Abstract
The physicochemical properties of the optimized microemulsion and the permeating ability of oxyresveratrol in microemulsion were evaluated, and the efficacy of oxyresveratrol microemulsion in cutaneous herpes simplex virus type 1 (HSV-1) infection in mice was examined. The optimized microemulsion was composed of 10% w/w of isopropyl myristate, 35% w/w of Tween 80, 35% w/w of isopropyl alcohol, and 20% w/w of water. The mean particle diameter was 9.67 ± 0.58 nm, and the solubility of oxyresveratrol in the microemulsion was 196.34 ± 0.80 mg/ml. After accelerated and long-term stability testing, the microemulsion base and oxyresveratrol-loaded microemulsion were stable. The cumulative amount of oxyresveratrol permeating through shed snake skin from microemulsion at 6 h was 93.04 times compared to that of oxyresveratrol from Vaseline, determined at 20% w/w concentration. In cutaneous HSV-1 infection in mice, oxyresveratrol microemulsion at 20%, 25%, and 30% w/w, topically applied five times daily for 7 days after infection, was significantly effective in delaying the development of skin lesions and protecting from death (p < 0.05) compared with the untreated control. Oxyresveratrol microemulsion at 25% and 30% w/w was significantly more effective than that of 30% w/w of oxyresveratrol in Vaseline (p < 0.05) and was as effective as 5% w/w of acyclovir cream, topically applied five times daily (p > 0.05). These results demonstrated that topical oxyresveratrol microemulsion at 20–30% w/w was suitable for cutaneous HSV-1 mouse infection.
KEY WORDS: cutaneous infection in mice, herpes simplex virus, microemulsion, oxyresveratrol, therapeutic efficacy
INTRODUCTION
Herpetic infections by herpes simplex virus (HSV) type 1 (HSV-1) and type 2 (HSV-2) are one of the major public health problems worldwide. These infections are incurable and persistent as latent form in the host for a lifetime. In immunocompetent individuals, primary HSV infections are usually mild, and most clinical manifestations observed are herpes labialis and herpes genitalis from recurrent HSV-1 and HSV-2 infections, respectively. However, the viruses can cause severe and mortal multiorgan diseases, especially in immunocompromised patients. Among all antiviral drugs in clinical use, acyclovir (ACV) is the first-line drug in the treatment of herpes virus infection. However, the emergence of ACV-resistant virus strains is still one of the clinical concerns that urgently require further developments (1–5). Topical antiherpes formulations received increased attention in drug development, according to their advantages, over oral and systemic administration. Several topical preparations of ACV, penciclovir, foscarnet, as well as docosanol, are in current clinical use and research (3,6–9).
Oxyresveratrol (OR) (trans-2,4,3′,5′-tetrahydroxystilbene) is a polyphenolic stilbene abundantly derived from the heartwood of the Thai traditional plant, Artocarpus lakoocha Roxburgh (Moraceae) (10,11). OR showed inhibitory effect on the growth of HSV-1 and HSV-2 wild types, drug-resistant HSV-1 strains (12), clinical isolates of HSV-1 and HSV-2 (13), as well as various varicella zoster virus (VZV) strains including wild type, thymidine kinase-deficient and DNA polymerase VZV mutant in vitro (14). Various biological activities including tyrosinase-inhibitor (15), antioxidant (16) and anthelmintic activities (17) have been also reported. As previously reported, 30% w/w of OR in Vaseline topically applied five times daily provided better therapeutic efficacy than the oral treatment of OR in cutaneous HSV-1 infection in mice (12). Due to the large amount of OR required for therapeutic efficacy against HSV-1 infection from Vaseline preparation, suitable topical formulations need further development to reduce the concentration.
A microemulsion (ME) is a thermodynamically stable system composed of oil, water, and surfactant, frequently in combination with cosurfactant, which occurs spontaneously after gentle mixing. The appearance of ME is clear or translucent due to its droplet size usually in the range of 20–200 nm. In the pharmaceutical field, MEs have been used as drug carriers for percutaneous, ocular, oral, and parenteral administration according to their high solubilization capacity for lipophilic and hydrophilic compounds as well as protection of compounds from hydrolysis and oxidation. For topical formulation, MEs also enhance skin permeation of the drugs by various effects, including an increase of concentration gradient of drugs across the skin and permeation enhancement of some components in the systems such as surfactants, cosurfactants, and oils. The low interfacial tensions of MEs also provide excellent wetting properties ensuring good contact between membrane and formulations (18–20).
In this study, oxyresveratrol-loaded ME (ORME) was formulated. Its physicochemical properties and therapeutic efficacy in cutaneous HSV-1 infection in mice were evaluated.
MATERIAL AND METHOD
Material
The chemicals (pharmaceutical grade) used for ME preparation were isopropyl myristate (Fluka, USA), oleic acid (Merck, Germany), olive oil (Giralda, Australia), polyoxyethylene sorbitan monooleate (polysorbate 80 or Tween 80; Sigma, USA), sorbitan monooleate (Span 80; Sigma, USA), polyoxyl 40 hydrogenated castor oil (Cremophor RH 40; BASF, Germany), propylene glycol (Repsol, Spain), butanol (Fischer Scientific, UK), and isopropanol (Fischer Scientific, UK). The acetronitrile (Burdick & Jackson, Korea), methanol (Burdick & Jackson, Korea), trifluoroacetic acid (Fluka, USA), and water (Burdick & Jackson, Korea) used were HPLC grade.
OR was purified from the heartwood of A. lakoocha Roxburgh (Moraceae), and the method was previously reported (10,11). Briefly, heartwood of A. lakoocha was extracted in methanol. The active fraction was isolated from the methanol extract using vacuum liquid chromatography. The purified compound was analyzed as OR (trans-2,4,3′,5′-tetrahydroxystilbene) by spectroscopy. The chemical was assayed by HPLC and found to be greater than 99% pure. Amounts of 10–30% w/w of OR were incorporated in ME base and were used in physicochemical property study and in animal experiments.
HSV-1 7401H strain was used. The virus was propagated in African green monkey kidney cells (Vero cells) as previously reported (21–23). African green monkey kidney cells (Vero cells) were grown and maintained in Eagle’s minimum essential medium supplemented with 5% and 2% of fetal bovine serum for growth medium and maintenance medium. ACV cream 5% (Vilerm®) was purchased from Siam Bheasach Co. Ltd., Thailand. Petroleum jelly (Vaseline®) was used as ointment base (12).
Formulation of Microemulsions
Solubility Study
To determine solubility of OR in various oils, surfactants, and cosolvents, an excess amount of OR was added in isopropyl myristate, oleic acid, olive oil, Tween 80, Span 80, Cremophor RH40, isopropanol, butanol, or propylene glycol, and then, the mixtures were shaken reciprocally at 25°C for 48 h to reach equilibrium. Each tube was centrifuged at 15,000 rpm for 20 min, followed by filtering through a 0.45-μm membrane filter. OR concentration was determined by HPLC analysis at appropriate dilution with methanol (7,24). The partition coefficient of OR in each oil to water was presented as the logP value. The solubility was determined by three independent experiments.
Construction of Pseudoternary Phase Diagrams
To investigate the ME region, pseudoternary phase diagrams were constructed by the water titration method (7,25). Surfactant (Tween 80) was mixed with cosurfactant (isopropanol) at a weight ratio of surfactant to cosurfactants, varying between 1:1, 2:1, and 4:1. The oily mixtures with the weight ratio of oil (isopropyl myristate) to mixture of surfactant and cosurfactant at 5:95, 10:90, 15:85, 20:80, 25:75, 30:70, 35:65, 40:60, 45:55, 50:50, 55:45, 60:40, 65:35, 70:30, 75:25, 80:20, 85:15, 90:10, and 95:5 were prepared by mixing the oil (isopropyl myristate) into each surfactant mixture ratio. Water was added drop by drop to each oily mixture under magnetic stirring at room temperature. After being equilibrated, the mixture was evaluated visually and defined as ME when they appeared as clear liquid (26).
Preparation of Oxyresveratrol-Loaded Microemulsion Systems
The selected formulas based on phase diagram and OR solubility were prepared by mixing isopropyl myristate, Tween 80, and isopropanol together, and then, water was added and mixed using a vortex mixer for 5 min at room temperature. All MEs were stored to achieve equilibrium at room temperature, at least 24 h before further investigation (27). At least 30% w/w of OR that could be incorporated in ME formulation was the aim of this study, since 30% w/w of OR in Vaseline exhibited therapeutic efficacy against cutaneous HSV-1 infection in mice (12). To determine the amount of OR that can be loaded in ME, 10–30% w/w of OR was dissolved in each ME system under ultrasonication, and the end point was a clear solution after ultrasonication. For further permeation study and animal experiments, 10%, 20%, and 30% w/w of OR were dissolved in the selected ME system under ultrasonication. OR ointment was prepared by grinding 10%, 20%, and 30% w/w of OR in petroleum jelly (Vaseline™).
Characterization of Microemulsions
Physical properties of ME formulations and ORME 10%, 20%, and 30% w/w were characterized for pH, viscosity, and conductivity using a pH meter (Beckman, USA), Rotovisco 1 viscometer, and conductivity meter at 25 ± 2°C. The particle size and size distribution of the MEs were evaluated by laser light scattering technique (Marvern Zetasizer). The viscosity, conductivity, mean particle size, and polydispersity index were determined by three independent experiments. The appearance of all formulas was determined by visual inspection on clarity, turbidity, phase separation, color, and cross-polarized microscope (KHC Olympus, Japan).
Stability Test
All ME samples were stored in well-closed and light-protected glass bottles. Thermodynamic stability of ME base and ORMEs (10%, 20%, and 30% w/w) was studied by centrifugation at 13,000 rpm for 30 min at 25°C (28). Thereafter, the systems were investigated visually for phase separation. In addition, stability tests using accelerated condition by heating–cooling (4°C for 48 h and 40°C for 48 h) for six cycles (29) and long-term stability testing at room temperature for 6 months were performed. The clarity, phase separation, particle size, viscosity, and content of OR before and after the stability tests were recorded. OR was assayed by HPLC using Phenomenex Luna C18 column (250 × 4.6 nm, 5 μm). Aqueous acetronitrile (40%) containing 0.01% trifluoroacetic acid was used as the mobile phase at a flow rate of 1.0 ml/min. The absorbance was detected at 310 nm, as described by Chao et al. (30). All samples were determined in triplicate. The calibration curve of OR in the concentration range of 1.17–150 μg/ml was linear with the correlation coefficient of 0.9999. The intra- and inter-day percent relative standard deviations (percent RSD) were less than 1.14%.
In Vitro Skin Permeation Study
Shed snake skin of the black rat snake (Elaphae obsoleta) was used as a model membrane for the skin permeation study because of its similarity to human skin in lipid content and permeability (31). The shed snake skin was kindly provided by the Queen Saovabha Memorial Institute, Thai Red Cross Society. The whole skin was kept at −20°C, and the skin membrane was hydrated for 30 min and mounted on a Franz diffusion cell (effective diffusion area of 2.1 cm2) with receiver solution (phosphate-buffered saline with 30% ethanol) before use (31). The skin was placed on the diffusion cell with the dorsal surface side up. Then, the ORME (10%, 20%, or 30% w/w) or OR in Vaseline (10%, 20%, or 30% w/w), 0.5 g, was applied to the surface of the skin through the donor compartment, and the temperature was maintained at 37 ± 0.5°C with magnetic stirring at 600 rpm throughout the experiment. Receiver solution samples, 1.0-ml aliquots, were withdrawn periodically for 6 h (0, 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, and 6 h) through the receiver compartment. The cells were refilled with receiver solution to keep the volume in the receiver compartment constant during the experiment (7,32). The content of OR in the receiver solution at each sampling time was measured for UV absorbance at 310 nm using a spectrophotometer. The experiments were performed in triplicate. The assay was linear in the OR concentration range of 0.94–30 μg/ml with the correlation coefficient of 0.9993. The cumulative amount (Qn, in micrograms per square centimeter) of OR permeating through the shed snake skin in the receiver solution was determined based on the following equation (7):
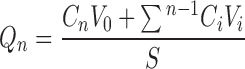 |
When Cn was the drug concentration of the receiver solution at each sampling time, Ci was the drug concentration of the sample at the time of zero, and V0 and Vi were the volumes of the receiver solution and the sample at each time period, respectively. S was the effective diffusion area.
Efficacy of Topical Oxyresveratrol Microemulsion in Cutaneous HSV-1 Infection in Mice
Female BALB/c mice (6 weeks old) were purchased from Sankyo Labo Service Co. Ltd., Tokyo, Japan. The right midflank of each mouse was clipped and depilated with a chemical depilatory, hair remover. Two or 3 days later, the naked skin was scratched using 27-guage needles and 5 μl of HSV-1 (7401H) suspension of 1 × 106 PFU was applied to the scarified area (12,22,23). OR was topically applied to mice (nine to ten mice per group) as follows: experiment 1: In order to find the formulation that was effective in mice, ORME, 10 or 20% w/w, or OR in petroleum jelly (Vaseline™), 30% w/w, was applied topically on the scratched area (5 mg/cm2/dose) 1 h after HSV-1 infection and three times daily for 7 days. ACV cream applied three times daily and mice without treatment were used as the control groups. Experiment 2: In order to find the frequency of application of ME that was effective in mice, ORME 10% w/w was applied topically on the scratched area (5 mg/cm2/dose) 1 h after HSV-1 infection three, four, or five times daily for 7 days. The mice without treatment were used as the untreated control. Experiment 3: In order to find the concentration of ORME that was effective in mice, ORME 10%, 15%, 20%, 25%, 30% w/w or OR in petroleum jelly (Vaseline™), 30% w/w, was applied topically on the scratched area (5 mg/cm2/dose) 1 h after HSV-1 infection five times daily for 7 days in each group. ACV, ME base, applied five times daily and mice without treatment were used as the control groups. In all experiments, the development of skin lesions and mortality were continuously observed three or five times daily for 10 days after HSV-1 infection and scored as follows: 0, no lesion; 2, vesicles in the local region; 4, erosion and/or ulceration in the local region; 6, mild zosteriform lesion; 8, moderate zosteriform lesion; and 10, severe zosteriform lesion and death. We conducted procedures conforming to the National Institute of Health Guide for the Care and Use of Laboratory Animals with the approval of the Animal Care Committee at the University of Toyama.
Statistical Analysis
Student’s t test was used to evaluate the significant differences in particle size, viscosity, OR content in stability tests, and permeation capacity of ORME. The repeated measures ANOVA as a multiple comparison was used to analyze the interaction between multiple groups in mean skin lesions for 3–10 days after infection. Significance of differences in mean survival times and mean times at which scored as 2, 4, or 6 after infection were also evaluated by Student’s t test. Statistical differences in the mortality were evaluated using the chi-square test. A p value of less than 0.05 was defined as statistically significant.
RESULT
Solubility Study
The solubility of OR in oils, surfactants, and cosurfactants was determined. For the solubility of OR in oils, the values were 0.65 ± 0.04, 0.31 ± 0.06, and 0.05 ± 0.01 mg/ml for isopropyl myristate, oleic acid, and olive oil, respectively. The values of log of partition coefficient determined were −0.20, −0.51, and −1.30 for isopropyl myristate, oleic acid, and olive oil, respectively. OR was solubilized in various surfactants, and the solubility in Tween 80, Cremophor RH 40, and span 80 was 71.01 ± 2.41, 44.24 ± 2.30, and 10.93 ± 0.52 mg/ml, respectively. In the cosurfactants, the solubility of OR in isopropaol, butanol, and propylene glycol was 140.95 ± 1.13, 127.30 ± 2.98, and 122.06 ± 0.92 mg/ml, respectively. The solubility of OR in water was 1.01 ± 0.01 mg/ml. Isopropyl myristate, Tween 80, and isopropanol that showed maximal solubilizing capacity for OR were selected for further ORME formulations.
Phase Diagrams
ME was prepared using isopropyl myristate as the oil phase, Tween 80 as surfactant, isopropanol as cosurfactant, and water. The pseudoternary phase diagrams of ME systems containing isopropyl myristate; Tween 80; isopropanol at fixed ratios (w/w) of 1:1, 2:1, and 4:1; and water after equilibrium at room temperature were presented in Fig. 1. The shade areas of the pseudoternary phase diagrams referred to the ME regions while the outside (white) areas indicated the turbid regions. All MEs were isotropic; transparent dispersion and no phase separation could be observed with all the ratios of oil/surfactant–cosurfactant/water after centrifugation at 13,000 rpm for 30 min at 25°C. In addition, there were no changes in the appearance of MEs and areas on the diagrams observed after 1 month storage. This indicated that ME formulated using isopropyl myristate, Tween 80, isopropanol, and water was stable. The ME regions existing in various weight ratios of surfactant and cosurfactant in pseudoternary phase diagrams were in the order of 1:1 > 2:1 > 4:1. The large ME regions were correlated with the ME systems containing cosurfactants.
Fig. 1.
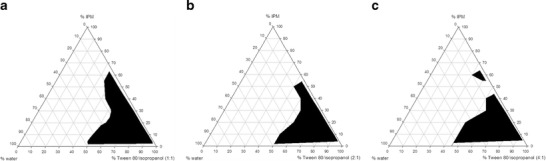
Pseudoternary phase diagram of isopropyl myristate/Tween80/isopropanol/water system at 1:1 (a), 2:1 (b), and 4:1 (c) w/w ratio of surfactant and cosurfactant
Physicochemical Properties of Microemulsions
Based on these pseudoternary phase diagrams, the ORME was selected from the systems using Tween 80/isopropanol with the ratio 1:1 which formed the largest single-phase region compared to other ratios. Several ME formulas were selected from the ME region in the phase diagram for the solubility test, and the formula that could incorporate OR at least 30% w/w with minimum concentration of surfactant and cosurfactant was chosen. As presented in Table I, among 16 formulas, 3 formulas of those with surfactant mixture between 40% and 50% w/w showed precipitation after incorporating more than 15% w/w of OR; five and four formulas of those with surfactant mixture between 40% and 50% w/w could solubilize 15–20 and 20–25% w/w of OR, respectively. There were four formulas with 5–10% w/w of oil and 70–90% w/w of surfactant mixture that could incorporate up to 30% w/w of OR. To avoid skin irritation from large amounts of surfactant and cosurfactant, the ME formula with the lowest amount of surfactant and cosurfactant mixture was selected from the latter four formulas. Thus, the selected ME was composed of isopropyl myristate (10% w/w), Tween 80 (35% w/w), isopropanol (35% w/w), with a surfactant/cosurfactant ratio of 1:1, and water (20% w/w).
Table I.
Composition of Various MEs and Their Oxyresveratrol Solubilizing Capacities
| Oil (% w/w) | Surfactant mixture (% w/w) | Water (% w/w) | Maximum OR loading capacity (% w/w) |
|---|---|---|---|
| 55 | 40 | 5 | 15 |
| 50 | 45 | 5 | 15 |
| 40 | 50 | 10 | 15 |
| 30 | 60 | 10 | 20 |
| 20 | 70 | 10 | 25 |
| 20 | 60 | 20 | 20 |
| 15 | 75 | 10 | 25 |
| 15 | 65 | 20 | 25 |
| 15 | 55 | 30 | 20 |
| 10 | 80 | 10 | >30a |
| 10 | 70 | 20 | >30a |
| 10 | 60 | 30 | 20 |
| 5 | 90 | 5 | >30a |
| 5 | 80 | 15 | >30a |
| 5 | 70 | 25 | 25 |
| 5 | 60 | 35 | 20 |
OR Oxyresveratrol
aThe formulations still showed clear solution after loaded with 30% w/w of OR (the solubilizing capacity at >30% w/w of OR was not determined)
Due to the dynamic nature and small size of surfactant aggregates, indirect measurement techniques, such as electrical conductivity and rheological studies, were employed to study the basic information of the internal structure (28). The selected ME base showed a light yellow and transparent appearance while ORME showed yellow and transparent appearance that was due to the color of the compound added. The parameters for physical characteristics of the optimized formula were indicated in Table II. The particle sizes (mean ± SD) determined by the laser light scattering technique were 9.67 ± 0.58, 44.00 ± 17.43, 179.90 ± 84.17, and 198.60 ± 55.62 nm for the ME base, ORME 10%, 20%, and 30% w/w, respectively. The increase in particle size may be affected by the incorporation of a high amount of OR in the formula and the property of the compound. However, the size was still ranged in 200 nm, and this system was chosen for further assays. All formulas showed one peak distribution of particle size, and the values of polydispersity indices (pI) were 0.04 ± 0.01, 0.24 ± 0.04, 0.34 ± 0.01, and 0.50 ± 0.09 for ME base, ORME 10%, 20%, and 30% w/w, respectively. The low values of pI indicated that all formulas had uniformity of size distribution. The ME base showed low viscosity that may result from the presence of low intrinsic viscosity compositions such as IPM and isopropanol. After OR had been added, the viscosity of MEs increased according to the increasing volume of the internal phase. ORME exhibited the viscosity values of 47.67–124.32 cP, pH values of 7.11–7.22, and the conductivity values of 10.39–12.83 μS/cm. The dark appearance of all formulas observed through a cross-polarized microscope showed the nonbirefringent property of ME. The solubility of OR in the selected ME was 196.34 ± 0.80 mg/ml. The results of conductivity showed that the MEs were in O/W systems.
Table II.
Physical Properties of Oxyresveratrol Microemulsions
| Formulations | pH | Viscositya (cP) | Conductivitya (μS/cm) | Particle sizea (nm) | Polydispersity indexa (pI) |
|---|---|---|---|---|---|
| Selected ME base | 7.43 | 16.10 ± 1.01 | 13.80 ± 1.49 | 9.67 ± 0.58 | 0.04 ± 0.01 |
| 10% ORME | 7.22 | 47.67 ± 0.83 | 12.83 ± 0.62 | 44.00 ± 17.43 | 0.24 ± 0.04 |
| 20% ORME | 7.19 | 87.18 ± 1.45 | 12.10 ± 0.65 | 179.90 ± 84.17 | 0.34 ± 0.01 |
| 30% ORME | 7.11 | 124.32 ± 1.88 | 10.39 ± 0.32 | 198.60 ± 55.62 | 0.50 ± 0.09 |
Selected ME was composed of isopropyl myristate (10% w/w), Tween 80 (35% w/w), isopropanol (35% w/w) with surfactant/cosurfactant ratio of 1:1, and water (20% w/w)
ME microemulsion, ORME oxyresveratrol-loaded ME
aMean ± SD determined from three independent experiments
In stability tests, ME base and ORME were stable in both conditions by heating–cooling for six cycles and long-term storage in ambient temperatures for 6 months (Table III). Incorporation of OR did not affect either the structure or the physical stability of the ME base, since no significant changes in clarity, precipitation, and phase separation were observed after long-term and accelerated conditions. Both selected ME base and ORMEs showed good physical stability after centrifugation at 13,000 rpm at 25°C for 30 min. In addition, ME base and all ORMEs showed good physical stability after the thermodynamic stability test since clear single-phase solutions were observed after centrifugation at 13,000 rpm at 25°C for 30 min. The color as well as transparency was still the same as those after being freshly prepared. No change in the size distribution graph was observed after ambient and accelerated stability conditions. There were no significant differences (p > 0.05) in viscosity of ME base and ORME that were determined after being freshly prepared (Table III) and storage in two conditions. The particle sizes of ME base, after accelerated and long-term storage, slightly increased (p < 0.05) when compared with those after being freshly prepared. However, there were no significant differences (p > 0.05) in particle size of ORME between fresh preparation and these two storage conditions. After accelerated and long-term stability testing, the OR content of formulations were 124.48.21 ± 6.07 (19.99% w/w) and 123.13 ± 8.07 mg/ml (19.83% w/w), respectively. The OR contents were not significantly different (p > 0.05) compared with that of freshly prepared 20% w/w ORME (123.92 ± 5.54 mg/ml). The results showed that OR was chemically stable in selected ME. This stability was better than that of OR 0.2 mg/ml in aqueous solutions, in which the amount of OR remaining after the same accelerated and long-term conditions were 83.02% and 76.78%, respectively.
Table III.
Stability Test of Oxyresveratrol Microemulsions
| Characteristics | Accelerated condition | Long-term condition | ||
|---|---|---|---|---|
| ME basea | ME + OR 20% | ME base | ME + OR 20% | |
| Appearance | ||||
| Color | Light yellow | Yellow | Light yellow | Yellow |
| Transparent | Yesb | Yes | Yes | Yes |
| Phase separation | Noc | No | No | No |
| Particle size (nm)d | 13.00 ± 0.01 | 213.00 ± 49.49 | 11.33 ± 0.60 | 186.33 ± 31.88 |
| Viscosity (cP)d | 16.02 ± 1.51 | 84.92 ± 0.93 | 16.05 ± 0.71 | 85.06 ± 1.94 |
ME microemulsion
aMicroemulsion base
bTransparent appearance was observed
cNo phase separation was observed after accelerated or long-term condition
dMean ± SD determined from three independent experiments
In Vitro Permeation of Oxyresveratrol Microemulsion
To study in vitro permeation of OR in formulations, the shed snake skin was used as a membrane, and the concentration of OR was 10%, 20%, and 30% w/w. A cumulative amount of OR (Qns) in the selected ME determined after 6 h of application was 2,079.13 ± 116.27, 5,773.07 ± 160.95, and 6,259.11 ± 472.00 μg/cm2, respectively (Fig. 2). A cumulative amount of OR (Qns) in Vaseline determined after 6 h of application was 23.31 ± 0.21, 44.55 ± 1.86, and 66.66 ± 3.54 μg/cm2, respectively (Fig. 2). The OR from the ORME showed greater permeation than the OR from the Vaseline preparation through the shed snake skins at every time point (p < 0.05). The cumulative amount of OR increased when increasing the concentration of OR in the formulations (p < 0.05). As indicated, the viscosity of the vehicle was one of the important factors in the permeation of OR into the skin. The viscosity of 20% w/w ORME (87.18 ± 1.45 cP) was much lower than that of 20% w/w of OR in Vaseline (2,408.35 ± 204.18 cP); therefore, the mobility of OR in ME was more facile.
Fig. 2.
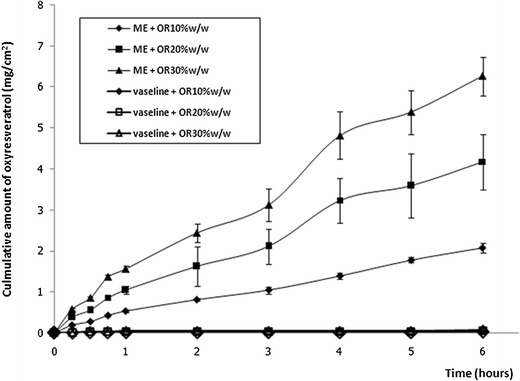
In vitro permeation of oxyresveratrol in microemulsion in shed snake skin (mean ± SD, n = 3)
Efficacy of Topical Oxyresveratrol Microemulsion on Cutaneous HSV-1 Infection in Mice
To evaluate the formulation that was effective, ORME at 10% and 20% w/w, and OR in Vaseline (30% w/w) topically applied three times daily for 7 days were tested. The mean time of the appearance of lesion score 2, 4, or 6; mean survival time; and percentage of mortality of mice were presented in Table IV (A). ORME at 20% w/w, OR in Vaseline (30% w/w), and ACV significantly delayed the development of skin lesions, prolonged mean survival times, and reduced the percentage of mortality of mice (p < 0.05) compared with the untreated control. ORME at 20% w/w showed significantly greater effectiveness (p < 0.05) compared with 30% w/w OR in Vaseline and did not show a significant difference compared with ACV cream. ORME 10% w/w significantly delayed the development and progression of skin lesions at a score 4 (p < 0.05) but did not significantly reduce the percentage of mortality of mice compared with the untreated control.
Table IV.
Effect of Formulation (A) and Frequency of Applications (B) of Oxyresveratrol Microemulsion in Cutaneous HSV-1 Infection in Mice
| Treatmenta | Mean timeb (days, mean ± SD) | % Mortalityc | |||
|---|---|---|---|---|---|
| Score 2 | Score 4 | Score 6 | Survival | ||
| A. Effect of formulation | |||||
| Untreated control | 5.65 ± 0.29 | 6.21 ± 0.50 | 7.02 ± 0.58 | 8.03 ± 0.42 | 100 |
| ORME 10%, ×3 | 6.88 ± 0.62d | 7.17 ± 0.63d | >7.80 ± 0.98 | >8.58 ± 0.78 | 88.89 |
| ORME 20%, ×3 | >7.96 ± 2.06d | >8.36 ± 1.61d | >8.95 ± 0.99d | >9.14 ± 0.81d | 55.56e |
| OR in Vaseline 30%, ×3 | >6.69 ± 1.38d | >6.79 ± 0.59d | >8.10 ± 0.95d | >8.87 ± 1.00d | 33.33e |
| ACV 5%, ×3 | >10d | >10d | >10d | >10d | 0e |
| B. Effect of frequency of applications | |||||
| Untreated control | 5.37 ± 0.31 | 6.49 ± 0.49 | 7.49 ± 0.30 | 8.03 ± 0.89 | 100 |
| ORME 10%, ×3 | 6.30 ± 0.82d | 6.78 ± 0.39 | >7.78 ± 0.93 | >8.10 ± 0.86 | 62.5e |
| ORME 10%, ×4 | >7.48 ± 1.59d | >7.73 ± 1.43d | >8.58 ± 1.19d | >8.83 ± 0.98 | 62.5e |
| ORME 10%, ×5 | >8.10 ± 1.60d | >8.18 ± 1.38d | >8.85 ± 1.16d | >9.15 ± 0.97d | 50e |
OR oxyresveratrol, ORME oxyresveratrol-loaded ME, ACV acyclovir, SD standard deviation
aORME was topically applied five times daily for 10 days after HSV-1 infection
bMean time at which score 2, 4, and 6 or survival was observed after infection, determined from nine to ten mice in each group (the mean time with symbol > referred to a group of mice that had mice showing score 0 at day 10)
cPercentage of mortality of mice tested, observed at day 10 after HSV-1 infection
dMean time was significantly prolonged (p < 0.05 vs. untreated control)
eMortality was significantly reduced (p < 0.05 vs. untreated control)
To determine optimal frequency of the treatment, ORME at 10% w/w was topically applied three, four, and five times daily for 7 days. As indicated in Table IV (B), ORME at 10% w/w applied five times daily significantly delayed the development and progression of skin lesions, prolonged mean survival times, and reduced the percentage of mortality of mice (p < 0.05) compared with the untreated control. ORME at 10% w/w applied four times daily significantly delayed the development of skin lesion scores 2, 4, and 6 (p < 0.05) compared with the untreated control. ORME at 10% w/w applied three times daily significantly delayed the development of skin lesion score 2 (p < 0.05) compared with the untreated control. ORME at 10% w/w applied four times and three times daily did not prolong the mean survival time compared with the untreated control.
The optimal concentration of ORME applied five times daily for 7 days was determined. The result was illustrated in Table V. It indicated that ORME acquired concentration-dependent efficacy, and the maximum efficacy observed on day 5 after infection was 15% w/w ORME (Fig. 3). The mean time of the appearance of lesion scores and mean survival time were significantly prolonged (p < 0.05) as compared with the untreated control for 10–30% w/w ORME, 30% w/w OR in Vaseline, and ACV. ORME at 20–30% w/w significantly delayed the development of skin lesions and reduced the percentage of mortality of mice (p < 0.05) compared with the untreated control. ORME at 25% and 30% w/w was significantly more effective than OR at 30% w/w in Vaseline (p < 0.05) and did not show a significant difference compared with ACV cream. Therefore, it was suggested that the therapeutic efficacy of 20–30% ORME could be obtained.
Table V.
Effect of Concentration of Oxyresveratrol Microemulsion in Cutaneous HSV-1 Infection in Mice
| Treatmenta | Mean timeb (days, mean ± SD) | % Mortalityc | |||
|---|---|---|---|---|---|
| Score 2 | Score 4 | Score 6 | Survival | ||
| Untreated control | 5.81 ± 0.59 | 6.27 ± 0.53 | 7.18 ± 0.40 | 7.55 ± 0.79 | 100 |
| ME base, ×5 | 6.29 ± 0.59 | 7.02 ± 0.95 | 7.62 ± 0.81 | 8.11 ± 0.76 | 100 |
| ORME 10%, ×5 | 6.80 ± 0.82d | 7.10 ± 0.82d | 7.72 ± 0.51d | 7.92 ± 0.43d | 100 |
| ORME 15%, ×5 | 7.06 ± 1.27d | 8.10 ± 1.16d | >8.29 ± 0.95d | >8.66 ± 0.98d | 70 |
| ORME 20%, ×5 | >8.30 ± 1.68d | >8.64 ± 1.37d | >9.07 ± 0.91d | >9.30 ± 0.78d | 30e |
| ORME 25%, ×5 | >8.90 ± 1.51d | >9.18 ± 1.11d | >9.22 ± 1.03d | >9.20 ± 1.0d | 40e |
| ORME 30%, ×5 | >9.34 ± 1.28d | >9.40 ± 1.15d | >9.48 ± 1.01d | >9.50 ± 0.99d | 30e |
| OR in Vaseline 30%, ×5 | >7.84 ± 2.05d | >8.27 ± 1.71d | >8.69 ± 1.25d | >9.02 ± 1.05d | 40e |
| ACV 5%, ×5 | >10d | >10d | >10d | >10d | 0e |
OR oxyresveratrol, ME microemulsion, ORME oxyresveratrol-loaded ME, ACV acyclovir, SD standard deviation
aORME was topically applied five times daily for 10 days after HSV-1 infection
bMean time at which score 2, 4, and 6 or survival was observed after infection, determined from nine to ten mice in each group (the mean time with symbol > referred to a group of mice that had mice showing score 0 at day 10)
cPercentage of mortality of mice tested, observed at day 10 after HSV-1 infection
dMean time was significantly prolonged (p < 0.05 vs. untreated control)
eMortality was significantly reduced (p < 0.05 vs. untreated control)
Fig. 3.
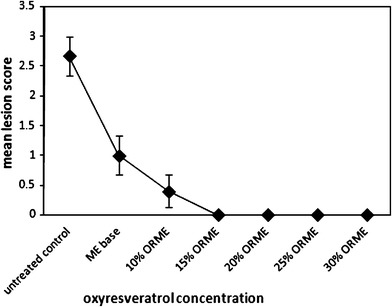
Concentration-dependent efficacy of oxyresveratrol microemulsion in cutaneous HSV-1 infection in mice observed at day 5 after infection. These results were part of the same experiment as that of Table V (data represented the mean ± SE and were determined from nine to ten mice in each group)
DISCUSSION
In formulation, the ME formula was selected on the basis of their particle sizes and maximum loading of OR of at least 30% w/w. The selected ME formula consisted of isopropyl myristate (10% w/w), Tween 80 (35% w/w), isopropanol (35% w/w), and water (20% w/w). It was reported that at optimum ratio of surfactant and cosurfactant, the cosurfactant was arranged exactly between surfactant molecules and resulted in formation of ME with maximum solubilizing capacity (33). Isopropanol acted as cosurfactant resulting in an increase in flexibility of the interfacial film of surfactant and also acted as permeation enhancers (27,34). It was observed that the particle sizes of ORMEs were increased after incorporation of OR. However, the sizes were still in the ME range. In addition, MEs with particle sizes of 200–800 nm were reported to exhibit good stability and increase the transdermal flux of drugs in vitro (35). The low values of pI, a measure of particle homogeneity of the selected ME and ORMEs, indicated that all formulas had a narrow size distribution (36). The mean particle size of ME slightly increased after storage. As reported, this might be caused by the high free energy of interfacial film together with some interactions and resulted in temporal aggregation of droplets (37–39). The selected ME was o/w type, and this was in accordance with previous studies. It was reported that o/w ME type showed high conductivity in the range of 10–100 μS/cm while w/o type showed lower conductivity levels (32,40,41). For the stability test, the HPLC peak of OR did not show any degradation peaks. However, the freshly prepared formulations were used in each animal study to ensure the efficacy of OR in ME and Vaseline formulations. There are a few reports concerning the stability of OR. OR possesses low bioavailability and is easily oxidized by prooxidant agents (42).
In the in vitro permeation, the cumulative amount of OR (Qns) in the selected ME was higher than that of OR in Vaseline. This may be affected by the ME formula with a permeation enhancer in reducing the diffusion barrier (32,40,41). As a commonly used transdermal enhancer, isopropyl myristate has been supposed to be able to increase permeation by fluidizing intercellular lipids in the stratum corneum or to increase drug solubility at the lipid part of the stratum corneum (42,43). In addition, surfactant including Tween 80 enhanced the flux of drug across the skin by incorporating in skin lipid bilayer and causing more permeable arrangement or increasing the fluidity of the membrane (44). As indicated, the lower viscosity of ORME compared to that of OR in Vaseline may affect the increase in permeation. The higher concentration of ORME resulted in the higher amount of OR permeating through the skin. The permeation rate has been reported to be correlated with a loading dose of various drugs in ME according to Fick’s first diffusion law (35,45,46). The shed snake skin was used as membrane for evaluation of permeating capacity, and it was reported that some compounds penetrating through the shed snake skin and human stratum corneum were at similar rates (31,47–49).
As indicated in cutaneous HSV-1 infection in mice, the progression of lesion scores in the ME base-treated group was not significantly different (p > 0.05) compared with the untreated control group. This indicated that the ME base did not possess antiviral activity. It was reported that the topical anti-HSV agent, n-docosanol, may inhibit early events in viral replication (50). The cream formulation was previously studied, and the application of 10–20% w/w OR cream five times daily was effective compared with the untreated control (13). The residence time of ME at the application site would be very low compared to semisolid preparations due to very low viscosity, and ME with higher viscosity should be investigated. In infected mice with repeated application of ORME, the weights of the mice were not significantly different (p > 0.05) compared with the mice before treatment. There was no skin irritation observed by visual erythema; the toxicity and skin irritation test of ORME in the non-infected mice will be further investigated.
CONCLUSION
In the present study, the optimized ORME that demonstrated efficacy in mice was composed of 10% w/w of isopropyl myristate, 35% w/w of Tween 80, 35% w/w of isopropyl alcohol, and 20% w/w of water. The ORME was characterized and showed the suitable capacity in solubilizing OR, particle size and viscosity. It also showed good physicochemical stability after accelerated and long-term conditions. In cutaneous HSV-1 infection in mice, it was indicated that ORME 20–30% w/w topically applied five times daily was effective. Therefore, ORME should have the potential for topical treatment of HSV infection.
ACKNOWLEDGMENTS
We gratefully thank the Japan Society for the Promotion of Science 2009, University of Toyama, Chulalongkorn University Centenary Academic Development Project (2008–2009) and Chulalongkorn University Graduate Scholarship Commemorating the 72nd Anniversary of His Majesty King (2008–2010) for the support of this work. We thank Tomoko Okuda for her excellent assistance.
REFERENCES
- 1.Naesens L, De Clercq E. Recent developments in herpesvirus therapy. Herpes. 2001;8(1):12–16. [PubMed] [Google Scholar]
- 2.Whitley R, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 3.De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis and management. J Am Acad Dermatol. 2007;57(5):737–763. doi: 10.1016/j.jaad.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Greco A, Diaz JJ, Thouvenot D, Morfin F. Novel targets for the development of anti- herpes compounds. Infect Disord Drug Targets. 2007;7(1):11–18. doi: 10.2174/187152607780090766. [DOI] [PubMed] [Google Scholar]
- 6.Balfour HH. Antiviral drugs. N Engl J Med. 1999;340(16):1255–1268. doi: 10.1056/NEJM199904223401608. [DOI] [PubMed] [Google Scholar]
- 7.Zhu W, Yu A, Wang W, Dong R, Wu J, Zhai G. Formulation design of microemulsion for dermal delivery of penciclovir. Int J Pharm. 2008;360(1–2):184–190. doi: 10.1016/j.ijpharm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Shishu, Rajan S, Kamalpreet Development of novel microemulsion-based topical formulations of acyclovir for the treatment of cutaneous herpetic infections. AAPS Pharm Sci Tech. 2009;10(2):559–565. doi: 10.1208/s12249-009-9242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treister NS, Woo SB. Topical n-docosanol for management of recurrent herpes labialis. Expert Opin Pharmacother. 2010;11(5):853–860. doi: 10.1517/14656561003691847. [DOI] [PubMed] [Google Scholar]
- 10.Sritularak B, De-Eknamkul W, Likhitwitayawuid K. Tyrosinase inhibitors form Artocarpus lakoocha. Thai J Pharm Sci. 1998;22:149–155. [Google Scholar]
- 11.Likhitwitayawuid K, Sritularak B, Benchanak K, Lipipun V, Mathew J, Schinaz RF. Phenolics with antiviral activity from Millettia erythrocalyx and Artocarpus lakoocha. Nat Prod Res. 2005;19:177–182. doi: 10.1080/14786410410001704813. [DOI] [PubMed] [Google Scholar]
- 12.Chuanasa T, Phromjai J, Lipipun V, Likhitwitayawuid K, Suzuki M, Pramyothin P, Hattori M, Shiraki K. Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antiviral Res. 2008;80(1):62–70. doi: 10.1016/j.antiviral.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Lipipun V, Sasivimolphan P, Yoshida Y, Daikoku T, Sritularak B, Ritthidej G, Likhitwitayawuid K, Pramyothin P, Hattori M, Shiraki K. Topical cream-based oxyresveratrol in the treatment of cutaneous HSV-1 infection in mice. Antiviral Res. 2011;91(2):154–160. doi: 10.1016/j.antiviral.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Sasivimolphan P, Lipipun V, Likhitwitayawuid K, Takemoto M, Pramyothin P, Hattori M, Shiraki K. Inhibitory activity of oxyresveratrol on wild-type and drug-resistant varicella-zoster virus replication in vitro. Antiviral Res. 2009;84(1):95–97. doi: 10.1016/j.antiviral.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Kim YM, Yun J, Lee CK, Lee H, Min KR, Kim Y. Oxyresveratrol and hydroxystilbene compounds: inhibitory effect on tyrosinase and mechanism of action. J Biol Chem. 2002;227(18):16340–16344. doi: 10.1074/jbc.M200678200. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz P, Roychowdhury S, Engelmann M, Wolf G, Horn TFW. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: effect on nitrosative and oxidative stress derived form microglial cells. Nitric Oxide. 2003;9:64–76. doi: 10.1016/j.niox.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Saowakon N, Tansatit T, Wanichanon C, Chaakul W, Reutrakul V, Sobhan P. Fasciola gigantica: anthelmintic effect of the aqueous extract of Artocarpus lakoocha. Exp Parasitol. 2009;122(4):289–298. doi: 10.1016/j.exppara.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery system. Adv Drug Deliv Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 19.Santos P, Watkinson AC, Hadgraft J, Lane ME. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol Physiol. 2008;21:246–259. doi: 10.1159/000140228. [DOI] [PubMed] [Google Scholar]
- 20.Azeem A, Khan ZI, Aqil M, Ahmad FJ, Khar RK, Talegaonkar S. Microemulsions as a surrogate carrier for dermal drug delivery. Drug Dev Ind Pharm. 2009;35(5):525–547. doi: 10.1080/03639040802448646. [DOI] [PubMed] [Google Scholar]
- 21.Shiraki K, Rapp F. Effects of caffeine on herpes simplex virus. Intervirology. 1988;29:235–240. doi: 10.1159/000150050. [DOI] [PubMed] [Google Scholar]
- 22.Kurokawa M, Ochiai H, Nagasaka K, Neki M, Xu H, Kadota S, Sutardjo S, Matsumoto T, Namba T, Shiraki K. Antiviral traditional medicines against herpes simplex virus (HSV-1), poliovirus, and measles virus and their therapeutic efficacies for HSV-1 infection in mice. Antiviral Res. 1993;22(2–3):175–188. doi: 10.1016/0166-3542(93)90094-Y. [DOI] [PubMed] [Google Scholar]
- 23.Lipipun V, Kurokawa M, Suttisri R, Taweechotipatr P, Pramyothin P, Hattori M, Shiraki K. Efficacy of Thai medicinal plant extracts against herpes simplex virus type 1 infection in vitro and in vivo. Antiviral Res. 2003;60(3):175–180. doi: 10.1016/S0166-3542(03)00152-9. [DOI] [PubMed] [Google Scholar]
- 24.Kuehl PJ, Stratton SP, Powell MB, Myrdal PB. Preformulation, formulation, and in vivo efficacy of topically applied apomine. Int J Pharm. 2009;382(1–2):104–110. doi: 10.1016/j.ijpharm.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YB, Lin YH, Lu TM, Wang RJ, Tsai YH, Wu PC. Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. Int J Pharm. 2008;349(1–2):206–211. doi: 10.1016/j.ijpharm.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Derle DV, Sagar BSH, Pimpale R. Microemulsion as a vehicle for transdermal permeation of nimesulide. Indian J Pharm Sci. 2006;68:622–625. doi: 10.4103/0250-474X.29630. [DOI] [Google Scholar]
- 27.Boonme P, Krauel K, Graf A, Rades T, Junyaprasert VB. Characterization of microemulsion structure in the pseudoternary phase diagram of isopropyl palmitate/water/brij 97:1-butanol. AAPS Pharm Sci Tech. 2005;7(2):E1–E6. doi: 10.1208/pt070237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Djordjevic L, Primorac M, Stupar M. In vitro release of diclofenac diethylamine from caprylocaproyl macrogolglycerides based microemulsions. Int J Pharm. 2005;296:73–79. doi: 10.1016/j.ijpharm.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Bali V, Ali M, Ali J. Nanocarrier for the enhanced bioavailability of a cardiovascular agent: in vitro, pharmacodynamic, pharmacokinetic and stability assessment. Int J Pharm. 2001;403(1–2):46–56. doi: 10.1016/j.ijpharm.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Chao J, Yu MS, Wang M, Chang RCC. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic Biol Med. 2008;45(7):1019–1026. doi: 10.1016/j.freeradbiomed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Itoh T, Xia J, Magavi R, Nishihata T, Rytting H. Use of shed snake skin as a model membrane for in vitro percutaneous penetration studies: comparison with human skin. Pharm Res. 1990;7(10):1042–1047. doi: 10.1023/A:1015943200982. [DOI] [PubMed] [Google Scholar]
- 32.Chandra A, Sharma PK, Irchhiaya R. Microemulsion-based hydrogel formulation for transdermal delivery of dexamethasone. Asian J Pharm. 2009;3(1):30–36. doi: 10.4103/0973-8398.49172. [DOI] [Google Scholar]
- 33.Kawakami K, Yoshikawa T, Moroto Y, Kanahashi K, Nishihara Y, Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drug. I. Prescription design. J Contr Release. 2002;81(1–2):65–74. doi: 10.1016/S0168-3659(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 34.Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Ad Coll Interf Sci. 2006;123–126:369–385. doi: 10.1016/j.cis.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Tsai YH, Chang JT, Chang JS, Huang CT, Huang YB, Wu PC. The effect of component of microemulsions on transdermal delivery of buspirone hydrochloride. J Pharm Sci. 2011;100(6):2358–2365. doi: 10.1002/jps.22474. [DOI] [PubMed] [Google Scholar]
- 36.Joshi M, Patravale V. Formulation and evaluation of nanostructured lipid carrier (NLC-based gel of valdecoxib. Drug Dev Ind Pharm. 2006;32(8):911–918. doi: 10.1080/03639040600814676. [DOI] [PubMed] [Google Scholar]
- 37.Hashem FM, Shaker DS, Ghorab MK, Nasr M, Ismail A. Formation, characterization, and clinical evaluation of microemulsion containing clotrimazole for topical delivery. AAPS Pharm Sci Tech. 2011;12(3):879–886. doi: 10.1208/s12249-011-9653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang SR, Lim SJ, Park JS, Kim CK. Phospholipid-based microemulsion formulation of all-trans-retinoic acid for parenteral administration. Int J Pharm. 2004;276(1–2):175–183. doi: 10.1016/j.ijpharm.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Park KM, Kim CK. Preparation and evaluation of flurbiprofen-loaded microemulsion for parenteral delivery. Int J Pharm. 1999;181(2):173–179. doi: 10.1016/S0378-5173(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 40.Baboota S, Alam S, Sharma S, Sahni JK, Kumar A, Ali J. Nanocarrier-based hydrogel of betamethasone dipropionate and salicylic acid for treatment of psoriasis. Int J Pharma Investig. 2011;1(3):139–147. doi: 10.4103/2230-973X.85963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baroli B, Lopez-Quintela MA, Delgado-Charro MB, Fadda AM, Blanco-Mendez J. Microemulsions for topical delivery of 8-methoxsalen. J. Contr Release. 2000;69(1):209–218. doi: 10.1016/S0168-3659(00)00309-6. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Bonilla P, Lopez-Nicolas JM, Garcia-Carmona F. Use of reversed phase high pressure liquid chromatographyl for the physicochemical and thermodynamic characterization of oxyresveratrol/β-cyclodextrin complexes. J Chromatorgr B Anal Technol Biomed Life Sci. 2010;878(19):1569–1575. doi: 10.1016/j.jchromb.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Mei ZN, Chen HB, Weng T, Yang YJ, Yang XL. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur J Pharm Biopharm. 2003;56:189–196. doi: 10.1016/S0939-6411(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 44.Imanidis G, Hartner KC, Mazer NA. Intestinal permeation and metabolism of a model peptide (leuprolide) and mechanisms of permeation enhancement by non-ionic surfactants. Int J Pharm. 1995;120(1):41–50. doi: 10.1016/0378-5173(94)00407-V. [DOI] [Google Scholar]
- 45.Chen H, Chang X, Weng T, Zhao X, Gao Z, Yang Y, Xu H, Yang X. A study of microemulsion systems for transdermal delivery of triptolide. J Contr Release. 2004;98(3):427–436. doi: 10.1016/j.jconrel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Zhao X, Liu JP, Zhang X, Li Y. Enhancement of transdermal delivery of theophylline using microemulsion vehicle. Int J Pharm. 2006;327(1–2):58–64. doi: 10.1016/j.ijpharm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 47.Rigg PC, Barry BW. Shed snake skin and hairless mouse skin as model membranes for human skin during permeation studies. J Invest Dermatol. 1990;94:235–240. doi: 10.1111/1523-1747.ep12874561. [DOI] [PubMed] [Google Scholar]
- 48.Harada K, Murakami T, Kawasaki E, Higashi Y, Yamamoto S, Yata N. In vitro permeability to salicylic acid of human, rodent and shed snake skin. J Pharm Pharmacol. 1993;4:414–418. doi: 10.1111/j.2042-7158.1993.tb05567.x. [DOI] [PubMed] [Google Scholar]
- 49.Takahash K, Tamagawa S, Katagi T, Rytting JH, Nishihata T, Mizuno N. Percutaneous penetration of basic compounds through shed snake skin as a model membrane. J Pharm Pharmacol. 1993;45:882–886. doi: 10.1111/j.2042-7158.1993.tb05613.x. [DOI] [PubMed] [Google Scholar]
- 50.Pope LE, Marcelletti JF, Katz LR, Lin JY, Katz DH, Parish ML, Spear PG. The anti- herpes simplex virus activity of n-docosanol includes inhibition of the viral entry process. Antivir Res. 1998;40(1–2):85–94. doi: 10.1016/S0166-3542(98)00048-5. [DOI] [PubMed] [Google Scholar]


