Abstract
Appropriate prioritization during dual-task walking is necessary to achieve task goals and maintain walking stability. We examined the effects of increased walking task difficulty on dual-task walking prioritization in healthy young adults. Walking under simple usual-base conditions was similar between equal-focus and cognitive-focus instructions, but these differed from walking-focus instructions, consistent with cognitive task prioritization. In contrast, narrow-base walking was similar between equal-focus and walking-focus instructions, but these differed from cognitive-focus instructions. This shift in prioritization with increasing walking task difficulty suggests that prioritization is dynamic and flexible.
Keywords: Attention, Instructions, Dual task, Gait, Narrow-base walking
Introduction
During dual-task walking, tasks must be prioritized appropriately to achieve goals while maintaining safety. This requires flexible allocation of cognitive resources like attention [1, 2]. The posture-first hypothesis suggests that postural tasks are prioritized at the expense of concurrent tasks to maintain stability and prevent falls, though evidence for this is conflicting [3, 4]. Shumway-Cook and colleagues proposed that posture-first is not an invariant strategy, noting that “the allocation of attention during the performance of concurrent tasks is complex, depending on many factors including the nature of both the cognitive and postural task, the goal of the subject, and the instructions” [3]. This implies that task prioritization is flexible and depends on a variety of individual, task, and environmental factors. This study examined how increased walking task difficulty affects prioritization during dual-task walking in healthy young adults (HYA). We anticipated cognitive task prioritization during simple usual-base walking and walking prioritization during more challenging narrow-base walking.
Methods
Fifteen HYA (mean [SD] age: 26.4 [4.3] years; 6 male) participated. Informed consent was obtained in accordance with institutional review board procedures.
An auditory Stroop test [5, 6], consisting of the words “high” and “low” said in a high or low pitch, was performed with instructions to “state the pitch as quickly and accurately as possible.” After training, three blocks (20 stimuli/block) of seated single-task and two blocks of each dual-task condition were performed. Outcomes were response latency (time from stimulus onset to response onset) and response accuracy (percentage of total responses that were correct).
Participants walked with a usual-base (UB) and a narrow-base (NB) of support (50% pelvic width) [7]. Instructions were “walk as quickly as possible” for UB walking and “walk as quickly and accurately as possible” for NB walking. A Qualisys Motion Capture system (Qualisys, Gothenburg, Sweden) recorded the position of markers on the feet, legs, pelvis, and trunk. Gait speed was measured for both conditions. NB step accuracy was the percentage of total steps that were accurate (the ankle marker, at heel strike, was on or within the path boundary).
Dual-task conditions were: 1) equal-focus (DTequal): “focus on both tasks equally;” 2) cognitive-focus (DTcog): “focus on the cognitive task;” and 3) walking-focus (DTwalk): “focus on walking.” The DTequal condition was performed first to eliminate an influence of instructions, with randomization of walking task (UB, NB) order between participants. For the remaining conditions, the order of walking task (UB, NB) and instructions (DTwalk, DTcog) was randomized.
The dual-task effect measures relative change in dual-task compared to single-task performance [8, 9]. A negative value represents a dual-task cost (decrement in dual-task compared to single-task performance). Composite dual-task effects were calculated for the cognitive task and walking to account for potential within-task trade-offs [8]. Response latency and response accuracy dual-task effects were summed for the cognitive dual-task effect. Gait speed defined the UB walking dual-task effect, while both speed and step accuracy dual-task effects were summed for the NB walking dual-task effect.
Prioritization was first assessed by comparing DTequal to single-task performance. Cognitive performance was assessed using analysis of variance (ANOVA; SPSS Statistics 17.0, Chicago, USA) with one factor, condition (single-task, UB, NB). Gait speed was assessed using ANOVA with two factors, condition (single-task, dual-task) and walking task (UB, NB). NB step accuracy in single-task versus DTequal conditions was assessed using a t-test. Second, DTequal was compared to DTcog and DTwalk performance. The effects of instructions and walking task were examined using ANOVA with two factors, instructions (DTequal, DTcog, DTwalk) and walking task (UB, NB). NB step accuracy was assessed using an ANOVA with one factor, instructions. Significance was set at α=.05, with Bonferroni adjustment for multiple post-hoc comparisons.
Results
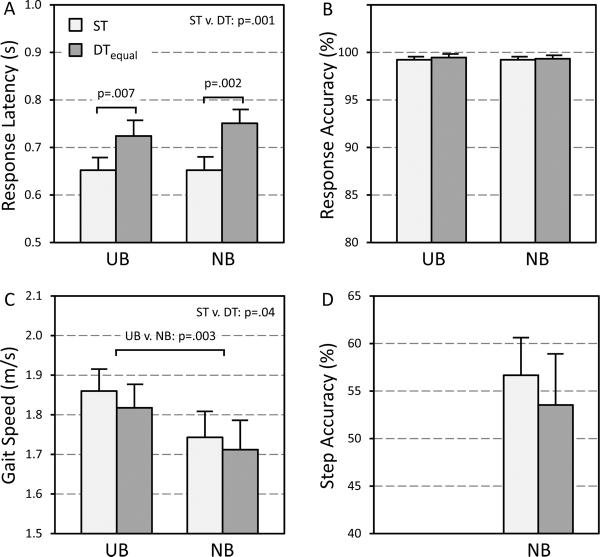
Condition influenced response latency (main effect: F(2,28)=9.659; p=.001), with shorter latencies under single-task compared to both UB DTequal (Fig. 1A; post-hoc: p=.007) and NB DTequal walking (post-hoc: p=.002). Condition did not affect response accuracy (Fig. 1B; main effect: p=.88). Gait speed was faster in single-task versus DTequal conditions (Fig. 1C; main effect: F(1,14)=4.961; p=.04) and for UB versus NB walking (main effect: F(1,14)=12.634; p=.003), with no interaction (p=.63). NB step accuracy was similar in single-task and DTequal conditions (Fig. 1D; p=.25).
Fig. 1.
Cognitive task response latency (A), cognitive task response accuracy (B), gait speed (C) and NB step accuracy (D) under single-task and dual-task walking conditions, with instructions to focus on both tasks equally (DTequal). Symbols represent means, and bars represent standard errors (note: standard errors for response accuracy were <1% in all cases). ST = single task condition; DT = dual task equal focus condition; UB = usual base walking; NB = narrow base walking.
Instructions affected response latency (main effect: F(2,28)=26.600; p<.001). DTequal latencies were longer than DTcog (post-hoc: p=.001) and shorter than DTwalk (post-hoc: p<.001). Walking task did not affect response latency (main effect: F(1,14)=3.479; p=.08), and there was no interaction (p>.44). Neither instructions (main effect: p>.35) nor walking task (main effect: p=.18) influenced response accuracy, with no interaction (both p=.51).
Both instructions (main effect: F(2.28)=5.549; p=.009) and walking task (main effect: F(1,14)=10.377; p=.006) affected gait speed, with an interaction (F(2,28)=5.939; p=.007). For UB walking, DTequal speed was similar to DTcog (post-hoc: p=.38) but slower than DTwalk (post-hoc: p=.008). Instructions did not affect NB speed (p>.11). Instructions influenced NB step accuracy (main effect: F(2,28)=4.598; p=.02). DTequal step accuracy was similar to DTwalk (p=.46) but higher than DTcog (post-hoc: p=.01).
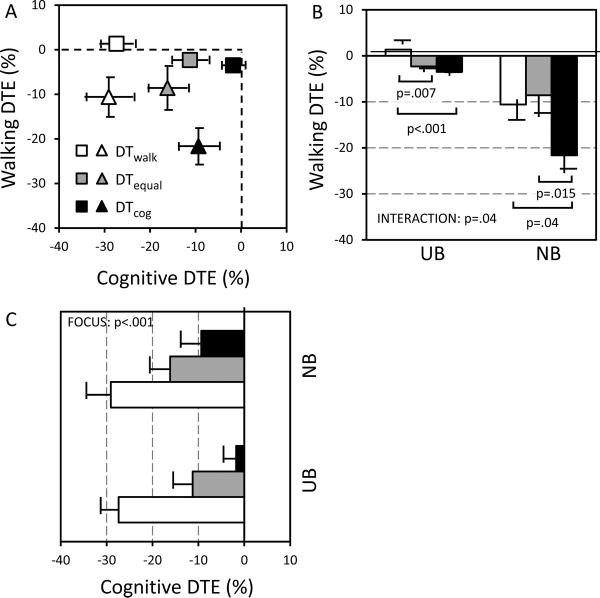
Instructions influenced cognitive dual-task effects (main effect: F(2,28)=26.061; p<.001) but walking task did not (main effect: F(1,14)=3.988; p=.07), with no interaction (Fig. 2A, C; p=.37). The cognitive dual-task cost in the DTequal condition was greater than DTcog (post-hoc: p=.001) and smaller than DTwalk (post-hoc: p<.001). Walking dual-task effects were influenced by instructions (main effect: F(2,28)=6.251; p=.006) and task (main effect: F(1,14)=13.628; p=.002), with a significant interaction (Fig. 2A, B; F(2,28)=3.713; p=.04). For UB walking, the dual-task cost in the DTequal condition was similar to DTcog (post-hoc: p=.35) but greater than DTwalk (post-hoc: p=.007), consistent with cognitive task prioritization. NB walking showed the opposite pattern. The DTequal dual-task cost was similar to DTwalk (post-hoc: p=.62) but smaller than DTcog (post-hoc: p=.02), consistent with walking prioritization.
Fig. 2.
Plot of walking versus cognitive dual-task effects (A) demonstrating the effect of instructed focus and walking task difficulty on dual task effects (DTE) for walking (B) and the cognitive task (C). In (A), squares represent usual base (UB) walking and triangles represent narrow base (NB) walking. Symbols represent means, and bars represent standard errors. DTwalk = dual task, walking focus; DTequal = dual task, equal focus, DTcog = dual task, cognitive task focus.
Discussion
These results indicate that the cognitive task was prioritized during simple UB walking while walking was prioritized during more complex NB walking, consistent with the concept of dynamic prioritization.
A number of methodological choices should be noted. First, instructions were to focus equally on both tasks rather than providing no instructions. Our results were similar to previous research using non-instructed conditions, suggesting similar effects [10]. Secondly, participants walked at their fast-as-possible versus self-selected speed to maximally challenge walking performance for optimal sensitivity to change. Finally, unlike NB walking, UB walking had no imposed accuracy constraints. The composite NB walking dual-task effect accounted for within-task trade-offs, allowing a direct comparison between UB and NB walking.
Inappropriate prioritization during dual-task walking can adversely affect functional mobility and may contribute to increased fall risk in geriatric or neurologic populations. Future research should continue to examine factors that influence dual-task prioritization since this may be critical to the delivery of effective dual-task interventions.
Highlights.
We assessed the effect of complex walking on dual-task performance in young adults.
Walking and the cognitive task were compared during usual- and narrow-base walking.
Usual-base walking performance was consistent with cognitive task prioritization.
Narrow-base walking performance was consistent with walking prioritization.
Performance changes suggest that prioritization is dynamic and flexible.
Table 1.
Effects of dual task performance, instructed focus, and task difficulty on absolute and relative measures of cognitive task and walking performance. Values represent the mean (standard deviation).
| Usual base walking |
Narrow base walking |
|||||||
|---|---|---|---|---|---|---|---|---|
| Single task | Equal focus | Dual task Walking focus | Cognitive focus | Single task | Equal focus | Dual task Walking focus | Cognitive focus | |
|
|
|
|
|
|||||
| Cognitive task | ||||||||
| Response Latency (s) | 0.65 (0.10) | 0.72 (0.12) | 0.82 (0.12) | 0.66 (0.10) | 0.65 (0.10) | 0.75 (0.11) | 0.83 (0.14) | 0.71 (0.13) |
| Response latency DTE (%) | --- | −11.5 (14.4) | −27.0 (14.6) | −2.2 (9.6) | --- | −16.3 (16.8) | −28.7 (19.9) | −8.8 (16.3) |
| Response accuracy (%) | 99.2 (1.1) | 99.5 (1.5) | 98.8 (2.3) | 99.7 (0.9) | 99.2 (1.1) | 99.3 (1.2) | 98.8 (2.4) | 98.7 (2.1) |
| Response accuracy DTE (%) | --- | 0.3 (2.1) | −0.4 (2.1) | 0.5 (1.1) | --- | 0.1 (1.7) | −0.4 (2.8) | −0.6 (2.1) |
| Cognitive task DTE (%) | --- | −11.2 (15.7) | −27.4 (14.6) | −1.8 (10.0) | --- | −16.2 (16.5) | −29.1 (20.0) | −9.4 (16.9) |
| Walking | ||||||||
| Gait speed (m/s) | 1.86 (0.21) | 1.82 (0.22) | 1.88 (0.22) | 1.80 (0.22) | 1.74 (0.25) | 1.71 (0.28) | 1.74 (0.26) | 1.74 (0.26) |
| Gait speed DTE (%) | --- | −2.3 (4.7) | 1.4 (3.9) | −3.5 (3.4) | --- | −2.0 (3.9) | 0.0 (3.6) | −0. (4.4) |
| Step accuracy (%) | --- | --- | --- | --- | 56.7 (15.4) | 53.5 (20.9) | 51.4 (19.8) | 45.1 (15.9) |
| Step accuracy DTE (%) | --- | --- | --- | --- | --- | −6.5 (19.4) | −10.6 (17.7) | −21.6 (15.0) |
| Walking DTE (%) | --- | −2.3 (4.7) | 1.4 (3.9) | −3.5 (3.4) | --- | −8.6 (18.3) | −10.6 (16.5) | −21.6 (14.9) |
Acknowledgments
This research was supported by the University of Washington Stolov Research Fund and the National Institutes of Health, National Institute of Child Health and Human Development (K01HD052018). The authors gratefully acknowledge R. Price for engineering support, L. Flexner for recording the stimuli, and A. Schang, R. Hashish, J. Eastman, A. Nowak, A. Fish, S. Sirois, S. Woldemariam, and A. Gillespie for assistance with data collection and analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest There are no personal or financial conflicts of interest for any of the authors.
References
- [1].Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16(1):1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- [2].Fraizer EV, Mitra S. Methodological and interpretive issues in posture-cognition dual-tasking in upright stance. Gait Posture. 2008;27(2):271–279. doi: 10.1016/j.gaitpost.2007.04.002. [DOI] [PubMed] [Google Scholar]
- [3].Shumway-Cook A, Woollacott M, Kerns KA, Baldwin M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J Gerontol A Biol Sci Med Sci. 1997;52(4):M232–240. doi: 10.1093/gerona/52a.4.m232. [DOI] [PubMed] [Google Scholar]
- [4].Teasdale N, Stelmach GE, Breunig A. Postural sway characteristics of the elderly under normal and altered visual and support surface conditions. J Gerontol. 1991;46(6):B238–244. doi: 10.1093/geronj/46.6.b238. [DOI] [PubMed] [Google Scholar]
- [5].Morgan AL, Brandt JF. An auditory Stroop effect for pitch, loudness, and time. Brain Lang. 1989;36(4):592–603. doi: 10.1016/0093-934x(89)90088-6. [DOI] [PubMed] [Google Scholar]
- [6].Siu KC, Chou LS, Mayr U, Donkelaar P, Woollacott MH. Does inability to allocate attention contribute to balance constraints during gait in older adults? J Gerontol A Biol Sci Med Sci. 2008;63(12):1364–1369. doi: 10.1093/gerona/63.12.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kelly VE, Schrager MA, Price R, Ferrucci L, Shumway-Cook A. Age-associated effects of a concurrent cognitive task on gait speed and stability during narrow-base walking. J Gerontol A Biol Sci Med Sci. 2008;63(12):1329–1334. doi: 10.1093/gerona/63.12.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kelly VE, Janke AA, Shumway-Cook A. Effects of instructed focus and task difficulty on concurrent walking and cognitive task performance in healthy young adults. Exp Brain Res. 2010;207(1–2):65–73. doi: 10.1007/s00221-010-2429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bock O. Dual-task costs while walking increase in old age for some, but not for other tasks: an experimental study of healthy young and elderly persons. J Neuroeng Rehabil. 2008;5:27. doi: 10.1186/1743-0003-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yogev-Seligmann G, Rotem-Galili Y, Mirelman A, Dickstein R, Giladi N, Hausdorff JM. How does explicit prioritization alter walking during dual-task performance? Effects of age and sex on gait speed and variability. Phys Ther. 2010;90(2):177–186. doi: 10.2522/ptj.20090043. [DOI] [PMC free article] [PubMed] [Google Scholar]