Abstract
Purpose
We examined differences in time to diagnosis by race/ethnicity, the relationship between time to diagnosis and stage, and the extent to which it explains differences in stage at diagnosis across racial/ethnic groups. Our analytic sample includes 21,427 non-Hispanic White (White), Hispanic, non-Hispanic Black (Black) and non-Hispanic Asian/Pacific Islander (Asian) women diagnosed with stage I to IV breast cancer between January 1, 2000 and December 31, 2007 at one of eight National Comprehensive Cancer Network centers. We measured time from initial abnormal mammogram or symptom to breast cancer diagnosis. Stage was classified using AJCC criteria. Initial sign of breast cancer modified the association between race/ethnicity and time to diagnosis. Among symptomatic women median time to diagnosis ranged from 36 days among Whites to 53.6 for Blacks. Among women with abnormal mammograms median time to diagnosis ranged from 21 days among Whites to 29 for Blacks. Blacks had the highest proportion (26%) of Stage III or IV tumors. After accounting for time to diagnosis, the observed increased risk of stage III/IV breast cancer was reduced from 40% to 28% among Hispanics and from 113% to 100% among Blacks, but estimates remained statistically significant. We were unable to fully account for the higher proportion of late-stage tumors among Blacks. Blacks and Hispanics experienced longer time to diagnosis than Whites, and Blacks were more likely to be diagnosed with late-stage tumors. Longer time to diagnosis did not fully explain differences in stage between racial/ethnicity groups.
Keywords: BREAST CANCER, MINORITY HEALTH, DIAGNOSIS DELAY, DISPARITIES
Introduction
Stage at diagnosis is a strong predictor of breast cancer mortality. Five-year survival rates are better for early stage (88% for women diagnosed at stage I and78% for diagnoses at stage II) than for advanced stage (15% for diagnoses at stage IV).[1] Non-Hispanic Whites and Asians are more likely to be diagnosed at early stages than Hispanics and Blacks. While 62.3% of Whites and 62.5% of Asian/Pacific Islanders are diagnosed with localized stage tumors, only 53.8% of Hispanics and 51.9% of Blacks have the same diagnostics.[1]
Longer time between the onset of symptoms or detection of a breast abnormality via mammography has been hypothesized to be associated with later stage diagnosis.[2, 3] Yet, few studies assessing delays in diagnosis have included racial/ethnic groups other than Blacks and Whites.[4–9] Studies have also focused on limited geographical areas and used patients diagnosed in the 1980’s and 1990’s. [10] There have been important reforms in coverage of breast cancer screening and treatment since the 1990s, including expansion of National Breast and Cervical Cancer Early Detection Program, which provides care for lower income women and has set goals for timely diagnostic resolution of abnormal mammograms. It is important to examine the relationship between delay, stage and race among more recently diagnosed women. Racial or ethnic variation in delay, or whether delay explains variation in stage and ultimately survival by race/ethnicity, has not been addressed. [11, 12] We examined the relationship between time to diagnosis and race/ethnicity, and associations between these factors and stage of disease in a multiethnic population in the U.S.
Patients and Methods
Study population and data collection
The National Comprehensive Cancer Network (NCCN) Breast Cancer Outcomes Database has collected prospective data on patient and tumor characteristics, sociodemographic information, treatment and outcomes for women receiving care for newly diagnosed breast cancer since 1997. The study population includes 25,510 women with new stage I-IV breast cancer diagnoses[13, 14] who presented and received primary care at one of eight comprehensive cancer centers between January 1, 2000 and December 31, 2007: The Ohio State University Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute, Columbus, OH; City of Hope Comprehensive Cancer Center, Duarte, CA; Dana- Farber Cancer Institute, Boston, MA; Fox Chase Cancer Center, Philadelphia, PA; H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; The University of Texas M.D. Anderson Cancer Center, Houston, TX; Roswell Park Cancer Institute, Buffalo, NY; and University of Michigan Comprehensive Cancer Center, Ann Arbor, MI. The Institutional Review Board (IRB) at each center approved the study, data collection process, data transmission methods, and data storage protocols.
Tumor characteristics including estrogen and progesterone hormone (ER/PR) status and grade and treatment information are gathered from tumor registries and chart review.[13, 15–17] At most centers, patients also complete an intake survey, which elicits information about the initial sign of breast cancer, race/ethnicity, employment status, educational status and menopausal status. Study abstractors fill in missing survey data based on chart review. Data quality assurance processes have been documented.[18]
We identified 25,131 Stage I-IV patients who presented with a new diagnosis of breast cancer and received primary therapy at one of the NCCN institutions between January 1, 2000 and December 31, 2007. We excluded anyone with: a previous cancer diagnosis (n=1,913), missing date of initial symptom/sign or reported date after patient’s diagnosis of breast cancer (n=434), missing description of or indicated “other” initial sign/symptom (n=966), and missing, American Indian Aleutian/Eskimo, or ‘other’ race (N=391). Our final sample included 21,427 women.
Using survey data, women were classified as Caucasian (White), African-American (Black), Asian or Pacific Islander. Ethnicity was classified as Non-Hispanic, Hispanic or Unknown. If ethnicity was unknown, women were assumed to be non-Hispanic. We then cross-classified race and ethnicity to create four categories: non-Hispanic White (White), non- Hispanic Black (Black), non-Hispanic Asian or Pacific Islander (Asian) and Hispanic.
Exposure and Outcome Assessment
Time to diagnosis was defined as the time from date of initial sign of breast cancer to date of breast cancer diagnosis. Date of diagnosis was date of first biopsy or diagnostic procedure. Times longer than one year were truncated at 365 days. Initial sign was dichotomized as either abnormal screening mammography or symptom (e.g., bloody nipple discharge, discovery of a breast lump, axillary mass, inverted nipple, or breast pain/discomfort). Stage at diagnosis was characterized according to the American Joint Committee on Cancer Staging Manual (5th Edition thru January 1, 2002 or 6th edition thereafter).
Statistical Analysis
We present age-standardized percentages of patient and clinical characteristics at study intake, stratified by racial/ethnic group. Multinomial logistic (categorical variables) and binomial logistic (binary variables) regression models were used to generate age-adjusted p-values. We used the Generalized Extreme Studentized Deviate Test for Outliers to examine time to diagnosis for extreme values.[19] We calculated Kaplan-Meier survival curves to examine the unadjusted relationship between time to diagnosis and race/ethnicity by initial sign (abnormal mammogram or symptom). We used multivariable Cox proportional hazards regression to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the relationship between time to diagnosis and race/ethnicity stratified by initial sign or symptom, after testing the proportional hazards assumption. HRs below one represent lower diagnosis rates and longer times to diagnosis; HRs above one represent higher diagnosis rates and shorter times to diagnosis.
We used multinomial logistic regression models to examine the association between time to diagnosis, race/ethnicity and breast cancer stage with stage I as the reference group. Time to diagnosis was categorized as follows: <30 days, 30–59 days, 60–89 days, 90–179 days, and ≥180 days. Likelihood ratio tests were used to assess interactions between time to diagnosis, race, and initial sign or symptom. P-values are two-sided with an alpha level of 0.05. Analyses were performed using SAS version 9.2.
We adjusted for the following: age at diagnosis (in years), body mass index at presentation (kg/m2; <18.5, 18.5–25, 25–29, ≥30), ER/PR status (ER+/PR+, ER+/PR−, ER−/PR+, ER−/PR−), tumor histologic/nuclear grade (high, intermediate, low), Charlson comorbidity index score (0, ≥ 1)[20, 21], educational attainment (high school or less, some college, college graduate, graduate school), employment status (employed or student, retired or homemaker, unable to work/unemployed, other), menopausal status at diagnosis (premenopausal, postmenopausal), insurance status (managed care, Medicaid, indemnity, Medicare, other/unknown), and NCCN institution.
Results
Of the 21,427 women included, 82.6% were White, 6.4% Black, 8.0% Hispanic and 3.0% Asian (Table 1). Time to diagnosis of less than one month occurred in the majority of NH-White women (52%), compared to 39% in Hispanics, 42% of Blacks, and 45% of Asians (p <0.0001). Whites were more likely to have abnormal screening mammogram as their first sign of breast cancer (46%), than Asians (32%) or Hispanics and Blacks (35%) (p< 0.0001) (Table 1). Although most women were diagnosed with stage I or II breast cancer (73% in Blacks to 85% in Whites), Blacks had the highest proportion (26%) diagnosed with Stage III or IV breast cancer (p <0.0001).
Table 1.
Age-Standardized characteristics at diagnosis according to race/ethnicity among women diagnosed with breast cancer, NCCN, 2000–2007, (n=21,427)
| CHARACTERISTIC | WHITE N=17,696 |
HISPANIC N=1,363 |
BLACK N=1,718 |
ASIAN N=650 |
P- VALUE |
|---|---|---|---|---|---|
| Age at diagnosis [mean (SD)]1 | 55.5 (12.6) | 51.4 (12.2) | 53.8 (12.8) | 50.6 (11.7) | <0.0001 |
| Time to diagnosis (days), % | <0.0001 | ||||
| 0–29 | 52 | 39 | 42 | 45 | |
| 30–59 | 24 | 24 | 24 | 24 | |
| 60–89 | 8 | 11 | 10 | 10 | |
| 90–179 | 8 | 14 | 12 | 10 | |
| ≥180 | 8 | 12 | 11 | 11 | |
| Menopausal status, % | 0.02 | ||||
| Pre | 39 | 46 | 41 | 53 | |
| Post | 61 | 54 | 59 | 47 | |
| Comorbidity score [mean(SD)] | 0.30 (0.7) | 0.2 (0.2) | 0.5 (0.5) | 0.2 (0.1) | |
| Body Mass Index (kg/m2), % | <0.0001 | ||||
| < 18.5 | 1 | 1 | 1 | 5 | |
| 18.5–25 | 40 | 31 | 18 | 63 | |
| 25–30 | 30 | 34 | 27 | 26 | |
| ≥ 30 | 28 | 34 | 54 | 6 | |
| Histologic/nuclear grade, % | <0.0001 | ||||
| Low | 15 | 12 | 7 | 12 | |
| Intermediate | 42 | 43 | 29 | 46 | |
| High | 43 | 45 | 64 | 42 | |
| Hormone receptor status, % | <0.0001 | ||||
| ER+PR+ | 62 | 58 | 42 | 61 | |
| ER+PR− | 14 | 15 | 17 | 12 | |
| ER−PR+ | 2 | 3 | 3 | 2 | |
| ER−PR− | 22 | 24 | 38 | 25 | |
| Educational status, % | <0.0001 | ||||
| High school or less | 31 | 48 | 41 | 25 | |
| Some college | 26 | 21 | 30 | 17 | |
| College graduate | 25 | 18 | 17 | 34 | |
| Graduate school | 18 | 12 | 12 | 25 | |
| Insurance status, % | <0.0001 | ||||
| Managed care | 65 | 56 | 57 | 72 | |
| Medicare | 21 | 10 | 19 | 8 | |
| Medicaid | 3 | 21 | 15 | 9 | |
| Indemnity | 7 | 2 | 5 | 5 | |
| Other/unk./miss | 3 | 11 | 4 | 7 | |
| Employment status, % | <0.0001 | ||||
| Employed/student | 53 | 51 | 53 | 57 | |
| Homemaker/retired | 38 | 40 | 27 | 35 | |
| Unable to work/unemp./other | 8 | 9 | 20 | 8 |
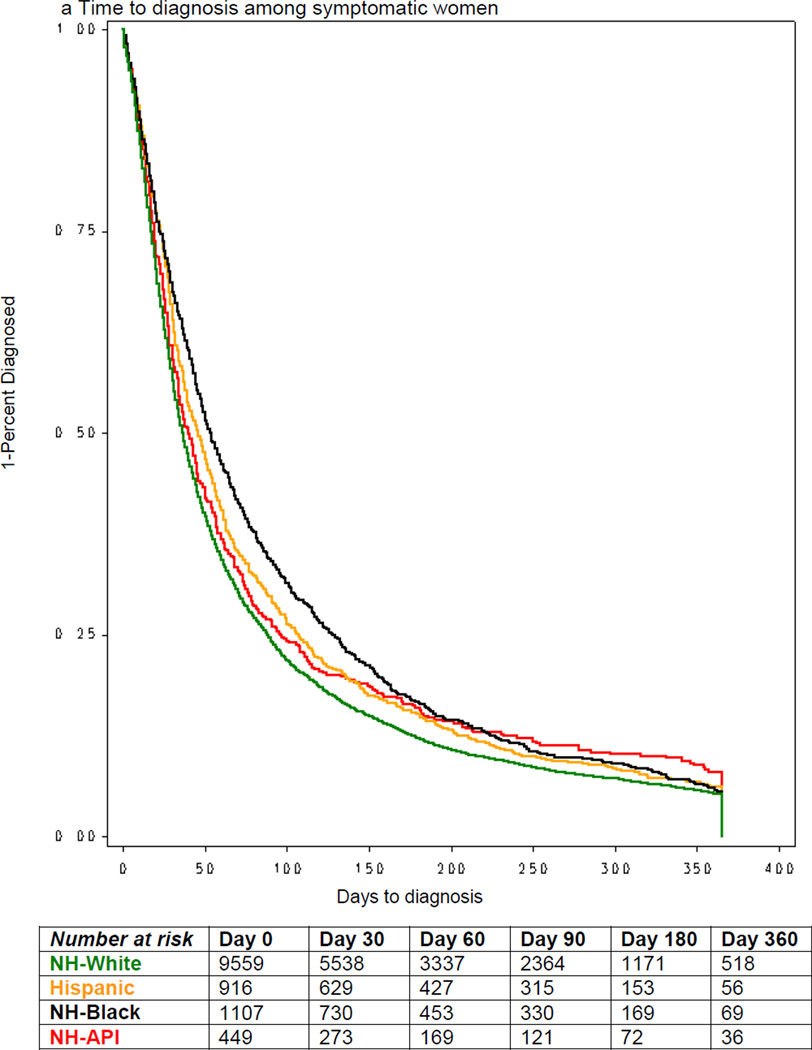
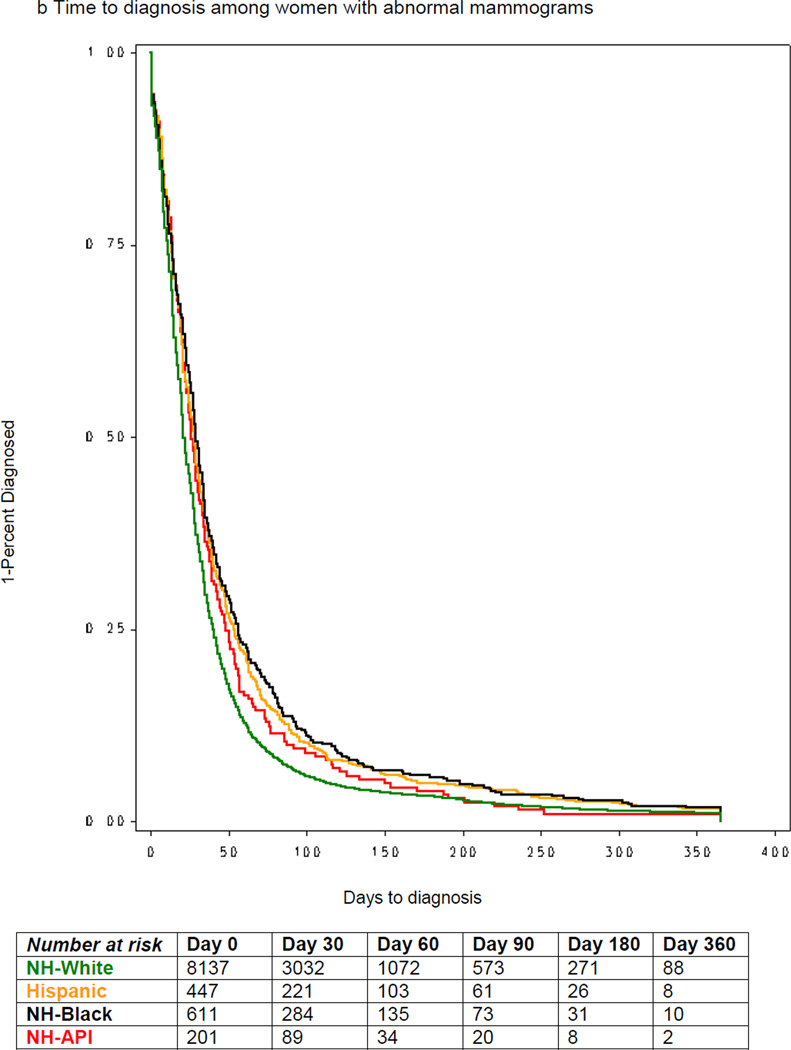
Across all racial/ethnic groups, women with symptoms had longer median time to diagnosis than women with abnormal mammograms (Figures 1a & 1b). Among symptomatic women, the median time to diagnosis was 36 days among Whites, 53.6 days for Blacks, 40 days for Asians and 46 days among Hispanics (Figure 1a). Among women with abnormal mammogram, the median time to diagnosis was 21 days among Whites, 29 days for Blacks, 28 days for Asians and 26 days among Hispanics (Figure 1b).
Figure 1.
Time to diagnosis by race/ethnicity and initial sign/symptom, among women diagnosed with breast cancer, NCCN, 2000–2007.
The association between race/ethnicity and time to diagnosis varied by type of initial sign of breast cancer (p-interaction=0.01; Table 2), thus we present stratified estimates. There was no difference in time to diagnosis between Asians and Whites regardless of initial sign. Among symptomatic women, after adjusting for sociodemographic and health characteristics there was no difference between Hispanics and Whites, while Blacks had 10% longer times to diagnosis (HR=0.92; 95% CI: 0.87, 0.98). Among women with an abnormal screening mammogram, Hispanics and Blacks had 14% (HR=0.86, 95% CI, 0.78–0.95) and 20% (HR=0.80; 95% CI, 0.73–0.87) longer time to diagnosis after adjustment for covariates.
Table 2.
Age-Standardized characteristics at baseline according to race/ethnicity among women diagnosed with breast cancer, NCCN, 2000–2007, (n=21,427) continued
| Initial symptom/sign, % | <0.0001 | ||||
| Abnormal mammogram2 | 46 | 35 | 35 | 32 | |
| Lump in breast (patient detected) | 42 | 50 | 50 | 53 | |
| Lump in breast (MD detected) | 5 | 5 | 5 | 6 | |
| Bloody nipple discharge or inversion | 2 | 3 | 2 | 1 | |
| Axillary mass | 2 | 2 | 3 | 1 | |
| Breast pain/discomfort | 3 | 5 | 5 | 6 | |
| Stage, % | <0.0001 | ||||
| I | 46 | 34 | 32 | 40 | |
| II | 39 | 44 | 41 | 44 | |
| III | 11 | 17 | 20 | 13 | |
| IV | 4 | 5 | 6 | 3 |
Note: Values are means (SD) or percentages and are standardized to the age distribution of the study population.
Value is not age-adjusted;
Screening mammogram only
Medicaid insurance was associated with 21% longer time to diagnosis (HR=0.79; 95% CI, 0.74–0.84) as compared to those with managed care (Table 2, Model 3). Women with a college education had 6% shorter time to diagnosis than women with a high school degree or less (HR=1.06; 95% CI, 1.02, 1.10). We did not find evidence of any statistical interactions between race and insurance status, tumor characteristics, education or employment status. Additional adjustment for tumor characteristics did not materially change the results (data not shown).
Table 3 shows estimates for the association between race/ethnicity and time to diagnosis with late stage breast cancer. Due to statistical interaction (p=0.003) we present estimates for time to diagnosis stratified by initial sign of breast cancer. Time to diagnosis did not explain differences in risk of stage II breast cancer among Hispanics and Blacks as compared to Whites. Simultaneously accounting for time to diagnosis and race/ethnicity yielded slightly attenuated estimates for race/ethnicity, but Hispanics and Blacks remained at higher risk of stage II breast cancer (Table 3, Model 2). For example, the OR for Blacks decreased from 1.40 to 1.37. After accounting for insurance status, education and other factors (Table 3, Model 3), Hispanics were no longer at increased risk, while Blacks continued to have a higher risk for stage II breast cancer (OR= 1.17, 95% CI: 1.03–1.32). In data not shown, adjustment for hormone receptor status and tumor grade, attenuated the risk of stage II vs. stage I among Blacks compared to Whites (OR= 1.11, 95% CI: 0.98–1.26).
Table 3.
Hazard ratios for time to diagnosis and race/ethnicity among women diagnosed with breast cancer by type of initial sign/symptom
| Characteristic | Age and Center Adjusted HR (95% CI)1 |
Multivariable Adjusted HR (95% CI) 2> |
|---|---|---|
| Among symptomatic women: N=12,031 | ||
| Race/ethnicity* | ||
| White | 1.00 (REF) | 1.00 (REF) |
| Hispanic | 0.88 (0.82, 0.95) | 0.93 (0.87, 1.00) |
| Black | 0.89 (0.84, 0.95) | 0.92 (0.87, 0.98) |
| Asian | 0.94 (0.85, 1.04) | 0.95 (0.86, 1.05) |
| Among women with abnormal mammograms3: N=9,396 | ||
| Race/ethnicity* | ||
| White | 1.00 (REF) | 1.00 (REF) |
| Hispanic | 0.83 (0.74, 0.91) | 0.86 (0.78, 0.95) |
| Black | 0.77 (0.71, 0.84) | 0.80 (0.73, 0.87) |
| Asian | 0.91 (0.77, 1.03) | 0.90 (0.78, 1.04) |
| Among all women: N=21,427 | ||
| Insurance Status* | ||
| Managed care | 1.00 (REF) | |
| Medicaid | 0.79 (0.74, 0.84) | |
| Indemnity | 0.97 (0.91, 1.03) | |
| Medicare | 0.92 (0.88, 0.98) | |
| Other/unk./miss | 0.89 (0.83, 0.96) | |
| Educational status* | ||
| High school or less | 1.00 (REF) | |
| Some college | 1.00 (0.97, 1.04) | |
| College grad | 1.06 (1.02, 1.10) | |
| Graduate school | 1.08 (1.04, 1.13) | |
| Employment status^ | ||
| Employed/student | 1.00 (REF) | |
| Homemaker/retired | 1.01 (0.98, 1.05) | |
| Unable to work/unemp./other | 1.00 (0.95, 1.04) | |
| Body Mass Index (kg/m2) | ||
| < 18.5 | 0.96 (0.86, 1.08) | |
| 18.5–25 | 1.00 (REF) | |
| 25–30 | 1.01 (0.98, 1.05) | |
| ≥ 30 | 1.02 (0.98, 1.05) | |
Note: HRs below one represent lower diagnosis rates and longer times to diagnosis; HRs above one represent higher diagnosis rates and shorter times to diagnosis
Age and Center. Adjusted for age at diagnosis and NCCN center;
Multivariate Model. Adjusted for age and center plus employment status, educational status, insurance status, comorbdity score, BMI at presentation, and menopausal status at diagnosis.
Screening mammogram only;
Type 3 p-value <0.0001;
Type 3 p-value <0.05;
There was no significant association between time to diagnosis and stage II breast cancer among symptomatic women (90–179 days OR=1.09, 95% CI: 0.94–1.25; ≥ 180 days OR=1.02, 95% CI: 0.89–1.18) (Table 3, Model 3). Longer time to diagnosis was associated with lower risk of stage II breast cancer among women with abnormal mammograms. Compared to diagnosis within 30 days, women diagnosed between 90–179 days after initial sign/symptom were 37% less likely to have stage II breast cancer (OR=0.63, 95% CI: 0.49, 0.81 (Table 3, Model 3).
The second section of Table 3 displays estimates for risk of stage III/IV breast cancer as compared to stage I. There were no differences in risk of stage III/IV at diagnosis among NHAPI women compared to Whites across any of our models. Time to diagnosis did not eliminate differences in risk of late stage disease between Whites, Hispanics and Blacks (Table 3, Model 2). Estimates for Hispanics and Blacks decreased after accounting for time to diagnosis, but remained significant. The OR for Hispanics went from 1.40 (95% CI: 1.18–1.64) to 1.28 (95% CI: 1.09–1.51). For Blacks estimates decreased from 2.13 (95% CI: 1.86–2.44) to 2.00 (95% CI: 1.75–2.30) (Table 3,Model 2). In fully adjusted models Hispanic women were no longer at increased risk of late stage breast cancer relative to Whites (OR=0.97, 95% CI: 0.82–1.16) (table 3, model 3). However NH-Black women had 50% higher risk of stage III/IV breast cancer as compared to NH-White women (OR=1.50, 95% CI: 1.29–1.74) (Table 3, Model 3). In data not shown, higher risk of stage III/IV among NH-Black women persisted (OR= 1.34, 95% CI: 1.16–1.56) after further adjustment for tumor grade and hormone receptor status.
Among symptomatic women, time to diagnosis ≥60 days was associated with increased risk of stage III/IV diagnosis. Women with 60–89 days between first symptom and diagnosis were 22% more likely than women with 0–30 days to have stage III/IV breast cancer and those with ≥180 days were 92% more likely (60–89 days OR=1.22, 95% CI:1.03–1.46; ≥180 days OR=1.92, 95% CI: 1.63– 2.26) (Table 3,Model 3). Among women with abnormal mammograms, only time to diagnosis ≥180 days was associated with increased risk of III/IV tumors (OR=1.53, 95% CI: 1.07–2.20) (Table 3, Model 3).
Discussion
We found that Blacks, Hispanics and Asians, experienced longer times from initial sign of breast cancer to definitive diagnosis, and were more likely to present with symptoms vs. detected through screening mammography. Blacks and Hispanics were also more likely to be diagnosed with stage II &III/IV breast cancer when compared with Whites. Time to diagnosis did not explain racial/ethnic variation in stage at diagnosis. Accounting for Sociodemographic factors eliminated differences between Hispanics and Whites, but higher risk of late stage breast cancer persisted among Blacks. Our results are consistent with studies showing that racial/ethnic minorities have a lower likelihood of detection through mammography and longer times to diagnosis than Whites in the U.S. [4, 22, 23]
Women presenting with symptoms had longer times to diagnosis and were diagnosed at later stages than women with abnormal mammograms. The difference by detection method may be due to the tendency of mammography to detect tumors of smaller size and with more favorable characteristics than tumors detected via symptoms. [24] Additionally, the start date for the calculation of time to diagnosis is likely later in the tumor development process among symptomatic women vs. those with abnormal mammograms. This is consistent with our finding that time to diagnosis of ≥60 days was associated with increased risk of stage III/IV disease among women with symptoms while ≥180 days was the threshold for women with abnormal mammograms.
Access to care, individual, and health system factors may also contribute to time to diagnosis in symptomatic vs. screen-detected cancers.[6, 25–27] Paradoxically, among women with abnormal mammograms, those with longer times to diagnosis had lower risk of stage II diagnosis. A possible explanation for this observation might be that diagnostic follow-up occurs more quickly for high risk groups (e.g., those with a family history of breast cancer) while women with longer time to diagnosis are those suspected by providers to have benign or less aggressive disease.[3]
National Breast and Cervical Cancer Early Detection Program (NBCCEDP) guidelines aim for all women to be diagnosed within 60 days of an abnormal mammogram. Efforts to address system and individual barriers to timely follow-up include case-management and patient navigation programs. Case management within NBCCEDP was associated with a 35% decreased risk of diagnostic delay and this effect did not differ by race/ethnicity.[28] Patient navigation strategies may be an effective strategy for decreasing delay and proportion of advanced stage diagnoses.[29, 30] In a randomized intervention trial, Ferrante et al., 2008 found that women receiving navigation services had shorter diagnostic resolution times, lower mean anxiety scores and higher mean satisfaction scores than control participants.[31] Navigation programs may help women with abnormal mammograms, but more work must be done to reduce the burden of among symptomatic women. Seven of eight centers included in this analysis have implemented patient navigation programs, though none were in operation during the entire period covered by this analysis. Had our study been conducted in centers without such programs, it is possible we could have observed even greater racial/ethnic disparities.
We were unable to account for the burden of late stage breast cancer among NH-Black women as compared to Whites. Previous studies that have examined the relationship between breast cancer stage at diagnosis and race/ethnicity suggest that differences may involve socioeconomic status, tumor biology and their interaction.[32–34] Blacks are generally diagnosed at younger ages and with later stage, higher grade and hormone-receptor negative tumors.[35–37] Blacks are also more likely to live in poverty and to have lower educational attainment relative to Whites, factors that are associated with breast cancer stage at diagnosis. Lifestyle factors and environmental exposures may be associated with tumor biology.[38, 39] When we accounted for ER, PR and tumor grade in our stage at diagnosis analysis, Blacks were still at increased risk of late stage tumors, though the risk was somewhat attenuated. However, there are other markers that we were unable to account for and tumor biology may still play an important role in racial differences in stage at diagnosis.
We could not adjust for prior mammography or other screening history (e.g., MRI, ultrasound use) in our analysis. We have stratified by initial sign as time to diagnosis may be differentially misclassified by initial sign. Date of abnormal mammogram is abstracted from medical records while date of first symptom is subject to recall. However, misclassification is unlikely to be differential with respect to our primary exposure of interest, race/ethnicity. Nondifferential misclassification among symptomatic women may explain why associations were stronger for women with abnormal mammograms than for symptomatic women (table 2). To reduce recall bias, women were interviewed within weeks or months of their diagnosis and patient reports were confirmed through record review when possible. Finally, the NCCN database is not population-based, and the experiences of women in this study may not be generalizable to all women with breast cancer. Patients receiving care at NCCN centers may be better at accessing the health system than the general population. They may also represent a more aggressive case mix since local providers may be more likely to refer them to large centers for care.
Educational attainment and employment status were used as surrogates of socioeconomic status. While residual confounding cannot be ruled out, it is unlikely to fully explain our results. First, higher educational attainment was associated with earlier diagnosis, and lower risk of late stage breast cancer. Second, education and employment explained variation in stage at diagnosis among Hispanics and misclassification of these factors is not expected to vary by race/ethnicity. Despite these limitations, our study used recent data from over 20,000 women across the United States to examine time to diagnosis. Most prior studies on this topic have focused on black and white women; inclusion of Hispanics and Asians is another important contribution. We were also able to examine whether time to diagnosis explains variation in stage at diagnosis by race ethnicity.
We were unable to fully account for the higher risk of late stage diagnosis among NHBlack women in our study. The factors that contribute to this may include tumor characteristics which vary by race/ethnicity, biologic responses and individual risk behavior, and social and institutional factors.[40] Future research to identify effective solutions will require moving beyond examining proximal causes of disease and creating interventions targeting these different factors.
Table 4.
Odds ratios for race/ethnicity, time to diagnosis and risk of advanced Stage breast cancer, 2000–2007
| Stage II vs. Stage I N=8,546 |
Stage III/IV vs. Stage I N=3,514 |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Model 1: Age and center OR (95% CI) |
Model 2: Race and time to Diagnosis OR (95% CI) |
Model 3: Multivariable OR (95% CI) |
Model 1: Age and Center OR (95% CI) |
Model 2: Race and time to diagnosis OR (95% CI) |
Model 3: Multivariable OR (95% CI) |
| Race/ethnicity | ||||||
| White | 1.00 (REF)* | 1.00 (REF)* | 1.00 (REF)@ | 1.00 (REF)* | 1.00 (REF)* | 1.00 (REF)@ |
| Hispanic | 1.31 (1.14, 1.48) | 1.27 (1.12, 1.45) | 1.10 (0.96, 1.28) | 1.40 (1.18, 1.64) | 1.28 (1.09, 1.51) | 0.97 (0.82, 1.16) |
| Black | 1.40 (1.25, 1.57) | 1.37 (1.22, 1.54) | 1.17 (1.03, 1.32) | 2.13 (1.86, 2.44) | 2.00 (1.75, 2.30) | 1.50 (1.29, 1.74) |
| Asian | 1.15 (0.96, 1.37) | 1.14 (0.95, 1.36) | 1.12 (0.93, 1.35) | 0.92 (0.73, 1.17) | 0.89 (0.70, 1.14) | 0.90 (0.70, 1.16) |
| Time to diagnosis | Symptomatic women N=5,840 | Symptomatic women N=2,872 | ||||
| 0–30 (days) | 1.00 (REF)* | 1.00 (REF)* | 1.00 (REF)* | 1.00 (REF)* | 1.00 (REF)* | 1.00 (REF)* |
| 30–59 | 0.97 (0.87, 1.08) | 0.96 (0.86, 1.07) | 0.95 (0.85, 1.06) | 1.05 (0.91, 1.20) | 1.03 (0.90, 1.18) | 1.02 (0.89, 1.17) |
| 60–89 | 0.92 (0.79, 1.06) | 0.91 (0.78, 1.05) | 0.90 (0.77, 1.04) | 1.27 (1.07,1.51) | 1.25 (1.05, 1.49) | 1.22 (1.03, 1.46) |
| 90–179 | 1.09 (0.94, 1.25) | 1.08 (0.93, 1.24) | 1.06 (0.92, 1.22) | 1.71 (1.46, 2.01) | 1.68 (1.43, 1.98) | 1.62 (1.37, 1.91) |
| ≥ 180 | 1.02 (0.89, 1.18) | 1.01 (0.87, 1.16) | 1.01 (0.88, 1.17) | 1.96 (1.67, 2.30) | 1.93 (1.64, 2.26) | 1.92 (1.63, 2.26) |
| Time to diagnosis | Women with abnormal mammograms N=2,706 | Women with abnormal mammograms N=642 | ||||
| 0–30 (days) | 1.00 (REF)* | 1.00 (REF)* | 1.00 (REF)* | 1.00 (REF)* | 1.00 (REF)* | 1.00 (REF)* |
| 30–59 | 0.83 (0.75, 0.93) | 0.83 (0.74, 0.93) | 0.82 (0.74, 0.92) | 0.66 (0.53, 0.82) | 0.64 (0.52, 0.80) | 0.63 (0.50, 0.78) |
| 60–89 | 0.83 (0.69, 1.01) | 0.82 (0.67, 0.99) | 0.81 (0.66, 0.98) | 0.79 (0.55, 1.14) | 0.73 (0.51, 1.06) | 0.73 (0.51, 1.05) |
| 90–179 | 0.65 (0.51, 0.84) | 0.64 (0.50, 0.82) | 0.63 (0.49, 0.81) | 1.09 (0.75, 1.59) | 1.01 (0.69, 1.48) | 1.00 (0.68, 1.46) |
| ≥ 180 | 0.90 (0.70, 1.17) | 0.89 (0.69, 1.15) | 0.89, (0.69, 1.15) | 1.62 (1.13, 2.32) | 1.53 (1.07, 2.20) | 1.53 (1.07, 2.20) |
Model 1. Estimates come from two models: 1) included race/ethnicity, age at diagnosis and NCCN center; 2) included time to diagnosis, age at diagnosis and NCCN center.; Model 2. Includes age at diagnosis, NCCN center with mutual adjustment for race/ethnicity and time to diagnosis; Model 3: Includes 2 plus employment status, educational status, body mass index at presentation, insurance status, and menopausal status at diagnosis.
Type 3 p-value <0.0001;
Type 3 p-value=0.0001
Acknowledgements
The authors would like to thank Graham A. Colditz, Bernard A. Rosner and Julie R. Palmer for their review of early drafts of the manuscript. We also thank the participants in the NCCN breast cancer outcomes database. Erica T. Warner was supported by National Cancer Institute grant number 5T32CA009001-36 and National Institute of General Medical Sciences grant number 5R25GM055353-14. This paper was also supported by the National Comprehensive Cancer Network.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.National Cancer Institute, Surveillance Research Program. Fast Stats: An interactive tool for access to SEER cancer statistics. 2011 [Google Scholar]
- 2.Tartter PI, Pace D, Frost M, et al. Delay in diagnosis of breast cancer. Ann Surg. 1999;229:91. doi: 10.1097/00000658-199901000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arndt V, Stürmer T, Stegmaier C, et al. Provider delay among patients with breast cancer in Germany: a population-based study. Journal of clinical oncology. 2003;21:1440. doi: 10.1200/JCO.2003.08.071. [DOI] [PubMed] [Google Scholar]
- 4.Gorin SS, Heck JE, Cheng B, et al. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166:2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 5.Caplan LS, Helzlsouer KJ. Delay in breast cancer: a review of the literature. Public Health Rev. 1992;20:187–214. [PubMed] [Google Scholar]
- 6.Caplan LS, Helzlsouer KJ, Shapiro S, et al. System delay in breast cancer in whites and blacks. Am J Epidemiol. 1995;142:804. doi: 10.1093/oxfordjournals.aje.a117719. [DOI] [PubMed] [Google Scholar]
- 7.Wujcik D, Fair AM. Barriers to diagnostic resolution after abnormal mammography: a review of the literature. Cancer Nurs. 2008;31:E16–E30. doi: 10.1097/01.NCC.0000305764.96732.45. [DOI] [PubMed] [Google Scholar]
- 8.Wujcik D, Shyr Y, Li M, et al. Delay in diagnostic testing after abnormal mammography in low-income women. Oncol Nurs Forum. 2009;36:709–715. doi: 10.1188/09.ONF.709-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 10.Richardson J, Langholz B, Bernstein L, et al. Stage and delay in breast cancer diagnosis by race, socioeconomic status, age and year. Br J Cancer. 1992;65:922. doi: 10.1038/bjc.1992.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards MA, Smith P, Ramirez AJ, et al. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999;79:858. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards MA, Westcombe AM, Love SB, et al. Influence of delay on survival in patients with breast cancer: a systematic review. The Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL. AJCC cancer staging manual. Springer Verlag; 2002. [Google Scholar]
- 14.Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. Journal of clinical oncology. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Weeks J. Outcomes assessment in the NCCN: 1998 update. National Comprehensive Cancer Network. Oncology (Williston Park) 1999;13:69–71. [PubMed] [Google Scholar]
- 16.Weeks JC. Outcomes assessment in the NCCN. Oncology (Williston Park) 1997;11:137–140. [PubMed] [Google Scholar]
- 17.Niland JC. NCCN outcomes research database: data collection via the Internet. Oncology (Williston Park) 2000;14:100–103. [PubMed] [Google Scholar]
- 18.Christian CK, Niland J, Edge SB, et al. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction: A study of the National Comprehensive. Cancer Network. Ann Surg. 2006;243:241. doi: 10.1097/01.sla.0000197738.63512.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25:165–172. [Google Scholar]
- 20.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Adams SA, Smith ER, Hardin J, et al. Racial differences in follow-up of abnormal mammography findings among economically disadvantaged women. Cancer. 2009;115:5788–5797. doi: 10.1002/cncr.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerner JF, Yedidia M, Padgett D, et al. Realizing the promise of breast cancer screening: clinical follow-up after abnormal screening among Black women. Prev Med. 2003;37:92–101. doi: 10.1016/s0091-7435(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 24.Taplin SH, Ichikawa L, Yood MU, et al. Reason for late-stage breast cancer: absence of screening or detection, or breakdown in follow-up? J Natl Cancer Inst. 2004;96:1518. doi: 10.1093/jnci/djh284. [DOI] [PubMed] [Google Scholar]
- 25.Bish A, Ramirez A, Burgess C, et al. Understanding why women delay in seeking help for breast cancer symptoms. J Psychosom Res. 2005;58:321–326. doi: 10.1016/j.jpsychores.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Smith LK, Pope C, Botha JL. Patients' help-seeking experiences and delay in cancer presentation: a qualitative synthesis. The Lancet. 2005;366:825–831. doi: 10.1016/S0140-6736(05)67030-4. [DOI] [PubMed] [Google Scholar]
- 27.Burgess C, Ramirez A, Richards M, et al. Who and what influences delayed presentation in breast cancer? Br J Cancer. 1998;77:1343. doi: 10.1038/bjc.1998.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobb R, Allen JD, Emmons KM, et al. Timely care after an abnormal mammogram among low-income women in a public breast cancer screening program. Arch Intern Med. 2010;170:521–528. doi: 10.1001/archinternmed.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabram SGA, Lund MJB, Gardner J, et al. Effects of an outreach and internal navigation program on breast cancer diagnosis in an urban cancer center with a large African-American population. Cancer. 2008;113:602–607. doi: 10.1002/cncr.23568. [DOI] [PubMed] [Google Scholar]
- 30.Ell K, Vourlekis B, Lee PJ, et al. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44:26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Ferrante JM, Chen PH, Kim S. The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: a randomized controlled trial. Journal of Urban Health. 2008;85:114–124. doi: 10.1007/s11524-007-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baquet CR, Mishra SI, Commiskey P, et al. Breast cancer epidemiology in blacks and whites: disparities in incidence, mortality, survival rates and histology. J Nat Med Assoc. 2008;100:480. doi: 10.1016/s0027-9684(15)31294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lantz PM, Mujahid M, Schwartz K, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96:2173. doi: 10.2105/AJPH.2005.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandelblatt J, Andrews H, Kao R, et al. Impact of access and social context on breast cancer stage at diagnosis. J Health Care Poor Underserved. 2010;6:342–351. doi: 10.1353/hpu.2010.0449. [DOI] [PubMed] [Google Scholar]
- 35.Ademuyiwa FO, Edge SB, Erwin DO, et al. Breast Cancer Racial Disparities: Unanswered Questions. Cancer Res. 2011;71:640–644. doi: 10.1158/0008-5472.CAN-10-3021. [DOI] [PubMed] [Google Scholar]
- 36.Ademuyiwa FO, Olopade OI. Racial differences in genetic factors associated with breast cancer. Cancer Metastasis Rev. 2003;22:47–53. doi: 10.1023/a:1022259901319. [DOI] [PubMed] [Google Scholar]
- 37.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 38.Gordon NH. Socioeconomic factors and breast cancer in black and white Americans. Cancer Metastasis Rev. 2003;22:55–65. doi: 10.1023/a:1022212018158. [DOI] [PubMed] [Google Scholar]
- 39.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 40.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98:1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]