Abstract
In the present study, grafting technology has been used to develop novel grafted hydrogel beads as controlled drug delivery carriers. The chemical crosslinking and grafting of polyacrylamide onto sodium alginate has been found to be efficient method for the development of new polymeric carrier. The successful crosslinking has been confirmed by Fourier transformed infrared spectroscopy, thermogravimetric analysis, and elemental analysis. The polymeric network of sodium alginate–co-polyacrylamide (NaAlg-g-PAM) has been interlinked by covalent and hydrogen bonds which also strength the gel network. Simple ionotropic gelation method has been used for the preparation of NaAlg-g-PAM hydrogel beads. Its swelling and gelation were dependent on monomer and crosslinker concentrations. Entrapment of the drug moiety (famotidine; an antiulcer drug) within the grafted beads has been confirmed by X-ray powder diffraction and differential scanning calorimetry. More than 75% of drug loading in beads occurred with the increase of monomer and crosslinker concentration. In vitro drug release was found to be sustained up to the 12 h with 80% drug release.
Key words: crosslinking, grafting, hydrogel beads, mechanical strength, polyacrylamide
INTRODUCTION
Alginates are linear natural polysaccharide isolated from marine-origin brown seaweeds and algae, forming a group of copolymers of β-d mannuropyranosyluronic acid and α-l-gulopyranosyluronic acid units. These units are interlinked by β-1,4 and α-1,4 glycosidic bonds to form a chain. Due to the equatorial–equatorial bonding, mannuropyranosyluronic region looks flat and ribbon-like, the same as cellulose conformation, whereas gulopyranosyluronic acid regions have a wrinkled conformation due to axial–axial glycosidic bonds. Alginates consist of large number of free hydroxyl and carboxyl groups distributed along its backbone which makes it a better candidate for chemical modification (1). Hydrogels made up of natural polymers have weak mechanical strength which restricts their applications as drug delivery carrier (1); thus, polymer blending (2), polymerization, crosslinking, and co-grafting (3) are attractive methods to improve its mechanical and functional properties. Synthetic polymers are combined to impart better physical and mechanical properties of the synthetic components onto natural polymers (3). Modification or derivatization of alginates or chemically co-grafting could alter its solubility, hydrophobicity, and physicochemical and biological characteristics. Yang et al. have summarized an excellent report on chemical modification of alginate (1). Chemical modification of free hydroxyl group of alginates could be done by oxidation, reductive amination, sulfation, copolymerization, and cyclodextrin conjugation whereas chemical modification of free carboxyl group can be done by esterification, Ugi reaction, and amidation. Alginates contain different proportions of 1,4′-linked β-d-mannuronic acid and α-l-guluronic acid which constitute gel-forming ability in the presence of divalent (Ca2+) or trivalent (Al3+) cations. Gel formation phenomenon takes place by attachment of guluronic group with the free divalent ions (Ca++). The free chains of polysaccharides are bound together at a junction of α-l-guluronic acid blocked by available divalent ions and forms immobile networks (1). The stack of such networks can then dimerize to form a gel (Fig. 1a, b).
Fig. 1.
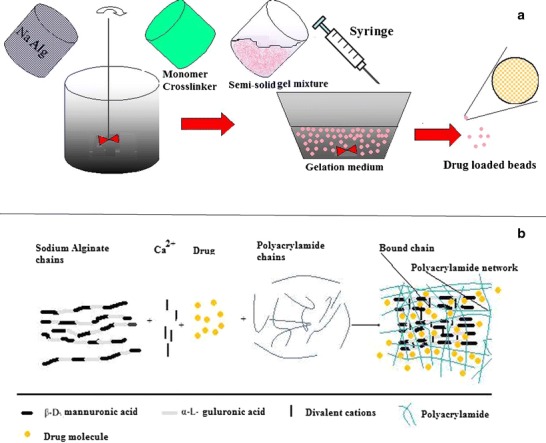
a Schematic representation of ionotropic gelation process for the preparation of drug loaded NaAlg-PAM beads and b mechanism of drug entrapment during polymerization/crosslinking of PAM with NaAlg
Unfortunately, instability and rapid disintegration of gel are two most common disadvantages of physical crosslinking (4). Modification of natural polymers by grafting technique is a promising tool to produce relatively stable gels. Grafting enables introduction of special property in the polymer and explores their potential application for drug delivery. Polymer modifications have attracted polymer, cosmetic, and drug delivery scientists from all across the globe. Grafting provides in situ encapsulation of two or more different polymers in a single carrier system to provide combination of both properties. Grafting and crosslinking are the special chemical treatments by which modification of natural polymer can be done that contain abundance of polysaccharides. Some of the best interpenetrating polymeric networks (IPN) systems contain polysaccharides (5). Huge numbers of techniques have been reported in the literature for graft copolymerization in which IPN provides excellent opportunity to entrap the drugs like progesterone (6), verapamil hydrochloride, nifedipine (7), 5-florouracil (8), tetracycline (9), ibuprofen (10), and improve swelling property of hydrogel network (11). Such IPNs take advantages of two or more polymers to modify drug release, thus it could be used as potential drug delivery carrier (12).
Hydrogels (hydro = water) are balanced networks which swell and retain enormous amounts of water or solvent. Their three-dimensional structure is made of polymeric chains which are strongly bonded with the cohesive and dispersive forces and provides excellent shape stability during swelling. These cohesive forces are interrelated with covalent bonding (in chemical gels)/electrostatic forces or dipole–dipole bond formation (physical gels) and gives additional mechanical strength as well as structural integrity to the gels apart from covalent linkage between their polymeric chains (11). Nature of the polymer, polymer–solvent congruous, degree of polymerization/crosslinking, and polymer elasticity are the dependent variables for the overall swelling and uncoiling performance of hydrogels (9,11).
Neutral hydrogels [acrylamide (AAm)-based hydrogels] have gathered great interest in the field of pharmaceuticals and biomedicals as these have not yet been explored much as drug delivery carrier (10,13,14). Ferreira et al. have incorporated acetylsalicylic acid in the polyacrylamide gel and found that crosslinking density has controlled diffusion of drug (15). In another in vivo study, neutral hydrogels (acrylamide based) were found useful for pulsatile delivery of ketoprofen in the stomach of rats (16). These gels are nontoxic, biodegradable, and inert in nature (17). In a study, Hussain et al. have prepared a sustained-release gel containing ibuprofen with varying ratios of AAm and N,N methylene bisacrylamide (MBA). They found that 4% w/w of AAm and 0.5% w/w of MBA would be able to generate transparent thin jelly, and gradual increase in ratio (concentration-dependent) could produce more thick or opaque acrylamide gels (10). The compactness of the gel depends either on the concentration of monomer or crosslinker, or initiator or free divalent cations, and/or combination of all. The dispersed drug gets entrapped within these gel networks (18).
Famotidine was selected as model drug due to its low solubility (aqueous solubility; 0.1% w/v) and low permeability (BCS Class-IV) (19). Famotidine is a widely used third-generation H2-receptor antagonist for the treatment of gastrointestinal ulcers, Zollinger Ellison syndrome, and gastro-esophageal reflux. The stomach is the site for its systemic absorption, and local availability at stomach-wall receptors ameliorates effectively the suppression of acid secretion (20). Its oral administration is incompletely absorbed from gastrointestinal tract with variable oral bioavailability (20–45%). The recommended dose of famotidine is 20 mg twice in a day (21). Thus, sustained release could be a better alternative to enhance its bioavailability. In this study, the sodium alginate was chosen due to its abundant availability and low cost. We have modified sodium alginate by grafting it with polyacrylamide (PAM) to develop a sustained-release gastroretentive hydrogel carrier system for the delivery of famotidine, an anti-ulcer drug.
MATERIALS AND METHOD
Drug (famotidine) was obtained as gift sample from Lupin Research Park (Pune, India). AAm was purchased from Merck Specialities Private Limited (Mumbai, India). MBA and ammonium persulphate (APS) were obtained from SISCO Research Laboratories Pvt. Limited (Mumbai, India). Sodium alginate (NaAlg) was purchased from Central Drug House (New Delhi, India); sodium bi carbonate and calcium chloride were procured from LOBA Chemie Pvt Ltd (Mumbai, India). All other chemicals were of analytical grade and used as obtained.
Synthesis and Development of Sodium Alginate–Polyacrylamide Polymeric Hydrogel Beads (NaAlg-g-PAM)
The graft copolymers have been synthesized by grafting of PAM onto NaAlg by free radical polymerization method in aqueous system using varying concentrations of AAm (1.45× 10−1, 2.89× 10−1, and 4.35 10−1 mol/L), MBA (9.72× 10−3, 19.45 × 10−3, and 29.18 × 10−3 mol/L), and APS (1.09×10−2). NaAlg was placed under constant magnetic stirring (100 rpm) in a beaker containing distilled water (10 ml) at its phase transition temperature 25 ± 2°C (22). Firstly, the required amount of APS was dissolved in an aqueous system. APS undergoes breakdown of –O–O– bond (peroxide bond) and produces hydroxyl ions after reacting with water. Homolytic fission would occur in the APS of peroxide bond (–O–O–) which further provides (SO4)− radical as anion. This anion reacts with water to form hydroxyl radical. These active initiator ions were further utilized for initiation of process of polymerization by pouring it into a beaker containing viscous mucilaginous solution of NaAlg, sodium bicarbonate, AAM, and MBA. Drug (famotidine) was added and mixed thoroughly in this mucilaginous mixture.
Ionotropic Gelation Method
Different grafted NaAlg-g-PAM beads could be obtained simply by dropping a solution of these modified alginates into calcium chloride (Table I). Drug-loaded viscous mucilage was added drop-wise from a distance of 6 in. into a beaker containing hydrochloric acid solution (HCL; 1% v/v) of calcium chloride (CaCl2; 4% w/v), i.e., gelation medium. The gelling solution was kept under constant magnetic stirring (30 rpm) during drop-wise addition of mucilage. The 19 G syringe was used to produce spherical drug-loaded floatable beads of NaAlg-g-PAM hydrogel (Fig. 1a, b). The buoyancy could be achieved due to imbibition of HCL solution within beads, which reacts to sodium bicarbonate and liberates CO2 (23). The gas gets entrapped in the network of beads, allows the system to float, and is useful for stomach targeting. These hydrogel beads were washed thrice with distilled water and kept overnight at 25°C. The prepared crosslinked hydrogels beads were further washed with distilled water to remove uncrosslinked polymer and unreacted substances. These hydrogel beads then dried at 25°C. These dried hydrogels were used to study release characteristics of the drug from drug-loaded floating hydrogel beads and to evaluate its application for floating drug delivery carriers. To obtain appropriate sustained-release hydrogel beads of famotidine, it is necessary to optimize its properties like swelling and gelation parameters.
Table I.
Formula for the Preparation of Polymeric Beads
| S. no. | Ingredients used | ||||
|---|---|---|---|---|---|
| Batch | Drug (mg) | NaAlg (mg) | AAm (mol/L) | MBA (mol/L) | APS (mol/L) |
| FT-0 | 100 | 300 | 1.45× 10−1 | 3.24×10−3 | 1.09 ×10−2 |
| FT-1 | 100 | 300 | 1.45× 10−1 | 19.45 ×10−3 | 1.09 ×10−2 |
| FT-2 | 100 | 300 | 2.89× 10−1 | 9.72 ×10−3 | 1.09 ×10−2 |
| FT-3 | 100 | 300 | 2.89× 10−1 | 19.45 ×10−3 | 1.09 ×10−2 |
| FT-4 | 100 | 300 | 2.89× 10−1 | 29.18 ×10−3 | 1.09 ×10−2 |
| FT-5 | 100 | 300 | 4.35 10−1 | 9.72 ×10−3 | 1.09 ×10−2 |
| FT-6 | 100 | 300 | 4.35 10−1 | 19.45 ×10−3 | 1.09 ×10−2 |
| FT-7 | 100 | 300 | 4.35 10−1 | 29.18 ×10−3 | 1.09 ×10−2 |
Sodium bicarbonate, 100 mg, and water, 10 ml, were used for the preparation of each batch
Characterizations
Fourier Transform Infrared Spectroscopy
The pellets were prepared using KBr press. The Fourier transformed infrared spectroscopy (FTIR) spectra were recorded over the wave number range of 4,000–400 cm−1 using Shimadzu-8400 S (Japan) FTIR equipment.
Thermogravimetric Analysis
The DTA and thermogravimetric analysis (TGA) thermograms of NaAlg and NaAlg-g-PAM were obtained using Lybsys TG-DTA 16 (SETARAM Instrumentation, Caluire, France) thermal analyzer at a heating rate of 20°C/min in nitrogen atmosphere. The TGA thermograms were recorded over a temperature range from ambient to 650°C. The aluminum crucible was used with the capacity of 100 μl. The weight of the samples used for each analysis was 15–20 mg.
Elemental Analysis
Elemental analysis of NaAlg and crosslinked NaAlg-g-PAM were made using a CHN analyzer (Exeter Analytical, Chelmsford, MA), and the percent estimation of nitrogen, carbon, and hydrogen was calculated.
X-ray Diffraction Study
The X-ray powder diffraction (XRD) patterns of pure drug, NaAlg, and NaAlg-g-PAM were recorded on a Bruker, D8 ADVANCE goniometer (Bruker Corporation, Tokyo, Japan). Samples were analyzed between 5° and 60° (2θ). Experimental conditions were as follows—2.2 kW sealed X-ray tube (Cu-Kα), LynxEye ID-PSD (gain of ~200 wrt a single detector); 40 kV and 40 mA; scattering and receiving slit, 4.2° and 0.3 mm, respectively. The scan speed was 2.5°/min, and graphite filter was used during continuous-scan mode.
Differential Scanning Calorimetry
The differential scanning calorimetry (DSC) thermograms were recorded by the use of a DSC 2910-modulated DSC (TA instrument, New Castle, DE, USA). DSC was calibrated with metallic indium (99.9% purity). Drug, NaAlg, and drug-loaded NaAlg-g-PAM beads were tested in crimped aluminum pans at a heating rate of 10°C/min under dry N2 gas (25 ml/min) over a temperature range from 30°C to 200°C. Melting temperature was taken as the peak of the melting endotherm. The error in each measurement was estimated to be ±0.5°C.
Bead Size Measurement
The measurements were carried out using digital Vernier calipers (ABSOLUTE DIGIMATIC, Mitutoyo Corp., Japan) having an accuracy of 0.001 mm. Individually, 20 beads were subjected to size measurement, and the average was calculated.
Determination of Percent Gelation
The hydrogel beads were then dried at 25°C until a constant weight was reached. The gelation occurred due to strong interpenetrating network formation between NaAlg and PAM. Percent gelation of hydrogel beads (NaAlg-g-PAM) was calculated using following equation (5)
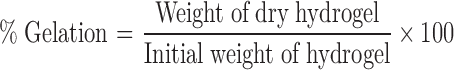 |
Swelling of Beads
Swelling studies of these polymeric beads were carried out in an aqueous medium by gravimetric method. To attain equilibrium swelling, known weights of polymers were immersed in excess of distilled water for 24 h at 37°C temperature, and then these beads were taken out at different time intervals (0.5, 1, 2, 3, 6, 9, 12, 18, and 24 h), wiped with tissue paper to remove excess of water, and weighed immediately. The equilibrium percent swelling (Ps) of the polymeric network was calculated as follows:
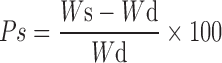 |
Where, Ws and Wd are weights of swollen polymers and dried polymers. Swelling behavior of the polymeric networks prepared with different monomers and crosslinker concentrations were studied as function of time (24).
Drug Loading
An accurately weighed amount of 40 mg of famotidine-loaded NaAlg-g-PAM beads were dissolved in 250 ml (acidic buffer; pH = 1.2) by stirring for 12 h using magnetic stirrer. The resulting solution was then filtered using 0.45 μm syringe filter (Pall Corporation, USA) and diluted suitably. Famotidine content was determined spectrophotometrically at 264 nm using a double-beam UV–visible spectrophotometer (Shimandzu-UV-1800, Japan). Drug loading was calculated by the following equation, and measurement was done in triplicate.
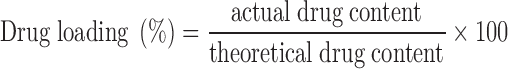 |
Mechanical Strength of Hydrogel Beads
A new modified device was developed in our laboratory as shown in Fig. 2, to measure mechanical strength of beads. This device consists of a hypodermic LUER MOUNT borosilicate glass syringe (PERFEKTUM, Hindustan syringes Pvt Ltd, Faridabad, India) of 5 ml. From the delivery end, needle hub was removed precisely from the unit. The thumb rest was opened from the upper end to make the plunger hollow. Gradually predetermined weights of mercury (35, 60, 80, and 100 g) were poured within this hollow plunger. Weight of mercury was enough to produce force to move the plunger within syringe, so the beads would get pressed down. This modified syringe device was placed vertically on a sieve (#40 mesh) as shown in Fig. 2. Twenty wet beads were taken within the syringe and pressed down under the weight exerted by plunger containing mercury. Pre- and post-diametrical changes of beads were recorded through digital Vernier calipers. Gradually, weights were increased by pouring additional mercury in the plunger, and diametrical changes were used for calculation of mechanical strength of beads.
Fig. 2.
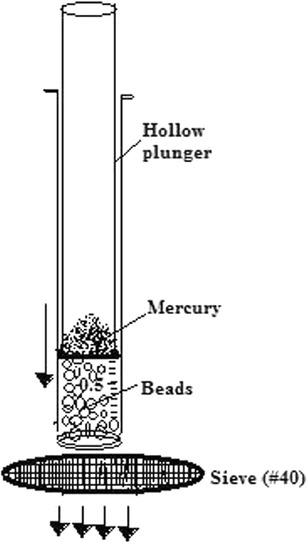
Schematic representation of in-house-developed modified syringe method for the measurement of mechanical strength
In Vitro Drug Release
The in vitro drug release studies (in triplicate) were carried out in the media (buffer pH1.2, gastric pH). The drug-loaded beads (equivalent to 40 mg famotidine) were placed into dissolution vessel filled with 900 ml of release medium incubated at 37°C and stirred at 50 rpm (Electrolab-TDT 06P, Mumbai, India). At appropriate intervals, 5 ml of the aliquot was taken and replaced by fresh medium. The samples were assayed spectrophotometrically at 264 nm. The drug released amount was calculated from calibration curve and represented cumulative percentage drug release versus time (25).
Mathematical Modeling of In Vitro Release Kinetics
Pharmacokinetic models were assessed by fitting in vitro release data into different mathematical models to ascertain drug release mechanism involved from the hydrogels beads. Zero-order release kinetics, first-order release kinetics, and Higuchi and Korsmeyer–Peppas kinetics were employed by using following set of equations.
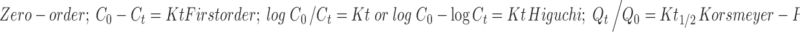 |
Where C0 is the initial concentration of drug (that is to be released outside), Ct is concentration at time t present in the solution (that is released outside the hydrogels), K is the rate constant, t is time, Qt/Q0 is the fraction of drug released, and n is release exponent. The value of n is used to indicate different release mechanisms involved in Korsmeyer–Peppas model. Value of n = 0.5 indicates Fickian (case I) release, >0.5, but < 0.89 for non-Fickian (anomalous) release, n ≈ 1 indicates case-II (zero order) transport, and >1 indicates super case II type of release (23).
RESULTS AND DISCUSSION
FTIR Spectra
FTIR spectrum of NaAlg, NaAlg-g-PAM, drug-loaded NaAlg-g-PAM, and drug were carried out (Fig. 3) to find out changes in peak or peak shifting that could reveal bonding or interaction between atoms. FTIR stretches were observed at 3,435 and 3,404 cm−1 in NaAlg, NaAlg-g-PAM, and drug-loaded NaAlg-g-PAM correspond to presence of –O–H stretching vibration of the hydroxyl group in the alginate backbone. More broadening of 3,435 cm−1 stretch clearly states intermolecular hydrogen bond formation within NaAlg-g-PAM and drug-loaded NaAlg-g-PAM. Usually, hydrogen bonding occurred in between proton donor and proton acceptor atoms. The lone pair of electrons at –O– of PAM could interact with –H atom of NaAlg and formed intermolecular hydrogen bond. Asymmetrical stretching vibration at 1,600 cm−1 in NaAlg showed presence of –COO group. The characteristic band at 1,647 cm−1 was due to amide group (–CONH2) of PAM (>C=O stretching) (26). The bands at 2,931 and 2,918 cm−1 were due to –C–H stretching of –CH2 group of PAM. Conversion of 1,094 and 1,030 cm−1 stretching band of NaAlg into a singlet 1,041 cm−1 absorption stretch in NaAlg-g-PAM denotes the formation of secondary alcohol (characteristic peak of –CH–OH in cyclic alcohol C–O stretch) during the process of grafting. This shift towards lower frequency suggests weakening of –C–C– and –C–O– bonds (5,27). Stretching vibration adsorption at 1,456 cm−1 for the C–N group can be observed in the FTIR spectrum of drug-loaded NaAlg-g-PAM, which did not appear in the spectrum of NaAlg (28). The intermolecular hydrogen bonding provides an additional mechanical strength to the grafted polymer. These differences supported the successful grafting of acrylamide onto sodium alginate.
Fig. 3.
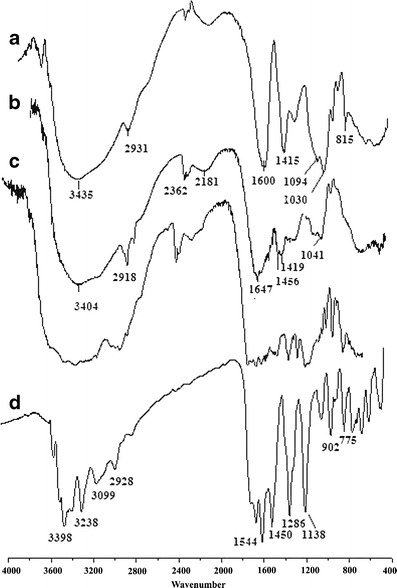
FTIR graph: a NaAlg, b NaAlg-PAM, and c drug-loaded NaAlg-g-PAM hydrogel beads and d drug
Thermal Analysis
During the process of grafting, initiator generates free radical sites on NaAlg and AAm. These reactive sites were crosslinked together due to presence of multifunctional property of MBA. Thus, crosslinkage forms three-dimensional networks termed as sodium alginate–grafted-polyacrylamide (NaAlg-g-PAM) hydrogel network at its phase transition temperature. The thermograms of NaAlg and grafted NaAlg-g-PAM are shown in Fig. 4a, b. The TGA-DTA graph of NaAlg shows sharp distinct peaks for NaAlg at temperatures 241.98°C, 351.99°C, 512.83°C, and 576.65°C. NaAlg is a polysaccharide and contains sugar units, galactose and mannose. The moisture loss occurred below 100°C. The major degradation of NaAlg occurring at 241.98°C (weight loss of 42.4%) and subsequently at 351.99°C (weight loss of 18.75%) are due to loss of volatile components, rupture of chain, and fragmentation of sodium alginate. Decomposition of NaAlg occurring at 512.83°C with the weight loss of 19.4% depicts fragmentation of NaAlg into monomers (sugar unit), followed by its conversion into carbonate as a byproduct (29).
Fig. 4.
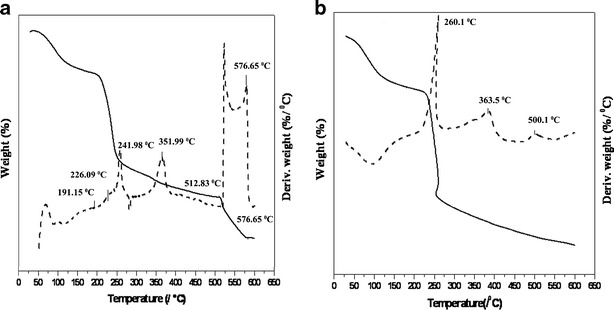
DTA-TGA thermogram: a NaAlg and b NaAlg-g-PAM hydrogel (solid line TGA, broken line DTA)
The NaAlg-g-PAM thermogram shows weight loss at between 50–200.14°C and 200.14–254.5°C were 22.6% and 49.25% due to moisture loss, dissociation of chemical crosslinkage, and bonding between PAM with NaAlg. When comparing both DTA graphs (Fig. 4a, b), peaks of sodium alginate at 241.98°C and 351.99°C were shifted to 260.2°C and 383.5°C, respectively, indicating transformation which further provided thermal stability to NaAlg. Absence of any other peaks in DTA graph (Fig. 4b) revealed that the PAM was also able to protect sugar moieties of NaAlg from further degradation. Furthermore, PAM is a polymer of chemical origin produced by the reaction of monomer, crosslinker, and intitiator whereas NaAlg is a natural biocompatible polymer of marine origin (30). Hydroxyl group of NaAlg could form hydrogen bonding with PAM. Thermal analysis data are presented in Table II; it has been observed that the mechanisms of decomposition in both NaAlg and NaAlg-g-PAM were different, respectively (31).
Table II.
Thermal Analysis of NaAlg and NaAlg-g-PAM
| Compound | Process | ∆T/°C | %Wt loss | Inference |
|---|---|---|---|---|
| NaAlg | Dehydration | 82.58–191.15 | 14.5 | Moisture loss |
| Decomposition | 191.15–254.68 | 42.41 | Chain fragmentation | |
| 254.68–512.83 | 18.75 | |||
| Carbonate formation | 512.83–576.65 | 19.4 | Na2CO3 formation | |
| NaAlg-g-PAM | Dehydration | 50.07–200.14 | 22.6 | Moisture loss |
| Decomposition | 200.14–254.5 | 49.25 | Dissociation and rupture of bonds |
Elemental Analysis
The elemental analysis data are presented in the Table III. The NaAlg holds lesser amount of nitrogen at 6.98% due to presence of more sugar (polysaccharide moieties). Presence of –CONH2 in NaAlg-g-PAM proves crosslinking of PAM with NaAlg (26). However, grafted NaAlg-g-PAM contains 16.58% of nitrogen, revealing conjugation of –CONH2 group of acrylamide with NaAlg during polymerization process. These results were also supported by FTIR, thermal analysis, and percent gelation. The percent of carbon of NaAlg and NaAlg-g-PAM was found to be 24.55% and 24.4%, respectively, with no significant change in carbon percentage. However, a slight increment in the hydrogen content from 7.58% to 8.07% in NaAlg-g-PAM has proven that structural integrity of NaAlg was not impaired during the formation of NaAlg-g-PAM.
Table III.
Elemental Analysis Data
| Sample | % Nitrogen | % Carbon | % Hydrogen | % Elements remaining |
|---|---|---|---|---|
| NaAlg | 6.98 | 24.55 | 7.58 | 60.88 |
| NaAlg-g-PAM | 16.58 | 24.4 | 8.07 | 50.95 |
XRD Spectra
XRD spectra shown in Fig. 5 exhibit the characteristic peaks of drug, NaAlg, and drug-loaded NaAlg-g-PAM hydrogel beads. Famotidine is crystalline in nature; however, its crystalline nature gets masked within the NaAlg-g-PAM beads as these hydrogel beads of NaAlg-g-PAM show amorphous pattern of XRD spectra. It revealed that crystalline drug molecules get entrapped within the pockets of hydrogel networks of NaAlg-g-PAM. There was also a decrease in crystallinity of pristine sodium alginate due to the formation of new polymer carrier system. XRD of NaAlg exhibiting characteristic peaks at 19°, 28°, 28.9°, 32.5°, and 33.5° 2θ while, in the case of NaAlg-g-PAM beads, no such sharp peaks were observed, suggesting complete transformation of NaAlg into new polymer. The FTIR spectrum also supported the grafting of PAM onto NaAlg within the drug-loaded beads. Addition of more bulky groups, i.e., PAM onto the backbone of NaAlg could reduce the overall crystallinity and also decrease the intermolecular hydrogen bonding (27).
Fig. 5.
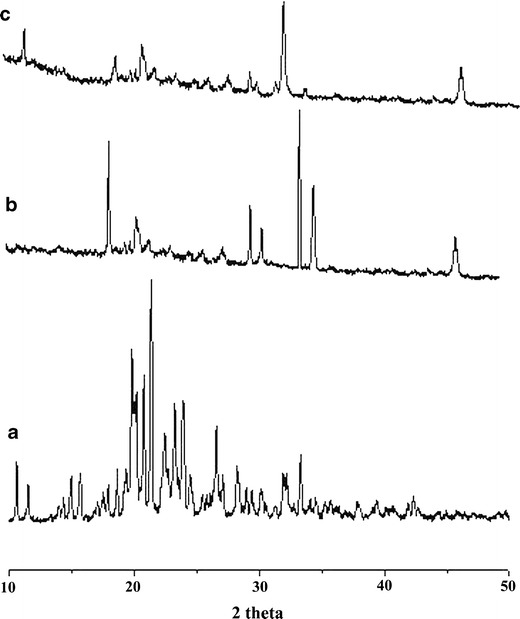
XRD graph: a drug, b NaAlg, and c drug-loaded NaAlg-g-PAM hydrogel beads
DSC Analysis
In the DSC graph (Fig. 6), sharp melting endotherm of drug was observed at 167°C (32). The graph of pristine sodium alginate exhibited a broad melting endotherm at 136.9°C. Whereas drug-loaded NaAlg-g-PAM has shown multiple endotherm peaks at 85°C, 185°C, and 339°C. In the NaAlg-g-PAM, there was shifting of peak of NaAlg from 136.9°C to 185°C and considerable thermal stability of NaAlg-g-PAM. This shifting might suggest addition of bulky groups as side chain and presence of amide bonds on NaAlg of NaAlg-g-PAM, which imparts rigidity to the NaAlg-g-PAM network (33). The appearance of melting endotherm at 339°C was uncertain. Thus, DSC supports the successful grafting of PAM onto NaAlg which could provide sufficient mechanical strength to the grafted polymer. But disappearance of drug peak in NaAlg-g-PAM grafted beads was due to entrapment of drug moiety in the grafted hydrogel beads.
Fig. 6.
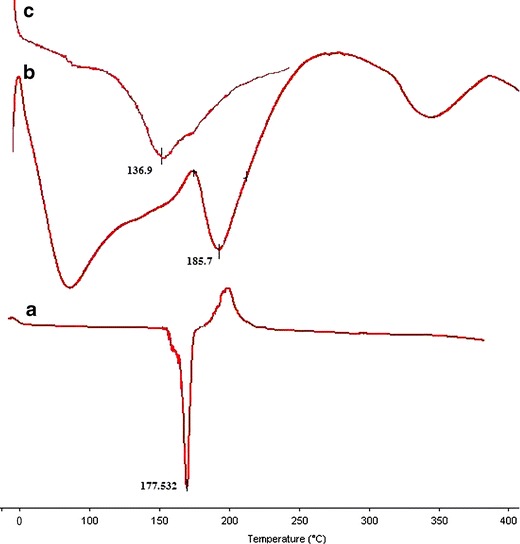
DSC thermograms: a drug, b NaAlg, and c drug-loaded NaAlg-g-PAM hydrogel beads
Percentage Gelation
The percentage gelation was increased with the gradual increase in the monomer and crosslinker concentrations. It could be seen from the Table IV that the percent gelation was found to be greater with the increase of monomer concentration. Batches FT-0 and FT-I showed that negligible polymerization occurred with liquid and 9% gelation product at the end of reaction, respectively. However, increase in monomer concentration supports the enhancement of percentage gelation. This was due to availability of more monomer, and crosslinker could promote more gel formation as in the batches FT-4 and FT-7; the percent gelation was found to be 86% and 95.6%, respectively. This indicates more polymerization and crosslinking has happened in beads of batch FT-4 and FT-7. The availability of monomer or crosslinker could affect the network formation (Fig. 1b). If lesser amounts of monomers and crosslinkers were present in the reaction mixture, less viscous gel formation occurred due to formation of weak and thin crosslinked polymeric strands whereas, at higher amount of monomer and crosslinker, gel became more viscous and thick in nature, attributed to the presence of thick and stronger crosslinked polymeric strands present within these networks. Stronger and thick polymeric strands might provide tight and strong bonding or crosslinking together with NaAlg.
Table IV.
Percent Gelation and Viscosity of Different Batches
| Batch | % Gelation | Viscosity |
|---|---|---|
| FT-0 | 9.01 ± 1.4 | + |
| FT-1 | 51.09 ± 4.3 | ++ |
| FT-2 | 52.61 ± 6.3 | ++ |
| FT-3 | 77.05 ± 4.6 | +++ |
| FT-4 | 86 ± 5 | ++++ |
| FT-5 | 75.2 ± 5.2 | +++ |
| FT-6 | 82.5 ± 6.6 | ++++ |
| FT-7 | 95.6 ± 4.5 | +++++ |
+ very poor; ++ poor; +++ moderate; ++++ good; +++++ excellent
Visually, it has been seen that viscosity of the gel network was also more in FT-4, FT-5, FT-6, and FT-7 as more polymerization reactions took place (Table IV). More crosslinking encountered lesser movement of polymeric strands due to rigid bond formation at the junction of PAM and NaAlg. Addition of diavalent cations (Ca2+) crosslinked the guluronic acid part of NaAlg more firmly and also restricts the movements of free NaAlg as well as NaAlg-g-PAM strands (Fig. 1b).
Effect of pH on Swelling
Swelling as function of AAm: Swelling of beads decreased with the increase in the monomer concentration within the beads (Fig. 7a). This could happen due to the polymerization and enhancement in the chain length. The excess of monomer present in the reaction mixture may propagate and produce long chains during polymerization and thus reduces the swelling capability. More monomer concentration in the reaction mixture could form more compact and dense network.
Swelling as function of MBA: The crosslinker plays crucial role in the polymerization reaction steps. They act as bridge between two or more monomers. The initial gel formation took place when pre-specified quantities of monomer (AAm), crosslinker (MBA), and initiator (APS) were mixed at controlled set of condition. As the gel formation occurred, more and more polymerization could strengthen the network of hydrogel. Swelling could get decreased with the addition of crosslinker in the reaction mixture (Fig. 7b).
Fig. 7.
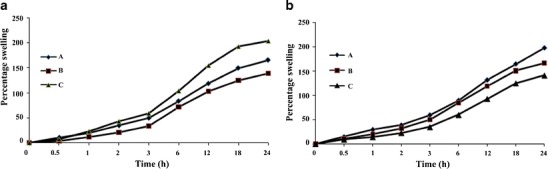
a Effect of concentration of AAm, A 1.45× 10−1, B 2.89× 10−1, and C 4.35 10−1 mol/L and b effect of concentration of MBA, A 9.72 ×10−3, B 19.45 ×10−3, and C 29.18 ×10−3 mol/L in the beads on swelling kinetics of NaAlg-g-PAM
Drug Loading
The grafting also affects the drug loading. Figure 8 shows percent drug loading in different batches of floating beads. The batch FT-I was in liquid state, and no gelation took place, thus determination of drug loading was difficult. More crosslinking provides better drug loading. Improvement in percent drug loading in batches FT-4 and FT-7 to the extent of 68.4% and 75%, respectively, revealed that the polymerization and crosslinking favored the entrapment of drug moiety within gel network. However, the bead diameters get reduced after drying (initially, the wet diameter of beads were almost same in all the batches prepared). The more crosslinking would strengthen the network and entrap more drug within gel network (Fig. 1b). Furthermore, due to more crosslinkage, the strong and thick strands of NaAlg-g-PAM could control the release of drug purely by slow uncoiling of polymer within the medium.
Fig. 8.
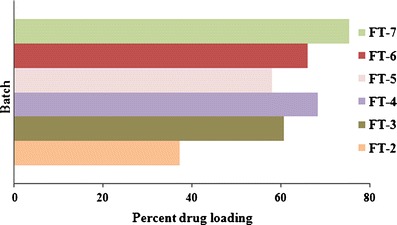
Percent drug loading in different batches
Mechanical Strength
The gel formation affects the swelling as well as mechanical property of different hydrogel beads. The increased concentration of monomer and crosslinker in the reaction solution increases the contacts between macromolecules, resulting in tighter gel networks with shorter segment lengths between cross-linkage. The stiffness of network gets increased with the increase of cross-linkage between polymer networks (Fig. 1b) (30,34,35). The graphs were presented in the Fig. 9a, b reveals the investigation about the mechanical strength of beads. Steep decrease in diameter of beads with the gradual increase in the concentration of AAm and MBA would have given the strength to the polymeric network (FT-1 > FT-3 > FT6: AAm and FT-5 > FT-6 > FT-7: MBA). The increase of mercury weight from 35 to 100 g compressed the beads gradually. The diametric changes with respect to weight applied were used to represent mechanical strength of network formed inside. It has been found that more complex polymeric network may resist more prominently at weight 60 g or more. This could reveal that the polymer chain/strands formed in the batches FT-4, FT-6, and FT-7 were more crosslinked compared with other batches. Occurrence of more polymerization was attributed to formation of tougher and complex networks. Thus, it was hypothesized that incorporation of drug moiety within these polymeric carriers is able to deliver drug in a sustained manner (35).
Fig. 9.
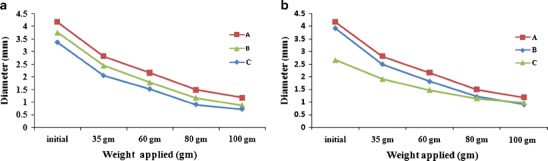
a Effect of concentration AAm, A 1.45× 10−1, B 2.89× 10−1, C 4.35 10−1 mol/L and b effect of concentration of MBA, A 9.72 ×10−3, B 19.45 ×10−3, C 29.18 ×10−3 mol/L on the mechanical strength of NaAlg-g-PAM beads
In Vitro Drug Release and In Vitro Buoyancy Study
The drug release through hydrogel formed by graft polymerization has followed sustained-release pattern. The pure drug dissolved in 10 min; however, the drug released from NaAlg-g-PAM hydrogel beads lasted till 18 h under acidic release media (pH 1.2). The drug molecules get entrapped within the gel matrix during polymerization in the highly crosslinked networks. Theoretically, mechanism consist of three different zones of hydration and uncoiling of hydrogel network in the bead; a water saturated swollen gel layer made of relaxed and hydrated chains, a thin layer into which the strands of polymeric network slowly uncoil due to constant solvent imbibition or penetration, and lastly, the third zone which consisted of dried, hard, and unswollen polymeric network remained uncoiled (36–39). In vitro release profiles shown in Fig. 10 clearly demonstrated that increase of monomer concentrations from 1.45 × 10−1, 2.89 × 10−1, and 4.35 × 10−1 mol/L, each having constant amount of crosslinker (19.45 × 10−3 mol/L), was able to sustain the release of drug till 3, 12, and 15 h, respectively. The leaching of drug from the polymeric network took place when water penetrated the beads and uncoiled their polymeric strands. In vitro release data of different hydrogel batches were fitted to various mathematical models to ascertain the kinetics of drug release and drug release exponent (n). Curve fitting into different set of equations suggested that drug release from all the batches of hydrogels is best suited to Korsmeyer–Peppas model, and values are presented in Table V. The release exponent for FT-4, FT-5, and FT-6 batches were found to be 0.28, 0.285, and 0.325 for Korsmeyer–Peppas model whereas highest value of release exponent was found to be 0.345, with higher sum of correlation coefficient (R2 = 0.995) for FT-7 batch. Thus, Korsmeyer–Peppas model not only gives insight of the exact drug release mechanism from the polymeric hydrogels beads; the values of the exponent (n) would indicate the extent of diffusion-controlled release mechanism. Relaxation of polymer and drug transport mechanism was associated to stresses and state-transition of grafted polymers which swells; thus dissolution followed by diffusion takes place through aqueous pathway. The swelling phenomenon is the dependent variable for the release of drug through hydrogel. NaAlg has enormous swelling capabilities, and crosslinking with polyacrylamide ameliorates its swelling ability maximally (9).
Fig. 10.
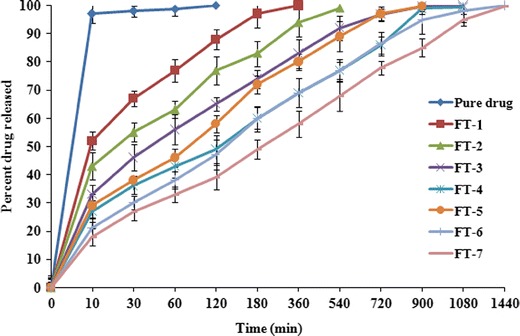
In vitro release profiles of different batches of NaAlg-g-PAM floating beads
Table V.
Fitting In Vitro Drug Release Data of Different Batches According to Various Mathematical Models
| Models | Zero-order | First-order | Higuchi | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| Batches | R 2 | K o | R 2 | K F | R 2 | K H | n | R 2 | K KP |
| FT-1 | 0.503 | 0.192 | 0.988 | 0.007 | 0.777 | 0.366 | 0.212 | 0.971 | −0.115 |
| FT-2 | 0.602 | 0.129 | 0.975 | 0.003 | 0.837 | 0.288 | 0.223 | 0.966 | −0.192 |
| FT-3 | 0.676 | 0.067 | 0.984 | 0.002 | 0.87 | 0.203 | 0.236 | 0.988 | −0.268 |
| FT-4 | 0.828 | 0.073 | 0.893 | 0.001 | 0.954 | 0.208 | 0.28 | 0.991 | −0.364 |
| FT-5 | 0.756 | 0.087 | 0.913 | 0.002 | 0.928 | 0.235 | 0.285 | 0.991 | −0.320 |
| FT-6 | 0.794 | 0.07 | 0.924 | 0.001 | 0.952 | 0.198 | 0.325 | 0.994 | −0.417 |
| FT-7 | 0.873 | 0.061 | 0.853 | 0.001 | 0.982 | 0.194 | 0.345 | 0.995 | −0.481 |
Drug release exponents (n), Korsmeyer–Peppas release constant (K KP), correlation coefficient (R 2), zero-order release rate constants (K 0), first-order release rate constant (K F) of different models
Drug release from batches FT-5, FT-6, and FT-7 (varying crosslinker concentrations, 9.72 × 10−3, 19.45 × 10−3, and 29.18 × 10−3 mol/L), each having constant amount of monomer (4.35 × 10−1 mol/L), revealed that with more crosslinkage, more grafting resulted, and formation of rigid gel networks retarded the drug release rate. Eighty percent drug was released from FT-5, FT-6, and FT-7 in the sixth, tenth, and twelfth hour, respectively. The drug release data and percentage swelling supported that crosslinking/grafting produced rigid and stiffer gel networks which further retarded the movement of drug molecule at a very early hour. Thus, co-grafting has imparted excellent physico-chemical properties to the hydrogel system to attain more sustained pattern release of drugs. It has been reported that the uncoiling of grafted chains depends on the pH of release media. It has also been well reported that more chain relaxation took place in the basic environment compared with acidic conditions (36,40). All the batches (except FT-0) have exhibited buoyancy up to 24 h in the in vitro release media during drug release study (Fig. 10). To bring these hydrogel beads floatable within gastric medium, floating mechanism has been employed. The sodium bicarbonate has been added within these beads so that the beads could remain buoyant within the stomach due to entrapment of CO2 gas in the polymeric network. CO2 gas is generated due to chemical reaction between sodium bicarbonate and the acidic media. Hydration of gel network simultaneously helps to entrap the gas and makes the system buoyant (23).
CONCLUSION
Hydrogel beads of NaAlg-g-PAM graft–co-polymer were prepared by ionotropic gelation method to produce sustained delivery of famotidine for the treatment of gastric ulcer. In vitro release studies suggest that cografting of NaAlg with PAM could be a useful tool for the sustaining the delivery of drugs within the stomach. FTIR, elemental analysis, and DTA-TGA has suggested the successful grafting (basically attachment of amide bond of polyacrylamide with caoboxyl group of NaAlg) of PAM on NaAlg. DSC and XRD data reveal the dispersion and amorphization of crystalline drug within the matrix of NaAlg-g-PAM beads. The in vitro drug release confirmed the release up to the 15 h, whereas beads were able to float up to 24 h. The increase of percentage grafting could reduce the release rate of drug; however, it increases the drug loading within the NaAlg-g-PAM grafted gastroretentive beads. The swelling of grafted NaAlg-g-PAM gel network in the beads are strongly interdependent on the crosslinking and polymerization process. The drug release depends upon the extent of gelation which was based on cross-linking and amount of graft copolymer used. This study could prove to be a platform to explore the possibility of grafting technology for the preparation of gastroretentive or hydrogel-based drug delivery system.
Acknowledgments
The first author is thankful to ICMR, New Delhi, for providing financial assistance in the form of SRF. Authors are thankful to IUC-UGC Consortium, Indore, for XRD and DSC analyses. We are also thankful to Department of Ceramic Engineering, IT-BHU, for thermal analysis.
Conflict of interest
There is no conflict of interest among authors. The manuscript has been prepared under the funding provided by ICMR, New Delhi, India.
References
- 1.Yang JS, Xie YJ, He W. Research progress on chemical modification of alginate: a review. Carbohyd Polym. 2011;84(1):33–39. doi: 10.1016/j.carbpol.2010.11.048. [DOI] [Google Scholar]
- 2.Liu J, Lin S, Li L, Liu E. Release of theophylline from polymer blend hydrogels. Int J Pharm. 2005;298(1):117–125. doi: 10.1016/j.ijpharm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Athawale VD, Rathi SC. Graft polymerization: starch as a model substrate. J Macromol Sci Polym Rev. 1999;39(3):445–480. doi: 10.1081/MC-100101424. [DOI] [Google Scholar]
- 4.Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58(12–13):1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Şolpana DL, Torun M. (Sodium Alginate/Acrylamide) semi-interpenetrating polymer networks and their usability on removal of lead, cadmium, nickel ions. J Macromol Sci A. 2005;42(10):1435–1449. [Google Scholar]
- 6.Mashak A. In vitro drug release from silicone rubber–polyacrylamide composite. Silicon Chem. 2008;3(6):295–301. doi: 10.1007/s11201-007-9031-1. [DOI] [Google Scholar]
- 7.Soppirnath KS, Aminabhavi TM. Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur J Pharma Biopharm. 2002;53(1):87–98. doi: 10.1016/S0939-6411(01)00205-3. [DOI] [PubMed] [Google Scholar]
- 8.Blanco MD, Garcia O, Trigo RM, Teijon JM, Katime I. 5-Fluorouracil release from copolymeric hydrogels of itaconic acid monoester. Biomaterials. 1996;17(11):1061–1067. doi: 10.1016/0142-9612(96)85906-0. [DOI] [PubMed] [Google Scholar]
- 9.Singh B, Chauhan GS, Sharma DK, Kant A, Gupta I, Chauhan N. The release dynamics of model drugs from the psyllium and N-hydroxymethylacrylamide based hydrogels. Int J Pharm. 2006;325(1–2):15–25. doi: 10.1016/j.ijpharm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Hussain MD, Rogers JA, Mehvar R, Vudathala GK. Preparation and release of ibuprofen from polyacrylamide gels. Drug Dev Ind Pharm. 1999;25(3):265–271. doi: 10.1081/DDC-100102170. [DOI] [PubMed] [Google Scholar]
- 11.Becerra-Bracamontes F, Sánchez-Diaz JC, González-Aĺvarez A, Ortega-Gudinõ P, Michel-Valdivia E, Martinnez-Ruvalcaba A. Design of a drug delivery system based on poly(acrylamide-co-acrylic acid)/chitosan nanostructured hydrogels. J Appl Polym Sci. 2007;106(6):3939–3944. doi: 10.1002/app.26992. [DOI] [Google Scholar]
- 12.Shin HS, Kim SY, Lee YM. Indomethacin release behaviors from pH and thermoresponsive poly(vinyl alcohol) and poly(acrylic acid) IPN hydrogels for site-specific drug delivery. J Appl Polym Sci. 1997;65(4):685–693. doi: 10.1002/(SICI)1097-4628(19970725)65:4<685::AID-APP7>3.0.CO;2-G. [DOI] [Google Scholar]
- 13.Satish CS, Satish KP, Shivakumar HG. Hydrogels as controlled drug delivery systems: synthesis, crosslinking, water and drug transport mechanism. Indian J Pharm Sci. 2006;68(2):133–140. doi: 10.4103/0250-474X.25706. [DOI] [Google Scholar]
- 14.Amine S, Rajabnezhad S, Kohli K. Hydrogels as potential drug delivery systems. Sci Res Assay. 2009;3(11):1175–1183. [Google Scholar]
- 15.Ferreira L, Vidal MM, Gil MH. Design of a drug delivery system based on polyacryalamide hydrogels: evaluation of structural properties. Chem Educ. 2001;6(2):100–103. doi: 10.1007/s00897010461a. [DOI] [Google Scholar]
- 16.Kulkarni RV, Sa B. Polyacrylamide-grafted-alginate-based pH-sensitive hydrogel beads for delivery of ketoprofen to the intestine: in vitro and in vivo evaluation. J Biomat Sci-Polym E. 2009;20(2):235–252. doi: 10.1163/156856209X404514. [DOI] [PubMed] [Google Scholar]
- 17.Boulard C, Lecroisey A. Rabbits were injected with slices of polyacrylamide gels containing entrapped insect proteins after separation by electrophoresis. J Immunol Methods. 1982;50(2):221–226. doi: 10.1016/0022-1759(82)90228-9. [DOI] [PubMed] [Google Scholar]
- 18.Omidian H, Rocca JG, Park K. Elastic, superporous hydrogel hybrids of polyacrylamide and sodium alginate. Macromol Biosci. 2006;6(9):703–710. doi: 10.1002/mabi.200600062. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda H, Mutsumoto S, Kaguragi K, Kurihara K, Tochigi K, Tomono K. Determination and correlation of solubilities of famotidine in water = co-solvent mixed solvents. Fluid Phase Equilib. 2011;302(1–2):115–122. doi: 10.1016/j.fluid.2010.09.029. [DOI] [Google Scholar]
- 20.Coffin MD, Parr AF. Ranitidine solid dosage form. US Patent. 1995;5:407–687. [Google Scholar]
- 21.Scarpignato C. New drugs to suppress acid secretion: current and future developments. Drug Discov Today Ther Strateg. 2007;4(3):155–163. doi: 10.1016/j.ddstr.2007.09.003. [DOI] [Google Scholar]
- 22.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58(15):1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Khan ZA, Tripathi R, Mishra B. Floating elementary osmotic pump tablet (FEOPT) for controlled delivery of diethylcarbamazine citrate: a water-soluble drug. AAPS PharmSciTech. 2011;12(4):1312–1323. doi: 10.1208/s12249-011-9699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh B, Bala R, Chauhan N. In vitro release dynamics of model drugs from psyllium and acrylic acid based hydrogels for the use in colon specific drug delivery. J Mater Sci-Mater M. 2008;19(8):2771–2780. doi: 10.1007/s10856-008-3406-5. [DOI] [PubMed] [Google Scholar]
- 25.Elmowafy EM, Awad GAS, Mansour S, El-Shamy AEA. Ionotropically emulsion gelled polysaccharides beads: preparation, in vitro and in vivo evaluation. Carbohyd Polym. 2009;75:135–142. doi: 10.1016/j.carbpol.2008.07.019. [DOI] [Google Scholar]
- 26.Soppimath KS, Kulkarni AR, Aminabhavi TM. Chemically modified polyacrylamide-g-guar gum-based crosslinked anionic microgels as pH-sensitive drug delivery systems: preparation and characterization. J Control Release. 2001;75(3):331–345. doi: 10.1016/S0168-3659(01)00404-7. [DOI] [PubMed] [Google Scholar]
- 27.Sutar PB, Mishra RK, Pal K, Banthia AK. Development of pH sensitive polyacrylamide grafted pectin hydrogel for controlled drug delivery system. J Mater Sci-Mater M. 2008;19(6):2247–2253. doi: 10.1007/s10856-007-3162-y. [DOI] [PubMed] [Google Scholar]
- 28.Xu K, Xu X, Ding Z, Zhou M. Synthesis and flocculability of sodium alginate grafted with acrylamide. China Particuology. 2006;4(2):60–64. doi: 10.1016/S1672-2515(07)60235-8. [DOI] [Google Scholar]
- 29.Parhi P, Ramanan A, Ray AR. Preparation and characterization of alginate and hydroxyapatite-based biocomposite. J Appl Polym Sci. 2006;102(6):5162–5165. doi: 10.1002/app.24706. [DOI] [Google Scholar]
- 30.Pourjavadi A, Samadi M, Ghasemzadeh H. Temperature sensitive superabsorbent hydrogels from poly(N-t-butyl acrylamide-co-acrylamide) grafted on sodium alginate. Macromol Symp. 2008;274(1):177–183. doi: 10.1002/masy.200851424. [DOI] [Google Scholar]
- 31.Swamy TMM, Ramaraj B, Siddaramaiah Sodium alginate and poly(ethylene glycol) blends: thermal and morphological behaviors. J Macromol Sci A. 2010;47(9):877–881. [Google Scholar]
- 32.Cheng WT, Lin SY. Famotidine polymorphic transformation in the grinding process significantly depends on environmental humidity or water content. Int J Pharm. 2008;357(1–2):164–168. doi: 10.1016/j.ijpharm.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 33.Sanli O, Ay N, Isiklan N. Release characteristics of diclofenac sodium from poly(vinyl alcohol)/sodium alginate and poly(vinyl alcohol)-grafted-poly(acrylamide)/sodium alginate blend beads. Eur J Pharm Biopharm. 2007;65(2):204–214. doi: 10.1016/j.ejpb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Trabbic-Carlson K, Setton LA, Chilkoti A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules. 2003;4(3):572–580. doi: 10.1021/bm025671z. [DOI] [PubMed] [Google Scholar]
- 35.Tang Q, Sun X, Li Q, Wu J, Lin J. Fabrication of a high-strength hydrogel with an interpenetrating network structure. Colloid Surf A. 2009;346(1–3):91–98. doi: 10.1016/j.colsurfa.2009.05.031. [DOI] [Google Scholar]
- 36.Kulkarni RV, Sa B. Evaluation of pH-sensitivity and drug release characteristics of (polyacrylamide-grafted-xanthan)-carboxymethyl cellulose-based pH-sensitive interpenetrating network hydrogel beads. Drug Dev Ind Pharm. 2008;34(12):1406–1414. doi: 10.1080/03639040802130079. [DOI] [PubMed] [Google Scholar]
- 37.Singh B, Sharma N. Modification of sterculia gum with methacrylic acid to prepare a novel drug delivery system. Int J Biol Macromol. 2008;43(2):142–150. doi: 10.1016/j.ijbiomac.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Sen G, Pal S. Microwave initiated synthesis of polyacrylamide grafted carboxymethylstarch (CMS-g-PAM): application as a novel matrix for sustained drug release. Int J Biol Macromol. 2009;45(1):48–55. doi: 10.1016/j.ijbiomac.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Alfrey T, Gurnee EF, Lloyd WG. Diffusion in glassy polymers. J Polym Sci C. 1966;12:249–261. doi: 10.1002/polc.5070120119. [DOI] [Google Scholar]
- 40.Leonard M, Rastello De Boisseson M, Hubert P, Dalençon F, Dellacherie E. Hydrophobically modified alginate hydrogels as protein carriers with specific controlled release properties. J Control Release. 2004;396(98):395–405. doi: 10.1016/j.jconrel.2004.05.009. [DOI] [PubMed] [Google Scholar]


