Abstract
Patients admitted to the intensive care unit (ICU) can develop a condition referred to as “ICU-acquired weakness.” This condition is characterized by profound weakness that is greater than might be expected to result from prolonged bed rest. Intensive care unit–acquired weakness often is accompanied by dysfunction of multiple organ systems. Individuals with ICU-acquired weakness typically have significant activity limitations, often requiring physical assistance for even the most basic activities associated with bed mobility. Many of these individuals have activity limitations months to years after hospitalization. The purpose of this article is to review evidence that guides physical rehabilitation of people with ICU-acquired weakness. Included are diagnostic criteria, medical management, and prognostic indicators, as well as criteria for beginning physical rehabilitation, with an emphasis on patient safety. Data are presented indicating that rehabilitation can be implemented with very few adverse effects. Evidence is provided for appropriate measurement approaches and for physical intervention strategies. Finally, some of the key issues are summarized that should be investigated to determine the best intervention guidelines for individuals with ICU-acquired weakness.
Intensive care unit–acquired weakness, as the name implies, develops in individuals who are admitted to the intensive care unit (ICU) for a variety of reasons, commonly including sepsis and acute respiratory distress syndrome (ARDS). Intensive care unit–acquired weakness may develop if an individual is on mechanical ventilation for as little as 4 to 7 days.1,2 Recent efforts have been directed at developing clear diagnostic criteria and characterizing the elements of physical rehabilitation while individuals are in the ICU.3 As such, it is timely to review the condition and physical rehabilitation strategies and to provide guidance regarding physical therapist examination, evaluation, intervention, and outcomes for individuals with ICU-acquired weakness.
The term “ICU-acquired weakness” is the most recent in a series of terms that have been used to describe this condition.3 Specific manifestations of this condition also are discussed under other terms, including “critical illness myopathy” (CIM), “critical illness polyneuropathy” (CIP), or a combination referred to as “critical illness polyneuromyopathy” (CIPNM or CINM).1,3 We use the term “ICU-acquired weakness” except when discussing literature related to specific diagnosis of CIP, CIM, and CINM.
Individuals with CIP have impairments of the neuromuscular system, including weakness, reduced deep tendon reflexes (DTRs), and impaired pain, temperature, and vibratory sense.1,4 Cranial nerves typically are spared; however, facial weakness is common.2,5,6 Individuals with CIM exhibit profound weakness, especially of proximal muscles. Deep tendon reflexes may be preserved or diminished. However, in contrast to CIP, sensation is intact. More often than not, patients exhibit mixed findings of both CIP and CIM, with affected individuals showing a mixture of the clinical, electrophysiological, and pathophysiologic changes of both CIP and CIM.1,2,7
Critical illness polyneuropathy is associated with abnormal nerve conduction studies; hence, electrodiagnostic testing is crucial for confirming the diagnosis and excluding other conditions that may mimic CIP.2,5 Specifically, reduction in sensory nerve action potential amplitudes and compound muscle action potential (CMAP) amplitudes reflect abnormal nerve excitability and axonal damage.2,5,8 Conduction velocities typically remain normal or are only mildly diminished. Focal slowing of conduction is not seen, and, if present, should raise concern for other problems such as acute inflammatory demyelinating polyneuropathy or traumatic neuropathies related to compression or direct injury due to invasive procedures.2,5
In patients with CIP, needle electromyographic (EMG) examination of affected muscles shows different changes depending on the timing of testing.2 Initially, recruitment of motor units is reduced, but this finding may be subtle in patients with mild weakness and often is difficult to assess early in disease due to poor cooperation in patients who have encephalopathy or are sedated. As CIP progresses, motor axon loss results in spontaneous activity. This loss is followed weeks to months later by small-amplitude reinnervation potentials, which develop into enlarged motor unit potentials as reinnervation continues over months.2,3
For patients with CIM, in contrast to those with CIP, nerve conduction studies demonstrate preserved sensory nerve action potentials.2 Compound muscle action potential amplitudes may be reduced, and the duration of the potentials may be prolonged, reflecting slowed muscle fiber conduction velocity.9 In some patients, muscle may be electrically inexcitable, and no muscle fiber action potentials can be recorded even with direct electrical stimulation of muscle.10 If the patient is able to activate the affected muscles, needle EMG examination shows nonspecific myopathic changes of small-amplitude, short-duration, polyphasic motor unit potentials that recruit in an early pattern.10 These morphological and recruitment changes are a reflection of the reduced force generated by unhealthy muscle fibers.
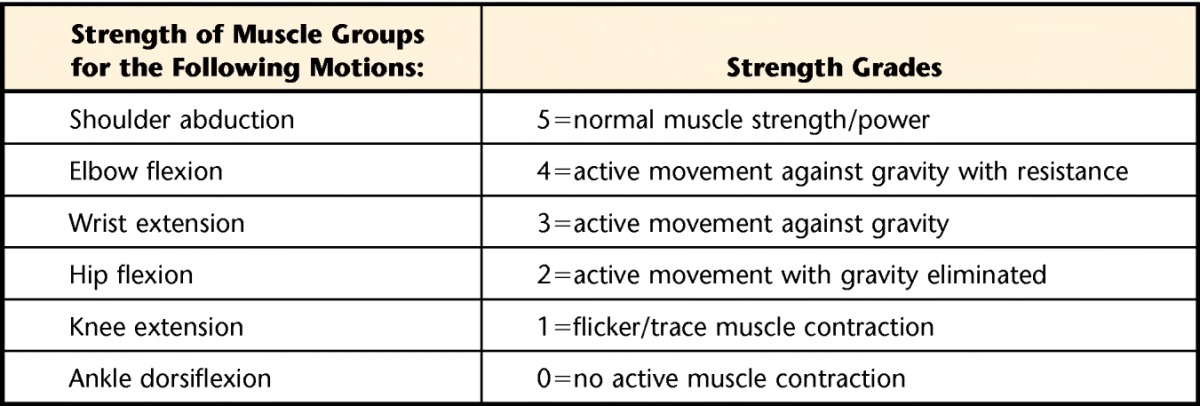
Changes in electrodiagnostic parameters during hospitalization may provide an early indication of evolving CIPNM.7 When electrodiagnostic testing is not available, ICU-acquired weakness has been inferred based on an average Medical Research Council (MRC) sum score of less than 48 (Tab. 1).3,11,12 This score is similar to an average manual muscle test (MMT) score of 4.0/5.0 based on the criteria of Kendall et al.14 The MRC has been validated and is reliable in individuals with peripheral and central nervous system dysfunction, as well as patients with critical illness.15 Weber-Carstens et al16 used CMAP following direct muscle stimulation to predict ICU-acquired paresis with a positive predictive value of 0.91, sensitivity of 83.3%, and specificity of 88.8%. These authors also used the CMAP of the peroneal nerve and found a positive predictive value of 0.69; pathological spontaneous activity of the tibialis anterior muscle had a positive predictive value of 0.94.16
Table 1.
Medical Research Council Scoring System13
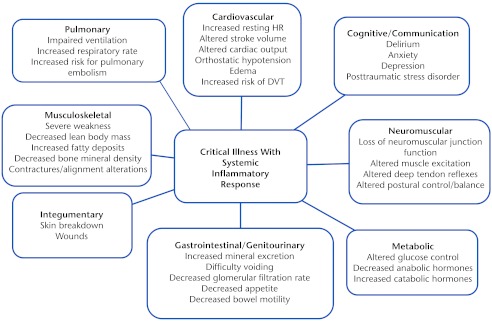
In addition to the neuromuscular and musculoskeletal systems, critical illness has the potential to affect multiple other systems, presumably because of the systemic inflammatory response associated with critical illness. Other systems that might be affected are the cardiopulmonary, cognitive, metabolic, genitourinary/gastrointestinal, and integumentary systems (Fig. 1).
Figure 1.
Potential body/structure effects of critical illness.1–3,6 HR=heart rate, DVT=deep vein thrombosis.
The incidence of ICU-acquired weakness varies substantially depending on the type of patients with critical illness studied, diagnostic criteria used, and timing of examination and diagnosis. De Jonghe et al17 found clinically significant ICU-acquired weakness in 25% of patients who were mechanically ventilated for at least 7 days. A prospective study of 50 patients who received mechanical ventilation for 7 days documented CIP in 58% of the individuals.18 Studies using cohorts restricted to sepsis and multi-organ failure have shown even higher incidences of neuromuscular disease, ranging from 50% to 100%.19–21
Medical Management
Although a variety of therapies may prevent the development of ICU-acquired weakness, no medical therapy is available once the condition has developed. Neuromuscular blocking agents and corticosteroids often are avoided to decrease the risk of ICU-acquired weakness, although there is no evidence supporting this recommendation.22 Insulin therapy alleviates the catabolic syndrome of prolonged critical illness because of its well-recognized anabolic properties, comprising stimulation of muscle protein synthesis and attenuation of protein breakdown.23 As part of a landmark intensive insulin therapy study of patients in the surgical ICU, weekly EMG studies were performed on patients who remained in the ICU for more than 7 days.23 Patients who received intensive insulin therapy were less likely to develop CIPNM (29%) than were those receiving conventional treatment (52%) (P<.001), and the condition resolved more rapidly in patients who did develop CIPNM. In a subsequent study of patients in the medical ICU exclusively, intensive insulin therapy again reduced the incidence of CIPNM from 51% to 39% (P=.02).24 However, in a large international randomized control trial of patients (N=6,020) in the ICU, Finfer et al25 reported that very tight glucose control was associated with increased mortality.
Sedative agents may mask symptoms or delay identification of ICU-acquired weakness by clinicians. In general, the use of daily interruption of sedative agents and limiting the administration and dosing of sedative agents improve the outcome of patients who are critically ill.26,27 Performing a neuromuscular examination while the sedative agents have been stopped may improve the clinician's ability to diagnose ICU-acquired weakness at an earlier time point.
Ouimet et al28 reported ICU delirium was linked to longer length of stay in both the ICU and hospital, as well as increased mortality for both. Hopkins and Jackson29 reviewed studies that evaluated neurocognitive outcomes following critical illness and found that associated cognitive changes negatively affected daily physical function, quality of life, and return to work. Schweickert et al30 reported that sedation vacation combined with early physical rehabilitation led to a shorter duration of ICU-associated delirium.
Evidence of delirium can be determined by the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU).31 The CAM-ICU uses nonverbal tasks, including picture recognition, vigilance levels, and response to simple questions and commands, and has excellent interrater reliability (κ=.96).31 The Richmond Agitation Sedation Scale also can be used to describe a patient's ability to interact.32 This 10-point continuous scale scores patients from −5 (unarousable) to +4 (combative). Interrater reliability is excellent (intraclass correlation coefficient=.92–.98), and the scale has been validated with the Ramsay Sedation Scale (r=−.78) and the Sedation Agitation Scale (r=.78).32
Prognosis
With regard to recovery of body systems, impairments of the respiratory, renal, and cardiovascular systems typically resolve.33,34 In contrast, recovery of neuromuscular impairments takes longer and may be incomplete. Even 6 months to 1 year after hospitalization, survivors frequently report weakness, fatigue, and cognitive changes.33,34 Fletcher et al35 followed a group of individuals with critical illness, all of whom were diagnosed with sepsis, had experienced failure of at least 2 organs, and were in the ICU for longer than 28 days. At follow-up (median=42.5 months), 6 of the 22 patients (27%) had sensory deficits, 4 (18%) had motor weakness, and 3 (14%) demonstrated both sensory and motor deficits.
Recovery of functional ability also takes time and may be incomplete. Indeed, substantial activity limitations are common and can persist for years after discharge from the ICU, as indicated by greatly reduced 6-minute walk distance. In 2 studies of survivors of ARDS, the 6-minute walk distance was around 66% of the expected value at 1 year33,36 even after pulmonary function tests had returned to normal levels; similarly, Cheung et al36 reported that 6-minute walk distance remained at 68% of the expected values even at 2 years.
Overall participation and health-related quality of life likewise may be compromised, and limitations may persist long after ICU discharge. Herridge et al33 reported that at 1 year the 36-Item Short-Form Health Survey (SF-36) domain of physical role was 25% of the age- and sex-predicted normal values, physical function was 60%, and vitality was 55%. Cheung et al36 reported that for survivors of ARDS, at 1 year the SF-36 physical role was 30% of age- and sex-predicted values and physical function was 67%. At 2 years, these values were 60% and 80% of predicted values. As long as 6 years after discharge from the ICU, Kaarola et al37 reported poorer quality of life on the RAND 36-Item Health Survey (a measure similar to the SF-36) for subscores of physical function, pain, and general health categories. Finally, Latronico and colleagues38 performed a meta-analysis of 36 smaller studies of adult patients with a clinical and electrophysiological diagnosis of CIPNM. Although each of these studies had a mean sample size of only 8 patients, long-term outcomes were available on a total of 263 patients, with a mean duration of follow-up of 3 to 6 months. Only 68% of the patients eventually reported complete functional recovery. Twenty-eight percent of the patients had persistent, severe disability that impeded independent ambulation. Milder abnormalities such as muscle atrophy and foot drop were common even in those patients with apparent complete functional recovery.
Considerable information still is needed to further understand the prognosis of patients who have ICU-acquired weakness. For example, it is possible that electrophysiological testing in the ICU will help to differentiate patients who have true ICU-acquired weakness from those who have simple deconditioning. By differentiating these 2 groups of patients, it will be possible to better understand prognosis and appropriate physical intervention for people with true ICU-acquired weakness. Furthermore, much remains to be learned regarding the impact on prognosis for functional change, both during the acute phase of the illness and in long-term follow-up. Two studies that are under way (National Institutes of Health [R01 NR-11051; ClinicalTrials.gov registration: NCT01058421] and the Australian and New Zealand Clinical Trials Network [ACTRN12605000776606]) have the potential to provide some of these answers.
In summary, long after resolution of the critical illness, individuals with ICU-acquired weakness clearly still may have substantial weakness and sensory deficits. These deficits are paralleled by limitations in activities, participation, and overall contextual factors. The extent of deficits is particularly troubling given the young age of many of these patients (mean age=45 years33–36). Of further concern is the lack of an accepted systematic approach to assist these individuals in regaining functional ability or adjusting to their altered circumstances in the absence of full recovery. This concern was discussed in a recent Society of Critical Care Medicine conference report,39 which described the need for greater awareness of ICU-acquired weakness for rehabilitation strategies across the continuum of care and for expansion of research and resources to address the long-term consequences experienced by patients and families.
Physical Management
A number of recent investigations provide guidance regarding rehabilitation strategies for people with ICU-acquired weakness. Although many questions yet remain and robust, randomized controlled physical intervention studies are needed, these investigations help to guide decisions regarding the following: (1) criteria for beginning physical rehabilitation, (2) measurement approaches, and (3) physical intervention strategies. Key findings are summarized below.
Criteria for Beginning Physical Rehabilitation
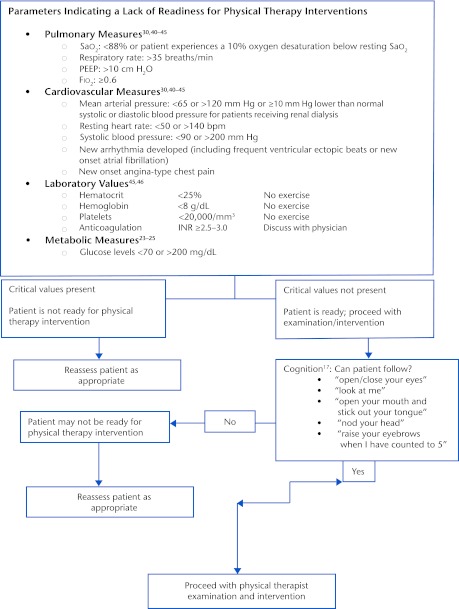
Sufficient evidence now is available to suggest that physical rehabilitation for individuals with ICU-acquired weakness can begin as soon as they have sufficient medical stability to accommodate the increased vascular and oxygen demands that accompany the physical examination and intervention.30,40–44 Figure 2, based on available reports, provides an algorithm for decisions throughout the encounter, beginning with the decision about when to begin rehabilitation and progressing through the intervention. The criteria are consistent with those in a recent recommendation by Hanekom et al,45 based on a recent synthesis of evidence and expert opinion.
Figure 2.
Decision algorithm. Sao2=oxyhemoglobin saturation, PEEP=positive end-expiratory pressure, Fio2=fraction of inspired oxygen, INR=international normalized ratio.
A number of studies have been conducted to examine the benefits and adverse effects of early physical rehabilitation for people in the ICU. As shown in Table 2, investigators used a wide range of criteria for including participants in their studies (eg, any patient in the ICU longer than 5 days,44 patients on mechanical ventilation for at least 72 hours,30,43 patients on mechanical ventilation longer than 4 days42). The incidence of adverse effects in these studies was very low, ranging from less than 1% of sessions41 to 16% of sessions,43 depending on whether only more serious adverse events or all events that led to terminating the intervention early were taken into consideration. Criteria for stopping rehabilitation procedures are similar across studies. Figure 2 summarizes the criteria, based on a number of these studies, as well as guidelines from the American Physical Therapy Association.46
Table 2.
Overall Examination Schema for Intensive Care Unit–Acquired Weakness and Related Conditions
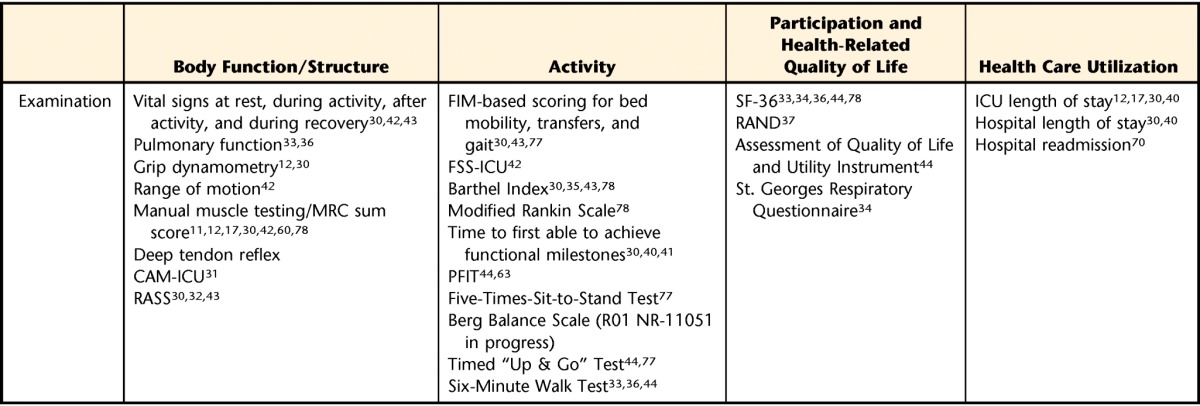
a FIM=Functional Independence Measure, SF-36=36-Item Short-Form Health Survey questionnaire, ICU=intensive care unit, FSS-ICU=Functional Status Score for the ICU, RAND=RAND 36-Item Health Survey, MRC=Medical Research Council, PFIT=Physical Function in the ICU Test, CAM-ICU=Confusion Assessment Method for the Intensive Care Unit, RASS=Richmond Agitations-Sedation Scale.
Based on the extent of multisystem involvement from the systemic inflammatory response, the medical acuity of patients in the ICU can fluctuate daily, hourly, and even by the minute. The condition can wax and wane, depending on the extent of the inflammatory responses. Hence, the first consideration in determining when to start physical rehabilitation is to ensure that the patient can tolerate rehabilitation from a physiological perspective. The moment-to-moment interpretation of a patient's physiological response to activity is termed “response-dependent management.”47 This approach recognizes that delivery of oxygen must equate to the consumption of oxygen by the body.47 Cellular research has demonstrated that even passive range of motion and upright positioning can increase the body's consumption of oxygen.48 Therefore, ongoing assessment of physiological responses (Fig. 2), is critical to determine that the amount of oxygen delivered is sufficient to meet the physical demands of bed level of activity and to establish whether the patient can tolerate the increased demands of movement into sitting and other upright positions.
While patients are critically ill and on mechanical ventilation, a number of respiratory values are continuously monitored, several of which are particularly important to the physical therapist, who proceeds with the physical examination and intervention only if the patient is sufficiently stable (Fig. 2). The fraction of inspired oxygen (Fio2) level is generally determined by how much oxygen is necessary to maintain a patient's oxyhemoglobin saturation (Spo2) at greater than 90%.49 Values for Fio2 range from 0% to 100%, with higher numbers indicative of greater oxygen needs by the patient. Because high levels of Fio2 are associated with risk for oxygen toxicity, it is important to balance patient safety with maintaining an Spo2 of greater than 90%.
Positive end-expiratory pressure (PEEP) refers to pressure required to prevent alveolar collapse at the end of expiration and usually will range from 0 to 24 cm H2O. Although there are no absolute contraindications regarding applied PEEP, there are risk factors such as increased intracranial pressure and intrapulmonary shunting.50 Additional ventilator settings may reflect degree of respiratory system compromise. Minute ventilation is a product of tidal volume and respiratory rate. When either high respiratory rate or large tidal volume is required to maintain oxygen demands, there is a risk of air trapping, which can lead to increased alveolar pressure at end of expiration. Higher tidal volumes are associated with barotraumas and increased risk of ventilator-associated pneumonia.51
The clinician also should be aware of mean arterial pressure, which is the amount of pressure required to maintain tissue perfusion. Due to cardiovascular system impairments, patients who are critically ill may require noradrenaline or vasopressor/inotrope medication.52 Currently, there is no established protocol for mobility during the use of these medications; however, physical therapists should be aware of the use of these medications to maintain adequate blood pressure and cardiac output.52 Denehy et al44 withheld physical rehabilitation if patients received >0.5 μg/min of noradrenaline, and Morris et al40 withheld intervention if a new vasopressor agent was begun. Korupolu et al53 recommended careful evaluation regarding risk and benefit of mobility when a new vasopressor agent is begun or an increase in dose occurs within 2 hours of planned activity.
Many patients with ICU-acquired weakness have been sedated to maintain hemodynamic and pulmonary stability, often resulting in compromised cognition (ie, ICU delirium).28,29 Physical examination and intervention can be initiated as long as patients can follow one-step commands3,11; however, they must be able to follow simple commands as outlined in Figure 2 in order to consider starting physical therapy interventions. If an individual is unable to maintain hemodynamic stability, active mobility may not be appropriate, although basic range of motion and bed mobility still are important.
Measurement Approaches
The elements of the examination are determined by the patient's physical functional activity. A core group of tests and measures have been reported in many investigations, including tests of body structure and function, activities, and participation. Many of these measures have been used with people diagnosed with ICU-acquired weakness, and others have been reported in related populations but should be considered for those with ICU-acquired weakness. These tests and measures have been reported by investigators, both of early rehabilitation and for survivors of up to 5 years, and were recently discussed in a review of instruments by Elliott and colleagues.54 They are summarized in Table 2 according to the International Classification of Functioning, Disability and Health (ICF)55 categories of body structure and function, activities, and participation, as well as health care utilization.
Body structure and function.
With regard to body structure and function, important measures include those related to strength, sensory status, DTRs, and respiratory status. Manual muscle testing13,15 and grip strength12 document the extent of muscle weakness. Handgrip also has been associated with increased risk of mortality,56 loss of independence,57 and longer hospital and ICU stays.58 Furthermore, grip strength is used in the diagnosis of patients with ICU-acquired weakness.12 Data from Ali et al12 suggest that grip strength of less than 7 kg for women and 11 kg for men is indicative of ICU-acquired weakness.
Intensive care unit–acquired weakness may affect respiratory muscles as well as peripheral muscles. In addition, mechanical ventilation for as little as 18 hours is associated with alterations in force production and muscle atrophy of the diaphragm, potentially further compromising the respiratory system.59 Furthermore, it is typically more difficult to wean patients with ICU-acquired weakness from mechanical ventilation.1,2,11 Thus, a careful assessment of the respiratory system is necessary, with implementation of appropriate treatment when indicated.
In addition to clinical assessment of breathing patterns and cough effectiveness, quantitative measures such as maximum inspiratory pressure (MIP) and maximum expiratory pressure (MEP) may be important indicators of the extent of neuromuscular involvement. De Jonghe et al60 found that MIP and MEP were poorly correlated (r=.31) to MRC sum score but were predictive of delayed extubation. Tzanis et al61 reported a MIP threshold of 36 cm H2O to be diagnostic of ICU-acquired weakness, with a sensitivity of 88% and a specificity of 76%. Maximal voluntary ventilation reflects the amount of air a patient can inhale and exhale in 1 minute and is helpful to gauge muscle endurance. This variable has not been reported by investigators to date but should be considered.
Assessment of DTRs is indicated because CIP and CIM both are associated with altered reflexes. Thus, assessment of DTRs can assist clinicians in interpreting whether a patient's weakness likely is associated with a primary dysfunction of the peripheral motor system or results from deconditioning due to bed rest or central nervous system damage. This distinction may be of importance in clinically differentiating patients who have critical care illnesses from those who are simply deconditioned, which, in turn, can assist the clinician in developing a realistic estimate of progress for motor and functional return and fall risk.
Assessment of the somatosensory system is not reported in most investigations, but is appropriate, given the impact of ICU-acquired weakness on that system. These findings assist the clinician in interpreting underlying causes of the patient's functional difficulties and may help with the interpretation of the patient's difficulties with specific activity limitations and in particular with difficulties of postural control.
Activity.
Several tests and measures provide insight into the patient's activity limitations and ability and are particularly useful for patients in the ICU. They include physical mobility components from the Functional Independence Measure (FIM),62 the Physical Function in the ICU Test (PFIT),63 and specific measures of gait and balance.54
Physical mobility components of the FIM30,42,43 have been used to monitor basic functional activities of people in the ICU. Tasks of the FIM are scored from 1 (total assist) to 7 (completely safe and independent).62 However, several items of the total FIM (eg, gait, stair negotiation) often cannot be assessed in an ICU setting; hence, a total score typically cannot be given. Although reliability of individual item scores has not been established, some authors have used items from the FIM to quantify functional mobility tasks relevant for patients in the ICU.30,42
The Functional Status Score for ICU (FSS-ICU) was developed specifically for use in this population, based on the FIM scoring system, but applied to tasks that are appropriate for patients in the ICU who are most limited.42 The tasks included in the FSS-ICU are rolling, supine-to-sit, sitting on edge of bed, sit-to-stand, and ambulation. Reliability and validity of this new measure have not yet been reported. The Acute Care Index of Function (ACIF) was developed to assess basic mobility and determine discharge locations for inpatients of neuromedical and surgical units.64 There are 4 subscales (mental status, bed mobility, transfers, and mobility), with intraclass correlation coefficients of .98 to 1.00 for the total score and the subscales. This scale may have utility for patients who have ICU-acquired weakness.
The PFIT was specifically developed for use with patients who are critically ill and consists of 4 items: amount of assistance for the sit-to-stand maneuver, strength for shoulder flexion and knee extension, marching in place, and an upper-extremity endurance task of arm elevation to 90 degrees of shoulder flexion.63 The amount of assistance required to stand is rated from 0 (no physical assistance required) to 3 (assistance of 3 people required). Strength for shoulder flexion and knee extension is rated on the Oxford Muscle Test Scale (1–5). For marching in place, the examiners record the number of steps taken and the time required to complete these steps. For the upper-extremity endurance component, the number of times both upper extremities are lifted above 90 degrees of shoulder flexion is recorded as well as the time to complete this task. This test63 can be used to guide exercise prescription and as an outcome measure.
Given the potential sensory and neuromuscular changes with ICU-acquired weakness, it is appropriate to measure balance. A number of tests have been used in related populations (eg, Berg Balance Scale,65 Timed “Up & Go” Test66), although outcome data have not yet been reported in patients with ICU-acquired weakness. In addition, given the long-term deficits associated with this disorder, the Five-Times-Sit-to-Stand Test,67 Two-Minute Walk Test68 (for people with substantial activity limitations), and Six-Minute Walk Test68 (for more active individuals) should be considered for this population.
An important consideration when choosing measures for people with ICU-acquired weakness is the variability in symptoms, both across individuals and within an individual across the episode of care. People with ICU-acquired weakness can have a continuum of deficits, sometimes changing rapidly over the course of hospitalization. Some patients are able to accomplish basic bed mobility, either independently or with assistance, but are not able to stand or walk for basic functional activities such as toileting. Other individuals have only minimal activity restrictions, such that they are able to walk independently or with standby assistance and are nearly ready for discharge to an assisted or independent living environment. Some measures, such as the components of the ACIF,64 FIM,30 and FSS-ICU,42 may be most appropriate for individuals with severe activity limitations who are not able to stand and walk, whereas other measures (eg, Six-Minute Walk Test,68 Berg Balance Scale65) are most appropriate for patients who function at a higher level. Because patient status can change rapidly over the course of recovery from critical illness, the clinician may incorporate measurement of progressively more difficult tasks over the course of rehabilitation. Likewise, the investigator may include a series of measures, some of which are not appropriate for patients at initial enrollment into a study but are appropriate as the patient's condition improves.
Participation and health-related quality of life.
A few measures of participation and health-related quality of life have been reported for people with ICU-acquired weakness. The SF-3633–36,69 is most frequently reported, although some investigators used the RAND 36-Item Health Survey.37 Recently, Denehy and colleagues44 included the Assessment of Quality of Life Utility Instrument in an ongoing randomized controlled intervention study.
Health care utilization.
No single set of outcome measures of health care utilization has been used across investigations of individuals with ICU-acquired weakness. Investigators have used a wide range of outcome measures, with lack of consistency across trials. Several investigators have focused on variables related to hospitalization (eg, length of stay, time on ventilation).40,70 Some investigators also have included measures of strength (eg, MRC scores),30,42 and more recently investigators have begun to report on functional outcomes using measures such as the FIM,30,43 the FSS-ICU,42 and specific functional tasks.30,43 Table 2 summarizes the measures reported in a number of key investigations.
Physical Intervention Strategies
A number of investigators have reported on intervention strategies for people with ICU-acquired weakness and related conditions. A primary focus of acute care physical therapy is to assist patients in regaining the ability to perform essential daily activities. Although function is the goal, patients who develop ICU-acquired weakness also require sufficient ability at the level of body systems in order to accomplish specific functional activities. As such, intervention strategies are indicated for the remediation of impairments of the cardiopulmonary, musculoskeletal, and neuromuscular systems. Specific strategies that have been reported for this population are summarized in Table 3. The choice of intervention is tailored, depending on whether the patient is fully awake, physiologically stable but functionally limited, or simply deconditioned.45,47
Table 3.
Physical Therapy Intervention Techniquesa
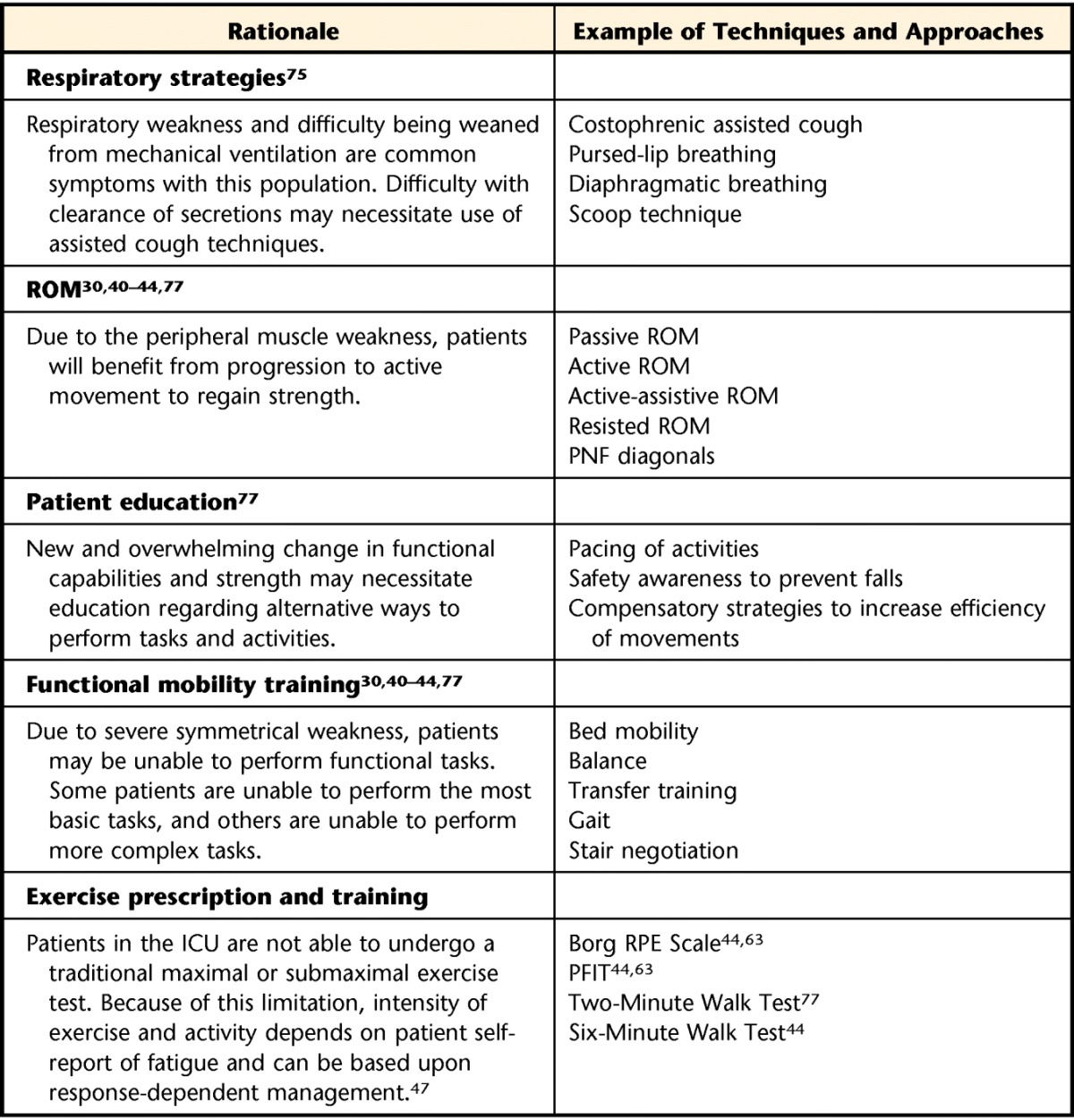
ROM=range of motion, PNF=proprioceptive neuromuscular facilitation, ICU=intensive care unit, RPE=Rate of Perceived Exertion, PFIT=Physical Function in the ICU Test.
In addition to the general intervention strategies, several specific interventions have been investigated to prevent muscle atrophy in patients who are sedated and unable to actively participate in rehabilitation. For example, Zanotti et al71 investigated the effects of electrical stimulation plus active exercises for a group of patients with chronic obstructive pulmonary disease who were mechanically ventilated and initially unresponsive. The patients who received electrical stimulation plus exercise demonstrated greater strength gains and lower respiratory rates and were able to transfer to a chair earlier than the patients who received exercise only. Two studies have examined the ability of electrical stimulation to prevent muscle atrophy72,73 and the development of CINM.73 Gerovsili et al72 found that individuals treated with daily electrical stimulation had less cross-sectional muscle loss of the quadriceps muscle than a control group. Routsi et al73 found that individuals who received daily electrical stimulation were less likely to develop CIPNM, and weaning them from mechanical ventilation required significantly less time.
Recently, cycle ergometry also has been utilized for unresponsive patients. Burtin et al74 reported that patients who received cycle ergometry plus standard physical therapy interventions during the ICU stay demonstrated greater gains in quadriceps muscle strength and greater 6-minute walk distances compared with patients who received standard physical therapy interventions only.
Several authors47,75,76 have described the use of respiratory techniques (eg, deep breathing, pursed-lip breathing, pacing of breathing, inspiratory muscle training, assisted cough, and airway clearance techniques) in related populations. These approaches have not been reported in people with ICU-acquired weakness, but may be useful in this population to address the respiratory effects of prolonged mechanical ventilation.59 One of the most important decisions when working with patients in the ICU relates to how much to challenge them physically. Several investigators have reported the safety and feasibility of early physical intervention for patients who are critically ill.30,40–44
Another consideration relates to the order of the intervention strategies. Many of the published approaches30,40–43 described interventions that began with the easiest exercises and progressed to the more demanding exercises. Schweickert et al30 and Morris et al40 described an exercise progression that began in the supine position and progressed to upright mobility, including sitting and standing activities, and ultimately to gait. These authors used standard therapeutic exercise principles, progressing from passive to active to active-resisted exercises as the patient's strength, endurance, and balance allowed. However, this progression may underestimate the patient's actual capacity. Fatiguing the patient during the least demanding tasks may make it impossible to practice the more demanding and functionally relevant tasks. For this reason, Denehy and colleagues44,63 developed a protocol in which patients performed the most demanding tasks (eg, marching in place) first. As time allowed, they proceeded to exercises that required less effort (eg, supine activities). There are merits to both approaches and insufficient data to determine which is more efficacious.
With regard to dose (intensity, duration, frequency), there is little available evidence to guide the physical therapist. Dean47 and Perme and Chandrahekar76 recommended shorter-duration and higher-frequency sessions. Perme and Chandrahekar76 described a treatment algorithm for patients in the ICU, not specifically with ICU-acquired weakness. For patients with the greatest acuity, they recommended 15 to 30 minutes, 1 to 2 times daily. Once patients move to a subacute level of medical care (eg, step-down units or medical floor), they can tolerate treatment sessions of longer duration. Perme and Chandrahekar76 advocated 30 to 60 minutes of physical therapy, 5 to 7 days per week.
Implications for Future Investigations
Recent studies have demonstrated the safety and feasibility of early intervention for patients while in the ICU and acute hospital.30,40–44 These studies also provide guidance for decisions regarding rehabilitation strategies, including both examination and intervention. A number of issues should be investigated to provide further guidance regarding the best physical rehabilitation strategies.
Given the acuity of these patients, physical therapists need to integrate information obtained from a systems-based examination with the medical acuity data. In addition to quantitative information, the physical therapist relies on moment-to-moment clinical reasoning skills to determine appropriateness for examination and intervention and to evaluate when and to what extent the patient can tolerate and benefit from increased activity load. Additional quantitative criteria are needed that can help guide these decisions.
With regard to the structure of the intervention, evidence is needed to determine whether intervention in the ICU should begin with the easiest or the most difficult tasks. With regard to discharge, several studies have shown that early physical rehabilitation leads to fewer days in the ICU40 and fewer overall hospital days30,40 and to fewer readmissions following hospital discharge.70 It is possible that people are discharged with more rehabilitation needs than was previously the case. This issue should be examined in future investigations. Additionally, evidence is needed to establish whether physical therapy interventions provided in the home setting have a positive impact on long-term functional outcomes.
Conclusions
Physical therapists play an important role in the restoration of function of people who have ICU-acquired weakness. This article reviews current evidence for physical therapy interventions with this population and discusses guidelines for safe implementation of physical therapy interventions and outcomes used to establish benefits. Evidence clearly indicates that physical rehabilitation can be implemented safely in people who have ICU-acquired weakness when appropriate guidelines are followed. A number of issues are identified that should be investigated in order for care to be most efficiently and appropriately implemented.
Footnotes
All authors provided concept/idea/project design and writing. Dr Moss provided project management.
The authors acknowledge Kyle Ridgeway, PT, DPT, and Alexander Benson, MD, for their assistance with the figures.
This work was made possible by support of the National Institutes of Health (R01 NR-11051).
References
- 1. Zink W, Kollmar R, Schwab S. Critical illness polyneuropathy and myopathy in the intensive care unit. Nat Rev Neurol. 2009;5:372–379 [DOI] [PubMed] [Google Scholar]
- 2. Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve. 2005;32:140–163 [DOI] [PubMed] [Google Scholar]
- 3. Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(10 suppl):S299–S308 [DOI] [PubMed] [Google Scholar]
- 4. Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Clinical review: critical illness polyneuropathy and myopathy. Crit Care. 2008;12:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolton CF, Laverty DA, Brown JD, et al. Critically ill polyneuropathy: electrophysiological studies and differentiation from Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 1986;49:563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zochodne DW, Bolton CF, Wells GA, et al. Critical illness polyneuropathy: a complication of sepsis and multiple organ failure. Brain. 1987;110:819–841 [DOI] [PubMed] [Google Scholar]
- 7. Kahn J, Harrison TB, Rich MM, Moss M. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology. 2006;67:1421–1425 [DOI] [PubMed] [Google Scholar]
- 8. Novak KR, Nardelli P, Cope TC, et al. Inactivation of sodium channels underlies reversible neuropathy during critical illness in rats. J Clin Invest. 2009;119:1150–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allen DC, Arunachalam R, Mills KR. Critical illness myopathy: further evidence from muscle-fiber excitability studies of an acquired channelopathy. Muscle Nerve. 2008;37:14–22 [DOI] [PubMed] [Google Scholar]
- 10. Rich MM, Teener JW, Raps EC, et al. Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology. 1996;46:731–736 [DOI] [PubMed] [Google Scholar]
- 11. De Jonghe B, Bastuji-Garin S, Sharshar T, et al. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30:1117–1121 [DOI] [PubMed] [Google Scholar]
- 12. Ali NA, O'Brien JM, Jr, Hoffman SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268 [DOI] [PubMed] [Google Scholar]
- 13. Kleyweg RP, Van Der Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14:1103–1109 [DOI] [PubMed] [Google Scholar]
- 14. Kendall F, McCreary E, Provance P, et al. Muscles: Testing and Function With Posture and Pain. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005 [Google Scholar]
- 15. Fan E, Ciesla ND, Truong AD, et al. Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med. 2010;36:1038–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weber-Carstens S, Koch S, Spuler S, et al. Nonexcitable muscle membrane predicts intensive care unit–acquired paresis in mechanically ventilated, sedated patients. Crit Care Med. 2009;37:2632–2637 [DOI] [PubMed] [Google Scholar]
- 17. De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867 [DOI] [PubMed] [Google Scholar]
- 18. Leijten FS, Harinck-de Weerd JE, Poortvliet DC, de Weerd AW. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA. 1995;274:1221–1225 [PubMed] [Google Scholar]
- 19. Witt NJ, Zochodne DW, Bolton CF, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99:176–184 [DOI] [PubMed] [Google Scholar]
- 20. Berek K, Margreiter J, Willeit J, et al. Polyneuropathies in critically ill patients: a prospective evaluation. Intensive Care Med. 1996;22:849–855 [DOI] [PubMed] [Google Scholar]
- 21. De Jonghe B, Cook D, Sharshar T, et al. Acquired neuromuscular disorders in critically ill patients: a systematic review. Intensive Care Med. 1998;24:1242–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deem S, Lee CM, Curtis JR. Acquired neuromuscular disorders in the intensive care unit. Am J Respir Crit Care Med. 2003;168:735–739 [DOI] [PubMed] [Google Scholar]
- 23. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 24. Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461 [DOI] [PubMed] [Google Scholar]
- 25. Finfer S, Chittock DR, et al. NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 26. Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477 [DOI] [PubMed] [Google Scholar]
- 27. Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomized trial. Lancet. 2010;375:475–480 [DOI] [PubMed] [Google Scholar]
- 28. Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73 [DOI] [PubMed] [Google Scholar]
- 29. Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130:869–878 [DOI] [PubMed] [Google Scholar]
- 30. Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients. validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA. 2001;286:2703–2710 [DOI] [PubMed] [Google Scholar]
- 32. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344 [DOI] [PubMed] [Google Scholar]
- 33. Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693 [DOI] [PubMed] [Google Scholar]
- 34. Heyland DK, Groll D, Caeser M. Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med. 2005;33:1549–1556 [DOI] [PubMed] [Google Scholar]
- 35. Fletcher SN, Kennedy DD, Ghosh IR, et al. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med. 2003;31:1012–1016 [DOI] [PubMed] [Google Scholar]
- 36. Cheung AM, Transey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–544 [DOI] [PubMed] [Google Scholar]
- 37. Kaarlola A, Tallgren M, Pettilä V. Long-term survival, quality of life, and quality-adjusted life-years among critically ill elderly patients. Crit Care Med. 2006;34:2120–2126 [DOI] [PubMed] [Google Scholar]
- 38. Latronico N, Shehu I, Seghelini E. Neuromuscular sequelae of critical illness. Curr Opin Crit Care. 2005;11:381–390 [DOI] [PubMed] [Google Scholar]
- 39. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509 [DOI] [PubMed] [Google Scholar]
- 40. Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243 [DOI] [PubMed] [Google Scholar]
- 41. Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145 [DOI] [PubMed] [Google Scholar]
- 42. Zanni J, Korupolu R, Fan E, et al. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25:254–262 [DOI] [PubMed] [Google Scholar]
- 43. Pohlman MC, Schweickert WD, Pohlman AS, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;38:2089–2094 [DOI] [PubMed] [Google Scholar]
- 44. Denehy L, Berney S, Skinner E, et al. Evaluation of exercise rehabilitation for survivors of intensive care: protocol for single blind randomised controlled trial. Open Crit Care Med J. 2008;1:39–47 [Google Scholar]
- 45. Hanekom S, Gosselink R, Dean E, et al. The development of a clinical management algorithm for early physical activity and mobilization of critically ill patients: synthesis of evidence and expert opinion and its translation into practice. Clin Rehabil. 2011;25:771–787 [DOI] [PubMed] [Google Scholar]
- 46. Acute Care Section–APTA Task force on lab values resource document. Available at: http://www.acutept.org/associations/11622/files/LabValuesResourceUpdate2012.pdf Accessed February 2012
- 47. Dean E. Mobilizing patients in the ICU: evidence and principles of practice. Acute Care Perspectives. 2008;17:1–9 [Google Scholar]
- 48. Verderber A, Gallagher KJ. Effects of bathing, passive range-of-motion exercises, and turning on oxygen consumption in healthy men and women. Am J Crit Care. 1994;3:374–381 [PubMed] [Google Scholar]
- 49. Kallet RH, Bransen RD. Respiratory controversies in the critical care setting: do the NIH ARDS clinical trials network PEEP/FI02 tables provide the best evidence-based guide to balancing PEEP and FI02 settings in adults? Respir Care. 2007;52:461–475 [PubMed] [Google Scholar]
- 50. Georgiadis D, Schwarz S, Baumgartner RW, et al. Influence of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure in patients with acute stroke. Stroke. 2001;32:2088–2092 [DOI] [PubMed] [Google Scholar]
- 51. Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema: respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164 [DOI] [PubMed] [Google Scholar]
- 52. De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789 [DOI] [PubMed] [Google Scholar]
- 53. Korupolu R, Gifford J, Needham DM. Early mobilization of critically ill patients: reducing neuromuscular complications after intensive care. Contemp Crit Care. 2009;6:1–12 [Google Scholar]
- 54. Elliott D, Denehy L, Berney S, Alison JA. Assessing physical function and activity for survivors of a critical illness: a review of instruments. Aust Crit Care. 2011;24:155–166 [DOI] [PubMed] [Google Scholar]
- 55. Jette AM. Toward a common language for function, disability, and health. Phys Ther. 2006;86:726–734 [PubMed] [Google Scholar]
- 56. Cook JW, Pierson LM, Herbert WG, et al. The influence of patient strength, aerobic capacity and body composition upon outcomes after coronary artery bypass grafting. Thorac Cardiovasc Surg. 2001;49:89–93 [DOI] [PubMed] [Google Scholar]
- 57. Sallinen J, Stenholm S, Rantanen T, et al. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc. 2010;58:1721–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vecchiarino P, Bohannon RW, Ferullo J, Maljanian R. Short-term outcomes and their predictors for patients hospitalized with community-acquired pneumonia. Heart Lung. 2004;33:301–307 [DOI] [PubMed] [Google Scholar]
- 59. Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med. 2009;37(10 suppl):S347–S353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. De Jonghe B, Bastuji-Garin S, Durand MC, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–2015 [DOI] [PubMed] [Google Scholar]
- 61. Tzanis G, Vasileiadis I, Zervakis D, et al. Maximum inspriatory pressure, a surrogate parameter for the assessment of ICU-acquired weakness. BMC Anesthesiol. 2011;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil. 1993;74:531–536 [DOI] [PubMed] [Google Scholar]
- 63. Skinner EH, Berney S, Warrillow S, Denehy L. Development of a physical function outcome measure (PFIT) and a pilot exercise training protocol for use in intensive care. Crit Care Resusc. 2009;11:110–115 [PubMed] [Google Scholar]
- 64. Roach KE, Van Dillen LR. Development of an acute care index of functional status for patients with neurologic impairment. Phys Ther. 1988;68:1102–1108 [DOI] [PubMed] [Google Scholar]
- 65. Berg K, Wood-Dauphinée S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27:27–36 [PubMed] [Google Scholar]
- 66. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly patients. J Am Geriatr Soc. 1991;39:142–148 [DOI] [PubMed] [Google Scholar]
- 67. Whitney SL, Wrisley DM, Marchetti GF, et al. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Phys Ther. 2005;85:1034–1045 [PubMed] [Google Scholar]
- 68. Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurment properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119:256–270 [DOI] [PubMed] [Google Scholar]
- 69. Skinner EH, Warrillow S, Denehy L. Health-related quality of life in Australian survivors of critical illness. Crit Care Med. 2011;39:1896–1905 [DOI] [PubMed] [Google Scholar]
- 70. Morris PE, Griffin L, Berry M, et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341:373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zanotti E, Felicetti G, Maini M, Fracchia C. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest. 2003;124:292–296 [DOI] [PubMed] [Google Scholar]
- 72. Gerovasili V, Stefanidis K, Vitzilaios K, et al. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care. 2009;13:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Routsi C, Gerovasili V, Vasileiadis I, et al. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care. 2010;14:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Burtin C, Clerckx B, Robbeets C, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37:2499–2505 [DOI] [PubMed] [Google Scholar]
- 75. Frownfelter D, Massery M. Facilitating ventilation patterns and breathing strategies. In: Frownfelter D, Dean E.Cardiovascular and Pulmonary Physical Therapy Evidence and Practice. 4th ed St Louis, MO: Mosby Elsevier; 2006:377–403 [Google Scholar]
- 76. Perme C, Chandrashekar R. Early mobility and walking program for patients in intensive care units: creating a standard of care. Am J Crit Care. 2009;18:212–221 [DOI] [PubMed] [Google Scholar]
- 77. Nordon-Craft A, Schenkman M, Ridgeway K, et al. Physical therapy management and patient outcomes following ICU-acquired weakness: a case series. J Neurol Phys Ther. 2011;35:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Intiso D, Amoruso L, Zarrelli M, et al. Long-term functional outcome and health status of patients with critical illness polyneuromyopathy. Acta Neurol Scand. 2011;123:211–219 [DOI] [PubMed] [Google Scholar]