Abstract
Different hydroxypropyl methylcellulose (HPMC)/anhydrous dibasic calcium phosphate (ADCP) matrix tablets have been developed aiming to evaluate the influence of both components ratio in the control release of a water-soluble drug (theophylline). In order to characterise the matrix tablets, swelling, buoyancy and dissolution studies have been carried out in different aqueous media (demineralised water, progressive pH medium, simulated gastric fluid, simulated intestinal fluid and simulated colonic fluid). The HPMC/ADCP ratio has turned out to be the determinant in the matrix behaviour: the HPMC characteristic swelling behaviour was modulated, in some cases, by the ADCP characteristic acidic dissolution. When the HPMC/ADCP ratio was ≥0.69, buoyancy, continuous swelling and low theophylline dissolution rate from the matrices (H1, H2 and H3) were observed in all dissolution media. Consequently, these formulations could be adequate as gastro-retentive drug delivery systems. Additionally, HPMC/ADCP ratio ≤0.11 (H5 and H6) induces a pH-dependent drug release which could be applied to design control drug release enteric formulations (with a suitable enteric coating). Finally, a HPMC/ADCP ratio between 0.11 and 0.69 (H4) yield a gastrointestinal controlled drug release, due to its time-dependent buoyancy (7 h) and a total drug delivery in 17 h in simulated colonic fluid.
Key words: anhydrous dibasic calcium phosphate, hydroxypropyl methylcellulose, matrix tablets, oral controlled release, theophylline
INTRODUCTION
Hydroxypropyl methylcellulose (HPMC), a semi-synthetic cellulose derivative, has been most frequently studied in the formulation of monolithic matrix tablets for controlled release, due to its hydrophilic gel-forming property, non-toxicity and cost-effectiveness (1–3). In addition, this polymer can be formulated with several drugs, being able to accommodate high levels of these molecules. On the other hand, it can be easily utilised to form matrix tablets by direct blending or granulation (4,5).
Once in contact with a liquid medium, this polymer would hydrate and swell, forming a hydrogel layer which regulates further penetration of the liquid into the tablet matrix and dissolution of the drug from the inside of the tablet (6). In consequence, the drug release from this polymeric matrix is achieved by diffusion, erosion or a combination of both processes (7,8).
Several factors, such as the polymer type and concentration, the drug particle size and the presence of additives or excipients in the formulations can modify the drug release from these matrices (9–11). In general, water-soluble drugs tend to follow a release mechanism based on a diffusion process, whereas in the case of water-insoluble compounds, the release would be controlled by the tablet erosion (12). On the other hand, other strategies aiming to modify the drug release rate are based on the addition of several diluents. Some studies have demonstrated that when HPMC was replaced by lactose, higher drug release rates were obtained with lower HPMC/lactose ratios (13). However, some precautions must be adopted before adding excipients to the dosage form considering that, although the addition of any amount of diluents or fillers generally increases the drug release rate (14), the inclusion of a non-soluble excipient like dibasic calcium phosphate decreased drug release rates (15) or resulted in a slower drug release with more linearity in the dissolution profile (16). On the other side, other studies found that the filler ratio was the most significant factor that affected the drug release (17), showing that the amount of HPMC played a dominant role in the dissolution behaviour. Therefore, Loftipour and co-workers have reported that the hydrophilicity or hydrophobicity of fillers did not have a significant effect on the drug release profile when it was controlled both by diffusion and erosion while at the same time the drug release was influenced by the type and concentration of the polymer (18).
Recently, Tavakoli and co-workers obtained favourable sustained release of acyclovir from tablets containing HPMC K4M, Na CMC and/or polyvinyl pyrrolidone (PVP) or Na alginate and gas-generating agent. The combination of a cellulosic polymer, HPMC, with at least one more synthetic or naturally derived polymer could achieve a compromise between floating lag time, total floating duration, drug release rates and mucoadhesion properties (19). Likewise, formulation containing HPMC K15M, dicalcium phosphate, citric acid and sodium bicarbonate were optimum from both duration of buoyancy and drug release point of view (total amount of drug release for 12 h in stomach). Thus, floating drug delivery system provides a method for sustaining the drug release of a highly soluble drug propranolol HCl in stomach (20). Another researches, reported that the combination of sodium bicarbonate and hexadecanol with HPMC K4M was found to achieve optimum in vitro release and floatability (the drug release at 12 h was more than 85%). Based on the in vivo performance, the developed formulation could prolong Tmax without increasing the fluctuation of plasma concentrations and decreasing the bioavailability (21).
In addition, the changing conditions along the gastrointestinal tract can potentially affect the tablet erosion for a gel matrix tablet and thereby alter the drug release rate (22). The dissolution testing procedure has been established as a quality control during the product development in pharmaceutical production. So, in order to mimic the physiological human conditions, and contributes to establish in vitro–in vivo correlations (23), it has been described as dissolution medium nature, medium ionic strength or temperature of the medium can affect the hydration of hydrophilic polymers, and in consequence the drug release from matrix systems could be affected (24). In this sense, Ebube and Jones have reported the significant impact of the pH of the dissolution medium on the drug release rate (3) while recent efforts have been undertaken to show that HPMC matrices were hydrolysed by a selective endoglucanase from Trichoderma reesei (25), suggesting that the study of the enzymes activity over HPMC formulations could be crucial in order to know the effects of physiological conditions.
On the other hand, The Food and Drug Administration (FDA) in 2005 warned of the possibility that the presence of alcohol in the medium, interacts with the modified-release oral formulations, accelerating the release of the drug. This phenomenon, known as “dose dumping”, is the consequence of tablet rupture or erosion and accumulation in the body (26). Single unit non-disintegrating dosage forms are liable to a higher risk of dose dumping (27). Despite that, matrix tablets have more uniform release profiles with a high resistance to dose dumping than other controlled release formulations (28).
Considering all this background, the aim of the present study was to study the influence of HPMC/anhydrous dibasic calcium phosphate (ADCP) ratio in the matrix tablets formulations able to release a water-soluble drug (theophylline) in different zones of the gastrointestinal tract.
MATERIALS AND METHODS
Materials
Theophylline anhydrous (THE; batch: 048K0709) was purchased from Sigma-Aldrich (Saint Louis, MO). Hydroxypropyl methylcellulose-Methocel® K15M CR PREMIUM (HPMC; lot: QJ9012N11) was a generous gift from Colorcon Ltd (Kent, England). Pepsin (batch: 8947200011) was provided by Guinama (Valencia, Spain). Anhydrous dibasic calcium phosphate (ADCP; batch: 1006; Emcompress® Anhydrous) was provided by Mendell (Bodenheim, Germany). Magnesium stearate PRS-CODEX (MgSt; batch: 85269ALP) was purchased from Panreac (Barcelona, Spain). Kollidon® 30 (PVP K30; batch: 98-0820) supplied by BASF (Ludwingshafen, Germany). Pancreatin USP (batch: A0257028) was manufactured by Acros Organic (Fair Lawn, NJ), and pectinolytic enzymes extracted from Aspergillus niger with an activity of 26,000 FDU/mL at pH 3.5 (Pectinex Ultra SP-L, batch: KRN05620) were kindly granted by Novozymes (Bagsvaerd, Denmark). All other reagents were of analytical grade. In all cases, demineralised water was used.
Preparation of Matrix Tablets
Matrix tablets containing, HPMC and ADCP with varying ratios (Table I) and 50 mg of THE as a model drug, were prepared. The granules were produced by addition of a PVP K30 ethanol solution onto a physical mixture of THE, HPMC and ADCP. The wet mass was then passed through a mesh size of 0.5 mm, which was dried at 40°C for 12 h. An amount of 2% MgSt (w/w) was added to the granules before the compression; 600 mg of the blend was weighed; and tablets were obtained using an eccentric machine Bonals® B 40 (Barcelona, Spain), with concave punches of 13 mm diameter, applying the maximum compression force accepted by the formulation. The compaction pressure was kept constant during the preparation of every batch. Furthermore, a control batch (C), without polymer, was prepared in the same conditions. The obtained tablets have fulfilled the hardness tests (Pharma Test-PTB 311; Hainburg, Germany), average weight, drug content and diameter and thickness.
Table I.
Composition of the Different Batches Manufactured
| Components (mg) | Formula code | ||||||
|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | H5 | H6 | C | |
| HPMC K15M | 440 | 300 | 200 | 100 | 50 | 30 | – |
| ADCP | 48 | 188 | 288 | 388 | 438 | 458 | 488 |
| Theophylline | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| PVP | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| MgSt | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
HPMC hydroxypropyl methylcellulose, ADCP anhydrous dibasic calcium phosphate, PVP polyvinyl pyrolidone, MgSt magnesium stearate
Swelling Characterisation
In order to study the influence of composition and pH media on HPMC/DCPA matrices behaviour, the swelling degree was determined in different media, according to a method described by Ruiz-Caro and Veiga (29).
Before the tablets were exposed to the aqueous solutions, they were glued onto metallic discs with a diameter of 20 mm. The discs where then placed in beakers that contained 500 mL of different media. These beakers were placed in a thermostated water shaking bath (Selecta® UNITRONIC320 OR, Barcelona, Spain) with an experimental temperature of 37 ± 0.1°C and a shaking rate of 42 U/min.
The media assayed were: demineralised water, progressive pH medium, simulated gastric fluid with pepsin (SGF) and simulated intestinal fluid with pancreatin (SIF). The progressive pH medium was composed of an aqueous mixture of 0.005 M hydrochloric acid (37%), 0.05 M ortho-phosphoric acid (85%) and 0.05 M glacial acetic acid with a pH value of 1.5, which was maintained during the first 2 h of the assays. After that, NaOH 10 M was added to the medium until pH increased to a value of 4.0, which was maintained for the following 2 h. Finally, another aliquot of NaOH 10 M was added to obtain a pH value of 6.8 which was maintained for 24 h. SGF and SIF were elaborated according to the US Pharmacopeia (30).
At specific time intervals, the tablets were extracted from the test medium and blotted with filter paper to absorb the excess of liquid on the sample surface before the weight evaluation. The swelling ratio (SR) of every sample was calculated according to Eq. 1 (31).
 |
1 |
where Ts and Td were the weights of the swollen and dried tablets, respectively. All swelling tests were performed in triplicate.
At t = 120, 240, 480 and 1440 min, photographs were taken with a digital camera (Panasonic® Lumix DMC-FZ28 10.0 Megapixels, Osaka, Japan), to observe the aspect and evolution of the tablets in contact with the different media.
In Vitro Buoyancy Study
All matrices tablets prepared were characterised in order to determine their buoyancy ability, using the paddle method at a rotation speed of 50 rpm with a Sotax® AT-7 dissolution apparatus (Basel, Switzerland) in 900 mL of the different media previously described in the swelling study at 37 ± 0.1°C.
The position (or location) of the matrix tablet during buoyancy test was visually checked every hour. All buoyancy tests were performed in triplicate.
Dissolution Test
A Sotax® AT-7 dissolution apparatus (Basel, Switzerland) with paddles was employed to carry out all tests. The dissolution media used were the same as those employed in the swelling studies. The paddle speed, volume of the dissolution medium and the experimental temperature were 50 rpm, 900 mL of each dissolution medium and 37 ± 0.1°C, respectively. Besides the four dissolution media used, another medium was assayed to simulate the mouth-to-colon transit. Before the tablets were exposed to the dissolution media, they were glued onto metallic discs with a diameter of 20 mm, in order to improve the manipulation of the tablets during the test. During the first 6 h, the tests were carried out in the same way as the dissolution tests in progressive pH medium (2 h at pH 1.5→2 h at pH 4→2 h at pH 6.8). After this period, the tablets were removed from a dissolution medium and immersed in beakers that contained 100 mL of simulated colonic fluid with pectinolytic enzymes (SCF; 4 mL/L), whose enzyme activity showed to be closely correlated with that of Bacteroides ovatus, the main colon producer of pectinolytic enzymes (32). These beakers were placed to a thermostated water shaking bath (Selecta® UNITRONIC 320 OR, Barcelona, Spain) with an experimental temperature of 37 ± 0.1°C and a shaking rate of 42 U/min.
At specific time intervals, samples of dissolution medium were withdrawn and filtered using Whatman® filter papers (type 42). The quantity of dissolved THE was determined by UV spectroscopy at a wavelength of 271 nm (Shimadzu® UV-1700 spectrophotometer; Kyoto, Japan). Three replicates of each dissolution test were carried out. No changes in the λmax of THE because of the presence of tablet excipients had previously been checked.
Dissolution Data Analysis
In order to compare the dissolution profiles from each batch under different dissolution conditions and to study the effect of composition batches on the drug release, two indices proposed by Moore and Flanner were used (33). This approach, model independent, uses mathematical indices to define difference f1 (Eq. 2) and similarity f2 (Eq. 3) factors, respectively, to compare complete dissolution profiles:
 |
2 |
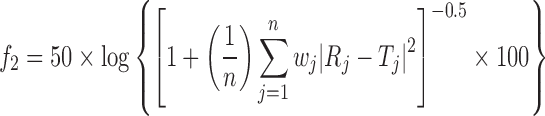 |
3 |
where Rj and Tj are the dissolved drug percentages at each time point for the reference (i.e. profile obtained from H1) and test product (i.e. profile obtained from H2), respectively; n is the number of samples for each dissolution test; t is the sample time index; and Wj is an optional weight factor (in the current work Wj = 1). In general, to ensure sameness between the profiles f1 should be in the range of 0–10 and f2 > 65 which denotes a similarity between profiles higher than 95% (34), meanwhile f2 < 65 represents non-similar profiles.
In order to investigate the drug release model from all matrix tablets, the dissolution data obtained from the assayed samples during dissolution tests, in all the different media, were analysed according to the following mathematical models: zero order (Mt ≤ 1.0) (Eq. 4) (35), first order (Mt ≤ 1.0) (Eq. 5) (35), Hixson–Crowell (Mt/M∞ = 1–Wt/W0 ≤ 1.0) (Eq. 6) (36), Hopfenberg (Mt/M∞ ≤ 1.0) (Eq. 7) (37,38), Higuchi (Mt/M∞ ≤ 0.6) (Eq. 8) (39) and Korsmeyer–Peppas (Mt/M∞ ≤ 0.6) (Eq. 9) (40) kinetic equations:
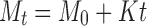 |
4 |
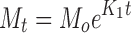 |
5 |
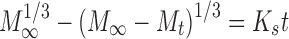 |
6 |
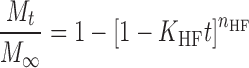 |
7 |
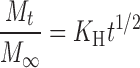 |
8 |
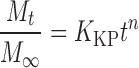 |
9 |
where Mt is the amount of drug dissolved in time t; M0 is the initial amount of drug in the solution (most times, M0 = 0); K is the zero-order release constant; K1 is the first-order release constant; M∞ is the initial amount of drug in the tablet; M∞–Mt is the remaining amount of drug in the tablet at time t; Ks is a constant incorporating the surface-volume relation; Mt/M∞ is the fraction of drug released at time t (the drug loading was considered as M∞); KHF is equal to K0/C0a0, being K0 equal to the erosion rate constant, C0 is the initial concentration of drug in the matrix and a is the initial radius of the matrix (for a sphere or cylinder or the half-thickness for a slab); the value of nHF is 1, 2 or 3 for a slab, cylinder or sphere, respectively; KH is the Higuchi dissolution constant; KKP is a constant incorporating structural and geometric characteristics of the drug dosage form; and n is the release exponent, which characterise the drug release mechanism and whose values depend on the shape of the tested matrix (41). Thus, for studying the release mechanism from cylinders, an n value of 0.45 corresponds to Fickian release (controlled release by diffusion), in case of case II transport (controlled release by relaxation) n = 0.89, meanwhile values of n between 0.45 and 0.89 respond to non-Fickian release kinetics (anomalous transport of drug corresponds to coupled diffusion/polymer relaxation). The optimum values for the present parameters in each equation were determined by linear or non-linear least-squares fitting methods with StatGraphics® Plus Version 5.1 software. When comparing models with different parameters, the adjusted coefficient of determination ( ) is more meaningful and was used in this work to test the applicability of the drug release models (35).
) is more meaningful and was used in this work to test the applicability of the drug release models (35).
RESULTS AND DISCUSSION
Swelling Studies
The SR from the formulations H1–H6 and C determined in different media are shown in Table II. Each positive SR value indicates that, at a given time, the swollen matrix weight was higher than that of the dry system weight (t = 0). On the other hand, each negative SR value indicated that the weight of the swollen system was lower than the weight of the dry system (t = 0).
Table II.
Values of SR from H1 to H6 and C Matrices at 2, 4, 8 and 24 h in Different Media
| Batch | 2 h | 4 h | 8 h | 24 h |
|---|---|---|---|---|
| SR in demineralised water | ||||
| H1 | 184.2 ± 4.2 | 251.9 ± 4.7 | 336.1 ± 3.2 | 485.8 ± 9.4 |
| H2 | 149.3 ± 2.2 | 205.2 ± 2.5 | 268.3 ± 1.4 | 350.2 ± 3.4 |
| H3 | 133.6 ± 2.9 | 176.6 ± 3.1 | 219.9 ± 1.7 | 280.0 ± 1.6 |
| H4 | 78.1 ± 1.1 | 105.3 ± 1.2 | 117.2 ± 1.8 | 169.6 ± 8.4 |
| H5 | 75.1 ± 0.9 | 90.0 ± 0.8 | 90.4 ± 1.5 | 99.5 ± 5.0 |
| H6 | 57.9 ± 1.3 | 65.1 ± 0.9 | 52.1 ± 2.2 | 37.3 ± 4.1 |
| C | 21.0 ± 3.5 | 27.0 ± 9.6 | 21.3 ± 3.4 | 19.2 ± 2.4 |
| SR in progressive pH medium | ||||
| H1 | 177.5 ± 1.6 | 240.9 ± 9.4 | 314.2 ± 8.2 | 482.7 ± 4.3 |
| H2 | 152.1 ± 3.2 | 202.7 ± 1.8 | 262.7 ± 3.6 | 368.5 ± 5.6 |
| H3 | 136.4 ± 1.3 | 174.4 ± 3.3 | 217.3 ± 1.2 | 282.4 ± 4.8 |
| H4 | 79.8 ± 1.6 | 101.1 ± 1.4 | 112.3 ± 0.8 | 183.2 ± 3.0 |
| H5 | 71.1 ± 1.1 | 80.3 ± 1.5 | 82.6 ± 3.4 | 72.5 ± 3.4 |
| H6 | 44.1 ± 1.2 | 49.1 ± 1.9 | 44.0 ± 2.0 | 21.9 ± 4.7 |
| C | −8.6 ± 0.6 | −18.5 ± 2.2 | −44.7 ± 5.6 | −46.9 ± 5.5 |
| SR in SGF | ||||
| H1 | 174.9 ± 1.3 | 239.6 ± 8.7 | 314.5 ± 5.8 | 463.9 ± 5.2 |
| H2 | 159.5 ± 1.8 | 217.3 ± 3.0 | 280.8 ± 7.0 | 357.3 ± 27 |
| H3 | 138.3 ± 2.5 | 180.4 ± 1.2 | 226.7 ± 0.8 | 189.9 ± 37 |
| H4 | 83.2 ± 2.2 | 109.1 ± 4.5 | 122.3 ± 9.8 | −33.8 ± 5.3 |
| H5 | 74.9 ± 0.8 | 72.6 ± 3.9 | −36.5 ± 9.5 | – |
| H6 | 53.6 ± 4.1 | 25.4 ± 2.8 | – | – |
| C | −24.4 ± 3.4 | −61.8 ± 4.4 | −96.8 ± 0.6 | −98.1 ± 1.8 |
| SR in SIF | ||||
| H1 | 169.4 ± 6.4 | 237.6 ± 12 | 321.1 ± 8.6 | 509.3 ± 13 |
| H2 | 142.5 ± 0.7 | 196.5 ± 1.6 | 252.6 ± 3.0 | 370.7 ± 8.3 |
| H3 | 126.7 ± 2.4 | 167.1 ± 1.0 | 208.2 ± 2.5 | 230.4 ± 9.4 |
| H4 | 76.8 ± 1.2 | 104.1 ± 1.4 | 124.1 ± 3.6 | 115.6 ± 7.5 |
| H5 | 71.1 ± 0.5 | 85.9 ± 1.4 | 89.5 ± 1.4 | 97.8 ± 3.2 |
| H6 | 54.7 ± 0.9 | 60.6 ± 0.9 | 54.6 ± 0.6 | 41.6 ± 0.9 |
| C | 15.0 ± 1.2 | 17.3 ± 1.4 | 12.1 ± 4.5 | −4.5 ± 13.1 |
SR swelling ratio, SGF simulated gastric fluid with pepsin, SIF simulated intestinal fluid with pancreatin
In general, the SR results from all matrices studied in different media revealed a direct relationship between swelling value and HPMC amount in the formulation. Therefore, when the polymer concentration increased, the SR values increased too. This fact could be attributed to a slow erosion of the gelled layer and to a higher capability of water uptake of the tablets containing higher amounts of HPMC. According to the data (Table II), H1, H2 and H3 matrix tablets showed a continuous weight increase during the experiment (24 h) in all the different media. Considering all this, it can be deduced that in these matrix tablets with HPMC/ADCP proportions (ratio, ≥0.69) predominated the pH-independent characteristic swelling of HPMC.
H4 tablets presented similar SR values in demineralised water, progressive pH medium, SGF and SIF. However, in SGF the swelling data at 24 h were lower than those obtained in the rest of the media, showing even negative values. The swelling behaviour from H5 and H6 matrices were similar in demineralised water, progressive pH medium and SIF, but when the medium test was SGF, a partial (H5) and total disintegration (H6) at 8 h can be observed. These last results could be explained considering that in the formulations with HPMC/ADCP ratios lower than 0.69, the ADCP could act as a disintegrant, and it prevails over the HPMC hydrophilic gel-forming property and only a weak gel layer was formed, which was not able to prevent the matrix disintegration.
Due to the pH-dependent solubility of ADCP, the swelling data from C batch in all the media studied showed a clear pH-dependent behaviour, obtaining similar and positives values in demineralised water or SIF, and a constant erosion process (negative values) in SGF or progressive pH medium.
Visual observations (Fig. 1) confirm the swelling test results. The swelling behaviour was pH independent in H1, H2, H3 and H4 formulations, where the HPMC/ADCP ratio was higher than 0.25. On the contrary, H4, H5 and H6 formulations (HPMC/ADCP ratio, <0.12) showed a pH-dependent swelling behaviour, only detected after 4 h of the assay, producing an evident slow erosion process yielding a small matrix core (H5) or producing a total matrix disintegration (H6) after 8 h of the test. C matrix resulted completely eroded in acidic media and almost intact in neutral media.
Fig. 1.
Evolution of H1–H6 and C matrices aspect after immersion in different media: demineralised water (a), progressive pH medium (b), SGF (c) and SIF (d) after 2, 4, 8 and 24 h
Buoyancy Test
The in vitro buoyancy behaviour of every matrix tablets in all the different media depended also on HPMC/ADCP ratio. Consequently, H1, H2 and H3 showed good buoyancy properties, because all these systems floated immediately upon contact with the release medium and continued for more than 8 h. The matrices H4 showed a partial floatability, because they floated only during the first 8 h of the test. No lag times in buoyancy of matrices were observed. These results could be attributed to the manufacturing process; the ethanol used in the granulation step could be responsible of the HPMC swelling and therefore of the buoyancy of the tablets which agrees with Yamashita and co-workers’ observations (42). On the contrary, H5 and H6 did not float in any case. The C batch exhibits, as well, no buoyancy due to the absence of hydrophilic polymer.
In Vitro Dissolution Test
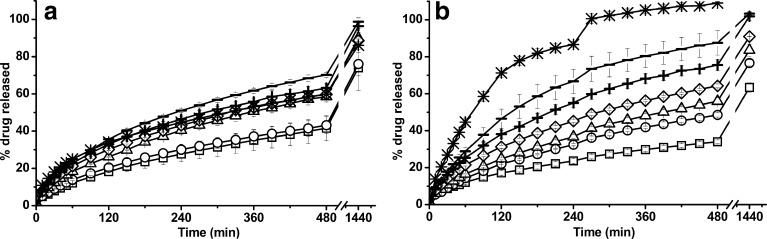
Figure 2 shows THE release profiles from H1–H6 and C batches in demineralised water (a) and progressive pH medium (b). In demineralised water, the THE dissolution profiles were influenced by the HPMC/ADCP ratio. As the content of HPMC was reduced, the ability to control the drug release decreased, yielding higher drug release values. Drug release dissolution patterns from H1 and H2 were similar, obtaining a sustained but incomplete THE release, less than 100% after 24 h of assay. On the contrary, H3, H4, H5 and H6 batches, displayed a controlled and almost complete drug release after a period of time of 24 h. The THE dissolution profile from C batch was intermediate because in demineralised water the acidic solubility of ADCP did not allow the tablet disintegration, as can be observed in Fig. 1a.
Fig. 2.
Dissolution profiles of H1 (squares), H2 (circles), H3 (triangles), H4 (diamonds), H5 (plus sign), H6 (minus sign) and C (asterisks) matrices in different media: demineralised water (a) and progressive pH medium (b). Error bars represent the standard deviation (in some cases, they are smaller than the symbol size)
In progressive pH medium, H1–H6 and C matrices showed drug release rates that clearly depended on the HPMC/ADCP ratios. Hence, when the concentration of HPMC increased, the THE release rate decreased (Fig. 2b). Furthermore, all the prepared formulations were able to efficiently control the drug release over a time period of 8 h, except for C matrix which resulted in a faster drug release (total drug released in 5 h) due to the tablet erosion in this pH medium (Fig. 1b).
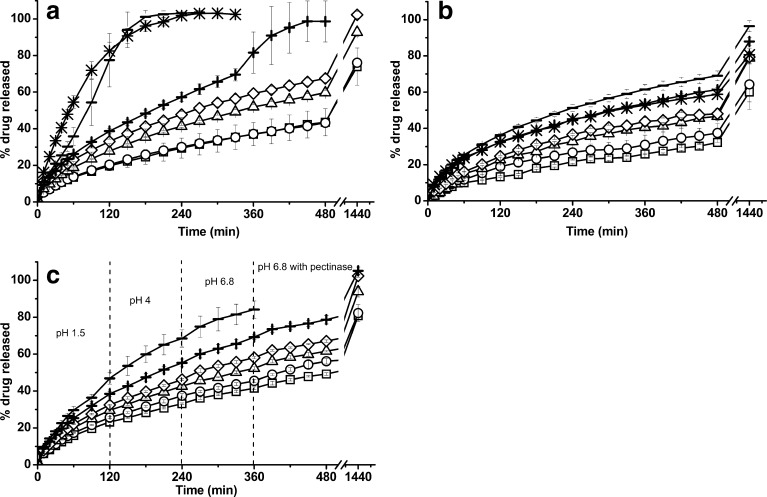
THE dissolution profiles in SGF from all the matrix tablets are displayed in Fig. 3a. H1, H2, H3 and H4 matrices showed slow drug releases: 43.5%, 49.6%, 59.7% and 67.5% of THE was dissolved at 8 h from H1, H2, H3, and H4, respectively. On the contrary, faster drug releases from H5 and H6 were obtained (100% of drug release after 8 h from H5 and in 3 h from H6), due to the high proportion of ADCP, which was soluble in acidic solution (43). The solubility of ADCP together with the reduction in the amount of the gelling agent justify the increase in the overall percentage release of THE, in accordance with the data reported by Chaturvedi and co-workers for other soluble drug (20).
Fig. 3.
Dissolution profiles from H1 (squares), H2 (circles), H3 (triangles), H4 (diamonds), H5 (plus sign), H6 (minus sign) and C (asterisks) matrices in different media: SGF (a), SIF (b) and SCF (c). Error bars represent the standard deviation (in some cases, they are smaller than the symbol size)
Figure 3b illustrates the THE release profiles from all batches in SIF. All the developed matrices were able to sustain the drug release. A direct relationship could be established between drug release rate again, and the HPMC proportion, yielding slower drug releases when the polymer concentration increased. These results differ to those obtained in demineralised water, probably due to the ionic nature of the dissolution medium, which could modulate the polymer–water interactions in non-ionic hydrophobically modified cellulose ethers such as HPMC, as was previously reported by other authors (3,44–49). Dissolution data from C batch were similar to those obtained in demineralised water, but different to the other two media, which confirm their pH-dependent drug release.
The dissolution profiles from H1, H2, H3, H4, H5 and H6 studied in SCF were displayed in Fig 3c. It was noticed that the presence of enzymes in the dissolution medium produced a change of the drug release rate of tablets H1, from a 49.2% at 8 h released in SCF to a 34% for the same period of time in the absence of enzymes. A similar observation was produced in H2 formulation, whose rate of drug release increased in this medium, producing a higher percentage of drug released at 8 h. However, this improvement in drug release rate could not be evidenced in H3, H4, H5 and H6 formulations. This behaviour suggests that the HPMC could be suffering a hydrolysis process by the hemicellulolytic activity of pectinase, in spite of the previous studies by Viriden and co-workers that showed only a specific HPMC hydrolysis by endoglucanases (25). Moreover, these results could be attributed to the modification in the dissolution method which ensured the complete immersion of the H1, H2 and H3 matrices within the dissolution medium.
Dissolution Data Analysis
The application of the similarity (f2) and different (f1) factors proposed by Moore and Flanner (33) and adopted by the CDER-FDA were the selected method for comparing the whole dissolution profiles obtained in all different media used in this work. According to data shown in Table III, when the dissolution medium was demineralised water, it was clearly observed that the dissolution profiles from H1 and H2 formulations should be considered similar (f1 < 15 and f2 > 65), but they were different to H3, H4, H5 and H6 matrices (f1 > 15 and f2 < 65). Moreover, H3, H4 and H5 drug release profiles were similar, and H5 THE profile was similar to H6, also. On the other hand, the f1/f2 test of whole profiles obtained in progressive pH medium showed that they were different, except H2 and H3 THE profiles which were similar (f1 < 15 and f2 > 65).
Table III.
Difference (f 1) and Similarity Factors (f 2) Values for the Release Profiles from H1 to H6 and C Matrices in Different Media: Demineralised Water (a), Progressive pH Medium (b), SGF (c), SIF (d) and SCF (e)
| R | P | a | b | c | d | e | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| f 1 | f 2 | f 1 | f 2 | f 1 | f 2 | f 1 | f 2 | f 1 | f 2 | ||
| H1 | H2 | 7.1 | 83.3 | 33.1 | 52.3 | 13.0 | 67.7 | 20.3 | 67.0 | 9.7 | 70.2 |
| H1 | H3 | 41.9 | 46.2 | 50.7 | 43.3 | 37.5 | 47.1 | 48.3 | 47.6 | 25.5 | 51.9 |
| H1 | H4 | 50.6 | 42.8 | 78.7 | 34.7 | 62.4 | 38.6 | 60.7 | 43.6 | 37.6 | 43.5 |
| H1 | H5 | 65.9 | 39.0 | 121 | 27.4 | 109 | 24.7 | 95.8 | 33.9 | 57.9 | 33.5 |
| H1 | H6 | 82.2 | 34.1 | 162 | 21.0 | 236 | 14.8 | 140 | 28.4 | 101 | 27.8 |
| H2 | H3 | 32.5 | 49.7 | 13.9 | 66.1 | 21.6 | 56.9 | 23.7 | 57.8 | 13.6 | 63.3 |
| H2 | H4 | 40.7 | 45.8 | 35.7 | 47.0 | 43.7 | 44.1 | 33.9 | 52.0 | 24.6 | 50.6 |
| H2 | H5 | 53.9 | 41.6 | 65.3 | 35.1 | 84.5 | 27.4 | 68.7 | 37.8 | 46.4 | 36.7 |
| H2 | H6 | 69.0 | 36.0 | 96.7 | 26.4 | 201 | 15.9 | 96.2 | 32.2 | 82.3 | 30.1 |
| H3 | H4 | 6.5 | 78.2 | 19.1 | 57.9 | 16.6 | 60.7 | 8.7 | 77.1 | 9.6 | 67.5 |
| H3 | H5 | 14.3 | 64.1 | 44.3 | 40.7 | 49.8 | 33.4 | 36.9 | 47.6 | 28.8 | 44.0 |
| H3 | H6 | 25.5 | 51.6 | 71.6 | 30.0 | 139 | 18.4 | 59.1 | 38.9 | 58.6 | 34.2 |
| H4 | H5 | 6.7 | 77.0 | 19.8 | 53.3 | 28.9 | 39.6 | 26.0 | 53.0 | 17.5 | 52.7 |
| H4 | H6 | 17.2 | 57.6 | 42.5 | 36.8 | 101 | 20.9 | 44.8 | 42.5 | 43.7 | 38.3 |
| H5 | H6 | 9.8 | 67.6 | 18.9 | 50.2 | 74.3 | 24.3 | 13.1 | 62.4 | 22.3 | 49.1 |
f 2 > 65 suggest that the dissolution profile are similar (95% confidence level). f 1 is near to zero when the test and reference profiles are identical (95% confidence level)
R the drug release profiles take as reference, P the drug release profiles take as problem
When SGF was employed as the dissolution medium, significant differences in drug release profiles from all batches were observed (f1 > 15 and f2 < 65), except for H1 and H2 profiles. Drug release profile from H1, H2, H3 matrices, evaluated in SIF, were different but H3 and H4, and H5 and H6 drug release profiles were similar among themselves, respectively (f1 < 15 and f2 > 65).
Finally, the f1 and f2 parameters obtained from the comparison of whole release profiles in SCF showed that the THE release behaviour from H1 and H2 were similar among themselves but different to the other formulations. Moreover, H3 and H4 profiles were similar among themselves and different to the other matrices.
To understand the drug release model from every formulations, all the data were treated according to zero order, first order, Hixson–Crowell, Hopfenberg, Higuchi and Korsmeyer–Peppas equations. Only the best fitting results are listed in Tables IV and V. In general, all the formulations were better adjusted to the Higuchi and Korsmeyer–Peppas equations.
Table IV.
Mathematical Modelling and Drug Release Kinetics from H1 to H6 and C Matrices Studied in Demineralised Water and Progressive pH Medium
| Batch | Hixson–Crowell | Hopfenberg | Higuchi | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| K 0 |
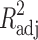
|
K s |
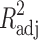
|
K H |

|
n | K KP |

|
|
| Demineralised water | |||||||||
| H1 | 0.0011 | 0.9876 | −0.1300 | 0.9852 | 0.0200 | 0.9982 | 0.5808 | 0.0114 | 0.9975 |
| H2 | 0.0012 | 0.9786 | −0.1625 | 0.9752 | 0.0206 | 0.9997 | 0.5617 | 0.0137 | 0.9989 |
| H3 | 0.0017 | 0.9882 | −0.1950 | 0.9844 | 0.0268 | 0.9996 | 0.5495 | 0.0192 | 0.9998 |
| H4 | 0.0018 | 0.9802 | −0.2277 | 0.9749 | 0.0282 | 0.9992 | 0.5288 | 0.0236 | 0.9986 |
| H5 | 0.0020 | 0.9749 | −0.2275 | 0.9684 | 0.0304 | 0.9983 | 0.5465 | 0.0232 | 0.9958 |
| H6 | 0.0023 | 0.9835 | −0.2600 | 0.9767 | 0.0343 | 0.9991 | 0.5574 | 0.0243 | 0.9973 |
| C | 0.0017 | 0.9725 | −0.195 | 0.9666 | 0.0257 | 0.9972 | 0.4370 | 0.0408 | 0.9989 |
| Progressive pH medium | |||||||||
| H1 | 0.0008 | 0.9839 | −0.0975 | 0.9821 | 0.0151 | 0.9950 | 0.4442 | 0.0211 | 0.9748 |
| H2 | 0.0014 | 0.9867 | −0.1625 | 0.9836 | 0.0240 | 0.9979 | 0.5964 | 0.0121 | 0.9971 |
| H3 | 0.0017 | 0.9913 | −0.1950 | 0.9882 | 0.0274 | 0.9975 | 0.5909 | 0.0147 | 0.9990 |
| H4 | 0.0020 | 0.9860 | −0.2600 | 0.9808 | 0.0312 | 0.9996 | 0.5622 | 0.0209 | 0.9993 |
| H5 | 0.0027 | 0.9851 | −0.3250 | 0.9684 | 0.0389 | 0.9993 | 0.6045 | 0.0206 | 0.9984 |
| H6 | 0.0037 | 0.9831 | −0.4225 | 0.9585 | 0.0501 | 0.9964 | 0.6682 | 0.0186 | 0.9993 |
| C | 0.0129 | 0.9239 | −0.845 | 0.9616 | 0.0718 | 0.9918 | 0.667 | 0.0286 | 0.9969 |
K
0 Hixson–Crowell constant, K
s Hopfenberg constant, K
H Higuchi kinetic constant, n release exponent, K
KP Korsmeyer kinetic constant,  adjusted coefficient of determination
adjusted coefficient of determination
Table V.
Mathematical Modelling and Drug Release Kinetics from H1 to H6 and C Matrices Studied in SGF, SIF and SCF
| Batch | Hixson–Crowell | Hopfenberg | Higuchi | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| K 0 |

|
K s |

|
K H |

|
n | K KP |

|
|
| SGF | |||||||||
| H1 | 0.0012 | 0.9896 | −0.1625 | 0.9873 | 0.0207 | 0.9981 | 0.5452 | 0.0148 | 0.9978 |
| H2 | 0.0014 | 0.9896 | −0.1625 | 0.9868 | 0.0241 | 0.9984 | 0.5749 | 0.0142 | 0.9978 |
| H3 | 0.0018 | 0.9893 | −0.2275 | 0.9855 | 0.0286 | 0.9995 | 0.5559 | 0.0195 | 0.9994 |
| H4 | 0.0021 | 0.9912 | −0.2600 | 0.9868 | 0.0315 | 0.9981 | 0.4981 | 0.0309 | 0.9916 |
| H5 | 0.0052 | 0.9112 | −0.5200 | 0.9419 | 0.0396 | 0.9991 | 0.5766 | 0.0243 | 0.9995 |
| H6 | 0.0218 | 0.8418 | −1.5925 | 0.961 | 0.0668 | 0.9508 | 0.7168 | 0.01924 | 0.9825 |
| C | 0.0129 | 0.9997 | −1.3325 | 0.9929 | –a | –a | –a | –a | –a |
| SIF | |||||||||
| H1 | 0.0008 | 0.9828 | −0.0975 | 0.9812 | 0.0149 | 0.9922 | 0.5694 | 0.0092 | 0.9931 |
| H2 | 0.001 | 0.9593 | −0.13 | 0.9558 | 0.0179 | 0.9941 | 0.618 | 0.00876 | 0.9831 |
| H3 | 0.0014 | 0.9669 | −0.1625 | 0.9622 | 0.024 | 0.9962 | 0.715 | 0.0064 | 0.9797 |
| H4 | 0.0014 | 0.9617 | −0.1625 | 0.9569 | 0.0243 | 0.9944 | 0.5956 | 0.0134 | 0.9922 |
| H5 | 0.0018 | 0.9784 | −0.2275 | 0.9727 | 0.0285 | 0.9984 | 0.5178 | 0.0262 | 0.9962 |
| H6 | 0.0022 | 0.9796 | −0.2600 | 0.9727 | 0.0335 | 0.9987 | 0.5375 | 0.0270 | 0.9974 |
| C | 0.0017 | 0.9697 | −0.195 | 0.9638 | 0.0264 | 0.9962 | 0.4701 | 0.0335 | 0.9966 |
| SCF | |||||||||
| H1 | 0.0013 | 0.9893 | −0.1625 | 0.9858 | 0.0236 | 0.9989 | 0.5512 | 0.0164 | 0.9994 |
| H2 | 0.0015 | 0.9849 | −0.1950 | 0.9808 | 0.0261 | 0.9978 | 0.5624 | 0.0170 | 0.9990 |
| H3 | 0.0018 | 0.9878 | −0.2275 | 0.9826 | 0.0295 | 0.9985 | 0.5574 | 0.0204 | 0.9990 |
| H4 | 0.0019 | 0.9803 | −0.2275 | 0.9731 | 0.0321 | 0.9992 | 0.5600 | 0.0278 | 0.9991 |
| H5 | 0.0025 | 0.9824 | −0.2925 | 0.9650 | 0.0388 | 0.9992 | 0.5874 | 0.0225 | 0.9992 |
| H6 | –a | –a | –a | –a | –a | –a | –a | –a | |
K
0 Hixson–Crowell constant, K
s Hopfenberg constant, K
H Higuchi kinetic constant, n release exponent, K
KP Korsmeyer kinetic constant,  adjusted coefficient of determination, SGF simulated gastric fluid with pepsin, SIF simulated intestinal fluid with pancreatin, SCF simulated colonic fluid with pectinolytic enzymes
adjusted coefficient of determination, SGF simulated gastric fluid with pepsin, SIF simulated intestinal fluid with pancreatin, SCF simulated colonic fluid with pectinolytic enzymes
aToo rapid release to allow calculation for <70% release
When demineralised water was the dissolution medium, the best kinetic adjustment for all the matrices was obtained in the Higuchi equations (Table IV (demineralised water)), showing similar values of KH for H1 and H2 dates (0.0200 and 0.0206), and near to 0.0300 from H3, H4, H5 and H6.
However, when progressive pH medium was employed (Table IV (progressive pH medium)), the better kinetic adjustment (Higuchi and Korsmeyer–Peppas equations), showed that the Higuchi constant (KH) increased as the HPMC amount decreased. It suggests that the polymer concentration modulated the drug release rate. Furthermore, the changes observed in the “n” values (0.44 to 0.66) from Korsmeyer–Peppas equation, suggested that the drug release mechanisms passed from diffusion to non-Fickian or anomalous transport mechanism when the polymers amount decrease. It confirms that the HPMC/ADCP ratio were the responsible of the changes in THE release rate. These observations agreed with other researches (14–16,18).
On the other hand, the kinetic adjustment of the matrices tested in SGF (Table V (SGF)) yields higher values of Higuchi (KH) and Korsmeyer–Peppas (KKP) constants compared with those observed in progressive pH medium. It suggests an anomalous diffusion for all the formulations tested in this medium. Likewise, the good fitting to Higuchi equation when the dissolution media was SIF (Table V (SIF)), indicated a drug release mechanism controlled mainly by a Fickian diffusion.
The dissolution profiles of H1, H2, H3, H4 and H5 matrices obtained in SCF were fitted best to Higuchi and Korsmeyer–Peppas equations (Table V (SCF)). The KH Higuchi constant obtained from H1, H2, H3, H4 and H5 formulations were 0.0236, 0.0261, 0.0295, 0.0321 and 0.0388, respectively, which suggest that the polymer amount was responsible of the drug release rate. However, the “n” values obtained from these matrices were around 0.55–0.59, suggesting that the mechanism of drug release from these formulations was produced by a Fickian diffusion, with a slight evidence of erosion process.
The kinetic constants obtained from the best fit to the different models were used to predict the theoretical time to obtain a complete drug release from all the matrix tablets in the different media (Table VI).
Table VI.
Theoretical Time Values Required to Reach the Complete THE Release from H1 to H6 and C Matrices Studied in Different Media: SGF, SIF and SCF
| Theoretical time for 100% drug released | |||
|---|---|---|---|
| Batch | SGF | SIF | SCF |
| H1 | 40.7 h | 78.0 h | 31.5 h |
| H2 | 30.8 h | 54.0 h | 25.9 h |
| H3 | 21.6 h | 31.8 h | 20.5 h |
| H4 | 17.1 h | 29.7 h | 16.9 h |
| H5 | 9.7 h | 20.4 h | NC |
| H6 | NC | 15.2 h | NC |
| C | NC | 22.9 h | NC |
NC values no calculated, SGF simulated gastric fluid with pepsin, SIF simulated intestinal fluid with pancreatin, SCF simulated colonic fluid with pectinolytic enzymes
The analysis of these data allow us to confirm that H1, H2 and H3 formulations can be used to provide a gastric controlled drug release, when only one dose of treatment is necessary since these formulations were able to release the drug in a period of time less than 48 h.
H4 formulation would be able to obtain a drug controlled release through the gastrointestinal tract for treatments when one dose every 18 h was required.
H5 and H6 matrices could be used for controlled drug release through the gastrointestinal tract. Moreover, if an adequate coating is applied onto these matrices, they can be used to provide an enteric or colonic drug release.
CONCLUSIONS
For a water-soluble drug (THE), the combination of a gel-forming excipient (HPMC) with a pH-dependent solubility material (ADCP) allows to obtain matrix tablets with different drug release profiles, which depend on the HPMC/ADCP ratio. When the HPMC/ADCP ratio was ≥0.69, floating matrix tablets with slow drug release in SGF were obtained (H1, H2 and H3), which could be applied as gastro-retentive drug delivery systems. A ratio between 0.11 and 0.69 allows to design a matrix tablet (H4) with partial buoyancy and controlled drug release in SGF and SIF, which could be suitable to obtain controlled drug release through the gastrointestinal tract. When the HPMC/ADCP ratio was ≤0.11 no floating matrix tablets with drug controlled release in SIF were obtained (H5 and H6), which could be considered as enteric or colonic delivery systems if an adequate enteric coating was applied.
Acknowledgement
Pseidy Luz Mamani Crispin is a recipient of a predoctoral scholarship from the Agencia de Española de Cooperación Internacional y Desarrollo (Spain) to do her Ph.D.
References
- 1.Lee BJ, Ryu SG, Cui JH. Formulation and release characteristics of hydroxypropyl methylcellulose matrix tablet containing melatonin. Drug Dev Ind Pharm. 1999;25:493–501. doi: 10.1081/DDC-100102199. [DOI] [PubMed] [Google Scholar]
- 2.Sako K, Sawada T, Nakashima H, Yokohama S, Sonobe T. Influence of water soluble fillers in hydroxypropylmethylcellulose matrices on in vitro and in vivo drug release. J Control Release. 2002;81:165–172. doi: 10.1016/S0168-3659(02)00067-6. [DOI] [PubMed] [Google Scholar]
- 3.Ebube NK, Jones AB. Sustained release of acetaminophen from a heterogeneous mixture of two hydrophilic non-ionic cellulose ether polymers. Int J Pharm. 2004;272:19–27. doi: 10.1016/j.ijpharm.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J Pharm Pharmacol. 2005;57:533–546. doi: 10.1211/0022357055957. [DOI] [PubMed] [Google Scholar]
- 5.Sung KC, Nixon PR, Skoug JW, Ju TR, Gao P, Topp EM, Patel MV. Effect of formulation variables on drug and polymer release from HPMC-based matrix tablets. Int J Pharm. 1996;142:53–60. doi: 10.1016/0378-5173(96)04644-3. [DOI] [Google Scholar]
- 6.Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optimal performance. Pharm Sci Technolo Today. 2000;3:198–204. doi: 10.1016/S1461-5347(00)00269-8. [DOI] [PubMed] [Google Scholar]
- 7.Lee PI, Peppas NA. Prediction of polymer dissolution in swellable controlled-release systems. J Control Release. 1987;6:207–215. doi: 10.1016/0168-3659(87)90077-0. [DOI] [Google Scholar]
- 8.Narasimhan B, Peppas NA. Molecular analysis of drug delivery systems controlled by dissolution of the polymer carrier. J Pharm Sci. 1997;86:297–304. doi: 10.1021/js960372z. [DOI] [PubMed] [Google Scholar]
- 9.Ford JL, Rubinstein ML, Hogan JE. Propranolol hydrochloride and aminophylline release from matrix tablets containing hydroxypropylcellulose. Int J Pharm. 1985;24:339–350. doi: 10.1016/0378-5173(85)90032-8. [DOI] [Google Scholar]
- 10.Feely LC, Davis SS. The influence of polymeric excipients on drug release from Hydroxypropylmethylcellulose matrices. Int J Pharm. 1988;41:83–90. doi: 10.1016/0378-5173(88)90139-1. [DOI] [Google Scholar]
- 11.Hogan JE. Hydroxypropylmethylcellulose sustained release technology. Drug Dev Ind Pharm. 1998;15:975–999. doi: 10.3109/03639048909043660. [DOI] [Google Scholar]
- 12.Hardy IJ, Windberg-Baarup A, Neri C, Byway PV, Booth SW, Fitzpatrick S. Modulation of drug release kinetics from hydroxypropyl methyl cellulose matrix tablets using polyvinyl pyrrolidone. Int J Pharm. 2007;337:246–253. doi: 10.1016/j.ijpharm.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Gao P, Nixon P, Skoug J. Diffusion in HPMC gels. II Prediction of drug release rates from hydrophilic matrix extended-release dosage forms. Pharm Res. 1995;12:965–971. doi: 10.1023/A:1016246028338. [DOI] [PubMed] [Google Scholar]
- 14.Williams RO, III, Reynolds TD, Cabelka TD, Sykora MA, Mahaguna V. Investigation of excipient type and level on drug release from controlled release tablets containing HPMC. Pharm Dev Technol. 2002;7:181–193. doi: 10.1081/PDT-120003486. [DOI] [PubMed] [Google Scholar]
- 15.Vargas CI, Ghaly ES. Kinetic release of theophylline from hydrophilic swellable matrices. Drug Dev Ind Pharm. 1999;25:1045–1050. doi: 10.1081/DDC-100102268. [DOI] [PubMed] [Google Scholar]
- 16.Jamzad S, Tutunji L, Fassihi R. Analysis of macromolecular changes and drug release from hydrophilic matrix systems. Int J Pharm. 2005;292:75–85. doi: 10.1016/j.ijpharm.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Rekhi GS, Nellore RV, Hussain AS, Tillman LG, Malinowski HJ, Augsburger LL. Identification of critical formulation and processing variables for metoprolol tartrate extended-release (ER) matrix tablets. J Control Release. 1999;59:327–342. doi: 10.1016/S0168-3659(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 18.Lotfipour F, Nokhodchi A, Saeedi M, Norouzi-Sani S, Sharbafi J, Siahi-Shadbad MR. The effect of hydrophilic and lipophilic polymers and fillers on the release rate of atenolol from HPMC matrices. Farmaco. 2004;59:819–825. doi: 10.1016/j.farmac.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Tavakoli N, Varshosaz J, Dorkoosh F, Motaghi S, Tamaddon L. Development and evaluation of a monolithic floating drug delivery system for acyclovir. Chem Pharm Bull. 2012;60:172–177. doi: 10.1248/cpb.60.172. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi K, Umadevi S, Vaghani S. Floating matrix dosage form for propranolol hydrochloride based on gas formation technique: development and in vitro evaluation. Sci Pharm. 2010;78:927–939. doi: 10.3797/scipharm.0909-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu L, Li L, Yang X, Liu W, Yang J, Jia Y, Shang Ch XH. Floating matrix dosage form for dextromethorphan hydrobromide based on gas forming technique: in vitro and in vivo evaluation in healthy volunteers. Eur J Pharm Sci. 2011;42:99–105. doi: 10.1016/j.ejps.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Abrahamsson A, Alpsten M, Bakec B, Larsson A, Sjögren J. In vitro and in vivo erosion of two different hydrophilic gel matrix tablets. Eur J Pharm Biopharm. 1998;46:69–75. doi: 10.1016/S0939-6411(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 23.Pillay V, Fassihi R. Unconventional dissolution methodologies. J Pharm Sci. 1999;88:843–851. doi: 10.1021/js990139b. [DOI] [PubMed] [Google Scholar]
- 24.Maderuelo C, Zarzuelo A, Lanao JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. J Control Release. 2011;154:2–19. doi: 10.1016/j.jconrel.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Viridén A, Larsson A, Schagerlöf H, Wittgren B. Model drug release from matrix tablets composed of HPMC with different substituent heterogeneity. Int J Pharm. 2010;401:60–67. doi: 10.1016/j.ijpharm.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 26.FDA Alert for Healthcare Professionals. Hydromorphone hydrochloride extended-release capsules (marketed as Palladone™), alcohol–palladone™ interaction, 2005.
- 27.EMEA, Committee for proprietary medicinal products (CPMP). Note for guidance on quality of modified release products. A: Oral dosage forms; B: Transdermal dosage forms. London: EMEA; 1999
- 28.Dow. Using dow excipients for controlled release of drugs in hydrophilic matrix systems. Midland: The Dow Chemical Company; 2006.
- 29.Ruiz-Caro R, Veiga MD. Characterization and dissolution study of chitosan freeze-dried systems for drug controlled release. Molecules. 2009;14:4370–86. [DOI] [PMC free article] [PubMed]
- 30.The United States Pharmacopeial/The National Formulary, USP 34/NF29. Philadelphia: National Publishing; 2011.
- 31.Haupt S, Zioni T, Gati I, Kleinstern J, Rubinstein A. Luminal delivery and dosing considerations of local celecoxib administration to colorectal cancer. Eur J Pharm Sci. 2006;28:204–211. doi: 10.1016/j.ejps.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Wakerly Z, Fell JT, Attwood D, Parkins DA. In vitro evaluation of pectin-based colonic drug delivery systems. Int J Pharm. 1996;129:73–77. doi: 10.1016/0378-5173(95)04251-2. [DOI] [Google Scholar]
- 33.Moore JW, Flanner HH. Mathematical comparison of dissolution profiles. Pharma Tec. 1996;20:64–74. [Google Scholar]
- 34.Shah VP, Tsong Y, Sathe P, Liu J. In vitro dissolution profile comparison-statistics and analysis of the similarity factor, f2. Pharm Res. 1998;15:889–895. doi: 10.1023/A:1011976615750. [DOI] [PubMed] [Google Scholar]
- 35.Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 36.Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation. Ind Eng Chem. 1931;239:923–931. doi: 10.1021/ie50260a018. [DOI] [Google Scholar]
- 37.Hopfenberg HB. Controlled release polymeric formulations. In: Paul DR, Harris FW, editors. ACS Symposium Series 33. Washington, DC: American Chemical Society; 1976. pp. 26–31. [Google Scholar]
- 38.Katzhendler I, Hofman A, Goldberger A, Friedman M. Modeling of drug release from erodible tablets. J Pharm Sci. 1997;86:110–115. doi: 10.1021/js9600538. [DOI] [PubMed] [Google Scholar]
- 39.Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 40.Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 41.Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23–26. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita K, Nakate T, Okimoto K, Ohike A, Tokunaga Y, Ibuki R, Higaki K, Kimura T. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int J Pharm. 2003;267:79–91. doi: 10.1016/j.ijpharm.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Morton RC. Calcium phosphate dibasic dehydrate. In: Weller PJ, Sheskey PJ, Rowe RC, editors. Handbook of pharmaceutical excipients. 4. London: Pharmaceutical Press; 2001. pp. 96–99. [Google Scholar]
- 44.Sarkar N. Thermal gelation properties of methyl and hydroxypropyl methylcellulose. J Appl Polym Sci. 1979;24:1073–1087. doi: 10.1002/app.1979.070240420. [DOI] [Google Scholar]
- 45.Doelker E. Cellulose derivatives. In: Peppas NA, editor. Advances in polymer science, vol. 107. Berlin: Springer; 1993. pp. 199–265. [Google Scholar]
- 46.Haque A, Morris ER. Thermogelation of methylcellulose. Part 1. Molecular structures and processes. Carbohydr Polym. 1993;22:161–173. doi: 10.1016/0144-8617(93)90137-S. [DOI] [Google Scholar]
- 47.Yuasa H, Nakano T, Kanaya Y. Suppression of agglomeration in fluidized bed coating I. Suppression of agglomeration by adding NaCl. Int J Pharm. 1997;158:195–201. doi: 10.1016/S0378-5173(97)00259-7. [DOI] [PubMed] [Google Scholar]
- 48.Nakano T, Yuasa H, Kanaya Y. Suppression of agglomeration in fluidized bed coating. III. Hofmeister series in suppression of particle agglomeration. Pharm Res. 1999;16:1616–1620. doi: 10.1023/A:1018921108172. [DOI] [PubMed] [Google Scholar]
- 49.Pygall SR, Kujawinski S, Timmins P, Melia CD. Mechanisms of drug release in citrate buffered HPMC matrices. Int J Pharm. 2009;370:110–120. doi: 10.1016/j.ijpharm.2008.11.022. [DOI] [PubMed] [Google Scholar]