus, the ratio of polymer to the sodium bicarbonate concentration was altered to get lowest possible lag time with the desired drug release (13).
Drug Release Studies
From the results of drug release studies, it was found that formulations T1–T3 did not give the desired release. T4 showed a desirable drug release of 98.8% at the 8 h but was found to show poor matrix integrity (Table III), and thus, the formulations T1–T4 were not considered for further studies. Formulation T5 also gave poor results for drug release studies. The formulation T5 containing 25 mg of citric acid and 100 mg of sodium bicarbonate showed 92.4% drug release at the end of 8 h with 50% drug release within 2 h. Thus, it did not meet the criteria of controlled release matrix (2), which required a drug release of 50% at the end of 3 h.
In the case of formulation T6, it was observed that 97.0% drug released in 8 h and 50% drug released within 3 h as is the criteria for controlled release matrix. T6 also showed optimum floating lag time, total floating time and excellent matrix integrity. Thus, the formulation T6 was considered as the optimized formulation
The optimized formulation T6 was subjected to various mathematical models to understand the release pattern. The value of the coefficient of regression was 0.9938 suggesting Korsmeyer–Peppas as best fit kinetic model. In the present study, diffusion value of exponent is 0.5212; therefore, release of the formulations is mainly by non-Fickian release (14). It indicates a coupling of the diffusion and erosion mechanism so-called anomalous diffusion and indicating that the drug release is controlled by more than one process.
Weight Gain and WU
As water ingresses from outer side to the tablet core, the outer gel layer starts to erode and volume decreases progressively followed by reduction in tablet weight. This erosion of polymer dominates over water sorption after 6 h. The test was performed to determine the swelling of a tablet which will ensure release of drug from the dosage form.
In Vivo Studies
From the results of animal studies and histopathological evaluation, it was found that pure amoxicillin marketed formulation gave percent inhibition of 30.59%. When combined with A. vera gel powder in the form of a bilayer tablet, this value increased to 74.77% indicating better activity against peptic ulcers.
CONCLUSION
In the present work, formulation T6 containing HPMC K4M and HPMC K100M in a ratio of 85:15 along with 1:4 ratio of effervescent agents gave better controlled drug release and floating properties in comparison to the other formulations. The most promising mechanism that the release patterns of the formulations was followed by non-Fickian diffusion or anomalous diffusion. In vivo studies showed that A. vera and amoxicillin in combination have gastric acid inhibitory properties and suggests its usage in peptic ulcer treatment.
REFERENCES
- 1.Fendrick AK, Forsch RT, Harrison RV, Scheiman JM. Peptic ulcer guideline team, University of Michigan Guidelines for Health System. 2005. http://www.stjames.ie/GPsHealthcareProfessionals/Newsletters/NMICBulletins/NMICBulletins1995/VOL1-3.pdf. Updated May 2005.
- 2.Hoffmana A, Danenbergb HD, Katzhendlera I, Shuvala R, Gilhar D, Friedman M. Pharmacodynamic and pharmacokinetic rationales for the development of an oral controlled-release amoxicillin dosage form. J Control Release. 1998;54:29–37. doi: 10.1016/S0168-3659(97)00165-X. [DOI] [PubMed] [Google Scholar]
- 3.Goddard AF. Review article: factors influencing antibiotic transfer across the gastric mucosa. Aliment Pharmacol Ther. 1998;12:1175–84. doi: 10.1046/j.1365-2036.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- 4.Cuna M, Alonso MJ, Torres D. Preparation and in vivo evaluation of mucoadhesive microparticles containing amoxicillin resin complexes for drug delivery to the gastric mucosa. Eur J Pharm Biopharm. 2001;51:199–205. doi: 10.1016/S0939-6411(01)00124-2. [DOI] [PubMed] [Google Scholar]
- 5.Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadiq Y, Agunu A, Diana M. The effect of Aloe vera A. Berger (Liliaceae) on gastric acid secretion and acute gastric mucosal injury in rats. J Ethnopharmacol. 2004;93:33–7. doi: 10.1016/j.jep.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Indian Pharmacopoeia. Ministry of Health and Family Welfare. Govt. of India, Controller of publication. Delhi; 2007:2:182–3.
- 8.Narang N. An updated review on: floating drug delivery system (FDDS) Int J Appl Pharm. 2011;3(1):1–7. [Google Scholar]
- 9.Badhan AC, Mashru RC, Shah PP, Thakkar AR, Dobaria NB, et al. Development and evaluation of sustained release gastroretentive minimatrices for effective treatment of H. pylori infection. AAPS PharmSciTech. 2009;10(2):459–67. doi: 10.1208/s12249-009-9231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umamaheswari M, Asokkumar K, Rathidevi R, Sivashanmugam AT, Subhadradevi V, Ravi TK. Antiulcer and in vitro antioxidant activities of Jasminum grandiflorum L. J Ethnopharmacol. 2007;110:464–70. doi: 10.1016/j.jep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Kumara A, Singhb V, Chaudhary AK. Gastric antisecretory and antiulcer activities of Cedrus deodara (Roxb.) Loud. in Wistar rats. J Ethnopharmacol. 2011;134:294–7. doi: 10.1016/j.jep.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 13.Nama M, Gonugunta CSR, Veerareddy PR. Formulation and evaluation of gastroretentive dosage forms of clarithromycin. AAPS PharmSciTech. 2008;9(1):231–7. doi: 10.1208/s12249-008-9038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulo C, Jose MSL. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]


