Abstract
The widespread use of indwelling medical devices has enormously increased the interest in materials incorporating antibiotics and antimicrobial agents as a means to prevent dangerous device-related infections. Recently, chlorhexidine-loaded polyurethane has been proposed as a material suitable for the production of devices which are able to resist microbial contamination. The aim of the present study was to characterize the in vitro release of chlorhexidine from new polymeric orthodontic chains realized with polyurethane loaded with two different chlorhexidine salts: chlorhexidine diacetate or chlorhexidine digluconate. The orthodontic chains constituted of three layers: a middle polyurethane layer loaded with chlorhexidine salt inserted between two layers of unloaded polymer. In vitro release of chlorhexidine diacetate and digluconate from orthodontic chains loaded with 10% or 20% (w/w) chlorhexidine salt was sustained for 42 days and followed Fickian diffusion. The drug diffusion through the polyurethane was found to be dependent not only on chlorhexidine loading, but also on the type of chlorhexidine salt. The antibacterial activity of 0.2% (w/w) chlorhexidine diacetate-loaded orthodontic chain was successfully tested towards clinically isolated biofilm forming ica-positive Staphylococcus epidermidis via agar diffusion test. In conclusion, the chlorhexidine salt-loaded chains could provide an innovative approach in the prevention of oral infections related to the use of orthodontic devices.
KEY WORDS: antibacterial activity, cariogenic treatment, chlorhexidine, in vitro release, orthodontic chains
INTRODUCTION
Polyurethane is a polymer widely used for medical device production due to its excellent physical and mechanical properties and good biocompatibility (1). Polyurethane elastomeric chains have been developed for orthodontic applications and are a suitable option to close space and correct rotations (2,3). Unfortunately, it has been shown that orthodontic treatment increases caries risk due to the plaque accumulation as well as bacterial flora modification (4).
Chlorhexidine is a bisbiguanide antiseptic widely used for the treatment and prevention of skin and mucosal infections. Furthermore, chlorhexidine was successfully used as oral antimicrobial and antiplaque agent (5,6). Recent studies have confirmed the chlorhexidine effectiveness and interest in dental use (7–9).
Lately, chlorhexidine-controlled release systems have been developed to broaden the possible applications of this antibacterial drug. Chlorhexidine diacetate has been associated with amorphous microporous silica for a potential application on dental implant to prevent infection in the earlier days following intervention (10,11). Antibacterial polyurethane nanocomposites using chlorhexidine diacetate have also been developed (12). In the same field, a previous study reported the preparation, physicochemical, and mechanical characterization of chlorhexidine diacetate-loaded polyurethane biomaterial for a local controlled delivery of chlorhexidine (13).
Prior studies showed that the application of chlorhexidine gel on orthodontic chains did not affect the mechanical properties of the orthodontic chains either in vitro or in vivo (14,15). However, the gel formulation is providing only a short-lived antibacterial action. Given the widespread use of elastomeric chains in orthodontic medical devices, in the present study, a new drug delivery system based on the chlorhexidine loading into polyurethane elastomer for sustaining release of antimicrobial drug during cariogenic orthodontic treatment is proposed. In this work, polyurethane orthodontic chains loaded with chlorhexidine diacetate or chlorhexidine digluconate were prepared. The release of chlorhexidine diacetate or chlorhexidine digluconate from polyurethane orthodontic chains was investigated in order to highlight the long-term release of chlorhexidine.
MATERIALS AND METHODS
Materials
Chlorhexidine diacetate dihydrate (CDA), chlorhexidine digluconate (CDG), sodium chloride, and sodium acetate were purchased from Sigma-Aldrich (Lyon, France). Tetrahydrofuran, glacial acetic acid, and acetonitrile were supplied by Carlo Erba (Val de Reuil, France) at high-performance liquid chromatography (HPLC) grade. Medical grade polyurethane (PU) was used as a polymer to incorporate chlorhexidine salts (3M Unitek, Cergy Pontoise, France). Tryptic soy agar was purchased from VWR International France (Fontenay-sous-Bois, France). Polycarbonate filters (pore diameter, 0.2 and 0.45 μm) were purchased from Fisher Bioblock (Illkirch, France).
Preparation of Chlorhexidine Salt-Loaded Polyurethane Orthodontic Chains
Orthodontic chains (OC) were prepared by casting and solvent evaporation following a method previously reported (13,16,17). Medical PU was dissolved in tetrahydrofuran to obtain a 6.7% (w/v) solution (solution A). A solution containing chlorhexidine was made by mixing solution A with a predetermined quantity of CDA powder or 20% w/v CDG aqueous solution (solution B). The orthodontic chain had a sandwiched structure and consisted of three layers (each layer approximately 0.05-cm thick) in which containing layer was between two layers of unloaded PU. The first unloaded PU layer was obtained by casting 0.25 mL of solution A in a vinylpolysiloxane laboratory-made mold (4.20 × 0.20 × 0.15 cm). The solvent was allowed to evaporate at about 25°C for 30 min forming a dry PU layer. The central layer of chlorhexidine salt-loaded PU was casted from 0.25 mL of solution B onto the PU layer. The third unloaded PU layer was obtained by casting 0.25 mL of solution A. After solvent evaporation, the systems were taken out from mold. The elastomeric chains were then weighed at regular intervals until constant weight corresponding to complete drying was attained and were wrapped in aluminum foil until used.
Three types of multilayered orthodontic chains were prepared:
CDA-loaded orthodontic chain (OC) at 0.2%, 10%, and 20% (weight CDA/weight PU)
CDG-loaded OC at 10% and 20% (weight CDG/weight PU)
Unloaded chains in which the central layer was chlorhexidine-free (unloaded OC)
In Vitro Drug Release Studies
Release studies were performed on 10% and 20% CDA-OC or 10% and 20% CDG-OC placed in a sealed glass vial containing 20 mL of 0.9% (w/v) NaCl aqueous solution (adjusted to pH 6.4), magnetically stirred at 150 rpm, at 25 ± 1°C (13). The release medium was completely changed after each sampling (1, 2, 4, 6, 13, 20, 27, and 35 days) to maintain sink conditions. Drug release studies were followed up to 42 days. All experiments were performed in triplicate. Samples were filtered through a polycarbonate membrane (pore diameter 0.45 μm) and analyzed for chlorhexidine content by previously developed HPLC method (18). Briefly, HPLC analysis was performed on Agilent 1200 series (Agilent, Massy, France) using the following conditions: column LiChrospher 100 RP-18, 4 × 125 mm, 5 μm (Agilent, Massy, France); mobile phase: acetonitrile and 30 mM sodium acetate aqueous solution, 50:50 (v/v), adjusted to pH 3.3 by glacial acetic acid; flow of 1.5 mL/min; injection volume of 40 μL; and UV detector at λ = 260 nm. The release profile of chlorhexidine released from the OC was expressed as the ratio of cumulative drug release to initial drug loading versus time (Eq. 1):
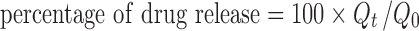 |
1 |
where Qt is the cumulative amount of drug released at time t and Q0 is the amount of drug loaded in the device.
Analysis of Release Data
The description of release profiles has been attempted using different release models. Data were evaluated according to the following equations:
 |
2 |
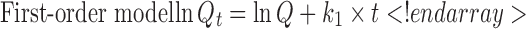 |
3 |
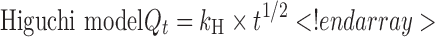 |
4 |
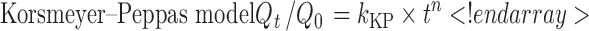 |
5 |
where Qt is the cumulative amount of drug released at time t, Q is the initial amount of drug present in the solution, k0 is the zero-order release constant, k1 is the first-order release constant, kH is the Higuchi release constant, kKP is the Korsmeyer–Peppas release constant, and n is the diffusional release exponent indicative of the release mechanism. The correlation coefficient (r2) was used as an indicator of the best fitting, for each of the considered models.
Antibacterial Activity
For the evaluation of antibacterial activity of chlorhexidine salt-loaded OC, 0.2% CDA-OC and unloaded OC were prepared as described previously (“Preparation of Chlorhexidine Salt-Loaded Polyurethane Orthodontic Chains” section). A 0.1-mL aliquot of bacteria suspension in 0.9% (w/v) NaCl aqueous solution (106 CFU/mL) was spread onto the surface of tryptic soy agar plates. Therefore, unloaded OC or 0.2% CDA-OC was placed at the center of these agar plates, which were then incubated at 37°C for 48 h. Finally, after 24 and 48 h, the growth inhibition zone around OC was measured from one to the opposite edge of the inhibition zone (including the diameter of the OC) by ImageJ software (National Institute of Health, Bethesda, USA). The experiment was performed in triplicate.
Statistical Analysis
Results are expressed as the mean ± standard deviation of three experiments. Data were statistically analyzed using Student’s t test and one-way ANOVA test. Significance was tested at the 0.05 level of probability.
RESULTS AND DISCUSSION
In Vitro Drug Release Studies and Kinetic Characteristics
For 10% and 20% CDA-OC and 10% and 20% CDG-OC, in vitro drug release studies were carried out for 42 days in 0.9% NaCl solution. The profile of drug released from 10% and 20% CDA-OC and 10% and 20% CDG-OC as a function of time is shown in Fig. 1. A remarkable sustained drug release, followed up to 42 days, could be maintained for chlorhexidine salts loaded with polyurethane orthodontic chains.
Fig. 1.
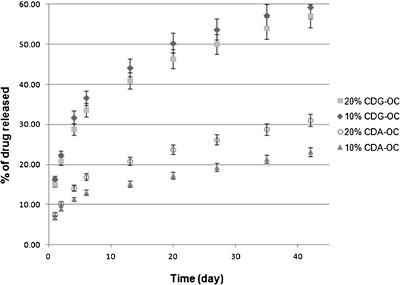
In vitro release of drug from 20% CDG-OC, 10% CDG-OC, 20% CDA-OC, and 10% CDA-OC as a function of time (n = 3)
Table I shows release rate parameters of CDA and CDG from 10% and 20% CDA-OC and from 10% and 20% CDG-OC. These parameters were obtained by fitting the curves of cumulative released amount in function of time to linear equation (KaleidaGraph 3.6, Synergy Software). For all the curves, the fitting was applied from 0 to 1 day and from 6 to 42 days because of an evident “burst effect” phase characterized by a fast and higher release of drug in the first 24 h, followed by a slowing down of the release with the establishment of a slower and protracted release from day 6 onwards. The linear fitting from 6 to 42 days was chosen because of the best fit coefficient (R2 = 0.999).
Table I.
Release Rate Parameters of Drug from 20% CDG-OC, 10% CDG-OC, 20% CDA-OC, and 10% CDA-OC (n = 3)
| Formulation | Drug loading (mg) | Release rate (mg/day) | |
|---|---|---|---|
| 0–1st day | 6–42nd day | ||
| 20% CDG-OC | 10.05 ± 0.01 | 1.51 ± 0.17a | 0.06 ± 0.01a |
| 10% CDG-OC | 5.02 ± 0.01 | 0.82 ± 0.09 | 0.03 ± 0.01 |
| 20% CDA-OC | 10.01 ± 0.04 | 0.76 ± 0.16b | 0.04 ± 0.01b |
| 10% CDA-OC | 5.00 ± 0.04 | 0.34 ± 0.01 | 0.01 ± 0.01 |
aSignificantly different compared to that of 10% CDG-OC (Student’s t test, p < 0.05)
bSignificantly different compared to that of 10% CDA-OC (Student’s t test, p < 0.05)
Earlier in vitro studies showed a limitation of CDA release from methacrylate copolymer in saline solution (0.013% to 0.06% w/v NaCl aqueous solution) because of the reduced CDA solubility in these media (19). This effect has to be carefully considered during the development of sustained-release formulation for long-term antisepsis (20,21). In saline solution, it has been reported that the formation of chlorhexidine dihydrochloride, whose solubility is 0.06% (w/v) at 20°C and 0.1% (w/v) at 37°C, was thermodynamically favored (19). Sink conditions of a release study performed on a chlorhexidine salt-loaded system could be affected by the presence of chloride ion in the release medium. The effect of chlorhexidine dihydrochloride solubility on the sink conditions, and consequently on release rates of CDA from polyurethane films, has been already reported and discussed (13). In the present study, release medium was clear and no precipitation was observed. Maximum concentration of formed chlorhexidine dihydrochloride during the first 24 h (0.007% (w/v) in the case of 20% CDG-OC) was about 10% of its solubility. After 24 h, the formed chlorhexidine dihydrochloride concentration between two sampling times was always below 10% of its solubility (maximum concentration observed in the case of 20% CDG-OC from 6th to 13th day was 0.004% w/v). Sink conditions were therefore constantly maintained during all the release studies.
Release mechanism from controlled release systems is essentially due to the penetration of the aqueous dissolution medium in the drug delivery system, dissolution of the drug, and subsequent release of the drug in the medium. The kinetics study of this process, evaluated by using the mathematical models, provide some insights about the release mechanisms presented by the delivery system. Polyurethane is a biocompatible delivery system but non-swellable and generally considered inert in aqueous media. This means that the drug is progressively released by the system, increasing the diffusional pathway necessary to the drug to be released in the surrounding fluid. In vitro drug release kinetic parameters from OC loaded with chlorhexidine salts are reported in Table II. In vitro kinetic parameters were obtained by fitting the curves of cumulative released amount in function of time to four different mathematical models. The higher correlation coefficient (r2) indicated a superiority of the release profile to the mathematical models. The studied OC show higher (r2) values for the Korsmeyer–Peppas model (r2 > 0.98, Table II). The diffusional exponent values (n) for the studied OC were lower than 0.45 (Table II)—a value that indicates purely Fickian diffusion mechanism for cylindrical systems (22,23). The lower diffusional exponent values found in the case of multilayer polyurethane systems loaded with chlorhexidine salts are probably to be attributed to the special geometry of the chains for orthodontic application. The drug diffusion through the PU is chlorhexidine salt-dependent: there is a statistical difference between the kKP of 20% CDG-OC and 20% CDA-OC (p < 0.05) and between the kKP of 10% CDG-OC and 10% CDA-OC (p < 0.05). Hiraishi et al. also observed that chlorhexidine release from chlorhexidine diacetate incorporated into resins was highly correlated with the degree of water uptake/sorption (24). The present in vitro release study has been performed in 0.9% (w/v) NaCl aqueous solution. Earlier studies stated that water mobility has a critical role in transport properties of molecules in polymers. Particularly in drug delivery systems, water state can affect drug pharmacokinetics (absorption, diffusion, release, etc.) (25,26). As reported by Farkas et al., water solubility (% w/v) of CDG is much higher than the one of CDA, >70.0 and 1.8, respectively (27). Even if the presence of chloride lowers chlorhexidine salt solubility, CDG solubility in 0.9% (w/v) NaCl aqueous solution stays higher than the one of CDA (19). As a result, higher percent of drug released and higher kKP were observed in the present study for CDG-OC.
Table II.
In Vitro Release Kinetic Parameters of Drug from 20% CDG-OC, 10% CDG-OC, 20% CDA-OC, and 10% CDA-OC (n = 3)
| Formulation | Zero-order model | First-order model | Higuchi model | Korsmeyer–Peppas model | |||||
|---|---|---|---|---|---|---|---|---|---|
| r 2 | k 0 | r 2 | k 1 | r 2 | k H | r 2 | k KP | n | |
| 20% CDG-OC | 0.295 ± 0.183 | 0.172 ± 0.009 | 0.029 ± 0.001 | 0.033 ± 0.001 | 0.799 ± 0.052 | 0.993 ± 0.057 | 0.989 ± 0.002 | 0.178 ± 0.019 | 0.316 ± 0.016 |
| 10% CDG-OC | 0.460 ± 0.115 | 0.091 ± 0.002 | 0.433 ± 0.006 | 0.055 ± 0.002 | 0.749 ± 0.033 | 0.529 ± 0.009 | 0.982 ± 0.004 | 0.198 ± 0.008 | 0.302 ± 0.009 |
| 20% CDA-OC | 0.238 ± 0.153 | 0.091 ± 0.009 | 0.482 ± 0.077 | 0.133 ± 0.061 | 0.869 ± 0.070 | 0.521 ±0.051 | 0.994 ± 0.004 | 0.086 ± 0.014 | 0.346 ± 0.032 |
| 10% CDA-OC | 0.350 ± 0.002 | 0.034 ± 0.001 | 0.973 ± 0.004 | 0.034 ± 0.001 | 0.783 ± 0.003 | 0.194 ± 0.002 | 0.992 ± 0.003 | 0.071 ± 0.001 | 0.308 ± 0.001 |
Antibacterial Activity
The antibacterial activity of chlorhexidine is already very well-documented (28–30). The purpose of this experiment was to evaluate the antibacterial activity of the chlorhexidine salt-loaded OC, even at a low chlorhexidine salt loading against a clinically relevant strain of Staphylococcus epidermidis. S. epidermidis is a significant opportunistic pathogen and has been identified as one of the predominant microorganisms responsible for nosocomial infections derived by the application of medical devices. The virulence of these bacteria in relation to medical devices has been correlated to their ability to form biofilms. The presence of the ica operon in the genome of Staphylococci encoding for polysaccharide intercellular adhesin and polymeric N-acetyl-glucosamine has been correlated to increased adherence to biomaterials, encapsulation, resistance to phagocytosis, and ability to form biofilms (31,32).
Agar diffusion test has been used to assess the antibacterial activity of the 0.2% CDA-OC. In this study, the unloaded MS demonstrated no inhibition of S. epidermidis (Fig. 2a), while 0.2% CDA-OC showed a clear zone of inhibition (Fig. 2b (24 h), c (48 h)). The growth inhibition zone (4.82 ± 0.28 cm2 including OC) observed at 24 h was significantly smaller than that observed after 48 h of experiment (7.60 ± 0.80 cm2 including OC) (Student’s t test, p < 0.05).
Fig. 2.

Typical pictures of agar plates showing the inhibition zones generated by 0.2% CDA-OC against clinical isolated S. epidermidis (106 CFU/mL) after 24 h (b) and 48 h (c) of incubation at 37°C, confirming the antimicrobial activity of the proposed system, whereas (a) unloaded OC shows no inhibition zone
In agar diffusion test, while the unloaded OC showed no inhibition of S. epidermidis growth, the inhibition efficacy of 0.2% CDA-OC proved that CDA could diffuse through the unloaded polyurethane layer to agar surface achieving a sufficient inhibitory concentration. The longer contact time, the larger inhibition zone (4.82 ± 0.28 and 7.60 ± 0.80 cm2 for 24 and 48 h, respectively, Student’s t test, p < 0.05 (Fig. 2)).
In a previous study, the prolonged activity against S. epidermidis of monolayer polyurethane disks containing CDA up to 35 days has been demonstrated (13). The monolayered films are less capable of controlling the release compared to the multilayered systems proposed in the present study because of the absence of the drug-free polyurethane layers and the presence of a much higher burst effect. As a consequence, a prolonged antibacterial activity from multilayered systems is assured by the more controlled and progressive release of drug. In the presented experiment, the author focused on a worst-case scenario with a system “depleted” and containing only a 0.2% of antibacterial drug to demonstrate that even at this low loading level the quantity of drug released is sufficient to inhibit S. epidermidis from colonizing the device. Further clinical studies will be necessary to validate the in vitro studies presented so far.
CONCLUSION
In this work, polyurethane orthodontic chains for local controlled release of chlorhexidine diacetate or chlorhexidine digluconate were prepared. The in vitro release of chlorhexidine diacetate or chlorhexidine digluconate from polyurethane orthodontic chains follows Fickian diffusion. The drug diffusion through the PU is dependent on chlorhexidine loading and also on chlorhexidine salt. In vitro release study showed a sustained drug release over an extended period of time, providing good basis for oral treatment modality. Agar diffusion test confirmed that chlorhexidine salt could diffuse through unloaded polyurethane wall. The polymeric chlorhexidine orthodontic chains proposed in this study appear to have all the prerequisites for clinical evaluation. Since this device is fabricated by molding, it enables customization with respect of future applications.
REFERENCES
- 1.Chauvel-Lebert DJ, Auroy P, Bonnaure-Mallet M. Biocompatibility of elastomers. In: Dumitriu S, editor. Polymeric biomaterials. New-York: Marcel Dekker; 2002. pp. 311–360. [Google Scholar]
- 2.Eliades T. Orthodontic materials research and applications: part 2. Current status and projected future developments in materials and biocompatibility. Am J Orthod Dentofac Orthop. 2007;131(2):253–262. doi: 10.1016/j.ajodo.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Renick MR, Brantley WA, Beck FM, Vig KWL, Webb CS. Studies of orthodontic elastomeric modules. Part 1: glass transition temperatures for representative pigmented products in the as-received condition and after orthodontic use. Am J Orthod Dentofac Orthop. 2004;126(3):337–343. doi: 10.1016/j.ajodo.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Opsahl Vital S, Haignere-Rubinstein C, Lasfargues J-J, Chaussain C. Caries risk and orthodontic treatment. Int Orthod. 2010;8(1):28–45. doi: 10.1016/j.ortho.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Jones CG. Periodontology 2000. Periodontology. 1997;15(1):55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 6.Reverdy ME. La chlorhexidine. In: Fleurette J, Freney J, Reverdy ME, editors. Antisepsie et désinfection. Paris: ESKA; 1995. pp. 135–168. [Google Scholar]
- 7.Becerik S, Turkoglu OYA, Emingil G, Vural C, Özdemir G, Atilla G. Antimicrobial effect of adjunctive use of chlorhexidine mouthrinse in untreated gingivitis: a randomized, placebo-controlled study. APMIS. 2011;119(6):364–372. doi: 10.1111/j.1600-0463.2011.02741.x. [DOI] [PubMed] [Google Scholar]
- 8.Menegon RF, Blau L, Janzantti NS, Pizzolitto AC, Corrêa MA, Monteiro M, et al. A nonstaining and tasteless hydrophobic salt of chlorhexidine. J Pharm Sci. 2011;100(8):3130–3138. doi: 10.1002/jps.22517. [DOI] [PubMed] [Google Scholar]
- 9.Stanislawczuk R, Reis A, Loguercio AD. A 2-year in vitro evaluation of a chlorhexidine-containing acid on the durability of resin-dentin interfaces. J Dent. 2011;39(1):40–47. doi: 10.1016/j.jdent.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Verraedt E, Pendela M, Adams E, Hoogmartens J, Martens JA. Controlled release of chlorhexidine from amorphous microporous silica. J Control Release. 2010;142(1):47–52. doi: 10.1016/j.jconrel.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Verraedt E, Braem A, Chaudhari A, Thevissen K, Adams E, Van Mellaert L, et al. Controlled release of chlorhexidine antiseptic from microporous amorphous silica applied in open porosity of an implant surface. Int J Pharm. 2011;419(1–2):28–32. doi: 10.1016/j.ijpharm.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 12.Fong N, Simmons A, Poole-Warren LA. Antibacterial polyurethane nanocomposites using chlorhexidine diacetate as an organic modifier. Acta Biomater. 2010;6(7):2554–2561. doi: 10.1016/j.actbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Huynh TTN, Padois K, Sonvico F, Rossi A, Zani F, Pirot F, et al. Characterization of a polyurethane-based controlled release system for local delivery of chlorhexidine diacetate. Eur J Pharm Biopharm. 2010;74(2):255–264. doi: 10.1016/j.ejpb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Catalbas B, Ercan E, Dalli M, Gelgor IE, Erdemir A. Does chlorhexidine affect the shear bond strengths of orthodontic brackets? J Dent Sci. 2011;6(2):76–81. doi: 10.1016/j.jds.2011.04.002. [DOI] [Google Scholar]
- 15.Dalli M, Ercan E, Zorba YO, Ince B, Sahbaz C, Bahsi E, et al. Effect of 1% chlorhexidine gel on the bonding strength to dentin. J Dent Sci. 2010;5(1):8–13. doi: 10.1016/S1991-7902(10)60002-5. [DOI] [Google Scholar]
- 16.Eliades T, Eliades G, Watts DC, Brantley WA. Elastomeric ligatures and chains. In: W.A. Brantley, T. Eliades (eds.) Elsevier, New-York 2001
- 17.Langlade M. Force élastique de classe 1, Optimisation des élastiques orthodontiques. Maloine ed. Paris 2000.
- 18.Lboutounne H, Chaulet J-F, Ploton C, Falson F, Pirot F. Sustained ex vivo skin antiseptic activity of chlorhexidine in poly([ε]-caprolactone) nanocapsule encapsulated form and as a digluconate. J Control Release. 2002;82(2–3):319–334. doi: 10.1016/S0168-3659(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 19.Nerurkar MJ, Zentner GM, Rytting JH. Effect of chloride on the release of chlorhexidine salts from methyl methacrylate: 2-hydroxyethyl methacrylate copolymer reservoir devices. J Control Release. 1995;33(3):357–363. doi: 10.1016/0168-3659(94)00104-3. [DOI] [Google Scholar]
- 20.Zeng P, Rao A, Wiedmann TS, Bowles W. Solubility properties of chlorhexidine salts. Drug Dev Ind Pharm. 2009;35(2):172–176. doi: 10.1080/03639040802220318. [DOI] [PubMed] [Google Scholar]
- 21.Zeng P, Zhang G, Rao A, Bowles W, Wiedmann TS. Concentration dependent aggregation properties of chlorhexidine salts. Int J Pharm. 2009;367(1–2):73–78. doi: 10.1016/j.ijpharm.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 22.Siepmann J, Göpferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv Drug Deliv Rev. 2001;48(2–3):229–247. doi: 10.1016/S0169-409X(01)00116-8. [DOI] [PubMed] [Google Scholar]
- 23.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv Drug Deliv Rev. 2001;48(2–3):139–157. doi: 10.1016/S0169-409X(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 24.Hiraishi N, Yiu CKY, King NM, Tay FR, Pashley DH. Chlorhexidine release and water sorption characteristics of chlorhexidine-incorporated hydrophobic/hydrophilic resins. Dent Mater. 2008;24(10):1391–1399. doi: 10.1016/j.dental.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinelli A, D’Ilario L, Francolini I, Piozzi A. Water state effect on drug release from an antibiotic loaded polyurethane matrix containing albumin nanoparticles. Int J Pharm. 2011;407(1–2):197–206. doi: 10.1016/j.ijpharm.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Schneider NS, Illinger JL, Karasz FE. Effect of water on the glass transition temperature of hydrophilic polyurethanes. J Appl Polym Sci. 1993;48(10):1723–1729. doi: 10.1002/app.1993.070481005. [DOI] [Google Scholar]
- 27.Farkas E, Zelkó R, Török G, Rácz I, Marton S. Influence of chlorhexidine species on the liquid crystalline structure of vehicle. Int J Pharm. 2001;213(1–2):1–5. doi: 10.1016/S0378-5173(00)00575-5. [DOI] [PubMed] [Google Scholar]
- 28.Cafiso V, Bertuccio T, Santagati M, Campanile F, Amicosante G, Perilli MG, et al. Presence of the ica operon in clinical isolates of Staphylococcus epidermidis and its role in biofilm production. Clin Microbiol Infect. 2004;10(12):1081–1088. doi: 10.1111/j.1469-0691.2004.01024.x. [DOI] [PubMed] [Google Scholar]
- 29.Kalaga A, Addy M, Hunter B. Comparison of chlorhexidine delivery by mouthwash and spray on plaque accumulation. J Periodontol. 1989;60:127–130. doi: 10.1902/jop.1989.60.3.127. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins S, Addy M, Newcombe RG. Dose response of chlorhexidine against plaque and comparison with triclosan. J Clin Periodontol. 1994;21:250–255. doi: 10.1111/j.1600-051X.1994.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 31.McKenney D, Hübner J, Muller E, Wang Y, Goldmann DA, Pier GB. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun. 1998;66(10):4711–4720. doi: 10.1128/iai.66.10.4711-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Eiff C, Jansen B, Kohnen W, Becker K. Infections associated with medical devices: pathogenesis, management and prophylaxis. Drugs. 2005;65(2):179–214. doi: 10.2165/00003495-200565020-00003. [DOI] [PubMed] [Google Scholar]


