Abstract
The objective of this investigation was to develop a thermosensitive vaginal gel containing raltegravir + efavirenz loaded PLGA nanoparticles (RAL-EFV-NPs) for pre-exposure prophylaxis of HIV. RAL-EFV-NPs were fabricated using a modified emulsion-solvent evaporation method and characterized for size and zeta potential. The average size and surface charge of RALEFV-NP were 81.8 ± 6.4 nm and −23.18 ± 7.18 mV respectively. The average encapsulation efficiency of raltegravir and efavirenz was 55.5% and 98.2% respectively. Thermosensitive vaginal gel containing RAL-EFV-NPs was successfully prepared using a combination of Pluronic F127 (20% w/v) and Pluronic F68 (1% w/v). Incorporation RAL-EFV-NPs in the gel did not result in nanoparticle aggregation and RAL-EFV-NPs containing gel showed thermogelation at 32.5°C. The RAL-EFV-NPs were evaluated for inhibition of HIV-1NL4-3 using TZM-bl indicator cells. The EC90 of RAL-EFV-NPs was lower than raltegravir + efavirenz (RAL-EFV) solution but did not reach significance. Compared to control HeLa cells without any treatment, RAL-EFV-NPs or blank gel were not cytotoxic for 14 days in vitro. The intracellular levels of efavirenz in RALEFV-NPs treated HeLa cells were above the EC90 for 14 days whereas raltegravir intracellular concentrations were eliminated within 6 days. Transwell experiments of NPs-in-gel demonstrated rapid transfer of fluorescent nanoparticles from the gel and uptake in HeLa cells within 30 min. These data demonstrate the potential of antiretroviral NP-embedded vagina gels for long-term vaginal pre-exposure prophylaxis of heterosexual HIV-1 transmission.
Keywords: raltegravir, efavirenz, pre-exposure prophylaxis, PLGA, nanoparticles, HIV, Pluronic, thermosensitive gel
1. Introduction
Prevention of HIV-1 infection to reduce the number of newly infected individuals is an international priority. Various modalities such as male circumcision (Warner et al., 2009), prophylactic HIV vaccines (Munier et al., 2009), vaginal microbicides (Kelly and Shattock, 2011) and oral pre-exposure prophylaxis (García-Lerma et al., 2010) have been explored to prevent sexual contraction of HIV. Prevention of HIV infection by using antiretroviral agents as vaginal microbicides has received more attention in recent years. However, clinical trials focusing on vaginal prophylaxis of HIV using topical microbicides have shown mixed results. Early-phase topical microbicides such as PRO 2000 (McCormack et al., 2010) and Carraguard™ (Skoler-Karpoff et al., 2008) have failed to show efficacy in clinical trials whereas coitally-dependent administration of 1% tenofovir gel has shown success in CAPRISA 004 trial (Abdool Karim et al., 2010). Conversely, the VOICE trial employing a coitus-independent, once daily administration of 1% tenofovir gel was halted due to lack of efficacy (Adams and Kashuba, 2012). The VOICE trial setback has prompted investigators to examine alternative strategies such as the use of a combination of antiretroviral agents acting on different stages of viral replication. Combination antiretroviral therapy (HAART) has already demonstrated success in the treatment of HIV infected individuals. Recently, gels containing a combination of tenofovir and UC-781 (a hydrophobic non-nucleoside reverse transcriptase inhibitor) are being evaluated (Kiser et al., 2012).
Efavirenz (EFV) is a FDA-approved non-nucleoside reverse transcriptase inhibitor with ability to inhibit HIV at nanomolar concentrations in vitro. The long elimination half-life (~40 h) of EFV could be useful for achieving long term pre-exposure prophylaxis of HIV-1 (Magiolo, 2009). Maurin et al., (2002) have demonstrated efavirenz has maximum stability at pH 4. Thus, efavirenz could have optimal stability in the vaginal environment if developed as a vaginal microbicide. Raltegravir (RAL) is the only HIV-1 integrase inhibitor currently approved by FDA. Recently, Clavel et al., (2011) reported that raltegravir-containing regimen results in penetration of raltegravir in the genital tract of HIV-1-infected women. Until today, there have been no attempts to evaluate potential of raltegravir and/or efavirenz as a vaginal microbicide.
The advent of nanotechnology has brought a paradigm shift in the treatment of all life-threatening diseases including HIV. In fact, a nanotechnology-based product (VivaGel®, Starpharma, Australia) has been developed for prophylaxis of HIV and other sexually transmitted infections and has undergone extensive preclinical and clinical safety evaluation (Price et al., 2011). Woodrow et al., (2009) have demonstrated that intravaginal administration of PLGA-siRNA nanoparticles can induce sustained gene silencing in vagina for 14 days. This investigation clearly indicated that PLGA nanoparticles could have potential for long-term vaginal prophylaxis of HIV. However, there are no reports describing use of PLGA nanoparticles containing combination antiretroviral drugs for vaginal prophylaxis.
The mode of delivery of vaginal microbicides is also very important aspect in HIV prophylaxis. Until today, majority of clinical trials have employed conventional gels for vaginal microbicide delivery. However, gel leakage, uneven distribution and messiness are major disadvantages of conventional gels for women (Adams and Kashuba, 2012). Thermosensitive gels have gained greater attention as an alternative to conventional gels in last decade. Thermosensitive gels are liquid at room temperature but form a highly viscous gel at 37°C once delivered inside the body (Ruel-Gariépy and Leroux, 2004). Thermosensitive gels are easy to handle and deliver as compared to conventional gels due to their liquid nature, can spread evenly on gelation whereas their high viscosity at body temperature can minimize chances of gel leakage (Roy et al., 2001). Furthermore, thermosensitive gels can also sustain the delivery of drugs (Balogu et al., 2011). The objective of this investigation was to evaluate potential of a thermosensitive gel containing raltegravir + efavirenz loaded PLGA nanoparticles (RAL-EFV-NPs) for vaginal prophylaxis of HIV.
2. Materials and Methods
2.1. Materials
Poly-lactide-co-glycolide (Avg. Mol. Wt. 52000 Da, Inherent viscosity: 0.59 dL/g in hexafluoroisopropanol) was purchased from Birmingham Polymers (Birmingham, AL). Raltegravir (RAL) and efavirenz (EFV) were purchased from Sequoia Research Ltd. (Pangbourne, UK). Potassium dihydrogen phosphate (HPLC grade), acetonitrile (HPLC grade), dimethyl sulfoxide (DMSO, AR Grade), ethyl acetate (AR grade), citric acid (AR grade), trisodium citrate (AR grade), polyvinyl alcohol (88% hydrolyzed; Mol. Wt. 88000) and Rhodamine 6G were purchased from Fischer Scientific Ltd (Pittsburg, PA, USA). Pluronic F127, Pluronic F68 (BASF Corp., Edison, NJ, USA) and N-methylpyrrolidone (Pharmasolv®, Ashland Inc, Wayne, NJ, USA) were received as gift samples. Ultrapure water was used for all the experiments.
2.2. Cell culture
Human cervical (HeLa) cells, T cells (H9 cells) and HIV indicator (TZM-bl) cells were used for multiple experiments. Culture methods and cell use are described in the supplementary materials.
2.3. Preparation of PLGA nanoparticles containing RAL and EFV combination (RAL-EFVNP)
Briefly, PLGA (50 mg) and Pluronic F127 (100 mg) were dissolved in 3 ml of ethyl acetate by heating at 40°C in an incubating shaker bath. RAL (5 mg) and EFV (5 mg) were dissolved in a mixture of DMSO (0.15 ml) and N-methylpyrrolidone (0.1 ml) by heating at 40°C in an incubating shaker bath. Ethyl acetate solution was added to the DMSO and N-methylpyrrolidone mixture containing RAL and EFV to obtain a homogenous solution (organic phase). The organic phase was emulsified in 10 ml of ultrapure water using a probe sonicator (UP100H; Hielscher USA, Inc., NJ, USA; Amplitude: 80% and Intensity: 0.8) for 15 min in an ice bath. The resultant oil-in-water emulsion was transferred to a 50 mL beaker and stirred at 700 rpm for 2 h using a magnetic stirrer to evaporate the ethyl acetate. Particle size, polydispersity index, and surface charge of resulting RAL-EFV-NPs were measured using dynamic light scattering (ZetaPlus instrument, Brookhaven Instruments Corp, NY, USA) as previously described (Shibata et al., 2012). All experiments were carried out in triplicate. RAL-EFV-NPs were sterile filtered through a 0.22 µm filter and used for further studies. For fabrication of fluorescent nanoparticles, Rhodamine 6G (1 mg) was dissolved in 3 mL ethyl acetate in place of antiretroviral drugs and processed as described above.
2.4. Imaging of RAL-EFV-NPs by SEM
RAL-EFV-NPs were placed on the slide surface and allowed to dry. The slide was sputter coated with 2% w/v uranyl acetate, dried, and then visualized by using a JEOL-40A (JEOL Ltd, Sheboygan, WI) scanning electron microscope (Shibata et al., 2012).
2.5. In vitro cytotoxicity studies
Long-term in vitro cytotoxicity of RAL-EFV-NPs was evaluated in HeLa cells. Briefly, cells were seeded in 12 well plates at a density of 1.0 × 104 cells/well in triplicate and allowed to attach to wells overnight. RAL-EFV-NPs were added to obtain a RAL and EFV concentration of 5 µg/mL in each well. Blank gel containing no nanoparticles (50 µL) was added to triplicate wells to determine cytotoxicity of this component. Cell viability was assessed on days 1, 2, 4, 7, 10 and 14 as per MTT method (Destache et al., 2009). The absorbance obtained for RAL-EFV-NPs was compared to control cells (no treatment). Assuming non-Gaussian distribution, nonparametric statistics were used to determine significance.
2.6. Anti-HIV activity
Activity of RAL-EFV-NPs and RAL-EFV solution against HIV-1NL4-3 was determined using TZM-bl HIV-1 indicator cells as per reported method with suitable modifications (Fletcher et al., 2009). Further description of these experiments is included in supplementary materials.
2.7. Studies on intra-cellular release of raltegravir and efavirenz from RAL-EFV-NPs
Intracellular release of RAL and EFV from RAL-EFV-NPs was studied using HeLa cells. HeLa cells (1 × 105 cells/well) were seeded onto 12-well plates and cultured overnight. RAL-EFV-NPs were added to wells with a combined starting raltegravir + efavirenz concentration of 10 ± 2 µg/well. Release of RAL and EFV in HeLa cells and in culture media was analyzed by HPLC on day 1, 2, 4, 5, 6, 7, 10 and 14. Further details are described in the supplementary materials.
2.8. Development of a thermosensitive vaginal gel
For development of a thermosenstive vaginal gel, RAL-EFV-NPs were prepared in pH 4.5 aqueous citrate buffer. Table 1 outlines the gel composition that was used for further experiments. Various quantities of Pluronic F127 and Pluronic F68 were used for identifying suitable composition of thermosensitive gel. Further description of the preliminary experiments to fabricate the thermosensitive gel is provided in supplementary information.
Table 1.
Composition of the RAL-EFV-NPs loaded thermosensitive gel
| Components | Quantity |
|---|---|
| Pluronic F127 | 2 g (20% w/v) |
| Pluronic F68 | 0.1 g (1% w/v) |
| DMSO | 0.15 ml (1.5% v/v) |
| N-methyl pyrrolidone | 0.1 ml (1% w/v) |
| RAL-EFV-NPs in citrate pH 4.5 buffer | 10 ml |
The pH 4.5 citrate buffer was prepared by dissolving citric acid (42 mg) and trisodium citrate dehydrate (59 mg) in the 1000 ml of ultra-pure water. RAL-EFV-NPs were preparedin pH 4.5 citrate buffer for preparation of thermosensitive gel.
2.9. Characterization of transfer of fluorescent PLGA nanoparticles from thermosensitive gel using transwells
HeLa cells were plated at 0.5 ×106 cells/ml on Poly-D Lysine precoated 12 mm BD BioCoat*coverslips (BD Biosciences, San Jose, CA). Cells were cultured overnight in DMEM plus 10% fetal calf serum at 37°C, 5% CO2. Thermosensitive gel (100 µl) containing Rhodamine 6G labeled fluorescent PLGA nanoparticles was placed on 0.4 µm transwell permeable supports (Corning Inc. Life Sci., MA, USA) at 37°C for 10 min to allow gel solidification. Transwells were placed above HeLa cells for 15, 30 min, 2 h, 12 h and 24 h. At the appropriate time, the HeLa cells were fixed and imaged using fluorescence microscopy to determine the transfer of the nanoparticles from the gel into the HeLa cells. This is described in supplementary information.
2.10. Statistical Analysis
Results are reported as mean ± SEM for all experiments. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. RAL-EFV-NP preparation and characterization
The PLGA nanoparticles containing RAL and EFV combination (RAL-EFV-NPs) had particle size of 81.8 ± 6.4 nm, polydispersity index of 0.15 ± 0.02 and the surface charge was −23.18 ± 7.18 mV (n=3). The entrapment efficiency of the RAL and EFV in the nanoparticles averaged (± SEM) 55.5 ± 5.61% and 98.2 ± 1.2% respectively (n=3). The RAL-EFV-NPs demonstrated physical and colloidal stability with no significant change in particle size and pH for at least 1 month. Scanning electron microscopy showed the presence of sub-100 nm RAL-EFV-NP (Figure 1) validating the results of dynamic light scattering. The osmolarity of RAL-EFV-NPs averaged 405.3 ± 0.57 mOsm/kg due to presence of 1.5% v/v DMSO and 1% v/v N-methylpyrrolidone.
Figure 1.
SEM image of the raltegravir + efavirenz loaded PLGA nanoparticles.
3.2. In vitro cytotoxicity studies
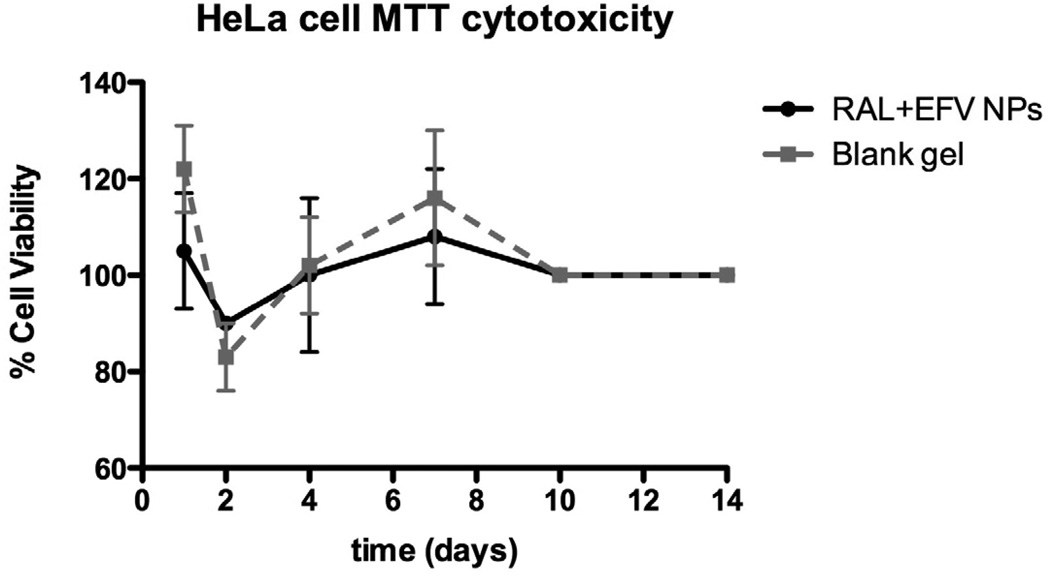
Long term in vitro cytotoxicity of RAL-EFV-NPs was carried out to ensure that RAL-EFV-NPs did not cause any cytotoxicity to HeLa cells during the intracellular drug release study (discussed in section 3.4.). It was observed that RAL-EFV-NPs at the RAL and EFV concentration of 5 µg/ml did not cause any significant toxicity as compared to control cells or blank gel (Figure 3).
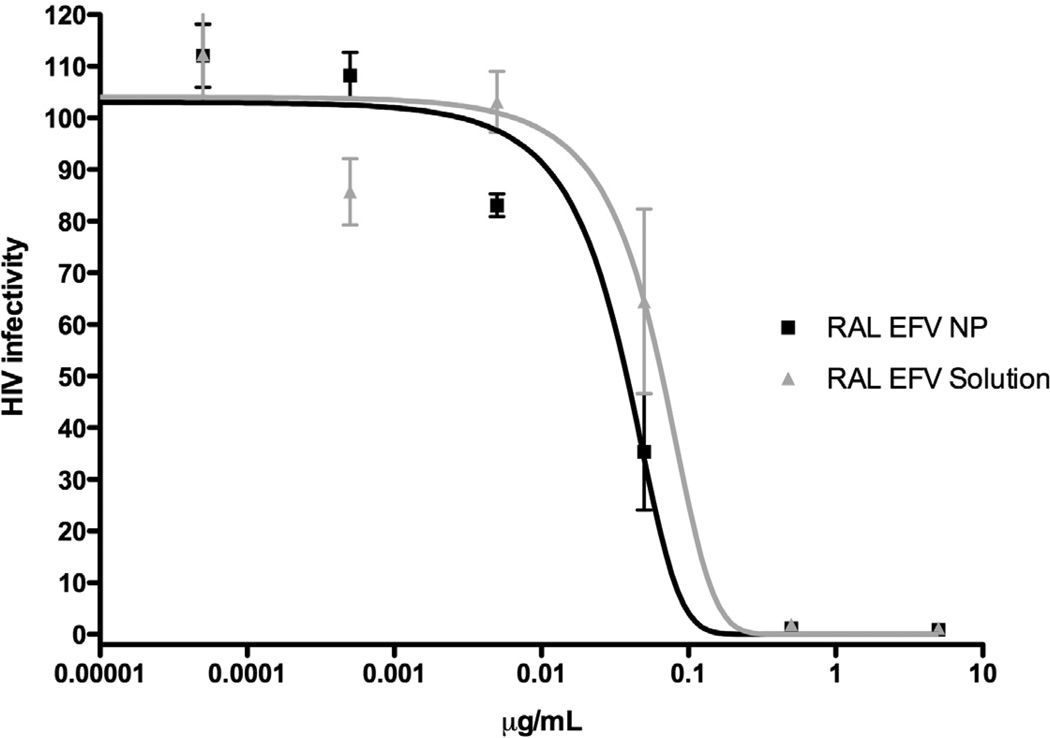
Figure 3.
Dose-response curves for RAL-EFV solution and RAL-EFV-NP. RAL-EFV solution and RAL-EFV-NP were incubated with HIV-1 indicator TZM-bl cells at different concentrations starting for RAL + EFV concentration of 10 µg/ml (n=9; data expressed as mean ± SEM).
3.3. Anti-HIV activity
The efficacy RAL-EFV-NPs was compared to RAL-EFV solution by evaluating their EC90 values against HIV-1NL4-3 strain (Figure 3). The inhibitory concentration for the RAL-EFV-NPs was less (mean ± MSE, NP formulation 90.3 ± 0.02, solution 144 ± 0.067 ng/mL; P > 0.05) than RAL-EFV solution.
3.4. Studies on intracellular release of RAL and EFV from PLGA nanoparticles
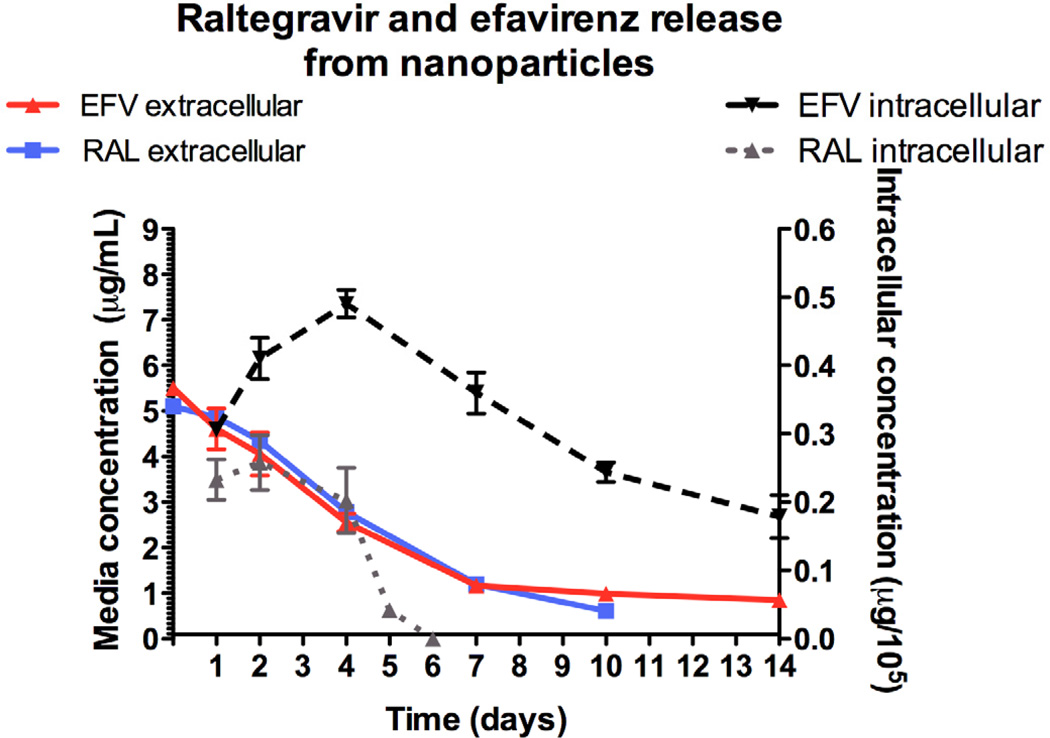
Intracellular and media concentrations of RAL and EFV released from RAL-EFV-NPs in HeLa cells are shown in Figure 4. The nanoparticle formulation offered sustained intracellular release of raltegravir and efavirenz. We have previously shown that intracellular efavirenz concentrations were undetectable from efavirenz solution after 2 days in vitro (Destache et al., 2009). The intracellular concentration of EFV on day 14 was > 150 ng/105 cells. The EC90 in our experiments averaged 90.3 ng/mL and therefore the concentration of efavirenz on day 14 was above the EC90 for the entire 14 days. Raltegravir intracellular concentration declined over the course of 6 days and was below the detectable limit of our assay on day 6. However, RAL was present in the media for 10 days, leading one to conclude that protection from HIV-1 infection could occur for 7 days with this combination product. Further experiments in vivo are necessary to confirm these results.
Figure 4.
Intra-cellular (HeLa cells) and media concentration of RAL and EFV released from RAL-EFV-NP over a period of 14 days (n=6; data presented as mean ± SEM).
3.5. Development of a thermosensitive vaginal gel
In the present investigation, we focused on developing thermosensitive gels to enable vaginal delivery of RAL-EFV-NP. Various compositions were studied to obtain a thermosensitive gel with optimal characteristics. The temperature of thermogelation obtained for various compositions is listed in supplementary materials (Table S1). The addition of Pluronic F68 had considerable effect on the thermogelation of the Pluronic F127. This is in agreement with others (Aka-Any-Grah et al., 2010). Pluronic F127 (20%) and Pluronic F68 (1%) showed optimal thermogelation in our investigation. The sol-gel transition curves for this composition and effect of temperature on the viscosity obtained using rheometry are provided in supplementary materials (Fig. S1 and S2). Figure S3 shows a photo of the RAL-EFV-NP gel before and after thermogelation.
3.6. Characterization of nanoparticle transfer from the thermosensitive gel using transwells
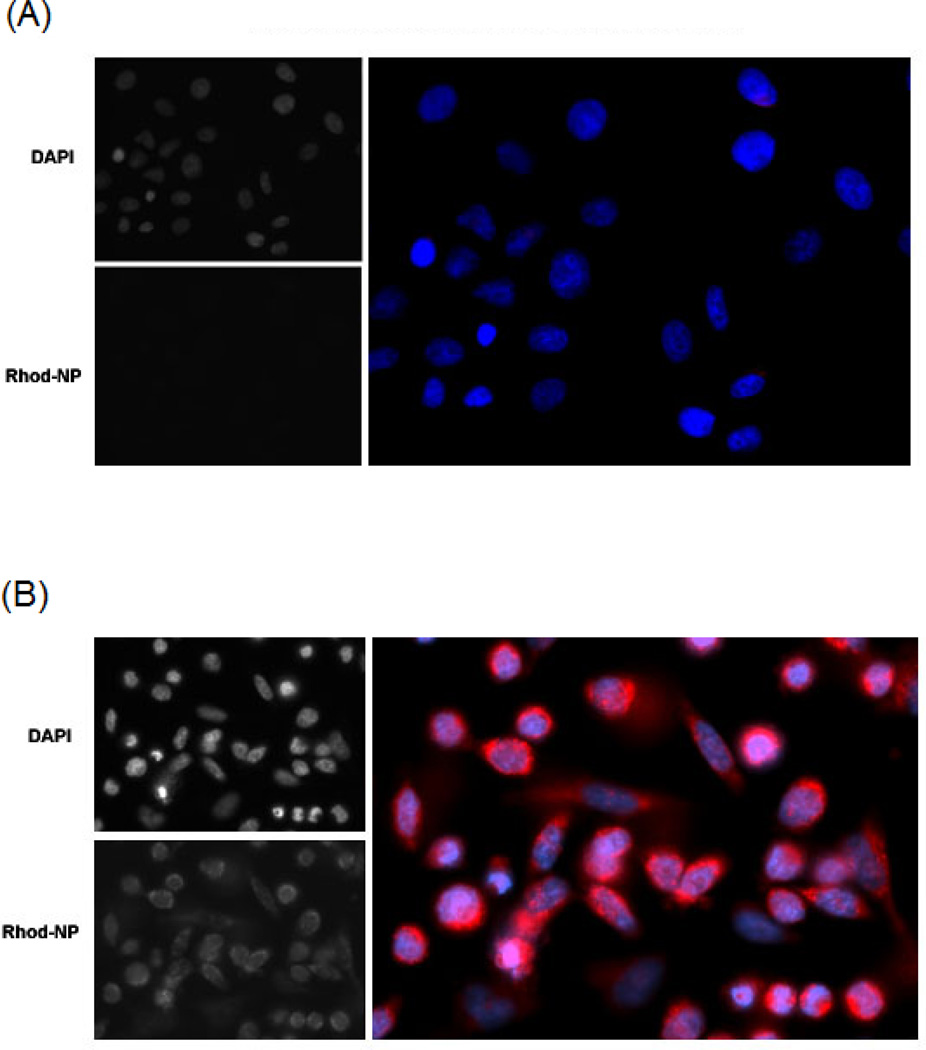
We sought to determine whether nanoparticles could be released from thermosensitive gel, move through a semi-permeable membrane and be taken up by HeLa cells. To perform these experiments, we utilized transwell experiments where the gel is placed on the top of the transwell support and the cells are adhered to the bottom of the culture well. We used fluorescent nanoparticles fabricated similarly to the RAL-EFV NPs to determine the time before the nanoparticles transfer to the cells. The results of these experiments are shown in Figure 5 A and B. Fluorescent nanoparticles were able to transfer through transwell membrane and were taken up by HeLa cells within 30 min.
Figure 5.
Transfer of Rhodamine 6G labeled PLGA nanoparticles from thermosensitive gel through transwell membrane and uptake by HeLa cells. Fluorescence imaging of HeLa cells after A)15 min and B) 30 min incubation with fluorescent nanoparticle containing gel in the transwell.
4. Discussion
The present investigation was focused on exploring the potential of polymeric nanoparticles for coital-independent vaginal prophylaxis of HIV. For successful vaginal delivery, the developed nanoparticles should preferably have small particle size and ability to rapidly penetrate through vaginal mucus in order to deliver the antiretroviral drug to the vaginal epithelium. We successfully developed sub-100 nm nanoparticles composed of PLGA, a FDA approved biodegradable polymer to deliver a combination of raltegravir and efavirenz (RAL-EFV-NPs). The Pluronic F127 was employed as a stabilizer for the development of RAL-EFV-NP. Recently, Yang et al., (2011) demonstrated rapid penetration of Pluronic F127 coated polystyrene nanoparticles through cervicovaginal mucus. Since, we employed Pluronic F127 for fabricating RAL-EFV-NPs, RAL-EFV-NPs were thought to have mucus-penetrating ability and a very recent study published by Hanes and coworkers proved our assumption (Ensign et al., 2012). Fabricated RAL-EFV-NPs demonstrated different entrapment efficiency for raltegravir and efavirenz. This difference can be attributed to difference in their physicochemical properties.
Raltegravir is a hydrophilic drug with negative log P value at pH 7.4 (Moss et al., 2012) whereas efavirenz is a lipophilic drug with positive log P value (Tanaka et al., 2008). During emulsification of the organic phase of the nanoparticle fabrication process, some amount of raltegravir would partition into aqueous phase due to its hydrophilicity while efavirenz would reside mainly in the organic phase due to its higher lipophilicity resulting in increased association with hydrophobic PLGA as compared to raltegravir.
Osmolarity is an important criterion for development of successful vaginal formulations. The 1% tenofovir gel used in the CAPRISA-004 trial was hyperosmolar (3111 mOsm/kg) and resulted in epithelial stripping of polarized explants (Dezzutti et al., 2012). Vaginal gels should have an osmolarity less than 1000 mOsm/kg to prevent mucosal irritation and damage to epithelial lining of the vagina (Friend 2010). Our studies showed that RAL-EFV-NPs had osmolarity less than 500 mOsm and are likely to be well tolerated compared to tenofovir 1% gel.
The long-term cytotoxicity experiments were designed to determine whether any of the components used for the fabrication of RAL-EFV-NPs containing gel were toxic to HeLa cells. We compared the RAL-EFV-NPs (at RAL and EFV concentration of 5 µg/mL) and blank gel to control cells without any treatment. The selection of drug concentration for the cytotoxicity experiment was based on the preliminary study which ensured that this concentration is completely nontoxic to HeLa cells (data not shown). The gel containing RAL-EFV-NPs did not show long-term toxicity to HeLa cells over the 14-day time period. The HeLa cells overgrew the wells after the 10-day time point. The MTT assay registered at the maximum limit of detection at these times and continued for the end of the 14-day experiment. This study also ensured that RAL-EFV-NPs did not affect HeLa cell viability during in vitro cell release studies.
It should be noted that the concentration-response experiments carried out in this investigation were adapted to establish the potential of RAL-EFV-NPs and RAL-EFV solution for prophylactic treatment. TZM-bl cells were treated with the RAL-EFV-NPs and RAL-EFV solution overnight, the media was removed, cells were washed, media was replaced with fresh media and the cells were infected with HIV after 24 h. Thus, cells would be infected with HIV-1 only if the treatment was unable to maintain effective concentrations. RAL-EFV-NPs were more active compared to the RAL-EFV solution. The lower EC90 of the nanoparticle formulation could be due to ability of nanoparticles to transport and maintain higher concentrations of raltegravir and efavirenz inside the cells.
The difference in intracellular concentrations of raltegravir and efavirenz is likely due to differences in their half-lives and metabolic profiles. Raltegravir has a half life of 7–12 h (Iwamoto et al., 2008) whereas efavirenz has a half life of 40–55 h (Bumpus et al., 2006). Raltegravir is metabolized by uridine diphosphate glucuronosyltransferase (UGT1A1, UGT1A3 and UGT1A9) mediated glucuronidation (Kassahun et al., 2007). Efavirenz is primarily metabolized by cytochrome P450 (CYP2B6) mediated hydroxylation (Bumpus et al., 2006). It has been reported that HeLa cells express UGT1A1 and UGT1A9 (Nakamura et al., 2008) whereas cytochrome P450 isoforms such as CYP3A4 are not present in HeLa cells (Rodríguez-Antona et al., 2003). The absence of CYP2B6 mRNA in cervical epithelia has also been reported (Farin et al., 1995). In view of this, it can be assumed that HeLa cells can readily metabolize raltegravir released from the nanoparticles whereas efavirenz may not undergo intracellular metabolism. This may explain the difference in the intracellular release of the raltegravir and efavirenz. We focused on the intracellular drug levels as this is the site of the drugs and HIV replication. However, the extracellular drug levels were also above the EC90 for the virus.
Development of suitable vehicle to enable vaginal delivery of RAL-EFV-NPs is an important aspect for bench-to-bedside translation. In the present investigation, we focused on developing thermosensitive gels for vaginal delivery of RAL-EFV-NPs. However, it is important to develop a thermosensitive gel that can remain liquid even in sub-tropical and tropical countries or in the zone IV as classified by the ICH guidelines where average temperature is usually greater than 30°C. At the same time, thermogelation temperature should not be too close to 37°C as body temperature shows variation as well. Thus, optimal thermogelation point of the gel was set to be between 30–33°C for this investigation. Pluronic F127 (20%) and Pluronic F68 (1%) showed optimal thermogelation in our investigation and the RAL-EFV-NPs did not show any visible signs of aggregation after incorporation in gel.
Finally, the transwell experiments were carried out to evaluate the transfer of the nanoparticles from the thermosensitive gel into the environment and cellular uptake. Interestingly, nanoparticles were taken up by HeLa cells within 30 minutes indicating release of nanoparticles from gel and rapid uptake of released nanoparticles by HeLa cells.
5. Conclusion
Combination antiretroviral PLGA nanoparticles containing raltegravir and efavirenz were successfully developed and incorporated into a thermosensitive gel suitable for vaginal delivery. Sustained intracellular release of raltegravir and efavirenz from the nanoparticles indicated that the developed gel-nanoparticle could have potential for long-term vaginal pre-exposure prophylaxis for prevention of heterosexual HIV transmission.
Supplementary Material
Figure 2.
Long-term cytotoxicity of RAL-EFV-NPs or blank gel incubated with HeLa cells over 14 days in vitro (n=3; data expressed as mean ± SEM). Data represents percent cell viability compared to HeLa cells without any treatment.
Highlights.
The PLGA nanoparticles (NPs) are < 100 nm with good encapsulation of raltegravir and efavirenz.
The gel was made allowing the gel to be liquid at room temperature and solid at body temperature.
The blank gel and raltegravir+efavirenz nanoparticles do not cause cytotoxicity over 14 days to HeLa cells.
Efavirenz had intracellular levels for 14 days and raltegravir had levels for 6 days by HPLC.
Fluorescent-loaded NPs transfer from the gel within 30 minutes and phagocytosized by HeLa cells.
Acknowledgement
The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: H9 cell-line from Dr. Robert Gallo, TZM-bl cells from Drs. John Kappes, Xiaoyun Wu, and Tranzyme, Inc. The authors would like to thank Dr. Han Chen of the Microscopy Core Research Facility at the University of Nebraska-Lincoln, for his assistance in SEM.
Financial Disclosure:
This work was supported by National Institute of Health grants 1R56AI095115-01 and a Clinical Translational Science Award from Creighton University (to C.J.D.), 5RO1AI080348 (to M. B.), a grant from the National Institute of General Medical Sciences (8 P30 GM103509-03), NIHINBRE grant and Creighton University Presidents Grant (to A.S.).
Glossary
- RAL
Raltegravir
- EFV
Efavirenz
- NP
nanoparticles
- NMP
N-methylpyrrolidone
- DMSO
Dimethylsulfoxide
- RAL-EFV-NP
Raltegravir + efavirenz loaded PLGA nanoparticles
- RAL-EFV solution
Raltegravir + efavirenz solution prepared with DMSO and water
- PLGA
Poly-lactide-co-glycolide
- ICH
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Miotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdool Karim SS, Baxter C. Overview of microbicides for the prevention of human immunodeficiency virus. Best Pract. Res. Clin. Obstet. Gynaecol. 2012;26:427–439. doi: 10.1016/j.bpobgyn.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams JL, Kashuba AD. Formulation, pharmacokinetics and pharmacodynamics of topical microbicides. Best Pract. Res. Clin. Obstet. Gynaecol. 2012;26:451–462. doi: 10.1016/j.bpobgyn.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aka-Any-Grah A, Bouchemal K, Koffi A, Agnely F, Zhang M, Djabourov M, Ponchel G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010;76:296–303. doi: 10.1016/j.ejpb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Apostolova N, Gomez-Sucerquia LJ, Moran A, Alvarez A, Blas-Garcia A, Esplugues JV. Enhanced oxidative stress and increased mitochondrial mass during efavirenz-induced apoptosis in human hepatic cells. Br. J. Pharmacol. 2010;160:2069–2084. doi: 10.1111/j.1476-5381.2010.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baloglu E, Karavana SY, Senyigit ZA, Hilmioglu-Polat S, Metin DY, Zekioglu O, Guneri T, Jones DS. In-situ gel formulations of econazole nitrate: preparation and in-vitro and in-vivo evaluation. J. Pharm. Pharmacol. 2011;63:1274–1282. doi: 10.1111/j.2042-7158.2011.01315.x. [DOI] [PubMed] [Google Scholar]
- 7.Bumpus NN, Kent UM, Hollenberg PF. Metabolism of efavirenz and 8- hydroxyefavirenz by P450 2B6 leads to inactivation by two distinct mechanisms. J. Pharmacol. Exp. Ther. 2006;318:345–351. doi: 10.1124/jpet.106.102525. [DOI] [PubMed] [Google Scholar]
- 8.Clavel C, Peytavin G, Tubiana R, Soulié C, Crenn-Hebert C, Heard I, Bissuel F, Ichou H, Ferreira C, Katlama C, Marcelin AG, Mandelbrot L. Raltegravir concentrations in the genital tract of HIV-1-infected women treated with a raltegravircontaining regimen (DIVA 01 study) Antimicrob. Agents Chemother. 2011;55:3018–3021. doi: 10.1128/AAC.01460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Destache CJ, Belgum T, Goede M, Shibata A, Belshan MA. Antiretroviral release from poly(DL-lactide-co-glycolide) nanoparticles in mice. J. Antimicrob. Chemother. 2010;65:2183–2187. doi: 10.1093/jac/dkq318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Destache CJ, Belgum T, Shibata A, Christensen K, Sharma A, Dash A. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC Infect. Dis. 2009;9:198. doi: 10.1186/1471-2334-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dezzutti CS, Rohan LC, Wang L, Uranker K, Shetler C, Cost M, Lynam JD, Friend D. Reformulated tenofovir gel for use as a dual compartment microbicide. J. Antimicrob. Chemother. 2012 doi: 10.1093/jac/dks173. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, Hanes J. Mucuspenetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci. Transl. Med. 2012;4:138ra79. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farin FM, Bigler LG, Oda D, McDougall JK, Omiecinski CJ. Expression of cytochrome P450 and microsomal epoxide hydrolase in cervical and oral epithelial cells immortalized by human papillomavirus type 16 E6/E7 genes. Carcinogenesis. 1995;16:1670. doi: 10.1093/carcin/16.7.1670. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher P, Harman S, Azijn H, Armanasco N, Manlow P, Perumal D, de Bethune MP, Nuttall J, Romano J, Shattock R. Inhibition of human immunodeficiency virus type 1 infection by the candidate microbicide dapivirine, a nonnucleoside reverse transcriptase inhibitor. Antimicrob. Agents Chemother. 2009;53:487–495. doi: 10.1128/AAC.01156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friend DR. Pharmaceutical development of microbicide drug products. Pharm. Dev. Technol. 2010;15:562–581. doi: 10.3109/10837450903369879. [DOI] [PubMed] [Google Scholar]
- 16.García-Lerma JG, Paxton L, Kilmarx PH, Heneine W. Oral preexposure prophylaxis for HIV prevention. Trends Pharmacol Sci. 2010;31:74–81. doi: 10.1016/j.tips.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto M, Kassahun K, Troyer MD, Hanley WD, Lu P, Rhoton A, Petry AS, Ghosh K, Mangin E, DeNoia EP, Wenning LA, Stone JA, Gottesdiener KM, Wagner JA. Lack of a pharmacokinetic effect of raltegravir on midazolam: in vitro/in vivo correlation. J. Clin. Pharmacol. 2008;48:209–214. doi: 10.1177/0091270007310382. [DOI] [PubMed] [Google Scholar]
- 18.Kassahun K, McIntosh I, Cui D, Hreniuk D, Merschman S, Lasseter K, Azrolan N, Iwamoto M, Wagner JA, Wenning LA. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab. Dispos. 2007;35:1657–1663. doi: 10.1124/dmd.107.016196. [DOI] [PubMed] [Google Scholar]
- 19.Kelly CG, Shattock RJ. Specific microbicides in the prevention of HIV infection. J. Intern. Med. 2011;270:509–519. doi: 10.1111/j.1365-2796.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- 20.Kiser PF, Mahalingam A, Fabian J, Smith E, Damian FR, Peters JJ, Katz DF, Elgendy H, Clark MR, Friend DR. Design of tenofovir-UC781 combination microbicide vaginal gels. J. Pharm. Sci. 2012;101:1852–1864. doi: 10.1002/jps.23089. [DOI] [PubMed] [Google Scholar]
- 21.Maggiolo F. Efavirenz: a decade of clinical experience in the treatment of HIV. J. Antimicrob. Chemother. 2009;64:910–928. doi: 10.1093/jac/dkp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurin MB, Rowe SM, Blom K, Pierce ME. Kinetics and mechanism of hydrolysis of efavirenz. Pharm. Res. 2002;19:517–521. doi: 10.1023/a:1015160132290. [DOI] [PubMed] [Google Scholar]
- 23.McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, Jentsch U, Pool R, Chisembele M, Kapiga S, Mutemwa R, Vallely A, Palanee T, Sookrajh Y, Lacey CJ, Darbyshire J, Grosskurth H, Profy A, Nunn A, Hayes R, Weber J. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376:1329–1337. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss DM, Siccardi M, Murphy M, Piperakis MM, Khoo SH, Back DJ, Owen A. Divalent Metals and pH Alter Raltegravir Disposition In Vitro. Antimicrob. Agents Chemother. 2012;56:3020–3026. doi: 10.1128/AAC.06407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munier CM, Andersen CR, Kelleher AD. HIV vaccines: progress to date. Drugs. 2011;71:387–414. doi: 10.2165/11585400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, Yokoi T. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab. Dispos. 2008;36:1461–1464. doi: 10.1124/dmd.108.021428. [DOI] [PubMed] [Google Scholar]
- 27.Notari S, Tommasi C, Nicastri E, Bellagamba R, Tempestilli M, Pucillo LP, Narciso P, Ascenzi P. Simultaneous determination of maraviroc and raltegravir in human plasma by HPLC-UV. IUBMB Life. 2009;61:470–475. doi: 10.1002/iub.181. [DOI] [PubMed] [Google Scholar]
- 28.Price CF, Tyssen D, Sonza S, Davie A, Evans S, Lewis GR, Xia S, Spelman T, Hodsman P, Moench TR, Humberstone A, Paull JR, Tachedjian G. SPL7013 Gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS One. 2011;6:e24095. doi: 10.1371/journal.pone.0024095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez-Antona C, Bort R, Jover R, Tindberg N, Ingelman-Sundberg M, Gómez-Lechón MJ, Castell JV. Transcriptional regulation of human CYP3A4 basal expression by CCAAT enhancer-binding protein alpha and hepatocyte nuclear factor-3 gamma. Mol. Pharmacol. 2003;63:1180–1189. doi: 10.1124/mol.63.5.1180. [DOI] [PubMed] [Google Scholar]
- 30. Roy S, Gourde P, Piret J, Désormeaux A, Lamontagne J, Haineault C, Omar RF, Bergeron MG. Thermoreversible gel formulations containing sodium lauryl sulfate or n-Lauroylsarcosine as potential topical microbicides against sexually transmitted diseases. Antimicrob. Agents Chemother. 2001;45:1671–1681. doi: 10.1128/AAC.45.6.1671-1681.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruel-Gariépy E, Leroux JC. In situ-forming hydrogels--review of temperaturesensitive systems. Eur J Pharm Biopharm. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Shibata A, McMullen E, Pham A, Belshan M, Sanford B, Zhou Y, Goede M, Date AA, Destache CJ. Combination antiretroviral nanoparticles for HIV-1 treatment. 2012 AIDS Human Retroviruses. 2012 Submitted. [Google Scholar]
- 33.Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, Maguire R, Lahteenmaki P. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka R, Hanabusa H, Kinai E, Hasegawa N, Negishi M, Kato S. Intracellular efavirenz levels in peripheral blood mononuclear cells from human immunodeficiency virus-infected individuals. Antimicrob. Agents Chemother. 2008;52:782–785. doi: 10.1128/AAC.01613-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warner L, Ghanem KG, Newman DR, Macaluso M, Sullivan PS, Erbelding EJ. Male circumcision and risk of HIV infection among heterosexual African American men attending Baltimore sexually transmitted disease clinics. J. Inf. Dis. 2009;199:59–65. doi: 10.1086/595569. [DOI] [PubMed] [Google Scholar]
- 36.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat. Mater. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M, Lai SK, Wang YY, Zhong W, Happe C, Zhang M, Fu J, Hanes J. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angew. Chem. Int. Ed. Engl. 2011;50:2597–2600. doi: 10.1002/anie.201006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.