Abstract
A major paradigm shift in cancer research is the emergence of multidisciplinary approaches to investigate complex cell behaviors to elucidate the regulatory mechanisms and to identify therapeutic targets. Recently, efforts are focused on the engineering of complex in-vitro models, which more accurately recapitulate the growth and progression of cancer. These strategies have proven vital for investigating and targeting the events that control tumor angiogenesis. In this review, we explore how the emerging engineering approaches are being utilized to unlock the complex mechanisms regulating tumor angiogenesis. Emphasis is placed on models employing natural and synthetic biomaterials to generate scaffolds mimicking the extracellular matrix (ECM) which plays a critical role in angiogenesis. While the models presented in this review are revolutionary, improvements are still necessary and concepts for advancing and perfecting engineering approaches for modeling tumor angiogenesis are proposed. Overall, the marriage between disparate scientific fields is expected to yield significant improvements of our understanding and treatment of cancer.
Keywords: Extracellular matrix, Scaffolds, Microfluidics, Micropatterning
Introduction
In order for tumors to grow and fuel their insatiable metabolic demands, ongoing formation of new blood vessels must occur, a process referred to as angiogenesis. Under non-pathological conditions, angiogenesis is strictly regulated by matrix proteins, cell-cell connections and the abundance of pro-and anti-angiogenic factors (1). During events associated with wound healing, for example, endothelial cells (ECs) transition from a quiescent to an activated state, degrading their underlying basement membrane which in turn, permits their migration and proliferation in the interstitial ECM (e.g. collagens and fibronectin) (2). At this point, the ECs align into cord-like structures, synthesizing a new, supportive basement membrane upon which they resume their quiescent state (2). While strictly regulated under homeostasis, these events become perturbed during tumorigenesis, wherein the uncontrolled formation of neovessels ensues. A process initially described by the pioneering work of Judah Folkman (3), angiogenesis is a requisite for tumors to grow beyond 1–2 mm3. As such, a greater understanding of the cellular and molecular mechanisms governing tumor angiogenesis has been of interest to those wishing to abrogate the process. The extracellular matrix (ECM), as discussed in more detail below, is a critical determinant regulating angiogenesis. In this manner, engineering approaches employing ECM-mimetic materials as scaffolds for cellular studies have provided invaluable insight into the events regulating tumor angiogenesis.
Throughout the years, in-vitro investigations centered mainly on the use of 2-dimensional (2D) or 3-dimensional (3D) spheroid cell culture systems to elucidate the cellular and molecular events governing angiogenesis, strategies that insufficiently mimic that which takes place in-vivo. In order to more accurately recapitulate the events promoting tumor angiogenesis, several strategies presently merge engineering principles with the life sciences. Specifically, to overcome the inherent limitations of traditional in-vitro cell culture systems, 3D ECM-inspired materials have been engineered to generate scaffolds to study cell behavior in an environment that more accurately corresponds to the in-vivo scenario. While such biomaterials may be derived and synthesized from biological sources, many are fabricated from synthetic materials. Scaffolds are designed to permit a myriad of cell behaviors and function as both a therapeutic entity, delivering cells to diseased and wounded tissues, and as a platform to investigate cell activities in-vitro. Focusing on the latter, one of the most important attributes of a engineered scaffold, aside from being non-toxic to the cells, is that it must permit cell attachment, typically by containing or incorporating cell adhesive entities such as the integrin binding Arg-Gly-Asp [RGD] motif (4). Furthermore, the scaffold must permit sufficient transport of nutrition and oxygen, as well as the movement of cells within the scaffold while maintaining sufficient mechanical strength to withstand cellular traction forces (4). In addition to these, other modifications can be addressed during scaffold fabrication such as surface chemistry (e.g. tethering of bioactive molecules for directed cell behavior), mechanical attributes (e.g. thermal-, photo- or chemically-induced crosslinking of polymers) and topography (e.g. introduction of cell-adhesive regions containing variations in height, width and shape). Together, these features allow the precise control over multiple cues and can be fine-tuned to elicit the desired cell response.
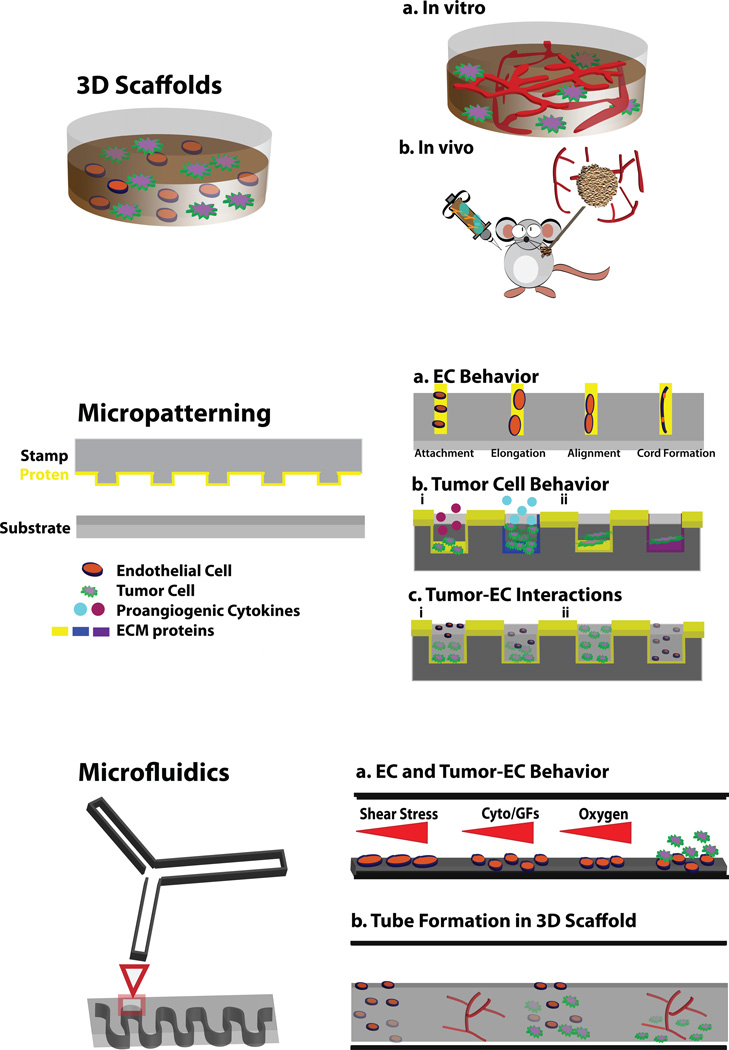
In the following sections, we discuss the employment of natural ECM matrices and the engineering of new scaffolds to study the cellular and molecular events regulating tumor angiogenesis in a biomimetic environment. These approaches have yielded significant advancements in our understanding of tumor angiogenesis and are changing the way in which scientists approach complex behaviors of tumor cells in their surrounding environment. Figure 1 provides an overview of the engineering-based approaches discussed in this review and their applications for investigating the independent and coordinate interactions taking place between vascular and tumor cells.
Figure 1. Overview of engineering-based approaches utilized for investigating angiogenesis.
Upper panel: 3D ECM scaffolds are utilized to investigate tumor-EC interactions (A) in vitro and (B) in-vivo. Middle panel: Micropatterning is utilized (A) to evaluate vascular cell behaviors on geometrically patterned arrays of ECM, (B) to investigate tumor cell behaviors on ECM proteins to evaluate (i) angiogenic cytokine secretion and (ii) adhesion and migration, and (C) to assess tumor-EC interactions including (i) tumor cell-directed invasion of ECs into gels and (ii) ECs and tumor cells co-culture in 3D ECM scaffolds. Lower panel: Microfluidic devices are utilized (A) to evaluate vascular cell responses to physical and chemical gradients, delineate the tumor-EC interactions taking place within a controlled environment and (B) to study vascular morphogenesis taking place in the presence and absence of tumor cells in 3D ECM scaffolds. Drawing not to scale.
ECM and its implications in angiogenesis and tumor angiogenesis
The ECM is a non-cellular, protein-rich entity which supports cell and tissue organization and controls cell quiescence, survival, growth and differentiation (5). The ECM contributes to these diverse cellular functions by providing attachment sites for cells, sequestering bioactive molecules and providing mechanical support to overlying cells (5). Regarding angiogenesis, it has been shown that fibrillar ECM proteins (e.g. fibronectin and collagens I and III) and basement membrane proteins (e.g. laminin and collagen IV) play distinct and overlapping roles in angiogenesis (6). Recently, we have shown that the collective ECM, deposited from co-cultured breast cancer cells and fibroblasts, support vascular morphogenesis of ECs (7). The most pronounced changes in EC capillary features were attributed to ECM deposited from metastatic breast cancer cells co-cultured with fibroblasts, suggesting that the tumor microenvironment may differentially affect vascular morphogenesis during tumor progression (7). Given its pro-angiogenic functions, researchers are aiming to better understand how specific ECM proteins, as well as ECM structure and degradability, govern the cellular and molecular processes ultimately leading to tumor angiogenesis.
In order to recapitulate the host environment, polymerized hydrogels derived from natural protein-based (e.g. Matrigel™, collagen and fibrin) materials are utilized to mimic the natural ECM. The benefits associated with natural protein-based materials is that they are more representative of that which occurs in the body, containing the appropriate spatiotemporal presentation of instructive cues necessary for directing a variety of cell behaviors. Thus, a great deal of effort has been placed on utilizing natural ECM matrices for investigating vascular cell and tumor-directed vascular cell behavior. One of the most extensively used natural ECM substrates for investigating angiogenesis is Matrigel™, a soluble basement membrane extracted from mouse tumors. Comprised primarily of laminin and collagen IV, Matrigel™ supports organization of ECs into complex networks of vascular structures (8) and has been widely utilized for investigations into the discovery of anti-angiogenic therapeutic targets and efficacy of newly developed anti-angiogenic agents (9–11). For instance, Mammoto et al (12) demonstrated that changes in the mechanical stiffness of Matrigel™, a feature of the tumor microenvironment, altered the expression of two Rho-activated transcription factors, Transcription Factor II-I and GATA2, known to regulate vascular endothelial growth factor (VEGF) receptor 2 expression. These factors and/or their pathways may serve as potential targets for anti-angiogenic therapies. In addition, it was shown that Matrigel™ encapsulated with immature dendritic cells (DCs) as opposed to Matrigel™ encapsulated with mature DCs, facilitated tumor angiogenesis when implanted adjacent to subcutaneous grown tumors (13), knowledge which may lend to development of targeted therapies. While Matrigel™ provides numerous benefits to evaluate the mechanisms controlling and abrogating angiogenesis, it has several drawbacks including batch-batch variations that reduce reproducibility of results, high cost and temperature sensitivity that limit matrix handling (14). As a result, additional sources of pure populations of ECM proteins (e.g. collagen, gelatin and fibrin) are increasingly being utilized as substrata for studying angiogenesis (15–17). Such pure ECM matrices allow investigators the opportunity to investigate how changes in the biological, structural and mechanical properties of the ECM affect capillary morphogenesis (18, 19). Pertaining to cancer, Ghosh et al (20) used fibrin gels to demonstrate that tumor-derived ECs exhibit abnormal mechano-sensing and angiogenesis. In addition, collagen I gels have been utilized to demonstrate that pro-angiogenic cytokines, secreted from spheroids of MDA-MB-231 breast cancer cells or OSCC-3 oral squamous carcinoma cells, promote formation of capillary sprouting and gel invasion (16, 21).
Although these and other studies have demonstrated the utility of collagen and fibrin-based scaffolds for investigating angiogenesis and tumor-induced angiogenesis, the restricted ability to modify certain chemical and mechanical properties, limits the modes, the range and the complexity of the ECM-driven cues that can be studied using such matrices. The opportunities to design a scaffold with desired physical and chemical properties is thus imperative for dissecting how various ECM-driven cues affect cell behavior.
Engineering approaches using ECM to investigate tumor angiogenesis
Similar to natural ECM matrices, scaffolds from biological or synthetic materials have been utilized to investigate diverse mechanisms controlling angiogenesis. Examples of ECM-mimetic hydrogels synthesized from biological sources include alginate, hyaluronic acid (HA), collagen, gelatin and fibrin (22). Commonly used synthetic scaffolds for studies of vascular regeneration include polycoprolactone (PCL), polyethylene glycol (PEG), polyglycolic acid (PGA), poly-l-lactic acid (PLLA), and poly(lactic-co-glycolic acid) (PLGA). (22, 23)
While these scaffolds have been primary utilized for engineering of healthy vasculature for regenerative medicine applications, researchers are increasingly applying these systems to investigate tumor angiogenesis in-vitro and in-vivo (Figure 1; top panel). Fischbach et al (24, 25) have demonstrated enhanced angiogenic factor secretion, interleukin 8 (IL8), from OSCC-3 tumor cells cultured in both biological (alginate coupled with RGD) and synthetic (PLG compounded with Matrigel™) 3D scaffolds. Conditioned media from such 3D cultures promoted capillary proliferation and elongation in-vitro and stimulated increased vascularity of tumors in-vivo over that observed for 2D cultures (24). Others have shown that human lung tumor cells grown in 3D HA hydrogels compounded with laminin and growth factors, promoted tumor vascularization in-vivo over those scaffolds not containing additional ECM or growth factor components (26). Recently, it was found that secreted factors from quiescent ECs, embedded in porous gelatin matrices, slowed the progression of lung tumor cells following their implantation adjacent to subcutaneously growing tumors (27). The authors attribute the use of the gelatin matrix to preserving the quiescent activity of the ECs which in turn, maintain their secretome (27). Together, these studies not only demonstrate that the 3D ECM environment participates in regulating tumor cell angiogenic cytokine secretion, but additionally illustrates the importance of choosing the appropriate ECM scaffold necessary for addressing the question at hand.
In addition to the use of bulk ECM-based biomaterials for 3D studies of tumor angiogenesis, micropatterning techniques are being utilized to investigate EC phenotypic changes occurring in the tumor microenvironment (Figure 1; middle panel). These approaches are desirable as they permit the precise control over the presentation of instructive environmental cues, including regulation of ECM spatial orientation, topography, and mechanics to guide cell behavior (28). Several groups have established the importance of controlling ECM spatiotemporal matrix features on EC behavior. Together, these groups outline how vascular cell behavior, including the switch from growth to apoptosis (29), differentiation (30), alignment and elongation (31–34) and cord formation (35) is controlled as a result of altering the geometry, spacing, and stiffness of patterned ECM. Recent studies have demonstrated the successful use of micropatterning for elucidating the micro-environmental features controlling tumor angiogenesis. In an effort to investigate patterns of pro-angiogenic factor secretion by tumor cells, single populations or clusters of squamous or prostate tumor cells were cultured on micro-printed, spatially defined fibronectin regions (36). It was found that profiles of VEGF, IL8 and basic fibroblast growth factor were markedly different based on tumor cell type and whether the cells were grown singularly, lacking cell-cell interactions or in clusters, permitting cell-cell interactions (36). This study not only illustrates how cell-cell interactions influence profiles of cytokine secretion, but demonstrates the importance of regulating the spatial presentation of cells on ECM to investigate patterns of cell behavior. In a similar study, it was found that IL8 secretion was increased in micro-printed stamps of OSCC-3 cells embedded in collagen (37). Increased IL8 secretion was further shown to promote tumor angiogenesis in-vitro via enhanced EC migration and invasion (37). As a proof of principle, Chiu et al (38) micropatterned tumor cells and ECs together in spatially and geometrically defined regions of fibrinogen, methodology the authors believe will not only allow for studies of tumor cell-directed EC migration and capillary formation, but will permit patterning of numerous cell types on the same substrate. Work in our lab has demonstrated the specific adhesion of breast cancer cells on micropatterned HA surfaces (39) and endothelial progenitor cell elongation and unidirectional network assembly on micropatterned fibronectin and fibrin gel stripes (32), suggesting the role of spatial presentation of specific ECM molecules in regulating tumor and vascular cell behaviors. Overall, through control over the geometry and spacing of ECM molecules, investigators will be better equipped to address the complex interactions taking place between cancer and vascular cells during angiogenesis in the tumor environment.
Microfluidic devices are an emerging engineering-based approach enabling investigators the precise control over several aspects of the tumor microenvironment (Figure 1; lower panel). An apparatus consisting of one or more micro- or nanometer sized channels, microfluidic devices allow the investigator to regulate the physical and chemical environment of cell population(s) of interest (40). Numerous groups have capitalized on the benefits afforded from microfluidic devices to investigate vascular cell behaviors in controlled microenvironments. For instance, microfluidic devices have been utilized to evaluate vascular cell responses to shear stress (41), migration in response to cytokine gradients in 2D (42) and 3D collagen gels (43). Additionally, groups are utilizing microfluidic devices to control the spatial and temporal distribution of oxygen, technology which will allow investigators the opportunity to probe cancer and vascular cells responses to low oxygen and to gradients of oxygen tension in a controlled microenvironment (44, 45). With specific relevance to angiogenesis Golden and Tien utilized microfluidics to create a series of interconnected channels in a collagen and fibrin-containing scaffold (46). The voided channels were subsequently seeded with ECs, forming a primitive vascular network (46). Utilizing a similar approach, a more recent publication demonstrates the use of microfluidics for the fabrication of living and biofunctional microvascular networks within collagen gels (47).
While the aforementioned studies have clear implications for the field of vascular biology, these concepts have been recently exploited to the study of tumor angiogenesis. In an attempt to elucidate how tumor cells regulate angiogenesis, Cross et al (16) utilized a microfluidic device to asses EC invasion and capillary and lumen formation in spatially defined micromolded collagen gels with and without tumor cells (16). It was found that ECs readily invaded collagen gels, a property attributed to tumor cell release of pro-angiogenic factors, producing networks of capillaries with defined lumens (16). Chung et al (48) have utilized microfluidic devices to demonstrate that different tumor cell populations, grown with and without smooth muscle cells (SMCs) in collagen gels, induced varying responses in ECs. Interestingly, in the absence of SMCs, MTln3 rat mammary tumor cells but not U87 glioblastoma cells, induced capillary formation of ECs (48). Toward miniaturizing the tumor environment, these and others demonstrate the feasibility of utilizing microfluidics for studying tumor angiogenesis.
Overall, these studies illustrate the importance of both relevant in-vitro bio-mimetic systems and controllable microenvironments to investigate vascular-tumor cell behaviors and interactions. These advanced cell culture platforms will undoubtedly become important tools for cancer researchers interested in interrogating the role of biochemical, physical, cell-to-cell and cell-ECM events driving tumorigenic progression.
Conclusions and Future Directions: The next generation of engineered systems
In order to understand the cellular, molecular, biochemical and physical events contributing to tumor initiation and progression, one must apply models, which most accurately recapitulate this in humans. Three-dimensional scaffolds allow one to interrogate the cell-cell and cell-ECM events regulating tumor angiogenesis. Micropatterning technologies allow one to control the spatial and temporal distribution of cells, enabling determination of how the geometry and topography of the confined surrounding influence tumor angiogenesis. Finally, microfluidic devices allow the investigator to precisely control the physical and chemical environment of cell population(s), enabling a better understanding of how micro-environmental insults regulate vascular cell behavior and tumor angiogenesis. While these techniques have and continue to yield great insight into tumor and vascular cell behaviors, it is foreseeable that as the technology progresses, cancer researchers will have platforms from which to gain a greater understanding of how tumors initiate, progress and respond to treatment.
A major area of focus for cancer researchers and engineers is on the development of scaffolds that better recapitulate the complex and dynamic tumor niche. One well-known aspect of the tumor niche is that it is “stiffer” compared to its normal tissue counterparts. For example, the mechanical stiffness of breast carcinomas has been reported to be as high as 42.5 kilopascals (kPa) for high grade invasive ductal carcinomas as opposed to 3.25 kPa reported for non-malignant mammary tissues (49). Similarly, lymph nodes harboring metastatic tumor foci had a mechanical stiffness of 3.35 ± 1.57 g/cm (e.g. 329 Pa) versus non-tumor bearing lymph nodes having a mechanical stiffness of 1.23 g/cm (e.g. 121 Pa) (50). To elucidate the putative role of stiffness during tumor development, polyacrylamide substrates were utilized to demonstrate the role of matrix stiffness in enhancing tumor cell proliferation and migration (51, 52) and resistance to chemotherapeutics (52). Similarly, it has been found that ECs change their behavior and morphology in a manner dependent on substrate stiffness (53, 54). Hence utilizing scaffolds fabricated to model the stiffness of the tissue/tumor of interest will be paramount for elucidating how the mechanically stiffer tumor contributes to pathological angiogenesis.
Engineering the mechanical stiffness of the scaffold, while decoupling it from other key properties such as cell adhesion sites, elasticity, composition and cytokine sequestering, may allow a better understanding of how the tumor’s physical environment contributes to cell phenotypic changes governing tumor angiogenesis and progression. In an attempt to utilize engineering approaches to understand the role of matrix stiffness on cellular behaviors, a recent publication employed a range of matrix stiffness, using two different hydrogel substrates, to investigate how substrate properties influence mesenchymal stem cells [MSCs] differentiation (55). By covalently linking collagen I to the hydrogel surface, the authors discovered that the stem cells respond to the mechanical feedback of the collagen anchored to the substrate (55). Hydrogels of different stiffnesses led to differences in anchoring densities and thereby altered the mechanical feedback of the collagen (55). Decoupling matrix stiffness from cell adhesiveness, the authors suggest that this approach more accurately models how cells receive information on mechanical changes in their environment via the ECM (55). In this manner, dissociating matrix stiffness from additional matrix-related cues will be imperative for addressing if and how the stiffened tumor environment facilitates tumor angiogenesis.
Advances in the technology are also envisioned to enable the better imitation of the dynamic surroundings. Currently, most technologies capture a specific makeup of the microenvironment and allow the study of vascular behaviors in response to a given cue/s. The ability to engineer dynamic environments in which the timing, length, and magnitude of the cues can be controlled and manipulated along the culture period, will be critical for unraveling how a complex tumor environment dictates angiogenesis. Furthermore, engineering an environment which responds to feedback provided from tumor and vascular cells and other cell types in the tumor microenvironment (e.g. fibroblasts, MSCs, immune cells, etc) will also allow for the analysis of vascular responses to the dynamically changing cancerous surroundings. An example is the fabrication of 3D scaffolds for investigating matrix metalloproteinase (MMP)-regulated angiogenesis. MMPs are zinc-dependent endopeptidases which digest various structural components of the ECM during tissue remodeling, including angiogenesis, where they degrade the vascular basement membrane and liberate matrix-sequestered pro-angiogenic factors (56). In addition to their homeostatic role, MMPs have also received widespread recognition for their ability to promote tumor angiogenesis (56). Since various 3D scaffolds can be coupled with MMP responsive groups, allowing controlled degradation of the scaffold for investigations of cell behavior and angiogenesis (57–60), this platform is translatable to studies of MMP-regulated tumor angiogenesis. Here, one may directly assess how the activity of one or more tumor-secreted MMPs regulates the degradation of the MMP-complexed scaffold, which in turn permits formation of vessels. This knowledge may allow for a greater understanding of how tumor-directed matrix-degradation controls tumor angiogenesis in a relevant 3D environment.
Merging the engineering-based approaches is envisioned to simultaneously provide the investigator with a platform from which to study tumorigenesis in a controlled environment harboring spatially defined regions of 3D cell constructs. Such platforms would enable the investigator unprecedented control over the spatial and temporal delivery of oxygen, solutes and potentially other cell populations to the 3D cell construct. For example, spatial control over vascular formation is essential for the generation of “desired patterns” of vascular networks. A recent study utilized confocal imaging combined with two photon laser scanning lithography to pattern hydrogels with multiple cell adhesive moieties (61). This approach enabled spatial control over 3D cell adhesion to guide the formation of well-organized vasculatures in PEG-based hydrogels (61). Another study demonstrated the utilization of a cytocompatible sacrificial template to generate vascular networks in various hydrogel systems (62). The endothelialized networks were subsequently perfused with blood, sustaining the metabolic activities of living cells within the hydrogels (62). While these innovative approaches were exploited to generate microvasculature networks of healthy tissue, it is conceivable that they may be employed to recapitulate the abnormal architecture of tumor vessels. Here, the re-created tumor vessels may be utilized to investigate how the tortuous nature of the tumor vasculature combined with aberrant blood flow patterns within the vessel, elicit changes in surrounding tumor cell behavior.
Another example is the use of micropatterning to arrange one or more cell populations in the same or different biologically-relevant hydrogel (63) or also in a microfluidic device. In this manner, one can evaluate how different tumor cell lines, representative of the various stages of tumorigenesis, differentially influence EC behavior and vascular morphogenesis in a spatially, chemically and physically controlled environment. Additionally, one may be able to ascertain how different cell types in the tumor microenvironment coordinate with tumor cells to direct capillary formation. Demonstrating the feasibility of such a system, Huang et al (64) utilized a microfluidic device in which they spatially patterned 3D hydrogels containing tumor cells or macrophages to investigate tumor-immune cell interactions. The authors report that Matrigel™-encapsulated macrophages readily invaded collagen I-encapsulated breast cancer cells (64), providing proof of principle that a merged engineered platform may be successfully utilized to elucidate tumor behaviors. As complexity is added to the system, through inclusion of various cell populations and/or establishment of physical and chemical gradients, investigators will not only have a platform from which to evaluate the biological events driving tumor angiogenesis, but will additionally have a system from which to reliably test the utility of known and emerging anti-angiogenic or chemotherapeutic drugs.
Altogether, recent engineering-based approaches are allowing investigators the ability to control key physicochemical and spatiotemporal features of the tumor environment. Specifically, these approaches have proven promising for investigations into vascular cell behavior in the cancerous environment. It is foreseeable that merging the technologies presented in this review will allow for unprecedented discoveries into not only tumor angiogenesis, but in the biology and treatment of tumors.
Acknowledgments
Grant Support
Funding for this work was supported by the National Cancer Institute Physical Sciences-Oncology Network (U54CA143868).
Footnotes
Disclosure of Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Pollina EA, Legesse-Miller A, Haley EM, Goodpaster T, Randolph-Habecker J, Coller HA. Regulating the angiogenic balance in tissues. Cell Cycle. 2008;7:2056–2070. doi: 10.4161/cc.7.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Ushida T, Tateishi T. Scaffold Design for Tissue Engineering. Macromolecular Bioscience. 2002;2:67–75. [Google Scholar]
- 5.Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5(Suppl 1):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 7.Hielscher AC, Qiu C, Gerecht S. Breast cancer cell-derived matrix supports vascular morphogenesis. Am J Physiol Cell Physiol. 2012;302:C1243–C1256. doi: 10.1152/ajpcell.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MS, Lee YM, Moon EJ, Kim SE, Lee JJ, Kim KW. Anti-angiogenic activity of torilin, a sesquiterpene compound isolated from Torilis japonica. Int J Cancer. 2000;87:269–275. [PubMed] [Google Scholar]
- 10.Ren X, Dai M, Lin LP, Li PK, Ding J. Anti-angiogenic and vascular disrupting effects of C9, a new microtubule-depolymerizing agent. Br J Pharmacol. 2009;156:1228–1238. doi: 10.1111/j.1476-5381.2009.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggeri BA, Robinson C, Angeles T, Wilkinson Jt, Clapper ML. The chemopreventive agent oltipraz possesses potent antiangiogenic activity in vitro, ex vivo, and in vivo and inhibits tumor xenograft growth. Clin Cancer Res. 2002;8:267–274. [PubMed] [Google Scholar]
- 12.Mammoto A, Connor KM, Mammoto T, et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fainaru O, Almog N, Yung CW, et al. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB J. 2010;24:1411–1418. doi: 10.1096/fj.09-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Yang G, Zhang H, Prestwich GD. Evaluating dual activity LPA receptor pan-antagonist/autotaxin inhibitors as anti-cancer agents in vivo using engineered human tumors. Prostaglandins Other Lipid Mediat. 2009;89:140–146. doi: 10.1016/j.prostaglandins.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen P, Melero-Martin J, Bischoff J. Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. J Tissue Eng Regen Med. 2011;5:e74–e86. doi: 10.1002/term.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross VL, Zheng Y, Won Choi N, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31:8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafleur MA, Handsley MM, Knauper V, Murphy G, Edwards DR. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs) J Cell Sci. 2002;115:3427–3438. doi: 10.1242/jcs.115.17.3427. [DOI] [PubMed] [Google Scholar]
- 18.Critser PJ, Kreger ST, Voytik-Harbin SL, Yoder MC. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res. 2010;80:23–30. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kniazeva E, Putnam AJ. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. Am J Physiol Cell Physiol. 2009;297:C179–C187. doi: 10.1152/ajpcell.00018.2009. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A. 2008;105:11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correa de Sampaio P, Auslaender D, Krubasik D, et al. A heterogeneous in vitro three dimensional model of tumour-stroma interactions regulating sprouting angiogenesis. PLoS One. 2012;7:e30753. doi: 10.1371/journal.pone.0030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan OF, Sefton MV. Endothelialized biomaterials for tissue engineering applications in vivo. Trends in Biotechnology. 2011;29:379–387. doi: 10.1016/j.tibtech.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravi S, Chaikof EL. Biomaterials for vascular tissue engineering. Regen Med. 2010;5:107–120. doi: 10.2217/rme.09.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischbach C, Chen R, Matsumoto T, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 25.Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci U S A. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Prestwich GD. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer. 2010;116:1739–1750. doi: 10.1002/cncr.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franses JW, Baker AB, Chitalia VC, Edelman ER. Stromal endothelial cells directly influence cancer progression. Sci Transl Med. 2011;3:66ra5. doi: 10.1126/scitranslmed.3001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito Y. Surface micropatterning to regulate cell functions. Biomaterials. 1999;20:2333–2342. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 29.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog. 1998;14:356–363. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- 30.Dike LE, Chen CS, Mrksich M, Tien J, Whitesides GM, Ingber DE. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev Biol Anim. 1999;35:441–448. doi: 10.1007/s11626-999-0050-4. [DOI] [PubMed] [Google Scholar]
- 31.Bettinger CJ, Zhang Z, Gerecht S, Borenstein JT, Langer R. Enhancement of In Vitro Capillary Tube Formation by Substrate Nanotopography. Adv Mater. 2008;20:99–103. doi: 10.1002/adma.200702487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickinson LE, Rand DR, Tsao J, Eberle W, Gerecht S. Endothelial cell responses to micropillar substrates of varying dimensions and stiffness. J Biomed Mater Res A. 2012;100:1457–1466. doi: 10.1002/jbm.a.34059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranjan A, Webster TJ. Increased endothelial cell adhesion and elongation on micron-patterned nano-rough poly(dimethylsiloxane) films. Nanotechnology. 2009;20 doi: 10.1088/0957-4484/20/30/305102. 305102. [DOI] [PubMed] [Google Scholar]
- 34.Uttayarat P, Toworfe GK, Dietrich F, Lelkes PI, Composto RJ. Topographic guidance of endothelial cells on silicone surfaces with micro- to nanogrooves: orientation of actin filaments and focal adhesions. J Biomed Mater Res A. 2005;75:668–680. doi: 10.1002/jbm.a.30478. [DOI] [PubMed] [Google Scholar]
- 35.Moon JJ, Hahn MS, Kim I, Nsiah BA, West JL. Micropatterning of poly(ethylene glycol) diacrylate hydrogels with biomolecules to regulate and guide endothelial morphogenesis. Tissue Eng Part A. 2009;15:579–585. doi: 10.1089/ten.tea.2008.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan CP, Seo BR, Brooks DJ, Chandler EM, Craighead HG, Fischbach C. Parylene peel-off arrays to probe the role of cell-cell interactions in tumour angiogenesis. Integr Biol (Camb) 2009;1:587–594. doi: 10.1039/b908036h. [DOI] [PubMed] [Google Scholar]
- 37.Verbridge SS, Choi NW, Zheng Y, Brooks DJ, Stroock AD, Fischbach C. Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis. Tissue Eng Part A. 2010;16:2133–2141. doi: 10.1089/ten.tea.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu DT, Jeon NL, Huang S, et al. Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. Proc Natl Acad Sci U S A. 2000;97:2408–2413. doi: 10.1073/pnas.040562297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickinson LE, Gerecht S. Micropatterned surfaces to study hyaluronic acid interactions with cancer cells. J Vis Exp. 2010 doi: 10.3791/2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanapalli SA, Duits MH, Mugele F. Microfluidics as a functional tool for cell mechanics. Biomicrofluidics. 2009;3:12006. doi: 10.1063/1.3067820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tkachenko E, Gutierrez E, Ginsberg MH, Groisman A. An easy to assemble microfluidic perfusion device with a magnetic clamp. Lab Chip. 2009;9:1085–1095. doi: 10.1039/b812184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barkefors I, Le Jan S, Jakobsson L, et al. Endothelial cell migration in stable gradients of vascular endothelial growth factor A and fibroblast growth factor 2: effects on chemotaxis and chemokinesis. J Biol Chem. 2008;283:13905–13912. doi: 10.1074/jbc.M704917200. [DOI] [PubMed] [Google Scholar]
- 43.Vickerman V, Blundo J, Chung S, Kamm R. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip. 2008;8:1468–1477. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abaci HE, Devendra R, Smith Q, Gerecht S, Drazer G. Design and development of microbioreactors for long-term cell culture in controlled oxygen microenvironments. Biomed Microdevices. 2012;14:145–152. doi: 10.1007/s10544-011-9592-9. [DOI] [PubMed] [Google Scholar]
- 45.Adler M, Polinkovsky M, Gutierrez E, Groisman A. Generation of oxygen gradients with arbitrary shapes in a microfluidic device. Lab Chip. 2010;10:388–391. doi: 10.1039/b920401f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Y, Chen J, Craven M, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009;9:269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- 49.Samani A, Zubovits J, Plewes D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol. 2007;52:1565–1576. doi: 10.1088/0031-9155/52/6/002. [DOI] [PubMed] [Google Scholar]
- 50.Miyaji K, Furuse A, Nakajima J, et al. The stiffness of lymph nodes containing lung carcinoma metastases: a new diagnostic parameter measured by a tactile sensor. Cancer. 1997;80:1920–1925. doi: 10.1002/(sici)1097-0142(19971115)80:10<1920::aid-cncr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 51.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci U S A. 2012;109:10334–10339. doi: 10.1073/pnas.1118073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schrader J, Gordon-Walker TT, Aucott RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–1205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanjaya-Putra D, Yee J, Ceci D, Truitt R, Yee D, Gerecht S. Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. J Cell Mol Med. 2010;14:2436–2447. doi: 10.1111/j.1582-4934.2009.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297:574–584. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 55.Trappmann B, Gautrot JE, Connelly JT, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:742. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 56.Rundhaug JE. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: A. C. Lockhart et al., Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin. Cancer Res., 9: 00-00, 2003. Clin Cancer Res. 2003;9:551–554. [PubMed] [Google Scholar]
- 57.Moon JJ, Saik JE, Poche RA, et al. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials. 2010;31:3840–3847. doi: 10.1016/j.biomaterials.2010.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241–244. [Google Scholar]
- 59.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 60.Hanjaya-Putra D, Bose V, Shen YI, et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood. 2011;118:804–815. doi: 10.1182/blood-2010-12-327338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Culver JC, Hoffmann JC, Poché RA, Slater JH, West JL, Dickinson ME. Three-Dimensional Biomimetic Patterning in Hydrogels to Guide Cellular Organization. Advanced Materials. 2012;24:2344–2348. doi: 10.1002/adma.201200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller JS, Stevens KR, Yang MT, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012 doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dickinson LE, Lutegebaucks C, Lewis D, Gerecht S. Patterning microscale extracellular matrices to study endothelial and cancer cell interactions in vitro. Lab Chip. doi: 10.1039/c2lc40819h. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang CP, Lu J, Seon H, et al. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip. 2009;9:1740–1748. doi: 10.1039/b818401a. [DOI] [PMC free article] [PubMed] [Google Scholar]