Abstract
The transport of proteins through skin can be facilitated potentially by using terpenes as chemical enhancers. However, we do not know about the effects of these enhancers on the stability and biological activity of proteins which is crucial for the development of safe and efficient formulations. Therefore, this project investigated the effects of terpene-based skin penetration enhancers which are reported as nontoxic to the skin (e.g., limonene, p-cymene, geraniol, farnesol, eugenol, menthol, terpineol, carveol, carvone, fenchone, and verbenone), on the conformational stability and biological activity of a model protein lysozyme. Terpene (5% v/v) was added to lysozyme solution and kept for 24 h (the time normally a transdermal patch remains) for investigating conformational stability profiles and biological activity. Fourier transform infrared spectrophotometer was used to analyze different secondary structures, e.g., α-helix, β-sheet, β-turn, and random coil. Conformational changes were also monitored by differential scanning calorimeter by determining midpoint transition temperature (Tm) and calorimetric enthalpy (ΔH). Biological activity of lysozyme was determined by measuring decrease in A450 when it was added to a suspension of Micrococcus lysodeikticus. The results of this study indicate that terpenes 9, 10, and 11 (carvone, l-fenchone, and l-verbenone) decreased conformational stability and biological activity of lysozyme significantly (p < 0.05) less than other terpenes used in this study. It is concluded that smaller terpenes containing ketones with low lipophilicity (log Kow ∼2.00) would be optimal for preserving conformational stability and biological activity of lysozyme in a transdermal formulation containing terpene as permeation enhancer.
Key words: conformational stability, lysozyme, penetration enhancers, protein, terpene
INTRODUCTION
Over the years, transdermal route has become one of the favored routes of delivering therapeutically effective drugs due its several advantages over conventional delivery methods such as bypassing the first-pass effect, sustained release of drugs over a period of time, and a better patient compliance (1). Transdermal patches containing hormones are being studied by scientists all over the world to exploit these advantages. A transdermal patch containing gestodene and ethinylestradiol is a convenient non-oral contraceptive which showed sustained release for a period of 7 days (2). Many cosmetic products available in the market contain various proteins, e.g., keratin, kinetin, interferon alpha, etc. as the active ingredient (3).
However, the delivery of protein/peptide-based therapeutics through the skin has been problematic due to the barrier functionality of top most layer of the skin, i.e., stratum corneum (4). The stratum corneum is made up of dead cells which mostly consisted of lipids and protein keratin. Therefore, these cells have the dual ability to absorb water as well as avoid loss of moisture. A drug has to pass through the continuous layer of intracellular lipids and intercellular proteins to reach the systemic circulation via the skin. Several methods have been used to overcome the barrier property of skin to accelerate the flow of proteins/peptides across the skin which involves the reversible and transient perturbation of barrier integrity of stratum corneum by using some kind of skin penetration enhancers (5–7). One of the techniques used to increase drug permeability through the skin is the use of “chemical penetration enhancers” (5). Various chemical penetration enhancers are used to increase the permeability of drugs through the skin barrier into the blood circulation. One of the most investigated substances is terpene penetration enhancers (8,9).
An ideal skin penetration enhancer should reversibly affect the structure of the skin (10). The various terpenes used to increase penetration include menthol, neomenthol, thymol, limonene, eugenol, carvone, farnesol, nerolidol, verbenone, l-fenchone, geraniol, etc. Terpenes have been used in transdermal patches as penetration enhancers to increase the permeation of various peptides and proteins such as the tripeptide thyrotropin-releasing hormone, luteinizing hormone-releasing hormone agonists, arginine vasopressin, and insulin (11–13). Terpenes act as penetration enhancer by disrupting the stratum corneum lipid structure (9). The terpenes have a high Poctanol/water value, thus the terpenes remain in the lipid portion of the stratum corneum; thus, fluidizing or perturbing the integrity of the barrier function of stratum corneum and thereby, facilitating the transport of drugs through skin.
Generally, the smaller terpenes are found to be more active permeation enhancers than the larger one such as sesquiterpenes. Hydrocarbon or nonpolar group containing terpenes such as limonene provide better enhancement for lipophilic drugs than do polar terpenes. Conversely, terpenes containing polar groups such as menthol and 1,8-cineole provide better enhancement for hydrophilic drugs (14). Therefore, terpenes are having potential for enhancing percutaneous absorption of therapeutic proteins from transdermal delivery systems which cannot be delivered orally due to its unique pharmacokinetic characteristics and stability issues (15). However, there is a dearth of data on effect of terpene on the stability and biological activity of proteins which must be protected to make a delivery system efficient and successful. Therefore, this project investigated the effects of terpene-based skin penetration enhancers, known to be nontoxic to skin (e.g., fenchone, carveol, menthol, cymene, terpineol, limonene, eugenol, carvone, farnesol, geraniol, and verbenone), on the conformational stability and biological activity of a model protein lysozyme so that we can have an understanding of the structural characteristics of terpenes optimal for use in a transdermal formulation of protein.
MATERIALS AND METHODS
Materials
Lysozyme (EC 3.2.1.17) from chicken egg white and Micrococcus lysodeikticus (Micrococcus luteus) were purchased from Sigma Chemical Company, St. Louis, Missouri. Micro bicinchoninic acid (BCA) protein assay reagent kit was purchased from Pierce Biotechnology, Inc., Rockford, Illinois. All the terpenes used in this study were purchased from Acros Organics, New Jersey, USA.
Methods
Preparation of Lysozyme Penetration Enhancers’ Formulations
Lysozyme was dissolved at a concentration of 1 mg/mL (0.07 mM) in the citrate–phosphate buffer (pH 4.4, 72.2 mM) (16). The terpene (5% v/v) was added to lysozyme solution and kept for 24 h (the time normally a transdermal patch remains) at 37°C which was used for investigating its conformational stability profiles by Fourier transform infrared (FTIR) spectrophotometer and differential scanning calorimeter (DSC) and biological activity by enzymatic assay using M. luteus as substrate. All the terpenes used in this study were assigned a number 1–11 as shown in Table I which was used to indicate a particular terpene in this study.
Table I.
List of Terpenes Used for Investigating Their Effect on Lysozyme
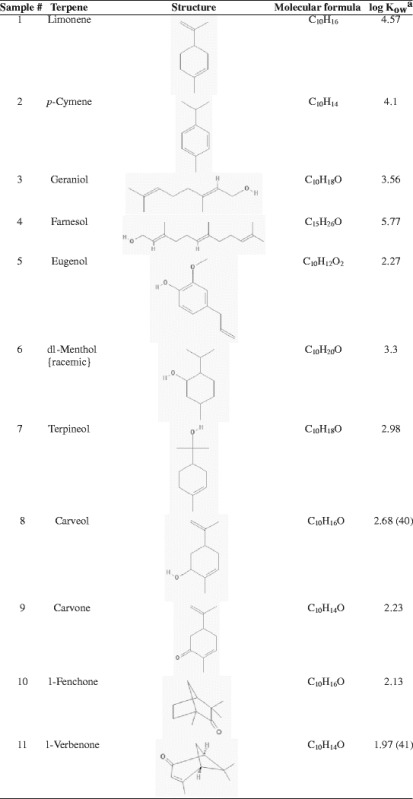
aAll log K ow values and structures are from PubChem database unless mentioned otherwise
Evaluation of Conformational Stability by Fourier Transform Infrared Spectrophotometer
Fifty microliters of the lysozyme samples was placed in the sample cell of IR Prestige FTIR (Shimadzu, Kyoto, Japan) spectrophotometer. All spectra analyzed represented an average of 15 scans with resolution of 4 cm−1. All spectra were taken in near infrared region which excluded the water band and entirely focused on amide bands of lysozyme under investigation. This had added advantage of being a nondestructive and noninvasive method. Amide I bands were derivatized and analyzed for quantitative estimation of different secondary structures like α-helix, β-sheet, β-turn, and random coil by using Shimadzu IR Solution 1.10 (Shimadzu, Kyoto, Japan) program.
Thermodynamic Stability Investigation by Differential Scanning Calorimeter
A sample containing lysozyme and terpene was centrifuged (4,229×g) for 20 min to remove any insoluble material. Supernatant was filtered through a 0.1-μm polytetrafluoroethylene filter (Millipore Corp., Bedford, MA). All samples and buffers were degassed by stirring under vacuum before loading into the sample and reference cells of an ultra-sensitive DSC (VP-DSC, MicroCal, Northampton, Massachusetts). The heat flow required for keeping the sample cell and reference cell thermally balanced was recorded from 10°C to 90°C using scan rate of 1.5°C/min. To ensure that the heat transition in protein is the only source of thermal difference between sample cell and reference cell, a baseline thermogram was obtained by loading the buffer in both sample cell and reference cell. This baseline was subtracted from the sample thermogram during data analysis. Midpoint transition temperature (Tm) and calorimetric enthalpy (ΔH) were used as conformational stability indicating thermodynamic parameters. A decrease in ΔH and Tm of the lysozyme was interpreted as an indication of destabilizing effect provided by different terpenes. All data manipulations were performed by using Origin software (MicroCal) provided with the DSC.
Determination of Biological Activity of Lysozyme
Biological activity of lysozyme was measured using a previously reported method (17). Briefly, a M. luteus stock suspension (0.01% w/v) was prepared in potassium phosphate buffer (0.66 mM and pH 6.24) which was suitably diluted so that it had an absorbance between 0.2 and 0.6 at 450 nm. Two and one half milliliters of this diluted M. luteus suspension was taken into a spectrophotometer cell and 0.1 mL of an appropriately diluted lysozyme sample/blank (200–400 units/mL) was added to it. The resulting rate of decrease of absorbance at 450 nm was monitored by UV spectrophotometer during a total incubation period of 5 min at 25°C. Slope (ΔA450 nm/min) of the linear portion of the curve between A450 nm and time was used to calculate the biological activity of lysozyme in enzyme unit (EU). A decrease of 0.001 A450 nm/min was defined as 1 EU. Biological activity of lysozyme in terms of EU per milliliter was determined by using following equation:
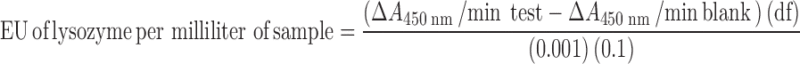 |
1 |
where df is the dilution factor; 0.001 is the change in absorbance at A450 nm as per the unit definition; and 0.1 is the volume (in milliliter) of the sample/standard used.
Amount of lysozyme in samples was determined by BCA method (18) and was used for the calculation of specific enzyme activity (in EU per milligram) of lysozyme by using the following equation:
 |
2 |
Data Analysis
Statistical comparisons were made using Student’s t test and analysis of variance. The level of significance was p < 0.5.
RESULT AND DISCUSSION
FTIR Spectroscopic Investigation of Lysozyme Samples Containing Terpenes
Table I shows the terpenes used in this study for their influence on conformational stability and biological activity of proteins using a model protein lysozyme. FTIR spectrophotometer was used to investigate the conformational changes in the lysozyme sample mixed with different terpenes. FTIR spectroscopy is extensively used for investigating changes in secondary structural organization of a protein (19,20). We focused on regions 1,600–1,700 cm−1 because different secondary protein structures absorb IR radiation in this region as shown in Table II (21). Table III shows the characteristic peaks in lysozyme control solution which represent various secondary structural components which have been used as baseline for comparing the locations and nature of corresponding peaks in lysozyme solutions exposed to terpenes. Peak splitting or shifting which could be either hypsochromic (shifting of peak towards lower wavelength, i.e., higher wavenumber) or bathochromic (shifting of peak towards higher wavelength, i.e., lower wavenumber) is related with protein instability (22). Table IV represents the β-sheet splitting and shift in β-turn in lysozyme samples containing 5% v/v terpenes for 24 h. β-sheet splitting, hypsochromic, shift, and absence of one of the peaks for β-turn were observed (Table IV) more prominently except samples 9 and 10 where all the three characteristic peaks for β-turn were observed. However, there were no β-sheet splitting in ten, whereas in nine, β-sheets were splitted into two peaks appearing at 1,620 and 1,631 cm−1. There is only one peak representative of β-turn present in sample 4 vs 3 in the control sample which might indicate greater damage to conformational integrity of lysozyme than other terpene samples which corroborated by the DSC and biological activity experimental results.
Table II.
A Range of Wavenumbers Characteristic of a Specific Secondary Structure in FTIR Spectrum of a Protein
| Secondary structure | Wavenumber (cm−1) |
|---|---|
| β-sheets | 1,620–1,640 |
| Random coils | 1,640–1,650 |
| α-Helices | 1,650–1,660 |
| β-Turns | 1,660–1,695 |
Table III.
Representative Secondary Structure Peaks in a Control Lysozyme Solution
| Peaks (cm−1) | Height (IU × 10−3) | Area (IU × 10−2) | Secondary structures |
|---|---|---|---|
| 1,624 | 425 | 4,708 | Beta-sheet |
| 1,649 | 518 | 4,327 | Random coil |
| 1,656 | 689 | 3,183 | Alpha-helix |
| 1,666 | 279 | 1,812 | Beta-turn |
| 1,680 | 760 | 6,806 | Beta-turn |
| 1,687 | 775 | 6,805 | Beta-turn |
IU independent unit
Table IV.
Beta-Sheet Splitting and Beta-Turn Shift in Lysozyme Solution Exposed to Terpene for 24 h
| Samples | Beta-sheet splitting (cm−1) | Beta-turn shift (cm−1) | Samples | Beta-sheet splitting (cm−1) | Beta-turn shift (cm−1) |
|---|---|---|---|---|---|
| 1 | 1,620 | 1,670 (H) | 7 | 1,616 | 1,678 (B) |
| 1,639 | 1,681 (H) | 1,631 | 1,689 (H) | ||
| 2 | 1,624 | 1,670 (H) | 8 | 1,635 | 1,674 (H) |
| 1,629 | 1,685 (H) | 1,681 (H) | |||
| 3 | 1,627 | 1,662 (B) | 9 | 1,620 | 1,666 (H) |
| 1,631 | 1,674 (B) | 1,631 | 1,685 (H) | ||
| 4 | 1,623 | 1,678 (H) | 1,697 (H) | ||
| 1,639 | 10 | 1,620 | 1,662 (B) | ||
| 5 | 1,622 | 1,670 (H) | 1,681 (H) | ||
| 1,629 | 1,685 (H) | 1,697 (H) | |||
| 6 | 1,618 | 1,666 (B) | 11 | 1,627 | 1,687 (H) |
| 1,627 | 1,689 (H) | 1,697 (H) |
Protein stability is reported to get increased with improvement in β-turns achieved by substituting other residues with Pro which is statistically preferred in β-turn structures (23). Beta-sheets are consisted of β-strands (extended polypeptide strands) connected by a network of hydrogen bonds and found widely in proteins. The intermolecular interactions between these hydrogen-bonding edges of beta-sheets are involved in biomolecular recognition, stabilization of protein quaternary structure, and protein aggregation. Moreover, the β-sheet interactions have been implicated in many diseases such as AIDS, cancer, Alzheimer’s, etc. (24). Therefore, we focused on these two secondary structural components—β-sheet and β-turn—of lysozyme while interpretations of FTIR spectra in presence of terpenes.
Differential Scanning Calorimetric Evaluation of Lysozyme Solution Containing Terpenes
DSC has been extensively used for investigating the effect of formulation components and process parameters on conformational stability of proteins (25). The characterization of protein unfolding, using several biophysical methods, has led to the notion that a loss in compact structure resulting in nonnative conformational change has a dramatic effect on aggregation (26), deamidation (27), and oxidation (28)—mechanisms involved in protein destabilization resulting in loss or decrease in associated biological activity and in some cases altogether alteration in activity. Biophysical studies have provided information about the relationship between protein unfolding and degree of stability (29). Thermodynamic parameters such as Tm and ΔH have been used in evaluating the stability of a protein in presence of its formulation components and its interaction with an excipient (30). Therefore, in this study, they have been used for investigating the effect of terpene on conformational stability.
Figure 1 represents a typical DSC thermogram of a freshly prepared lysozyme control solution. A peak appears when a transition from folded to unfolded state happens due to absorption of heat which is characterized by thermodynamic parameters Tm and ΔH. DSC data in Table V indicate significantly (p < 0.05) greater Tm and ΔH for sample containing terpenes 9, 10, or 11 in comparison to samples containing other terpenes which are indicative of greater conformational stability of existing secondary structure.
Fig. 1.
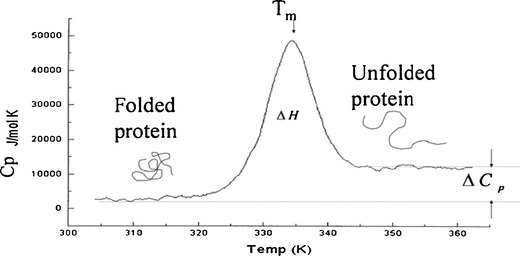
A typical DSC thermogram of a control solution of lysozyme
Table V.
Midpoint Transition Temperature and Calorimetric Enthalpy of Lysozyme Treated with Terpenes for 24 h
| Samples | Tm (°C) | Calorimetric enthalpy (Kcal) |
|---|---|---|
| (Mean ± SD; n = 3) | (Mean ± SD; n = 3) | |
| Control | 75.6 ± 2.8 | 95.9 ± 8.7 |
| 1 | 56.9 ± 1.9 | 40.3 ± 3.1 |
| 2 | 73.4 ± 1.1 | 41.1 ± 3.8 |
| 3 | 74.1 ± 1.9 | 43.5 ± 4.5 |
| 4 | 47.7 ± 0.5 | 13.5 ± 1.7 |
| 5 | 66.2 ± 0.7 | 64.7 ± 5.6 |
| 6 | 74.5 ± 1.6 | 45.8 ± 3.2 |
| 7 | 61.7 ± 1.2 | 49.8 ± 3.9 |
| 8 | 61.8 ± 0.9 | 65.6 ± 6.4 |
| 9 | 77.4 ± 1.2 | 81.6 ± 7.9 |
| 10 | 79.3 ± 1.8 | 89.8 ± 8.6 |
| 11 | 73.4 ± 1.2 | 79.8 ± 7.6 |
SD standard deviation
Biological Activity of Lysozyme Exposed to Terpenes
The buffered suspension of M. luteus is cloudy which exhibits high intensity absorbance at 450 nm. Lysozyme ruptures bacterial cell wall resulting in clearing of the cloudy suspension indicated by a decrease in absorbance. Thus, the measurement of rate of decrease in absorbance at 450 nm of M. luteus suspension is an indication of biological activity of lysozyme. A rate of decrease of 0.001 A450 nm/min was interpreted as 1 EU of lysozyme (17).
Figure 2 represents the specific enzyme activity of lysozyme solution mixed with different terpenes. We found significantly (p < 0.05) greater lysozyme activity in samples containing terpenes 9, 10, or 11 than other terpenes which indicate their greater compatibility with lysozyme. Terpenes 9, 10, and 11 contain ketone functional group and their log p values (2.23, 2.13, and 1.97, respectively) are lower than other terpenes (Table I) which may explain greater biological activity of lysozyme. Terpenes containing oxygen have been reported to oxidize methionine residues of a protein with greater oxidation by terpene containing oxygen in an epoxide ring (31). Terpenes 9, 10, and 11 contain oxygen as a ketone which is a weaker oxidant than terpenes containing hydroxyl or aldehyde functional groups. This may be the reason for greater biological activity determined for lysozyme samples containing terpenes 9–11.
Fig. 2.
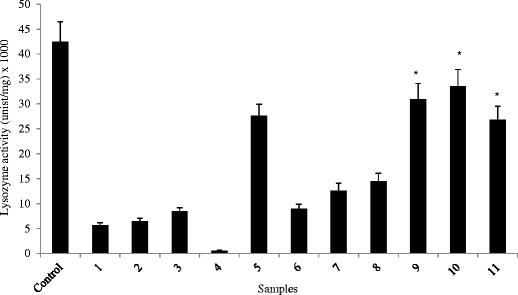
Biological activity of lysozyme containing 5% v/v terpene for 24 h. Samples containing terpenes 9, 10, and 11 were found to contain significantly (p < 0.05) greater biological activity than other samples
Although nonaqueous solvents generally destabilize protein, some of them with low lipophilicity can have an opposite effect, i.e., stabilize in low concentration (32). Therefore, terpene 4 which is most lipophilic than other terpenes used in this study (log Kow 5.31) and has a hydroxyl group significantly (p < 0.05) decreased the biological activity of lysozyme in comparison to rest of the terpenes. The percutaneous enhancement capacity of terpenes can be related with its structure and nature of permeates. Generally, terpenes with relatively greater lipophilicity provide better enhancement for lipophilic permeants while those with lower lipophilicity for hydrophilic permeants (14).
An aqueous solution of a folded protein has hydrophobic regions sequestered from and hydrophilic areas in contact with the aqueous environment. When the polarity of an aqueous solvent decreases by adding a nonaqueous solvent, protein hydrophobic cores tend to dissipate into the solvent, and the protein hydration shell may be disrupted which can cause destabilization and unfolding of protein (32,33). Therefore, terpenes 1–4 with greater lipophilicity than other terpenes caused greater destabilization of lysozyme resulting in significantly (p < 0.05) larger reduction in biological activity.
Lysozyme is the most widely studied and mechanistically best understood protein (34). Lysozyme is susceptible to conformational destabilization via various mechanisms such as aggregation, deamidation, and oxidation (35). Furthermore, lysozyme catalyzes the hydrolysis of its substrate, M. luteus, at a rate about 108-fold greater than that of the uncatalyzed reaction (36). Thus, it is uniquely suited for determining the influence of terpenes on its biological activity. Therefore, lysozyme was used in this study as a model protein for investigating the effect of various terpenes on its conformation stability and biological activity.
CONCLUSIONS
Although lipophilicity is a desirable property for enhancing percutaneous absorption (37) in view of predominantly hydrophobic make-up of stratum corneum, it may not be a favorable factor for preserving conformational stability as well as biological activity of a protein. Smaller sized terpenes such as d-limonene (C10H16) and 1,8-cineole (C10H18O) are reported to disrupt stratum corneum bilayer lipids, hence these potentially can work as an enhancer, whereas relatively large, long chain sesquiterpene such as nerolidol (C15H26O) reinforces the bilayers possibly by orienting alongside the stratum corneum lipids (14). Therefore, it is concluded that smaller terpenes containing ketones not alcohol such as 9, 10, and 11 with relatively lower lipophilicity are optimal for preserving conformation stability and biological activity of lysozyme while having potential of enhancing permeation through skin. Further studies utilizing more proteins varying in structural complexities are required to derive such a generalized effect for proteins.
Acknowledgments
This study was funded by the Health Future Foundation, Creighton University, Omaha, Nebraska.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Kalluri H, Banga AK. Transdermal delivery of proteins. AAPS PharmSciTech. 2011;12(1):431–441. doi: 10.1208/s12249-011-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sitruk-Ware R, Nath A. The use of newer progestins for contraception. Contraception. 2010;82(5):410–417. doi: 10.1016/j.contraception.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129(6):705–733. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson HA. Transdermal drug delivery: penetration enhancement techniques. Curr Drug Deliv. 2005;2(1):23–33. doi: 10.2174/1567201052772915. [DOI] [PubMed] [Google Scholar]
- 5.Wu F, Yang S, Yuan W, Jin T. Challenges and strategies in developing microneedle patches for transdermal delivery of protein and peptide therapeutics. Curr Pharm Biotechnol. 2012;13(7):1292–1298. doi: 10.2174/138920112800624319. [DOI] [PubMed] [Google Scholar]
- 6.Aungst BJ. Absorption enhancers: applications and advances. AAPS J. 2012;14(1):10–18. doi: 10.1208/s12248-011-9307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu T, Mitragotri S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc Natl Acad Sci U S A. 2011;108(38):15816–15821. doi: 10.1073/pnas.1016152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M, Ali A. Role of novel terpenes in transcutaneous permeation of valsartan: effectiveness and mechanism of action. Drug Dev Ind Pharm. 2011;37(5):583–596. doi: 10.3109/03639045.2010.532219. [DOI] [PubMed] [Google Scholar]
- 9.Sapra B, Jain S, Tiwary AK. Percutaneous permeation enhancement by terpenes: mechanistic view. AAPS J. 2008;10:120–132. doi: 10.1208/s12248-008-9012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purdon CH, Azzi CG, Zhang J, Smith EW, Maibach HI. Penetration enhancement of transdermal delivery-current permutations and limitations. Crit Rev Ther Drug Carrier Syst. 2004;21(2):97–132. doi: 10.1615/CritRevTherDrugCarrierSyst.v21.i2.20. [DOI] [PubMed] [Google Scholar]
- 11.Magnusson BM, Runn P, Karlsson K, Koskinen LD. Terpenes and ethanol enhance the transdermal permeation of the tripeptide thyrotropin releasing hormone in human epidermis. Int J Pharm. 1997;157(1):113–121. doi: 10.1016/S0378-5173(97)00235-4. [DOI] [Google Scholar]
- 12.Nair VB, Panchagnula R. The effect of pretreatment with terpenes on transdermal iontophoretic delivery of arginine vasopressin. Farmaco. 2004;59(7):575–581. doi: 10.1016/j.farmac.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Yerramsetty KM, Rachakonda VK, Neely BJ, Madihally SV, Gasem KA. Effect of different enhancers on the transdermal permeation of insulin analog. Int J Pharm. 2010;398(1–2):83–92. doi: 10.1016/j.ijpharm.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56(5):603–618. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Ruiz JM. Protein kinetic stability. Biophys Chem. 2010;148(1–3):1–15. doi: 10.1016/j.bpc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Stoll VS, Blanchard JS. Buffers: principles and practice. In: Deutscher M, Abelson J, editors. Guide to protein purification: methods in enzymology. London: Academic; 1990. pp. 24–37. [Google Scholar]
- 17.Shugar D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952;8(3):302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- 18.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 19.Du Z, Guan YX, Yao SJ, Zhu ZQ. Supercritical fluid assisted atomization introduced by an enhanced mixer for micronization of lysozyme: particle morphology, size and protein stability. Int J Pharm. 2011;421(2):258–268. doi: 10.1016/j.ijpharm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Petersen LK, Sackett CK, Narasimhan B. High-throughput analysis of protein stability in polyanhydride nanoparticles. Acta Biomater. 2010;6(10):3873–3881. doi: 10.1016/j.actbio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Vigano C, Manciu L, Buyse F, Goormaghtigh E, Ruysschaert JM. Attenuated total reflection IR spectroscopy as a tool to investigate the structure, orientation and tertiary structure changes in peptides and membrane proteins. Biopolymers. 2000;55(5):373–380. doi: 10.1002/1097-0282(2000)55:5<373::AID-BIP1011>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Brune DC, Gerola PD, Olson JM. Circular dichroism of green bacterial chlorosomes. Photosyn Res. 1990;24(3):253–263. doi: 10.1007/BF00032313. [DOI] [PubMed] [Google Scholar]
- 23.Fu H, Grimsley GR, Razvi A, Scholtz JM, Pace CN. Increasing protein stability by improving beta-turns. Proteins. 2009;77(3):491–498. doi: 10.1002/prot.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowick JS. Exploring beta-sheet structure and interactions with chemical model systems. Acc Chem Res. 2008;41(10):1319–1330. doi: 10.1021/ar800064f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen J, Arthur K, Chemmalil L, Muzammil S, Gabrielson J, Jiang Y. Applications of differential scanning calorimetry for thermal stability analysis of proteins: qualification of DSC. J Pharm Sci. 2012;101(3):955–964. doi: 10.1002/jps.22820. [DOI] [PubMed] [Google Scholar]
- 26.Singh SM, Molas JF, Kongari N, Bandi S, Armstrong GS, Winder SJ, et al. Thermodynamic stability, unfolding kinetics, and aggregation of the N-terminal actin binding domains of utrophin and dystrophin. Proteins. 2012;80(5):1377–1392. doi: 10.1002/prot.24033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suppavorasatit I, De Mejia EG, Cadwallader KR. Optimization of the enzymatic deamidation of soy protein by protein-glutaminase and its effect on the functional properties of the protein. J Agric Food Chem. 2011;59(21):11621–11628. doi: 10.1021/jf2028973. [DOI] [PubMed] [Google Scholar]
- 28.Lai M, Yam KC, Andrews D, Hill BC. Copper binding traps the folded state of the SCO protein from Bacillus subtilis. Biochim Biophys Acta. 2012;1824(2):292–302. doi: 10.1016/j.bbapap.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Phillips JJ, Javadi Y, Millership C, Main ER. Modulation of the multi-state folding of designed TPR proteins through intrinsic and extrinsic factors. Protein Sci. 2012;21(3):327–338. doi: 10.1002/pro.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein M, Aserin A, Ben Ishai P, Garti N. Interactions between whey protein isolate and gum Arabic. Colloids Surf B Biointerfaces. 2010;79(2):377–383. doi: 10.1016/j.colsurfb.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Maleknia SD., Adams MA. Reactions of oxygen-containing terpenes with peptides and proteins. Proceedings of the 4th International Peptide Symposium, Australian Peptide Association, Australia, 2007; 334–335.
- 32.Wang W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int J Pharm. 1999;185(2):129–188. doi: 10.1016/S0378-5173(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 33.Riske F, Hamilton A, Zhang C, Hayes M. Remodeling the oligosaccharides on β-glucocerebrosidase using hydrophobic interaction chromatography and applications of hydroxyl ethyl starch for improving remodeling and enhancing protein stability. Biotechnol Bioeng. 2012;109(5):1217–1227. doi: 10.1002/bit.24406. [DOI] [PubMed] [Google Scholar]
- 34.Merlini G, Bellotti V. Lysozyme: a paradigmatic molecule for the investigation of protein structure, function and misfolding. Clin Chim Acta. 2005;357(2):168–172. doi: 10.1016/j.cccn.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Singh J. Effect of polyols on the conformational stability and biological activity of a model protein lysozyme. AAPS PharmSciTech. 2003;4(3):E42. doi: 10.1208/pt040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voet D, Voet D. Biochemistry. New York: Wiley; 1995. pp. 381–383. [Google Scholar]
- 37.Kang L, Yap CW, Lim PF, Chen YZ, Ho PC, Chan YW, et al. Formulation development of transdermal dosage forms: quantitative structure-activity relationship model for predicting activities of terpenes that enhance drug penetration through human skin. J Contr Release. 2007;120(3):211–219. doi: 10.1016/j.jconrel.2007.05.006. [DOI] [PubMed] [Google Scholar]


