Abstract
Sustained-release matrix tablets based on Eudragit RL and RS were manufactured by injection moulding. The influence of process temperature; matrix composition; drug load, plasticizer level; and salt form of metoprolol: tartrate (MPT), fumarate (MPF) and succinate (MPS) on ease of processing and drug release were evaluated. Formulations composed of 70/30% Eudragit RL/MPT showed the fastest drug release, substituting part of Eudragit RL by RS resulted in slower drug release, all following first-order release kinetics. Drug load only affected drug release of matrices composed of Eudragit RS: a higher MPT concentration yielded faster release rates. Adding triethyl citrate enhanced the processability, but was detrimental to long-term stability. The process temperature and plasticizer level had no effect on drug release, whereas metoprolol salt form significantly influenced release properties. The moulded tablets had a low porosity and a smooth surface morphology. A plasticizing effect of MPT, MPS and MPF on Eudragit RS and Eudragit RL was observed via DSC and DMA. Solubility parameter assessment, thermal analysis and X-ray diffraction demonstrated the formation of a solid solution immediately after production, in which H-bonds were formed between metoprolol and Eudragit as evidenced by near-infrared spectroscopy. However, high drug loadings of MPS and MPF showed a tendency to recrystallise during storage. The in vivo performance of injection-moulded tablets was strongly dependent upon drug loading.
KEY WORDS: acrylates, controlled release, drug polymer interaction, drug release, injection moulding, matrix, metoprolol, physicochemical properties, solid state, sustained release, tablet
INTRODUCTION
Injection moulding (IM) originated from, and has been widely used, within the plastic processing industry, but has only recently been proposed as a novel drug delivery technology. IM is capable of mass-producing intricate polymeric parts with the aid of heat and pressure, in a wide variety of shapes with high dimensional precision. During the process, polymeric materials, functional excipients and active agents are gradually mixed and heated, and transferred by means of an injection step into a shape-specific mould. After sufficient cooling, the mould can be opened to recover the formed part.
Although IM has been routinely used within the plastics processing industry, it has been largely ignored as a drug delivery technology. Given the large number of advantages this process offers over traditional pharmaceutical manufacturing techniques, IM is now receiving increasing interest for biomedical and pharmaceutical applications (1–3). In this respect, injection moulding has been applied successfully to provide modified release drug delivery platforms via the homogeneous embedding of drug particles in release-modifying polymers. In these studies, drug release from IM tablets was sustained using ethylcellulose as matrix former and using various hydrophilic fillers (hydroxypropylmethylcellulose, low-substituted hydroxypropylcellulose, polyethylene oxide and xanthan gum) to control the release rate. Only the use of xanthan gum resulted in zero-order release; and for all these systems, drug was released by a combination of polymer swelling, Fickian diffusion and matrix erosion (4–7). In addition, hot melt methods (i.e. hot melt extrusion and injection moulding) offer the possibility to disperse active pharmaceutical ingredients in a polymeric carrier in a highly controlled manner, yielding solid dispersions/solutions (8). These dosage forms aim at an improved dissolution rate and hence bioavailability of poorly soluble drugs; an area of increasing importance (9). Moreover, hot melt methods offer a potential solution to problems encountered with traditional methods for preparing solid solutions/dispersions: the process itself inherently allows a better monitoring and standardisation, scale-up is more easily achieved, the complexities of solvent residuals and the need for solvent-proof equipment are negated (1). A further advantage of IM is the distinct possibility of mass producing drug delivery platforms in a single step, thus avoiding the need for additional processing steps; a problem often encountered with hot melt extrusion.
Although IM has significant potential as a novel drug delivery technology, a paradigm shift in pharmaceutical solid dosage form manufacturing will require a fundamental and extensive evaluation of this process to determine the effects of both process and formulations factors on product performance. Although IM is gaining increasing interest, the current scientific literature contains only a limited number of studies reporting the use of IM; mostly conducted by our group. In order to truly implement IM as a drug delivery technology, this deficit must be urgently addressed. The aim of this study was to evaluate the feasibility of polymethacrylates (Eudragit RL and RS) as matrix carriers in the development of IM sustained release matrix tablets containing different salt forms of metoprolol. In so doing, we aim to complement the current literature with novel information relating to the use of melt processed Eudragit RL/RS platforms (10,11). The influence of process temperature, plasticizer concentration and salt form of metoprolol (tartrate, fumarate and succinate) on the ease of processing and the in vitro drug release properties, were evaluated. Injection-moulded tablets were characterised with respect to polymer/drug miscibility, solid-state properties and physical stability. Finally, the in vivo performance of moulded tablets was determined after administration to dogs.
MATERIAL AND METHODS
Materials
Metoprolol tartrate (MPT, EQ Esteve, Spain) was selected as model drug. Other salts of metoprolol included metoprolol succinate (MPS) and fumarate (MPF; Polydrug Laboratories, India). Eudragit® RL PO and RS PO were kindly donated by Evonik (Darmstadt, Germany). Triethyl citrate was purchased from Sigma (TEC, USA). Slow-Lopressor® 200 Divitabs containing 200 mg metoprolol tartrate were purchased from Sankyo Pharma (Belgium).
Production and Storage of Injection-Moulded Tablets
An overview of the different formulations with respect to their processing conditions is given in Table I. In a preplasticizing step, triethyl citrate was mixed with Eudragit RL and RS using mortar and pestle, followed by homogenization in planetary mixer for 15 min at 90 rpm (Kenwood Major Classic, UK). This mixture was stored overnight to allow the plasticizer to interact with the polymer. Metoprolol was blended with (un)plasticized polymer for 15 min in a tumbling mixer prior to melt processing. These mixtures were extruded using a co-rotating twin-screw mini-extruder (Thermo scientific Haake MiniLab II Micro Compounder, Thermo Scientific, Germany) at a screw speed of 90 rpm employing a cylindrical die with a diameter of 2 mm. The molten extrudates were collected in a heated reservoir and immediately shaped into tablets using a lab-scale injection moulder (Haake MiniJet System, Thermo Electron, Germany) operating at the same temperature as the extruder. An injection pressure of 800 bar during an injection phase of 10 s followed by an after pressure of 600 bar, applied for a period 5 s, was used to prepare the matrix tablets. The temperature of the mould was set at 20°C. After cooling, biconvex tablets (diameter, 10 mm; height, 5 mm) with a mass of approximately 375 mg were obtained.
Table I.
Composition of Formulations and Injection-Moulding Process Parameters
| Drug load (%) | Metoprolol salt | Eudragit RL PO (%) | Eudragit RS PO (%) | TEC (% w/p) | Injection moulding temperature (°C) | Injection pressure (bar) | Optical appearance after storage | ||
|---|---|---|---|---|---|---|---|---|---|
| 0 Month | 2 Months | 12 Months | |||||||
| Influence of methacrylate type and process temperature | |||||||||
| 30 | MPT | 70 | – | 0 | 120 and 140 | 800 | T | T | T |
| 30 | MPT | – | 70 | 0 | 120 and 140 | 800 | T | T | T |
| Influence of plasticizer level | |||||||||
| 30 | MPT | 70 | – | 5 | 120 | 800 | T | T | Ta |
| 30 | MPT | 70 | – | 10 | 105 | 800 | T | T | Oa |
| 30 | MPT | 70 | – | 20 | 105 | 600 | T | O | Oa |
| 30 | MPT | – | 70 | 5 | 120 | 800 | T | T | Ta |
| 30 | MPT | – | 70 | 10 | 105 | 800 | T | T | Oa |
| 30 | MPT | – | 70 | 20 | 105 | 600 | T | O | Oa |
| Influence of metoprolol tartrate concentration | |||||||||
| 10 | MPT | 90 | – | 0 | 155 | 800 | T | T | T |
| 20 | MPT | 80 | – | 0 | 130 | 800 | T | T | T |
| 40 | MPT | 60 | – | 0 | 110 | 800 | C | C | C |
| 10 | MPT | – | 90 | 0 | 150 | 800 | T | T | T |
| 20 | MPT | – | 80 | 0 | 140 | 800 | T | T | T |
| 40 | MPT | – | 60 | 0 | 115 | 800 | C | C | C |
| Influence of metoprolol salt and concentration | |||||||||
| 10 | MPF | 90 | – | 0 | 150 | 800 | T | T | T |
| 20 | MPF | 80 | – | 0 | 135 | 800 | T | T | T |
| 30 | MPF | 70 | – | 0 | 125 | 800 | T | T | O |
| 40 | MPF | 60 | – | 0 | 120 | 800 | C | O | O |
| 10 | MPS | 90 | – | 0 | 145 | 800 | T | T | T |
| 20 | MPS | 80 | – | 0 | 120 | 800 | T | O | O |
| 30 | MPS | 70 | – | 0 | 112 | 800 | T | O | O |
| 40 | MPS | 60 | – | 0 | 110 | 800 | O | O | O |
MPT metoprolol tartrate, MPF metoprolol fumarate, MPS metoprolol succinate, T transparent, C cloudy, O opaque
aDeformation
In order to produce injection-moulded bars for dynamic mechanical testing and thermal analysis, the formulations were precompounded using a Thermo Haake twin screw extruder (Prism Eurolab 16 twin extruder, Thermo Scientific, Germany). Cooled extruded strands were cut into pellets (1–2 mm) via an in-line pelletiser (Prism Varicut 16 pelletiser, Thermo Scientific, Germany). Subsequently, these pellets were fed into an injection-moulding machine (Rondol High force 5, UK), equipped with a specific die (set at room temperature) to produce IM bars (length, 50.0 mm; width, 8.2 mm; height, 3.3 mm). Tablets were stored in open vials at 25°C ± 2°C/60% RH ± 5% for 12 months protected from light to determine the physical stability of the drug via thermal analysis.
In Vitro Drug Release
Dissolution testing (n = 3, each vessel containing one tablet) was performed using Apparatus 2 (USP27) on a VanKel VK7010 dissolution tester combined with a VK 8000 automatic sampling station (VanKel Industries, USA). Since Eudragit RL and RS offer a pH-independent drug release and preliminary work showed that MPT, MPF and MPS release was not influenced by pH (data not shown), demineralized water (900 ml) was used as dissolution medium. The paddle speed was set at 50 rpm, while the temperature of the medium was maintained at 37 ± 0.5°C. Samples of 5 mL were withdrawn at specific time points (0.5, 1, 2, 4, 6, 8, 12, 16, 20, and 24 h without media replacement) and spectro-photometrically assessed by means of a double beam spectrophotometer (UV-1650PC, Shimadzu, Belgium; λmax MPT, MPS and MPF was 222, 222 and 220 nm respectively). The MPT, MPF or MPS content in the samples was determined by linear regression. The drug release kinetics were determined by finding the best fit between the experimental data (amount drug released vs. time) and kinetic models for zero-order, first-order release and the semi-empirical Korsmeyer–Peppas model as described by Quinten et al. (3).
Liquid Uptake, Swelling and Erosion
Tablets (n = 3) were introduced into the dissolution medium and subjected to a dissolution test under the same conditions as described above. At predetermined time intervals, the tablets were withdrawn from the medium and weighed after excessive water was gently removed from the surface with paper towel. The liquid uptake, expressed as percentage weight gain of the total polymer content (Eq. 1), was calculated from the original weight, taking the amount of drug released at that particular time into account.
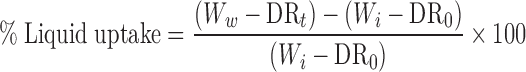 |
1 |
Ww is the weight of the matrix tablet at time t, Wi is the initial weight of the tablet before immersion (time 0), DR0 is the amount of drug in the tablet at time 0 and DRt is the amount of drug in the tablet at time t.
The radial and axial swelling of the matrices during dissolution was determined by measuring the individual diameter and height of the tablets using an electric digital calliper (Bodson, Belgium). In addition, the degree of erosion (expressed as percentage loss of polymer content; Eq. 2) was determined based on the weight difference between oven-dried matrices (40°C, 72 h) and the initial weight of the tablet, taken the amount of drug released at each time point into account.
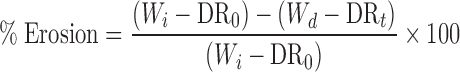 |
2 |
Wd is the dry weight of the matrix tablet at time t, Wi is the initial weight of the tablet before immersion (time 0), DR0 is the amount of drug in the tablet at time 0 and DRt is the amount of drug in the tablet at time t.
X-ray Diffraction
The X-ray patterns were determined using a D5000 Cu Kα diffractor (λ = 0.154 nm; Siemens, Germany) with a voltage of 40 kV and current of 40 mA in the angular range of 10° < 2θ < 60° using a step scan mode (step width = 0.02°, counting time = 1 s/step). To analyse crystallinity, X-ray diffraction was performed on the different individual components as well as on physical mixtures and IM tablets.
Scanning Electron Microscopy and Hot-Stage Microscopy
Scanning electron microscopy was used to study the morphology of the tablet surface. Tablets were coated with platinum by means of a sputter coater (Auto Fine Coater, JFC-1300, Jeol, Japan). Photomicrographs were taken with a scanning electron microscope (Jeol JSM 5600 LV, Jeol, Japan). A polarised-light microscope (Eclipse E400, Nikon, Japan) equipped with hot stage (LTS350, TP94, Linkam Scientific Instruments, England), digital camera (Digital Slight DS-L1, Nikon, Japan) and image analysis program (NIS-Elements Basic Research 2.30, Nikon, Japan) was used to investigate the drug/polymer miscibility. Cross-polarisation was applied to investigate crystallinity.
Thermal Analysis
Thermal analysis was done using a differential scanning calorimeter (Q2000 DSC, TA Instruments, UK) equipped with a refrigerated cooling system. The flow rate of dry nitrogen gas was 150 mL/min. Samples (n = 3, 5–10 mg) were run in hermetically sealed aluminium pans supplied by TA Instruments (Leatherhead, UK). Temperature and enthalpic calibration was done using indium as a standard. In order to determine the melt endotherm onset temperature (Tmo), melting point (Tm), and heat of fusion (ΔH) of the different individual components, physical mixtures and tablets; samples (5–10 mg) were cooled to −50°C, held isothermal for 5 min and heated to 180°C at a linear heating rate of 10°C/min.
Since the glass transition temperature was masked by an enthalpic relaxation endotherm on analysis with conventional DSC, modulated temperature DSC (MTDSC) was used to study the Tg of the individual components and physical mixtures according to a three-cycle analysis (heating, cooling, heating) from −50°C to 180°C. Injection-moulded bars were only subjected to one cycle. MTDSC was performed using a TA instruments Q2000 DSC. Samples (n = 3, ± 5 mg) were sealed in Tzero aluminium pans with lid. The DSC was cooled to −50°C, equilibrated for 5 min, followed by heating at an underlying heating rate of 2°C per minute, for which the temperature was modulated with an oscillation of 2°C every 60 s. The results were analysed using the TA Instruments Universal Analysis 2000 Software.
The experimental values of the glass transition temperature can be compared with calculated theoretical values to evaluate the miscibility of drug and excipients using the Gordon–Taylor equation (12). This relationship states that if drug and polymer are miscible, the mixture will show a single Tg that ranges between the Tg of pure components and depends on the relative portion of each component:
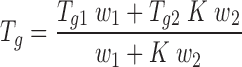 |
For which, Tg1 and Tg2 are the glass transition temperatures of the metoprolol salt and Eudragit RL, respectively, w1 and w2 are the weight fractions of metoprolol and Eudragit in the dispersion, and K is a constant that can be estimated using the Simha–Boyer rule (13):
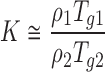 |
With ρ1 and ρ2 the true densities of the respective metoprolol salt and Eudragit RL as determined by means of helium pycnometry (AccuPyc 1330, Micromeritics Instruments, USA). Since the Gordon–Taylor addresses the densities of amorphous components, the density of amorphous metoprolol salts was estimated from the true density of their respective crystalline counterpart, reduced by 5% (14).
Dynamic Mechanical Analysis
A Perkin-Elmer DMA 8000 Dynamic Mechanical Analyser was used to study the glass transition temperature of injection-moulded bars (n = 3) in order to confirm and verify the results obtained with MTDSC. Experiments were performed by the application of a sinusoidal force resulting in a deformation of 10 μm at a frequency of 1 Hz. The amplitude of deformation, force and phase shift were recorded on bars clamped using a dual cantilever bending mode. The samples were cooled to −50°C at the start of the experiment and the temperature was ramped to 100°C at a rate of 3°C/min. The dynamic parameters, loss modulus (G″) and storage modulus (G′), were recorded as a function of temperature and time, and tan δ (G″/G′) was used to determine the Tg of the moulded bars.
Three-Dimensional Solubility Parameters
Three-dimensional solubility parameters were calculated using SPWin (Version 2.1, Breitkreutz), utilising an advanced parameter set combining the group contribution methods of Fedor and Van Krevelen/Hoftyzer, which was optimised by Breitkreutz (15). For polymeric excipients, determination of the solubility parameter was based on the average molecular weight. The solubility parameters of Eudragit RL and RS were compared to those of the APIs by observing the relative difference in the total solubility (δtotal), as well as partial Hansen solubility parameters (δd, contribution from dispersive forces; δp, contribution from polar forces; δh, contribution from hydrogen bonding).
NIR and Raman Spectroscopy
Diffuse reflectance near-infrared (NIR) spectra of physical mixtures, extrudates and injection moulded tablets containing Eudragit (RL or RS) and a metoprolol salt (MPT, MPF or MPS) were collected off-line, using a Fourier-transform NIR spectrometer (Thermo Fisher Scientific, Belgium), and Nicolet Antaris II near-IR analyzer equipped with an InGaAS detector and a quartz halogen lamp. Each spectrum was collected in the 10,000–4,500 cm−1 region with a resolution of 8 cm−1 and averaged over 16 scans. Data analysis was performed using the Result software (Version 3.0, Thermo Fisher Scientific, Belgium), SIMCA-P+ (version 12.0.1.0, Umetrics, Sweden), and Matlab (The Mathworks, version 7.7). All spectra were pre-processed using multiplicative signal correction, combined with smoothing of the spectra.
Raman spectra of physical mixtures, extrudates and injection-moulded tablets containing Eudragit (RL or RS) and a metoprolol salt (MPT, MPF or MPS) were collected off-line using a Raman Rxn1 spectrometer (Kaiser Optical Systems, Ann Arbor, MI, USA), equipped with an air-cooled CCD detector. The laser wavelength was the 785 nm line from a 785 nm Invictus NIR diode laser. All spectra were recorded with a resolution of 4 cm−1 and an exposure time of 2 s, using a laser power of 400 mW. Data collection and data transfer were automated using the HoloGRAMS™ data collection software, the HoloREACT™ reaction analysis and profiling software, the Matlab software (version 7.1, The MathWorks Inc., Natick, MA, USA) and SIMCA-P+ (version 12.0.1.0, Umetrics, Umeå, Sweden). The analysed spectral region was 0–1,800 cm−1, since this region contained all useful drug and polymer information. Prior to analysis, mean centering, standard normal variate pre-processing and smoothing were applied on the spectra.
In Vivo Study
All procedures were performed in accordance with the guidelines and after approval by the Ethics Committee of the Institute for Agricultural and Fisheries Research (Merelbeke, Belgium). The following formulations were administered during the in vivo evaluation: (a) injection-moulded tablet containing 30% w/w MPT with 70% Eudragit RS (formulation F1; MPT dose administered, 110 mg); (b) injection-moulded tablet containing 50% w/w MPT, and 50% Eudragit RS (formulation F2; MPT dose administered, 186 mg); (c) one half tablet Slow-Lopressor® 200 Divitabs® (reference formulation (Fref); MPT dose administered, 100 mg). Since the size of the moulded tablets was fixed (due to the specific dimensions of the mould) and as the MPT/Eudragit RS-ratio was constant for the formulations selected for the in vivo study, different MPT doses were administered during the in vivo study. However, the pharmacokinetic profile was normalised for administered dose as linear pharmacokinetics have been reported for MPT in a dose range between 50 and 400 mg (16). All formulations were administered to male mixed-breed dogs (weight, 20–40 kg) in a cross-over sequence with a wash-out period of at least 8 days. Dogs were fasted 12 h prior to the start of the experiments; however, water was available ad libitum during the course of the experiment. Samples were collected in dry heparinised tubes at 0.5, 1, 2, 4, 6, 8, 12 and 24 h after tablet intake followed by centrifugation at 1,500×g for 5 min. No food was given to the dogs during the initial 24 h of the test. Metoprolol tartrate plasma concentrations were determinate by a validated HPLC fluorescence method (2,17). The method was specific, linear (0.05–3.0 μg/mL; R2, 0.9972), precise: the intra-assay precision was 3.7–10.5% (within-day repeatability), and the intermediated precision was 1.7–11.6% (between-day repeatability). The method was accurate and 79.3–85% of MPT was recovered. The limit of detection was 0.03 μg/mL and the limit of quantification was 0.10 μg/mL.
Data Analysis
The peak plasma concentration, the extent of absorption (AUC0−24h) and the time needed to reach the highest plasma level were calculated using the MW-Pharm Program version 3.0 (Mediware 1987–1991, Utrecht, The Netherlands). AUC0–24h was calculated using logarithmic and linear trapezoidal rules. The relative bioavailability (expressed in percentage) was calculated as the AUC0–24h ratio between the test and the Fref, normalised for drug dose.
The effect of the formulation on the bioavailability was statistically evaluated by repeated-measures ANOVA (univariate analysis). To compare the effects of the different treatments on the pharmaco-kinetic parameters, a multiple comparison among pairs of means was performed using a Bonferroni post hoc test with p < 0.05 as significance level. The sphericity of covariances was tested with Mauchly’s test. If the assumption of sphericity was not fulfilled, the Huynh–Feldt correction was performed. All analyses were performed using SPSS 17 (SPSS Inc., USA).
RESULTS AND DISCUSSION
Processability of Injection-Moulded Eudragit RL/RS Formulations
Eudragit® RL and RS are thermoplastic water-insoluble methacrylic ester copolymers, containing different quantities of quaternary ammonium groups (10% and 5% functional ammonium groups, respectively), which provide these polymers a pH-independent water permeability. These polymers are frequently used for sustained release applications such as coating, pellets, films or tablets, and their thermoplastic properties make them good candidates for melt processing. An overview of the different formulations with respect to their processing conditions is presented in Table I.
Manufacturing Eudragit RL/RS tablets via IM was possible across a broad temperature range; however, as one of the main disadvantages of hot melt processing involves thermal stability, the production temperature was kept as low as possible to avoid degradation of drug and carrier. Previous studies have reported that Eudragit RL and RS are thermostable up to 167°C, and metoprolol is stable below 160°C, the process temperatures selected in this study were always below the onset of degradation of each component (3,18). In addition, DSC measurements were conducted up to 180°C on individual components and physical mixtures, and no evidence of thermodegradation was found in all thermograms.
Plasticizers are often included in IM formulations to improve flexibility and workability, as this allows lower production temperatures due to a decrease in melt viscosity and shear forces. In this study, the suitability of triethyl citrate (TEC) was assessed on processability and drug release. As seen in Table I, increasing the TEC concentration in the formulations allowed considerably lower production temperatures and injection pressures. However, 10% and 20% w/w TEC caused stability problems, as these tablets became gradually opaque/white during storage and were severely deformed after 1-year storage. These findings clearly indicated overplasticization, which was confirmed by DSC measurements. Eudragit RL plasticized with 5%, 10% or 20% w/p TEC and had a Tg of 43.6°C, 37.7°C and 22.8°C, respectively. This low glass transition temperature close to room temperature allowed the polymer chains to re-arrange due to increased mobility, resulting in sticky and opaque tablets and was responsible for the extensive deformation during storage. Thermal analysis (see “Thermal Analysis of Binary Mixtures and Injection-Moulded Samples” section) revealed that the conversion from transparent to opaque tablets was caused by a progressive recrystallization over time of the drug in the matrix, which was strongly influenced by the type of metoprolol salt.
Formulations containing low concentrations of metoprolol salts (10–20%) required processing around their respective melting point to ensure sufficient plasticization of the material, whereas higher drug concentrations (30–40%) allowed processing at lower temperatures indicating a plasticizing effect of the drug on the polymer (Table I). Solid-state plasticisation of polymers by pharmaceutically active agents has been previously reported during melt processing: the high level of mixing between molten polymer and drug and the elevated temperatures involved in extrusion facilitate intimate mixing between the two components, and can result in drug/polymer interactions allowing drug to occupy active sites along the polymer chain reducing polymeric inter-chain interactions, hence leading to reduced processing temperatures (7,11). This is also reflected in a decreased glass transition temperature of the polymer (see “Thermal Analysis of Binary Mixtures and Injection-Moulded Samples” section for a more detailed discussion).
Processing of these formulations resulted in the formation of transparent tablets, only tablets containing 40% MPS were opaque immediately after production. However, IM tablets containing 20%, 30% and 40% w/w MPS, and 40% MPF gradually became opaque during 2 months storage. After 1-year storage, only a limited number of IM tablets remained transparent (Table I). SEM images showed a smooth surface indicating a good coalescence of the melt after cooling (data not presented). To characterise the changes during storage and the interactions of metoprolol salts with Eudragit, the solid-state properties of these formulations were further analysed.
Physicochemical Properties and Solid-State Structure of Injection-Moulded Tablets
Solubility Parameter Calculation
Estimation of the solubility parameters is commonly done to predict the miscibility of drug and excipients. The solubility parameter is a measure of the cohesive energy densities of materials (19). Greenhalg et al. suggested that interactions between polar groups (δp) and hydrogen bonding (δh) significantly affect solubility and should be incorporated into the estimation of the total solubility parameter, which previously only accounted for dispersive forces (δd). As a consequence, most applications use the group contribution method described by Van Krevelen to determine the Hansen partial solubility parameter, that allows for an improved characterisation of more polar molecules (20). This method makes use of specific functional groups present in the molecular structure of the molecules under investigation to calculate the sum of intermolecular forces that form cohesive energy. Cohesive energy is the net effect of different types of intermolecular interactions, such as intermolecular or Van der Waals (dispersion) forces (δd), intermolecular polar forces (δp), and intermolecular hydrogen bonding (δh), and holds a substance together. By separate consideration of the sum of intermolecular forces, the ability of a molecule to interact with another one can be calculated. An overview of the solubility parameters is presented in Table II. The difference between the solubility parameters of two materials gives an estimation of the likelihood that these components will be miscible. Components with similar solubility parameters are likely to be miscible (Δδt < 7 MPa½) and components with Δδt > 10 MPa½ are likely to be immiscible. This is because the energy of mixing released by interactions within the component is balanced by the energy released by interactions between the components (20). All drug salts have similar solubility parameters and there is a high similarity in intermolecular dispersion forces and intermolecular hydrogen bonding between all drug salts and Eudragit RL and RS (Table II). As the difference in total solubility parameter is less than 4 MPa½ for all drug/polymer mixtures, these compounds show miscibility and are expected to form one-phase solid solutions, which were already observed via the formation of transparent tablets immediately after IM (21).
Table II.
Three-Dimensional Solubility Parameters of Drugs and Carriers
| Compound | δ d (MPa0.5) | δ p (MPa0.5) | δ h (MPa0.5) | δ t (MPa0.5) |
|---|---|---|---|---|
| MPT | 18.39 | 2.61 | 14.55 | 23.60 |
| MPS | 18.06 | 2.32 | 11.77 | 21.68 |
| MPF | 17.98 | 2.34 | 11.83 | 21.65 |
| Eudragit RL | 16.92 | 1.02 | 11.11 | 20.27 |
| Eudragit RS | 16.94 | 1.02 | 11.26 | 20.37 |
Intermolecular or Van der Waals (dispersion) forces (δ d), intermolecular polar forces (δ p), and intermolecular hydrogen bonding (δ h), total solubility parameter (δ t)
Thermal Analysis of Binary Mixtures and Injection-Moulded Samples
Thermal analysis was performed to ascertain miscibility between drug and matrix carrier, and to further elucidate the solid-state properties of injection-moulded samples. Thermal analysis also provided evidence for miscibility between Eudragit RL and all metoprolol salts (Table III): the melt onset temperature and heat of fusion decreased at higher Eudragit RL concentrations (22). In addition, independent of the metoprolol concentration and salt, part of the drug fraction dissolved in the polymer at temperature much lower than the melting point of the pure drug. These findings were confirmed using hot-stage microscopy, demonstrating that drug crystals were solubilized in the rubbery polymer below their respective melting point (Fig. 1).
Table III.
Melting Endotherm Onset Temperature, Melting Point, Heat of Fusion and Glass Transition Temperature of Metoprolol Salts, Eudragit RL and Various Binary Mixtures
| Formulation | Melting onset temperature (°C) | Melting point (°C) | Heat of fusion (ΔH, J g−1) | T g (°C) | Calculated T g by Gordon–Taylor equationa (°C) |
|---|---|---|---|---|---|
| Individual components | |||||
| Eudragit RL | – | – | – | 61.8 | – |
| MPT | 120.4 | 122.0 | 107.9 | 1.9 | – |
| MPS | 134.7 | 137.1 | 196.8 | 2.2 | – |
| MPF | 144.7 | 146.6 | 146.0 | 3.4 | – |
| Physical mixtures with MPT | |||||
| 10:90 | 105.8 | 115.0 | 9.9 | 58.0 | 54.4 |
| 20:80 | 107.8 | 116.9 | 18.6 | 49.4 | 47.4 |
| 30:70 | 110.6 | 117.2 | 28.0 | 34.2 | 40.7 |
| 40:60 | 112.8 | 118.5 | 35.2 | 30.6 | 34.4 |
| Physical mixtures with MPS | |||||
| 10:90 | 109.2 | 124.0 | 15.0 | 52.3 | 54.4 |
| 20:80 | 109.9 | 128.5 | 36.0 | 41.9 | 47.3 |
| 30:70 | 112.7 | 131.0 | 50.8 | 32.4 | 40.6 |
| 40:60 | 120.1 | 131.0 | 67.4 | 27.1 | 34.1 |
| Physical mixtures with MPF | |||||
| 10:90 | 118.3 | 139.9 | 15.1 | 53.2 | 54.4 |
| 20:80 | 118.6 | 139.5 | 25.9 | 41.4 | 47.9 |
| 30:70 | 129.9 | 141.5 | 41.6 | 34.5 | 40.7 |
| 40:60 | 133.7 | 142.9 | 49.9 | 30.0 | 34.3 |
aThe density of Eudragit RL, MPT, MPS and MPF was 1.1902, 1.2059, 1.2261 and 1.2086 g/cm3, respectively
Fig. 1.
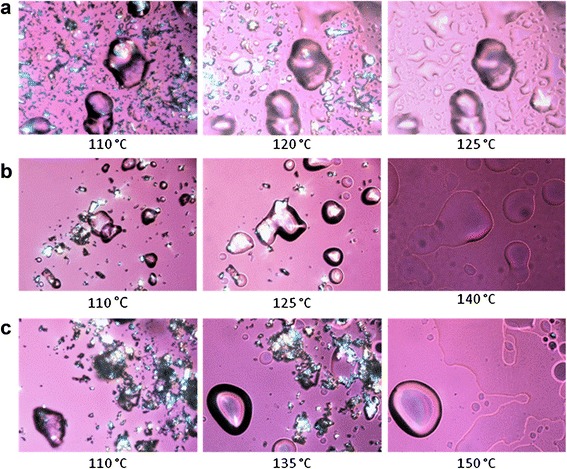
Hot-stage microscopy images of binary mixtures (ratio, 1:1) of Eudagit RL with metoprolol salts: tartrate (T m, 122°C; a), succinate (T m, 137°C; b) and fumarate (T m, 147°C; c)
In addition, DSC of binary mixtures showed a single Tg positioned between the glass transition temperatures of both components, indicating complete miscibility of drug and polymer at the given concentrations (23,24).
Although injection-moulded tablets were produced at temperatures below the melting point of the drug, a single Tg (data not presented) between the Tg of drug and carrier was also observed during thermo-analysis of the IM tablet. These findings illustrated that glass solutions of the drug were formed at production temperatures below the melting point of the metoprolol salt, due to the intensive mixing and high shear rates involved in the process, facilitating mixing and dispersion of drug and carrier at the molecular level.
At low drug concentrations (10–20% w/w), the experimental glass transition data corresponded with the theoretical values calculated by the Gordon–Taylor equation; however, for higher drug concentrations, negative deviations were observed, indicating a possible interaction between MP salts and Eudragit (Table III). The Gordon–Taylor equation is a theoretical approach to predict the Tg of binary drug/polymer mixtures of ideal amorphous one-phase systems. According to this equation, if the drug and polymer are miscible, the mixture will show a single Tg that ranges between the Tg of pure components and depends on the relative proportion of each component (weight fraction). The negative deviation observed at higher metoprolol concentration could be attributed to a plasticizing effect of drugs on the polymer, resulting in a change of free volume of the system (14). This plasticizing effect of the different metoprolol salts on Eudragit was also evident from the glass transition temperatures (Table III): a higher drug concentration in the physical mixtures lowered the glass transition temperature, demonstrating a solid-state plasticizing effect of drug on Eudragit.
These results were confirmed by dynamical mechanical analysis of injection-moulded bars, showing a clear reduction in Tg for increasing drug concentrations irrespective of the type of drug (Fig. 2). In addition, the type of metoprolol salt had a small influence on the Tg reduction, and similar results were obtained for Eudragit RS (data not shown). Glaessl et al. suggested that hydrophobic interactions between metoprolol base and the polymethacrylate backbone and/or ionic interactions between the different anions and the quaternary ammonium groups could possibly be the reason for this plasticizing effect (25). In this regard, Raman and NIR spectroscopy measurements were conducted to investigate more thoroughly the nature of these drug-polymer interactions.
Fig. 2.
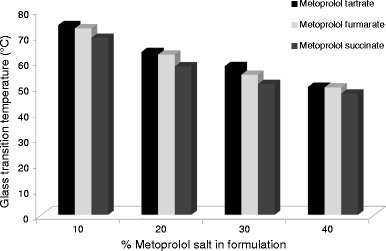
Glass transition temperature in function of drug load determined via dynamical mechanical analysis of injection moulded bars containing Eudragit RL with different amounts of metoprolol tartrate, succinate and fumarate
NIR and Raman Spectroscopy
The NIR spectra of the extrudates and IM tablets (Fig. 3) manifested a broad peak around 6,500 cm−1 (1,538 nm), which was absent in the spectra of physical mixtures. As hydrogen-bonded hydroxyl groups induce a broad peak in the 6,850–6,240 cm−1 region (which has been attributed to the first overtone of the bonded hydroxyl (26)), this observation indicated that hydrogen bonds were formed between drug and polymer during thermal processing, where the hydroxyl groups of the metoprolol salts act as proton donors. The intensity of this H-bond peak depended on the metoprolol salt, being the most intense in case of MPS and nearly non-existent for MPT, suggesting that the level of interaction between Eudragit RL and metoprolol was defined by the salt form (MPS > MPF > MPT). Interestingly, a correlation could be established between the extent of the hydrogen bonding and the glass transition temperature of these formulations, as the plasticizing effect of MPS on Eudragit was higher compared to MPF and MPT (Table III, Fig. 4). Similar results were established at a lower processing temperature (120°C), a lower metoprolol salt concentration (10%) and using Eudragit® RS PO as matrix former. It should be noted that also hydrophobic and ionic interactions between the various metoprolol salts and the quaternary ammonium group present in the acrylic backbone of the polymer are likely to occur (25).
Fig. 3.
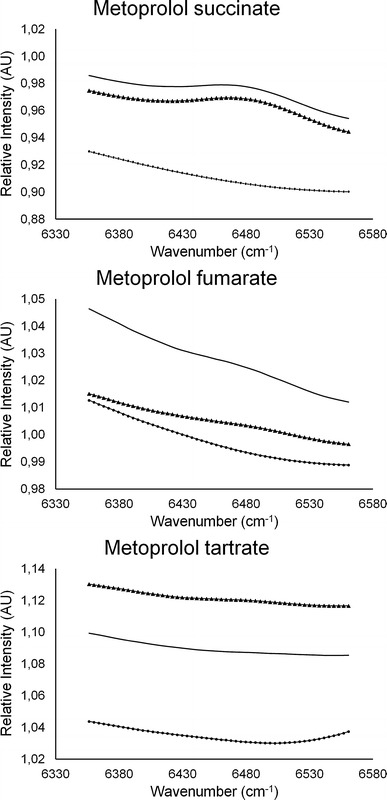
Detail of the off-line collected NIR spectra of a formulation containing Eudragit® RL PO and a metoprolol salt (ratio, 60/40; w/w): physical mixture (filled circle), extrudates (filled triangle), injection moulded tablets (straight line) processed at 140°C
Fig. 4.
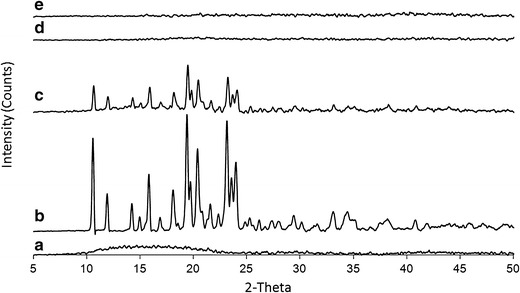
X-ray diffraction patterns of a Eudragit RL, b metoprolol tartrate and their binary mixture (70/30, w/w), c physical mixture, d injection moulded tablet at 120°C, e injection-moulded tablet at 140°C
In the Raman spectra, peak shifts larger than 4 cm−1 occurred only for extruded and IM mixtures processed at 140°C and containing a 40% drug loading. These peak shifts were more pronounced for formulations processes at 140°C when compared with formulations processed at 120°C, indicating that polymer–drug interactions were stronger when higher processing temperatures were used. Higher manufacturing temperatures result in a decrease in melt viscosity, increase in polymer free volume and mobility and could enhance the formation of H-bonds between the polymer backbone and the metoprolol salt.
As the peak shifts were only observed after thermal processing of the formulations, the molecular interactions and plasticizing effect of metoprolol salts on Eudragit polymers was also confirmed via Raman spectroscopy. The largest peak shifts were found in the spectra of MPS-containing tablets, highlighting the stronger plasticizing effect of the succinate anion on Eudragit (data not presented).
X-ray Diffraction
Diffractograms of Eudragit RL, metoprolol tartrate, physical mixtures and IM samples are displayed in Fig. 4. Metoprolol tartrate showed distinctive peaks due to its crystalline nature, whereas Eudragit RL was amorphous. Peaks corresponding to crystalline metoprolol tartrate were present in the physical mixture, however, absent in the diffractograms of transparent injection-moulded tablets produced at 120°C and 140°C. This demonstrated that the drug is present in an amorphous state in IM samples and confirmed the DSC results. Similar results were obtained for metoprolol succinate and fumarate (data not shown).
Storage Stability
Visual inspection of moulded samples revealed that some transparent tablets gradually turned cloudy/opaque during 1-year storage at ambient conditions (Table III). Evidence of recrystallization was found in these formulations as a melting peak of metoprolol was observed during thermo-analysis. The percentage of amorphous drug that had reverted to the crystalline state was estimated from the heat of fusion of similar physical mixtures (Table IV). Metoprolol tartrate provided the most stable formulations, as no recrystallization was observed during storage, even at high drug loadings. These formulations did not exhibit a melting transition and a single Tg was detected. In contrast, IM tablets containing metoprolol succinate and fumarate tended to recrystallize at higher drug load drug. These tablets possessed a melting endotherm and two glass transitions, indicating phase separation. These results suggested that molecular interactions between metoprolol salt and the methacrylate polymer have a significant impact in preventing drug recrystallization. These findings were confirmed with X-ray diffraction (XRD) measurements (data not shown).
Table IV.
Melting Endotherm Onset Temperature, Heat of Fusion and Glass Transition Temperature of Injection Moulded Tablets after 1-Year Storage at Ambient Conditions
| Formulation | Melting endotherm onset temperature (°C) | Heat of fusion (ΔH, J g−1) | T g (°C) | Crystallinity (%) |
|---|---|---|---|---|
| Injection moulded tablet containing MPT | ||||
| 10:90 | – | – | 54.7 | – |
| 20:80 | – | – | 47.8 | – |
| 30:70 | – | – | 26.3 | – |
| 40:60 | – | – | 21.2 | – |
| Injection moulded tablets containing MPS | ||||
| 10:90 | – | – | 51.6 | – |
| 20:80 | 111.4 | 1.2 | 44.0 | 3.2 |
| 30:70 | 114.2 | 15.7 | 9.9/56.6 | 30.8 |
| 40:60 | 117.9 | 47.2 | 3.5/59.6 | 70.0 |
| Injection moulded tablets containing MPF | ||||
| 10:90 | – | – | 51.8 | – |
| 20:80 | – | – | 40.0 | – |
| 30:70 | 119.4 | 3.5 | 4.2/60.4 | 32.7 |
| 40:60 | 126.6 | 34.9 | 3.6/63.6 | 69.9 |
In Vitro Drug Release
Influence of Matrix Composition and Production Temperature on Drug Release
Figure 5 present the drug release profiles of matrix tablets containing Eudragit RL or RS and 30% MPT. Both polymers have structurally the same composition but contain a different concentration of ammonium methacrylate units: Eudragit RL has approximately 5% (w/w, on dry substance), whereas Eudragit RL has 10%. Complete drug release was seen for all formulations after 24 h of dissolution, following time-dependent release kinetics. Drug release from tablets based on Eudragit RL was faster compared to Eudragit RS-based tablets. By blending Eudragit RL and RS (ratio 1:1), intermediate drug release profiles were obtained, indicating that drug release can be tailored using both methacrylates (data not shown).
Fig. 5.
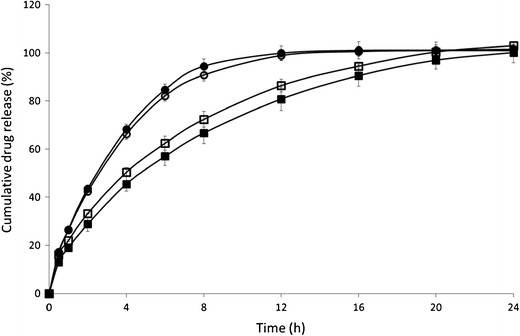
Influence of polymethacrylate type (Eudragit RL/RS) and process temperature (120/140°C) on metoprolol tartrate release. Mean dissolution profile (±SD) of injection-moulded tablets composed of MPT and Eudragit RL/RS (30/70, w/w). Eudragit RS-120°C (empty square), Eudragit RS-140°C (filled square), Eudragit RL-120°C (empty circle), and Eudragit RL-140°C (filled circle)
These difference in drug release are most likely attributed to the different amounts of quaternary ammonium groups, which account for differences in water permeability and hence dissolution properties. A matrix structure that is less water-permeable retards water influx and as a consequence drug dissolution and diffusion. The water uptake of matrices containing Eudragit RL (±315% after 24 h) was almost five times higher than Eudragit RS-based tablets (±66% after 24 h; Fig. 6). The radial swelling of Eudragit RS-based tablets was limited compared to Eudragit RL. For both Eudragit grades no significant erosion of the IM tablets was observed during dissolution. The drug release data provided a good fit with the Ritger–Peppas model and anomalous transport was depicted as the main drug release mechanism, confirming that both diffusion and swelling contributed to the overall drug release process.
Fig. 6.
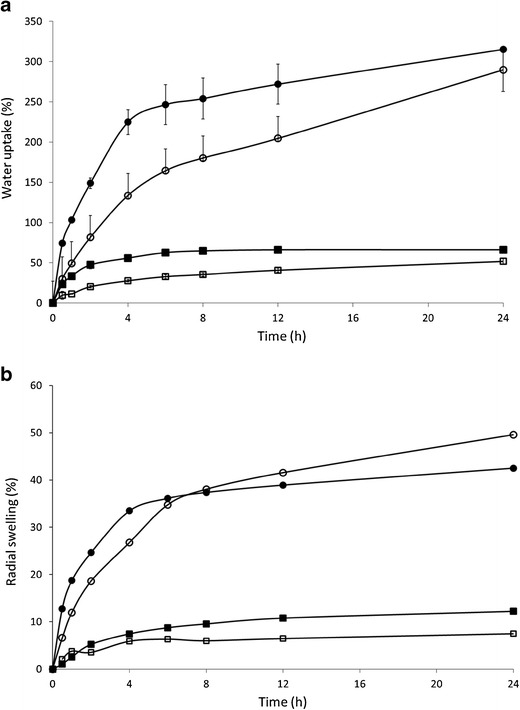
Influence of polymethacrylate type (Eudragit RL (circle)/RS (square)) with various metoprolol concentrations on water uptake (a) and radial swelling (b) of injection-moulded tablets. Closed symbols present formulation composed of MPT and Eudragit RL/RS (30/70, w/w), open symbols are formulations composed of MPT and Eudragit RL/RS (10/90, w/w)
In contrast to previous findings, using EC as matrix for IM tablets (2–5), the production temperature did not affect release from Eudragit-based tablets. As a higher production temperature reduced the polymer-free volume of the water-insoluble ethylcellulose and resulted in a denser and more tortuous matrix structure, drug release from EC matrices was incomplete and required a drug release modifying agent to promote drug release. In contrast, drug release from polymethacrylate-based tablets was complete due to their inherent water permeability, allowing drug diffusion to occur through the hydrated polymeric network. This was confirmed by Carli et al. who reported that drug release form Eudragit RL- or RS-based systems was controlled by intraparticle diffusion (27). Drug release from matrix tablets based on Eudragit RL and RS was not affected by TEC content (0–10%).
Influence of Drug Type and Concentration on Drug Release
The drug release profiles from Eudragit RL and RS formulations as a function of metoprolol concentration are presented in Fig. 7. In case of Eudragit RL (Fig. 7a), drug release was independent of drug loading. In contrast, tablets formulated with Eudragit RS showed faster drug release rates at higher MTP loading (Fig. 7b). Only formulations containing 30% and 40% MPT had a complete drug release, whereas only 77% and 30% MPT was released after 24 h dissolution from tablets containing 20% and 10% MPT, respectively. For Eudragit RS-based tablets, higher drug loadings also resulted in a more pronounced burst release. The water uptake from tablets containing 30% MPT was considerably higher compared to tablets with 10% MPT, especially during the first 8 h (Fig. 6), but the difference in swelling was limited. As a result, leaching of the highly soluble drug from the matrix towards the dissolution medium could have made the matrix more accessible to the dissolution medium by creating additional pores in the matrix structure. The impact of pore formation on drug dissolution is higher for Eudragit RS-based systems which are inherently less water permeable compared to Eudragit RL tablets, hence the effect of drug content on drug release from Eudragit RS tablets as more channel formation occurred at higher drug content.
Fig. 7.
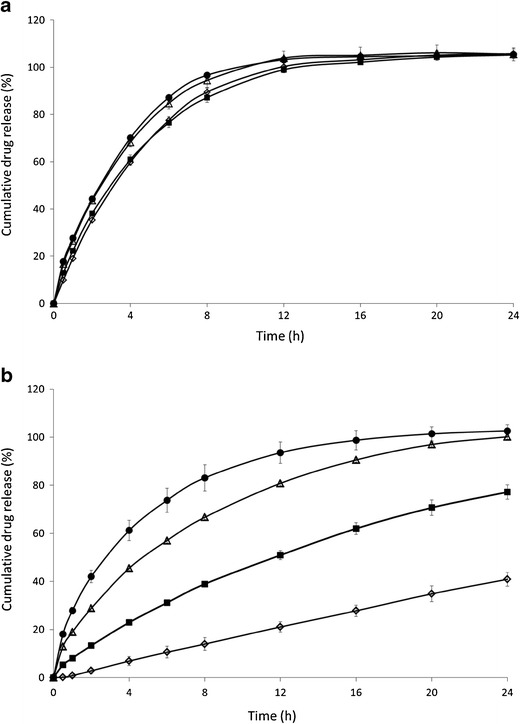
Influence of MPT concentration on drug release. Mean dissolution profile (±SD) of formulations containing Eudragit RL (a) or Eudragit RS (b) with various metoprolol tartrate concentrations: 10% (empty diamond), 20% (filled square), 30% (empty triangle), 40% w/w (filled circle) MPT
To investigate the influence of metoprolol salts on drug release, IM tablets containing 30% w/w metoprolol tartrate, fumarate and succinate were prepared. For both methacrylates, tablets composed of metoprolol tartrate provided the fastest drug release rates, whereas drug release from metoprolol fumarate was the slowest (Fig. 8). However, this difference in drug release was more pronounced for Eudragit RS-based tablets. In case of Eudragit RS formulations, only metoprolol tartrate was completely released after 24 h dissolution, whereas metoprolol succinate and fumarate release was only 88% and 68% after 24 h dissolution. These differences can not only be attributed to differences in intrinsic water solubilities (aqueous solubility at 37°C of metoprolol tartrate, fumarate and succinate is 3,630, 472 and 276 mg/ml, respectively) (28,29), since metoprolol fumarate is more water soluble than metoprolol succinate but provided slower release rates. However, it was reported that in the presence of anions, the chloride counterion of the quaternary ammonium group exchanged with these anions affecting the permeability of the polymer and hence the drug release profile (30). In addition, Wagner et al. demonstrated that the degree of polymer swelling and the permeability-enhancing effect depended on both the concentration and type of anions exchanging with chloride (31). From the results obtained in the current study (Fig. 8), faster drug release rates were seen for tartrate > succinate > fumarate. These anions exchanged with the chloride counterion and altered the permeability of the tablet and thus its hydratation, resulting in different release rates.
Fig. 8.
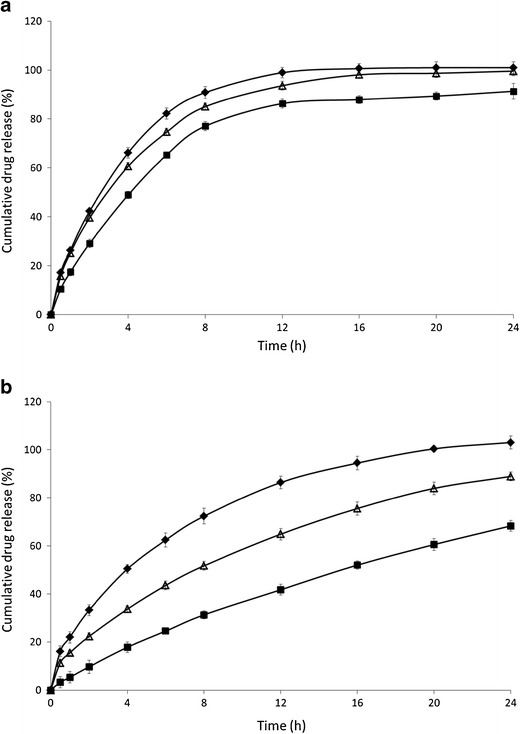
Influence of metoprolol salt on drug release. Mean dissolution profile (±SD) of formulations containing 70% Eudragit RL (a) or Eudragit RS (b) with 30% metoprolol salt: tartrate (filled diamond), succinate (empty triangle), fumarate (filled square)
Metoprolol Tartrate Bioavailability
Oral administration of IM tablets containing 30% MPT and 70% Eudragit RS (formulation F1) resulted in significantly lower pharmacokinetic parameters compared to the reference formulation (Fig. 9, Table V), yielding a relative bioavailability of only 42%. Intact tablets (which still contained 30.7 ± 8.4% of the MPT dose) were found in the faeces of the dogs, the low BA probably resulted from the fast GI transit time in dogs in combination with the limited fluids in the dog GI tract (thus limiting drug diffusion from these matrix tablets). To overcome these limitations, oral administration of a formulation containing a higher drug load (50/50% MPT/Eudragit RS, formulation F2) and having a faster in vitro drug release rate was also evaluated (100% release after 4 h), yielded similar pharmaco-kinetic parameters as reference formulation (Fig. 9, Table V), resulting in a relative bioavailability of 130%. Intact tablets were found in the faeces, but only 6.5 ± 2.5% of the initial dose MPT was recovered from these tablets, as the high drug load of these tablets promoted drug diffusion during passage in the GI tract.
Fig. 9.
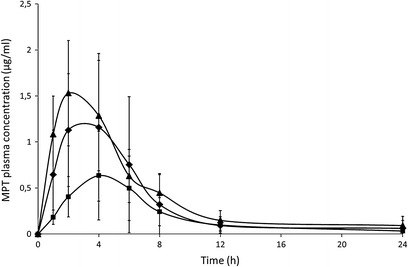
Mean plasma concentration-time profiles (±SD, n = 6, normalised for dose) after oral administration to dogs: one half tablet Slow-Lopressor® 200 Divitabs® (filled diamond), formulation 1 (filled square; 30% w/w MPT and 70% Eudragit RS), formulation 2 (filled triangle; 50% w/w MPT and 50% Eudragit RS)
Table V.
Mean Pharmacokinetic Parameters (±SD) of MPT after Oral Administration of Metoprolol Tartrate as Slow-Lopressor® 200 Divitabs® (F ref; dose, 100 mg), as Injection Moulded Tablet Composed of 30% w/w MPT and 70% Eudragit RS (F 1, dose: 110 mg), as Injection Moulded Tablet Composed of 50% w/w MPT and 50% Eudragit RS (F 2; dose, 185 mg) to dogs (n = 6)
| C max/D (μg/ml mg) | T max (h) | AUC0−24h/D (μg.h/ml.mg) | F rel (%) | |
|---|---|---|---|---|
| F ref | 1.3 ± 0.7 a | 2.8 ± 1.3 a | 9.6 ± 4.0 a | – |
| F 1 | 0.6 ± 0.3 b | 4.0 ± 0.8 b | 4.3 ± 2.8 b | 42.2 ± 22.3 a |
| F 2 | 1.5 ± 0.6 a | 2.0 ± 1.1 a | 11.1 ± 3.7 a | 130.5 ± 42.0 b |
The pharmacokinetic data were normalised for dose. Means in the same column with different superscript are different at the 0.05 level of significance
C max peak plasma concentration, T max time needed to reach the highest plasma level, F rel relative bioavailability
CONCLUSION
Despite being widely used in the plastic processing industry, injection moulding is a relative new technique to the pharmaceutical industry. The possibility of a continuous operation system with limited process processing steps, automation, and reduction in labour cost demonstrate that injection moulding is a versatile and promising production technology. This study showed that sustained release matrix tablets based on Eudragit RL and/or RS were successfully produced by means of injection moulding to control the drug release of different metoprolol salts. The drug release could be modulated by varying the matrix composition whereas the production temperature and plasticizer level did not affect drug release. All formulations showed first-order release kinetics and drug was released via a combination of swelling and diffusion (anomalous transport). The study showed that the metoprolol salt form had an impact on drug release, due to changes in matrix hydratation and permeability. Solubility parameters, thermal analysis, hot-stage microscopy and XRD ascertained that solid solution were formed after injection moulding, however, tablets containing high concentrations of metoprolol succinate and fumarate were not stable during storage, and re-crystallisation of the amorphous drug was seen. Metoprolol salts functioned as plasticizers for Eudragit RL/ RS, allowing lower processing temperatures thus making the process more economical in terms of energy input. Drug polymer interactions involved in these IM tablets included hydrophobic interactions, ionic interactions and H-bonding, offering valuable insights in the manufacturing of these dosage forms via hot-melt processing. The in vivo performance of injection moulded tablets depended on the drug dose, only in case of 50% MPT adequate drug plasma levels were observed.
REFERENCES
- 1.Quinten T, De Beer T, Remon JP, Vervaet C. Overview of injection molding as a manufacturing technique for pharmaceutical application. In: Kauffer PH, editor. Injection molding: process, design and application. New York: Nova; 2011. pp. 1–42. [Google Scholar]
- 2.Cheng L, Guo S, Wu W. Characterization and in vitro release of praziquantel from poly(ε-caprolactone) implants. Int J Pharm. 2009;377:112–9. doi: 10.1016/j.ijpharm.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Zema L, Loreti G, Melocchi A, Maroni A, Gazzania A. Injection molding and its application to drug delivery. J Control Release. 2012;159:324–31. doi: 10.1016/j.jconrel.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Quinten T, De Beer T, Onofre FO, Mendez-Montealvo G, Wang YJ, Remon JP, et al. Sustained-release and swelling characteristics of xanthan gum/ethylcellulose-based injection moulded matrix tablets: in vitro and in vivo evaluation. J Pharm Sci. 2011;100:2858–70. doi: 10.1002/jps.22480. [DOI] [PubMed] [Google Scholar]
- 5.Quinten T, De Beer T, Vervaet C, Remon JP. Evaluation of injection moulding as a pharmaceutical technology to produce matrix tablets. Eur J Pharm Biopharm. 2009;71:145–54. doi: 10.1016/j.ejpb.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Quinten T, Gonissen Y, Cnudde V, Masschaele B, Van Hoorebeke L, Remon JP, et al. Development of injection moulded matrix tablets based on mixtures of ethylcellulose and low-substituted hydroxypropylcellulose. Eur J Pharm Sci. 2009;37:207–16. doi: 10.1016/j.ejps.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Quinten T, De Beer T, Almeida A, Vlassenbroeck J, Van Hoorebeke L, Remon JP, et al. Development and evaluation of injection moulded sustained release tablets containing ethylcellulose and polyethylene oxide. Drug Dev Ind Pharm. 2011;37:149–59. doi: 10.3109/03639045.2010.498426. [DOI] [PubMed] [Google Scholar]
- 8.Andrews GP, Abudiak OA, Jones DS. Physicochemical characterisation of hot melt extruded bicalutamide-polyvinylpyrrolidone solid dispersions. J Pharm Sci. 2010;99:1322–35. doi: 10.1002/jps.21914. [DOI] [PubMed] [Google Scholar]
- 9.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 10.Schilling SU, Bruce CD, Shah NH, Malick AW, McGinity JW. Citric acid monohydrate as a release-modifying agent in melt extruded matrix tablets. Int J Pharm. 2008;361:158–68. doi: 10.1016/j.ijpharm.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Zhu YC, Shah NH, Malick AW, Infeld MH, McGinity JW. Solid-state plasticization of an acrylic polymer with chlorpheniramine maleate and triethyl citrate. Int J Pharm. 2002;241:301–10. doi: 10.1016/S0378-5173(02)00244-2. [DOI] [PubMed] [Google Scholar]
- 12.Gordon S, Taylor JS. Ideal copolymers and the second-order transitions of synthetic rubbers. I Non-crystalline copolymers. J Appl Chem. 1952;2:493–500. doi: 10.1002/jctb.5010020901. [DOI] [Google Scholar]
- 13.Suhma R, Boyer RF. General relation involving the glass transition temperature and coefficient of expansion of polymers. J Chem Phys. 1962;37:1003–7. doi: 10.1063/1.1733201. [DOI] [Google Scholar]
- 14.De Brabander C, Van Den Mooter G, Vervaet C, Remon JP. Characterization of ibuprofen as a nontradional plasticizer of ethyl cellulose. J Control Release. 2003;91:1678–85. doi: 10.1002/jps.10159. [DOI] [PubMed] [Google Scholar]
- 15.Breitzkreus J. Prediction of intestinal drug absorption properties by three-dimensional solubility parameters. Pharm Res. 1998;15:1370–5. doi: 10.1023/A:1011941319327. [DOI] [PubMed] [Google Scholar]
- 16.Blomqvist I, Westergren G, Sandberg A, Jonsson UE, Lundborg P. Pharmacokinetics and pharmacodynamics of controlled release metoprolol: a comparison with atenolol. Eur J Clin Pharmacol. 1988;33:S19–24. doi: 10.1007/BF00578408. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Semple HA, Song J. Determination of metoprolol, and its four metabolites in dog plasma. J Chromatogr B. 2004;809:9–14. doi: 10.1016/j.jchromb.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Lin SY, Yu HL. Microscopic Fourier transform infrared/differential scanning calorimetry systems used to study the different thermal behaviours of polymethacrylate copolymers of Eudragit RS, RL, E30D or E. J Appl Polym Sci. 2000;78:829–35. doi: 10.1002/1097-4628(20001024)78:4<829::AID-APP160>3.0.CO;2-2. [DOI] [Google Scholar]
- 19.Hancock BC, York P, Raymond CR. The use of solubility parameters in pharmaceutical dosage form design. Int J Pharm. 1997;148:1–21. doi: 10.1016/S0378-5173(96)04828-4. [DOI] [Google Scholar]
- 20.Greenhalgh D, Williams AC, Timmins P, York P. Solubility parameters as predictors of miscibility in solid dispersions. J Pharm Sci. 1999;88:1182–90. doi: 10.1021/js9900856. [DOI] [PubMed] [Google Scholar]
- 21.Chokshi RJ, Sandhu HK, Iyer RM, Shah NH, Malick AW, Zia H. Characterization of physico-mechanical properties of indomethacin and polymers to assess their suitability for hot-melt extrusion process as a means to manufacture solid dispersion/solution. J Pharm Sci. 2005;94:2463–74. doi: 10.1002/jps.20385. [DOI] [PubMed] [Google Scholar]
- 22.Forster A, Hempenstall J, Tucker I, Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int J Pharm. 2001;226:147–61. doi: 10.1016/S0378-5173(01)00801-8. [DOI] [PubMed] [Google Scholar]
- 23.Forster A, Hempenstall J, Rades T. Characterisation of glass solutions of poorly soluble drugs produced by melt extrusion with hydrophilic amorphous polymers. J Pharm Pharmacol. 2000;53:303–15. doi: 10.1211/0022357011775532. [DOI] [PubMed] [Google Scholar]
- 24.Chokski RJ, Shah NH, Sandhu HK, Malick AW, Zia H. Stabilisation of low glass transition temperature indomethacin formulations: impact of polymer-type and its concentration. J Pharm Sci. 2007;97:2286–98. doi: 10.1002/jps.21174. [DOI] [PubMed] [Google Scholar]
- 25.Glaessl B, Siepmann F, Tucker I, Siepmann J, Rades T. Characterisation of quaternary polymethacryalate films containing tartaric acid, metoprolol free base or metoprolol tartrate. Eur J Pharm Biopharm. 2009;73:366–72. doi: 10.1016/j.ejpb.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Weyer L, Lo S-C. Handbook of vibrotional spectroscopy: spectra—structure correlations in the near-infrared. New York: Wiley; 2006. [Google Scholar]
- 27.Carli F, Capone G, Colombo I, Magarotto L, Motta A. Surface and transport properties of acrylic polymers influence drug release from porous matrices. Int J Pharm. 1984;21:317–29. doi: 10.1016/0378-5173(84)90190-X. [DOI] [Google Scholar]
- 28.Glaessl B, Siepmann F, Tucker I, Rades T, Siepmann J. Deeper insight into the drug release machanism in Eudragit RL-based delivery systems. Int J Pharm. 2010;389:139–46. doi: 10.1016/j.ijpharm.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Ragnarsson G, Sandberg A, Johansson MO, Lindstedt B, SJögren J. In vitro release characteristics of a membrane-coated pellet formulation—influence of drug solubility and particle size. Int J Pharm. 1992;79:223–32. doi: 10.1016/0378-5173(92)90114-H. [DOI] [Google Scholar]
- 30.Ravishankar H, Patil P, Samel A, Petereit H-U, Lizio R. Controlled release by permeability alteration of cationic ammonio methacrylate copolymers using ionic interactions. Drug Dev Ind Pharm. 2006;32:709–18. doi: 10.1080/03639040600637556. [DOI] [PubMed] [Google Scholar]
- 31.Wagner KG, McGinity JW. Influence of chloride ion exchange on the permeability and drug release of Eudragit RS 30 D films. J Control Release. 2002;82:385–97. doi: 10.1016/S0168-3659(02)00145-1. [DOI] [PubMed] [Google Scholar]


