Abstract
Synthetic glucocorticoids (GC) have been used to promote lung development in preterm infants, thereby decreasing respiratory distress syndrome and mortality, yet, concern has arisen from reports that such treatment predisposes individuals to disease in adulthood. Given the variety of preclinical studies that show metabolic and behavioral abnormalities in adulthood following fetal exposure to synthetic GC, we examined the effect of in utero exposure to the synthetic GC, dexamethasone (DEX), on hypothalamic expression of thyrotropin-releasing hormone (TRH) a central neuropeptide involved in mediating behavior and metabolic balance. Pregnant Sprague-Dawley rats were administered 0.4 mg/kg DEX on gestational days 18–21. As adults (postnatal day (PD) 60), the offspring were fitted with temperature sensing transmitters allowing real-time monitoring of core body temperature (CBT) across the 24 hr light dark period. This revealed a significant decrease in CBT throughout the day in prenatal DEX-treated females on estrus and diestrus, but not in male offspring. The reduction in CBT by prenatal DEX exposure was accompanied by a significant decrease in the expression of Trh transcript in the paraventricular nucleus of the hypothalamus (PVN) of female rats at PD 60 and this effect was also present on PD7. There was also a female-specific reduction in the number of preproTRH -immunoreactive (ir) neurons in the PVN, with ppTRH-ir nerve fibers decreases that were present in both male and female offspring. No changes in thyroid hormone (triiodothyronine, T3; thyroxine, T4) were observed in adult offspring, but during development, both males and females (PD14) had lower T3 and T4 levels. These data indicate abnormal expression of TRH results from fetal DEX exposure during late gestation, possibly explaining the decreased CBT observed in the female offspring.
Keywords: Thyrotropin Releasing Hormone, Thyroid Hormone, Dexamethasone, Glucocorticoid, Core Body Temperature
1. Introduction
Synthetic glucocorticoids (GC) have been used since the late 1980’s to promote lung development and reduce morbidity and mortality in preterm infants, but concern over this practice has arisen due to the possibility that fetal GC exposure potentiates disease in adulthood [1–3]. This concern is supported by animal studies which document a higher incidence of abnormal metabolic and behavioral disorders in adulthood [4–7], some of which appear to be sex-specific [8]. Although links between adverse fetal environment, such as GC exposure, and adult disease are well established, neural mechanisms to explain this linkage remain unclear.
A large portion of daily energy expenditure goes towards the maintenance of core body temperature (CBT) in the face of changing external temperature [9]. Moreover, changes in body temperature are associated with changes in metabolic rate [9], both of which are controlled in part by neurons within the paraventricular nucleus of the hypothalamus (PVN) which express thyrotropin releasing hormone (TRH). The TRH neurons in the PVN influence metabolic rate and CBT through neuroendocrine effects on the hypothalamic-pituitary-thyroid (HPT) axis [10] as well as through non-neuroendocrine effects within the brain, although these latter pathways are poorly understood [11].
The current study was conducted to test the effects of fetal DEX exposure during late gestation on core body temperature (CBT) in adulthood. Because thyrotropin-releasing hormone (TRH) neurons in the PVN play a key role in regulating body temperature, we measured the expression of this gene in the PVN of offspring in early life (PD7) and in adulthood (PD60). Results indicate a significant decrease in CBT and an accompanying permanent decrease in Trh mRNA expression in female, but not male, rats. We also observed a female-specific decrease in the number of neurons in the PVN which express the TRH precuser peptide, preproTRH-immunoreactive (ppTRH). Nerve fibers within the PVN which expressed ppTRH-immunoreactive were also measured, and were decreased in both male and female offspring exposed to DEX during gestation. The data presented here indicate that fetal exposure to DEX reduces core body temperature in a sex-specific manner, and that abnormal expression of hypothalamic TRH may play a role in this phenomenon. The data presented in this manuscript thus document a novel metabolic abnormality in adulthood arising from fetal GC exposure, and indicate dysregulation of hypothalamic Trh as a potential mechanism behind this phenomenon.
2. Materials and Methods
2.1. Animals
Timed-pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) to arrive at gestational day (GD) 7. Because GD7 is prior to the birth of neurons destined for the hypothalamus, this period of gestation was selected to limit the stress from transport on the development of the PVN. Animals were handled daily beginning on GD14 to acclimate the rats to experimental manipulation, and DEX (0.4 mg/kg) or vehicle (2% absolute ethanol in safflower oil) was administered by subcutaneous injection on GD18–21. Our injections were performed between GD 18–21 due to similarities in the development the rat brain during this time when compared with development of the human brain in the late second/early third trimester [12]. Our DEX dose was selected because it falls within range of a typical human exposure (0.1–0.5 mg/kg) while remaining consistent with doses used in Sprague-Dawley rats which have resulted in abnormal neurodevelopment of the offspring (0.2–0.8 mg/kg) [13, 14]. Parturition occurred on GD22, and was designated as postnatal day (PD) 0. At birth, litters were thinned to 5 male/female pups per dam. All DEX-exposed offspring were randomly assigned to a DEX-injected dam, and vehicle-treated offspring were treated similarly. Cross-fostering of DEX-exposed offspring with a vehicle-injected Dam were not performed based on a study by Nyirenda et al. (2001) which reported that fetal GC exposure paradigms such as that used by our laboratory do not impact maternal nursing behavior [15]. Fetal DEX exposure was not observed to alter litter size nor male/female ratio, however, birth weights were significantly lower in DEX exposed offspring, regardless of sex [16]. An early-life cohort of male and female offspring which had been exposed to either DEX or vehicle were euthanized at PD7 by rapid decapitation, and trunk blood and brains were collected. However, due to extreme hemolysis of these blood samples, additional trunk blood samples were harvested from offspring at PD14. The remaining offspring were weaned at PD21 and subsequently maintained in same sex cages. Throughout the duration of the study, animals were maintained on a 12:12h light/dark cycle with food and water available ad libitum. Prior to euthanasia, female estrous cycle was recorded daily using vaginal swabs, confirming a 4 day cycle in all females, and female rats were killed only when in diestrus (PD 60–63) to limit any confounding effect of changing estradiol levels on measured parameters. Vaginal swabs did not reveal any effect of fetal DEX exposure on estrous cyclicity. All procedures were approved by the Arizona State University Institutional Animal Care and Use Committee, under subcontract from the University of Arizona College of Medicine - Phoenix and were in keeping with National Institutes of Health guidelines.
2.2. Core Body Temperature
Temperature sensitive E-mitters (Minimitter Co., Bend OR) were surgically implanted into the peritoneal cavity of the adult offspring of prenatal DEX or vehicle treated dams. Following surgery, animals were singly housed in shoebox type cages placed on receivers equipped to monitor the transmitter signals. Receivers were connected to ER-4000 data ports and information was transferred to a computer located within the animal housing room. After allowing one week for recovery, CBT was recorded every 15-minute over 7 days using Vital View Data Acquisition Software (Minimitter Co., Bend, OR). Female rats continued to be monitored for estrous cyclicity throughout this timeperiod. Each group consisted of between 3–4 animals, based on previous CBT studies performed by our laboratory [17]. Further, power is increased because of the repeated measures nature of sampling CBT throughout the day with this assay. Lastly, each animal sampled was taken from a different litter, thus care was taken to prevent false positives.
2.3. Gene Expression
Rats that were used for gene expression studies were killed by rapid decapitation, and brains were quickly harvested and snap-frozen using 2-methybutane chilled to −20°C. All brains were then stored at −80°C until microdissection and RNA extraction. Frozen brain were cut in 80 µm (PD7) or 150 µm (PD60) -thick sections using a Leica Model CM3050S Cryostat (Buffalo Grove, IL), and the PVN was harvested from sections containing the PVN (between Bregma −1.60 and −1.88) using a 1mm diameter tissue punch [18]. Total RNA was isolated from PVN tissue using a standard phenol/chloroform/isoamyl alcohol extraction [19], and RNA concentration and purity were confirmed spectrophotometrically using an Epoch Micro-Volume Spectrophotometer System (Winooski, VT). Reverse transcription was performed using an iScript cDNA Synthesis Kit (BioRad, Hercules, CA), and total cDNA was quantified using a Quant-iT Oligreen ssDNA Detection Kit (Molecular Probes, Eugene, OR). Gene expression was measured by real-time quantitative PCR (RT-qPCR) with a Roche 480 LightCycler and SYBR green chemistry using intron-spanning primers designed for rat Trh (Forward: 5’-ACCTTCCTGGCCTGGAGAATGTT-3’; Reverse: 5’-TGTCCTCCTCATCTGCCCATGAAT-3’). Because a suitable reference gene could not be identified, absolute target mRNA was determined using standard curves constructed from isolated PCR product. Gene expression is reported as a ratio of target gene (fg) to total cDNA per reaction (pg), thus controlling for variations in the amount of cDNA per PCR.
2.4. Immunohistochemistry
Adult rats used for immunohistochemical analysis of ppTRH were anesthetized by isoflurane (3.5%) and a blood sample was taken by cardiac puncture. Rats were then intracardially-perfused using ice-cold phosphate-buffered saline (PBS) supplemented with heparin (1000U/L), followed by 4% neutral-buffered paraformaldehyde in PBS. Blood samples were transferred to polystyrene culture tubes containing 100 µl 0.5 M EDTA and 200 µl 4 µg/ml aprotinin to prevent clotting and protein degradation and plasma was saved at −80c for later analysis of T3 and T4 levels by ELISA. Brains were post-fixed overnight at 4 C in 4% neutral-buffered paraformaldehyde in PBS, and transferred to 30% sucrose for cryoprotection. Formaldehyde-fixed brains were sectioned at 35 µm using a Leica Model CM3050S Cryostat (Buffalo Grove, IL), and IHC was performed using an antibody directed to preproTRH178–199 and previously validated for IHC and demonstrated to specifically detect ppTRH [20]. Neurons and fibers that contain ppTRH were visualized by the ABC method using 3-3 diaminobenzidine (DAB) as the chromagen. Neurons were visualized with a Zeiss Axioskop light microscope equipped with Neurolucida v.7 Software (MicroBrightField, Williston, VT) and soma were counted bilaterally throughout the PVN in every fourth section. Data are reported as an estimate of ppTRH neurons per PVN, however, these data were not corrected for double-counting errors as described by Abercrombie (1946) [21], nor were they generated using a stereological technique, such as that described by Coggeshal and Lekan (1996) [22]. Because the labeled neurons we counted did not appear to change in size, and section thickness did not vary between groups, systematic error should be identical for all groups. Our results are thus meant to provide relative data on the expression of ppTRH within the PVN, and not provide an estimate of absolute numbers of ppTRH neurons.
PreproTRH-ir fiber density was estimated as previously described by Suzuki et al., (2001) by drawing a straight line from medial to lateral near the middle the PVN, and counting the immunoreactive fibers which intersected this line over an 280 µm segment [20]. Fibers were estimated through the entire PVN using every fourth section, and are meant to provide a relative estimate of ppTRH-ir fiber density within the PVN.
2.5. Thyroid Hormone Measurements
Thyroxine (T4) and T3 were measured in plasma isolated from blood samples that were collected from rats at either PD14 (trunk blood) or PD60 (cardiac puncture). Blood was collected in polystyrene culture tubes containing EDTA (40 mM) and aprotinin (0.2 µg/ml) to prevent coagulation and proteolysis, respectively. Blood samples were placed on ice after collection, and plasma separated by centrifugation. All plasma samples were stored at −80°C until processing. Hormone concentrations were determined using commercially-available ELISA kits (Genway Biotech, Inc., San Diego, CA). The published detection limits for these assays are 0.05 ng/ml (T3) and 0.004 mg/ml (T4) which are well below the lowest T3 and T4 concentrations we detected in our plasma samples (0.41 ng/ml and 0.091 mg/ml, respectively). The intra-assay coefficient of variance calculated from replicate samples within each plate was 8.74% (T3) and 9.8% (T4).
2.6. Statistics
Core body temperatures were analyzed using a 2 (Prenatal Treatments) × 4 (Periods) × 24 (Time) ANOVA with repeated measures over the last two factors. A 2 (Prenatal Treatments) X 4 (6 hour period averages) ANOVA with repeated measures over the last factor was used to determine treatment differences in the average CBT across the light-dark cycle. Statistical comparisons of gene and protein expression, and plasma thyroid hormone (TH) measurements were performed using GraphPad Prism v5.0b (GraphPad Software, Inc., La Jolla, CA). Two-way ANOVA was used to measure effects of sex and fetal DEX exposure on these parameters, and post hoc comparison of the data was performed using the method of Bonferroni. In all cases, differences were deemed significant if p<0.05.
3. Results
3.1. Core Body Temperature
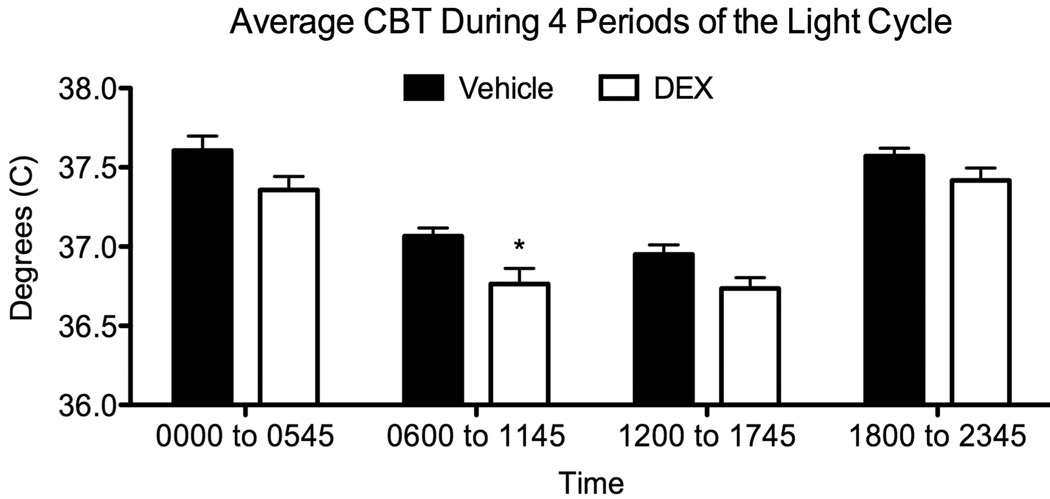
Temperature sensitive transmitters were used to record CBT in adult rats which had been exposed to DEX (or vehicle) during late gestation. Analysis of CBT in females, collapsed across the estrous cycle (Figure 1), showed a consistently lower CBT in DEX-exposed females compared to vehicle-treated counterparts (F[1,19]=13.65; p<0.002). The analysis also revealed a main effect of Period (F[3,57] = 103.75; p<0.0001), which was followed up with an analysis of the average CBT during each of the four 6-hour periods of the light cycle. This analysis revealed a main effect of Treatment (F[1,25] = 5.72; p<0.03) as well as Period (F[3,75] = 130.4; p<0.0001). Post hoc comparisons showed a significantly greater drop in CBT of DEX-exposed compared to Vehicle-exposed females during the first 6 hours of the light phase (p<0.03), which is shown in Figure 1.
Figure 1.
Average core body temperature of female DEX-treated offspring across 4 periods of the light cycle. CBT was collapsed across the estrous cycle and averaged across each of four 6-hr blocks of time. ANOVA showed a significant treatment effect (p<0.03). Bonferroni corrected comparisons for each period showed that the first 6 hours of the light cycle was significantly lower in female DEX treated offspring. CBT is reported as mean ± SEM of 12–15 measurements, with significance between vehicle and DEX-treated groups indicated by asterisk.
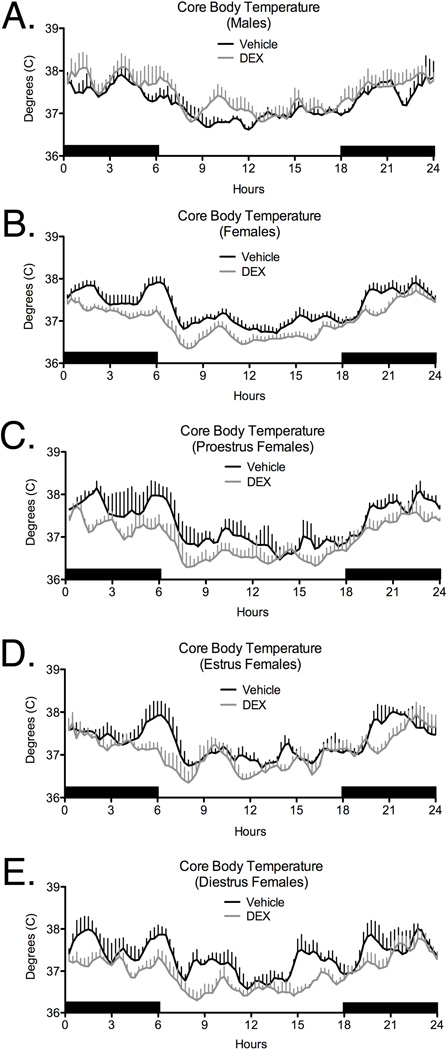
Analyses of CBT over the estrous cycle revealed that the decrease in CBT in DEX-exposed females was related to estrous cycle stage. CBT was significantly reduced in DEX-exposed females during estrus (Figure 2D; (F[1,5]=10.84; p<0.03) and diestrus (Figure 2E; (F[1,5]=6.58; p=0.05), but not during the proestrous stage, although there was a trend to significance on proestrus (Figure 2C; p<0.1). No significant effects of Prenatal Treatment on CBT were observed in males (Figure 2A).
Figure 2.
Effects of late gestation exposure to DEX on core body temperature (CBT) in adult male (A), and female (Panel B), offspring. For females, analysis of CBT during specific stages of the estrous cycle is also shown (Panels C–E). No effect of fetal DEX was seen on proestrus (C p<0.10), while CBT was decreased in DEX treated offspring during estrus (D, p<0.02) and diestrus (E, p<0.05). CBT was detected using temperature detecting transmitters that were inserted into the peritoneal cavity. Temperature was read remotely at 15 minute intervals throughout the day. Each data point represents the mean ± SEM of 3–4 animals.
3.2. Trh mRNA Expression in the PVN
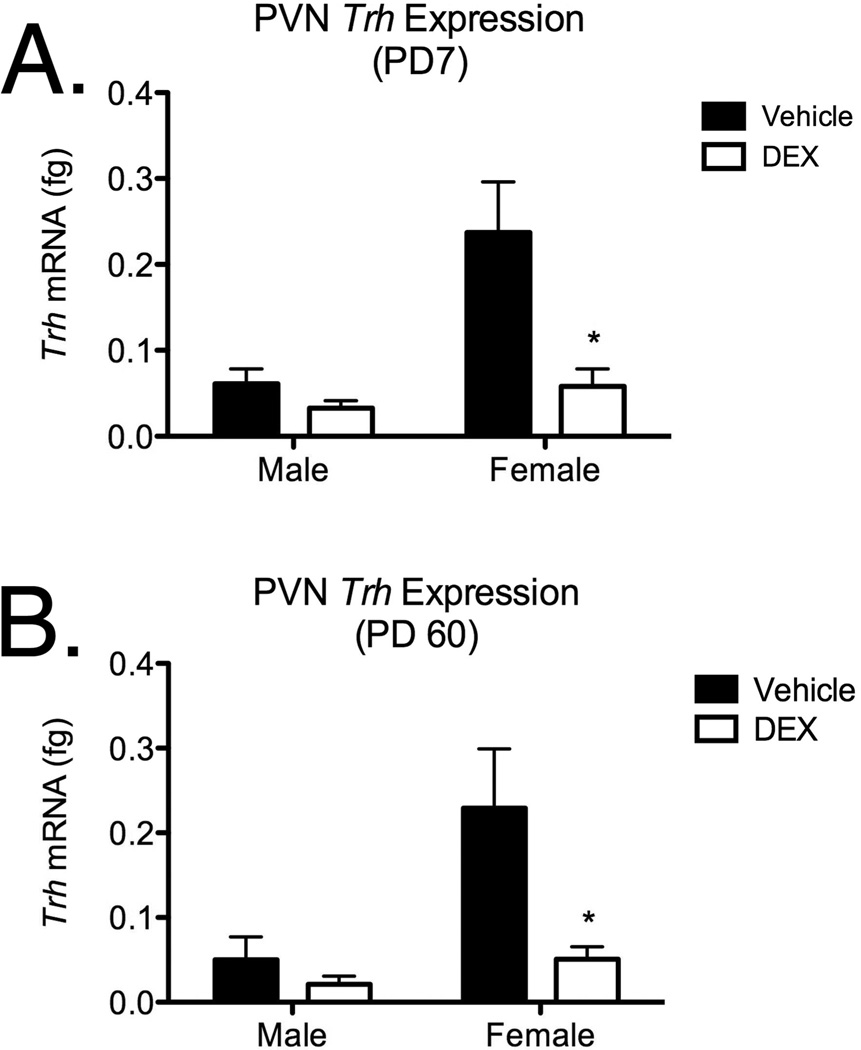
Real-time qPCR was used to measure the expression of Trh mRNA in the PVN from offspring harvested at PD7 (Figure 3A) and PD60 (Figure 3B), and expression was compared by two-way ANOVA (DEX exposure × Sex; Figure 3). Analysis of Trh expression in the PVN at PD7 revealed main effects of fetal DEX exposure (F[1, 23]=5.618; p=0.03) and sex (F[1, 23]=5.307; p=0.03), however, no interaction between these variables was observed. Post hoc analysis of Trh mRNA at PD7 revealed a significant decrease in the expression of this gene in female offspring which were exposed to DEX during fetal development, but this effect was not observed in the male offspring. Similar analysis of Trh mRNA from in the PVN from offspring harvested in adulthood (PD60; Figure 3B) also revealed main effects of fetal DEX exposure (F[1, 20]=5.493; p=0.03) and sex (F[1, 20]=5.577; p=0.03), with no interaction effect. Post hoc analysis of Trh mRNA expression in the PVN at PD60 revealed a significant decrease in the expression of this gene in female offspring which were exposed to DEX during fetal development, but this effect was not observed in the male offspring.
Figure 3.
Trh mRNA levels in the PVN following fetal DEX exposure during GD18–21. Animals were examined on PD7 (Panel A) and PD60 (Panel B). Data are mean ± SEM of 8–10 individuals, with significance (p<0.05) between vehicle and DEX-exposed groups indicated by asterisk.
3.3. Expression of ppTRH in the PVN
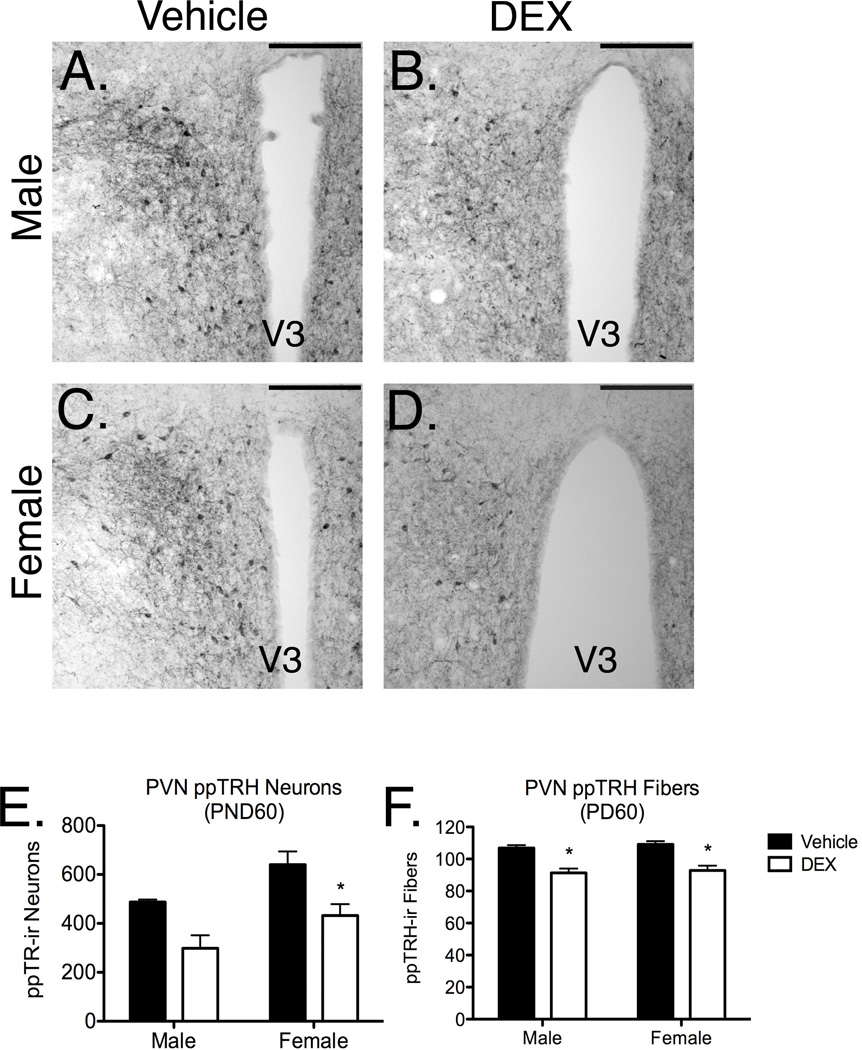
PreproTRH immunoreactivity (ir) was measured bilaterally through the PVN in brains harvested from adult rats that were exposed during fetal development to either DEX or vehicle. These estimates were compared between male vehicle-exposed (Figure 4A), male DEX-exposed (Figure 4B), female vehicle-exposed (Figure 4C) and female DEX-exposed (Figure 4D) offspring. Two-way ANOVA revealed a main effect of fetal DEX exposure (F[1, 16]=15.91; p=0.0011) and sex (F[1, 16]=8.260; p=0.0110) on ppTRH-expressing neurons within the PVN, however, a significant interaction between these variables was not observed (Figure 4E). Post hoc analysis of ppTRH ir neurons also revealed a significant decrease in this neuronal population in female, but not male, offspring exposed during late gestation to DEX (Figure 4E).
Figure 4.
ppTRH-ir neurons and fibers within the PVN in adult male and female offspring which were exposed during gestation to DEX (male, Panels A–B; female, Panels C–D; 20× objective, 100 µm scale bar). Bilateral neuron counts were taken through the PVN in female offspring which were exposed during late gestation to DEX (Panel E), while ppTRH-ir fiber counts were made through the PVN (Panel F). Neuron and fiber counts are reported as a mean ± SEM of 6 brains, with significance between vehicle and DEX-treated groups indicated by asterisk.
PreproTRH-ir fiber density was estimated within the PVN in male and female offspring (Figure 3A–D), and a main effect of fetal DEX exposure (F[1, 20]=42.12; p<0.0001), but not sex, on ppTRH-ir fiber counts was observed. No significant interaction between these variables was present, however, post hoc comparison of ppTRH-expressing fibers in the PVN revealed significantly lower density of immunopositive fibers in both male and female offspring which had been exposed to DEX during development (Figure 4F).
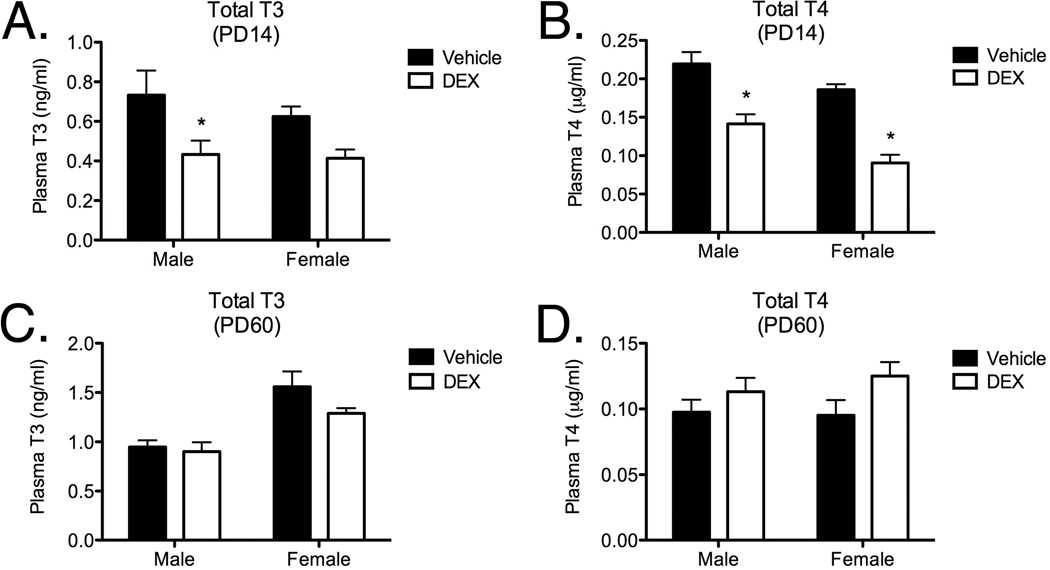
3.5. Total Plasma TH
Total plasma TH levels were measured by ELISA in plasma obtained from offspring at PD14 and PD60 which were exposed during late gestation to DEX or vehicle. Two-way ANOVA of total plasma T3 from PD 14 samples (Figure 5A) revealed a main effect of fetal DEX exposure (F[1, 18]=8.932; p=0.0079), but not sex (F[1, 18]=0.5615; p=0.4633). No interaction between these variables was present, however, post hoc analysis revealed a significant decrease in plasma T3 in male, but not female, offspring which were exposed to DEX during fetal development. Two-way ANOVA of total plasma T4 from offspring harvested at PD14 (Figure 5B) revealed main effects of both fetal DEX exposure (F[1, 20]=53.98; p<0.0001) and sex (F[1, 20]=12.76; p=0.0019), but no interaction was observed (F[1, 20]=0.5342; p=0.4733). Post hoc analysis of plasma T4 levels from offspring harvested at PD14 revealed a significant decrease in the amount of T4 in both male and female rats which were exposed to DEX during gestation.
Figure 5.
Effect of fetal DEX exposure on total plasma T3 levels (Panel A), and total T4 levels (Panel B) at PD14. Effect of fetal DEX exposure on T3 or T4 levels is also shown at PD60 (Panels C–D). Total T3 and T4 levels are reported as mean ± SEM of 6 brains, with significance between vehicle and DEX-treated groups indicated by asterisk.
Analysis of total plasma T3 levels from blood samples taken at PD60 (Figure 5C) did not reveal a main effect of fetal DEX exposure (F[1, 20] = 2.466; p = 0.1320), however, a main effect of sex (F[1, 20]=24.48; p<0.0001) was observed. No interaction effect (F[1, 20]=1.1221; p=0.2822) was observed, and post hoc analysis did not indicate any significant decrease in T3 in the adult animals which were exposed to DEX during gestation. Two-way ANOVA of total plasma T4 in adult rats (Figure 5D) indicated a main effect of fetal DEX exposure (F[1, 15]=4.626; p=0.0482), but not sex (F[1, 15]=0.2039; p=0.6581) on total plasma T4 levels. No interaction effect (F[1, 15]=0.4565; p=0.5095) was observed, nor were any significant changes in total T4 revealed by post hoc analysis of these data.
4. Discussion
Concern over fetal exposure to synthetic GC has arisen based on the results of studies examining a variety of animal models, which have documented an array of behavioral and metabolic disturbances later in life. Unfortunately, the mechanisms explaining the connection between early GC exposure and disrupted physiology in adulthood remain unclear [4, 5, 8, 24–26]. In the present studies, we demonstrate a sex-specific effect of fetal GC exposure on CBT, which was reduced in adult female, but not male, offspring following exposure to DEX during late gestation (GD 18–21). While CBT was decreased following fetal DEX exposure in female offspring when data were collapsed across the estrous cycle, further analysis indicated that this effect varied with the stage of the estrous cycle, such that a significant treatment (e.g. fetal DEX) effect was observed during estrus and diestrus, but not during proestrus. These data thus suggest that the mechanism behind decreased CBT in adulthood is secondary to fetal GC exposure, and is programmed in animals independent of estrous cycle. This concept is supported by a similar, though not significant, decrease in CBT during proestrus, during which suggests that the existing condition (e.g. decreased CBT) is partially mitigated by estrogen secretion. Furthermore, because decreased CBT was not observed in male offspring, our data indicate that the programming of CBT is sex-specific in that it affects female animals only, possibly due to the presence of testosterone in neonatal males which is elevated during the time of DEX exposure.
Fetal GC exposure is recognized to permanently alter gene expression of neuropeptides in the PVN [6]. Given the important role for TRH in regulating CBT, we hypothesized that the fetal DEX exposure used in these studies results in dysregulation of this peptide in the PVN. Within the PVN, TRH neurons are recognized for their role in regulation of the hypothalamic-pituitary-thyroid (HPT) axis, but evidence also suggests that TRH may act through non-neuroendocrine actions at distinct regions within the brain to regulate functions controlling energy balance, including food intake, thermoregulation, and locomoter activity [27–29]. Thus, TRH is thought to modulate CBT and other physiological functions through neuroendocrine and non-neuroendocrine pathways. Given the role for TRH in regulating CBT, we measured the expression of Trh mRNA in the PVN from both early life (PD7) and adult (PD60) cohorts to determine whether the expression of this gene is permanently altered in response to the fetal DEX exposure. Real-time qPCR demonstrated a decreased expression of Trh mRNA in the PVN from both PD7 and PD60 female animals whereas there was little change in expression of Trh in the male offspring following fetal exposure to DEX. Our data thus demonstrate a sex-specific effect of fetal DEX exposure to decrease the expression of Trh mRNA.
The expression of Trh is controlled in part by corticosterone signaling in the brain [30]. Because fetal exposure to excess GC has been reported to alter corticosterone signaling in adult rats [31], the possibility exists that the fetal DEX exposure paradigm employed in the present studies results in abnormal the expression of GR in the PVN. Alternatively, the fetal DEX exposure may alter the diurnal phase or dampen the amplitude of the corticosterone surge, which would likely result in abnormal Trh expression, however, further studies designed to record diurnal corticosterone rhythms are needed to confirm this hypothesis.
A second possibility explaining the permanent decrease in Trh expression is the potential for epigenetic modifications to this gene in response to fetal DEX exposure. Epigenetic programming of genes in response to adverse early-life events, such as stress, have been demonstrated several times. Murgatroyd et al., (2009), observed that stress in mice during a period of time where neurodevelopment is still occurring in rodents (PD1–10), resulted in long-term changes in arginine vasopressin (Avp) gene expression [32]. The changes in Avp expression were accompanied by abnormal methylation of the Avp gene promoter. Additionally, the possibility of sex differences in epigenetic programming has also been reported [33]. Given the permanent and sex-specific decrease in Trh signaling in the female PVN following late gestation exposure to DEX, the possibility exists that similar epigenetic changes, either through gene methylation or histone modification, may explain our findings.
In addition to examining Trh mRNA expression, we also employed IHC to measure the relative numbers of PVN neurons and fibers which express ppTRH, the precursor peptide to TRH. Our estimates of ppTRH-ir neurons in the PVN revealed that DEX exposure reduced the number of ppTRH-ir neurons in the PVN in female offpsring. A reduction in ppTRH-ir neurons in the male PVN was also suggested by our data, although this trend was not significant. However, we also observed a decrease in ppTRH-ir fibers in both male and female offspring. If these fibers originate in the PVN, then these latter observations support the claim that ppTRH-ir neuronal numbers are decreased in both male and female offspring following fetal DEX exposure. The observation that ppTRH expression may be reduced in both male and female offspring suggests that abnormal TRH expression may also exist in the male offspring, although potentially through a mechanism distinct from that occurring in females, based on our additional observations that Trh and GR expression were altered only in the female offspring.
Several possibilities also potentially explaining why high levels of DEX during development may permanently reduce ppTRH expression include the possibility that migration of ppTRH neurons destined for the PVN [34] may be disrupted, and that increased death of ppTRH neurons may occur in this region [25]. Because DEX exposure in these studies began on GD18, it is unlikely that migration was affected as the TRH-expressing neurons reportedly achieve their adult location and have undergone differentiation by GD15 in rats [35]. Moreover, recent studies have demonstrated that there are few apoptotic neurons in the PVN proper following prenatal DEX treatment [36]. A final possibility which explains the reduced number of ppTRH-ir neurons following fetal DEX exposure is that this peptide is present in the ppTRH neurons in the PVN, however, the expression levels are below our detection limits by IHC. This latter possibility is in keeping with likelihood that all neurons migrated correctly and did not undergo apoptosis, but rather that the expression of Trh is reduced through an epigenetic phenomenon, and this is reflected in the minimal detectable presence of ppTRH.
Based on the expression of ppTRH in the PVN, our data suggest that fetal DEX exposure may program a hypothyroid state in the female offspring. We therefore measured total plasma T3 and T4 levels in blood samples obtained from the offspring at PD14 and PD60. We observed a significant decrease in T3 in the fetal DEX-exposed male offspring at PD14 and total plasma T4 concentrations were reduced in both male and female offspring which exposed to DEX during gestation. Given the role for TRH in regulating HPT axis activity and in keeping with our gene expression data from the early-life cohort, these data suggest that additional dysregulation of the HPT axis may exist in both male and female offspring. However, total T3 and T4 were not altered by prenatal DEX treatment in adult male or female offspring. These data indicate that fetal DEX causes decreased TH levels early in life, however, by adulthood this deficit is resolved, despite a permanent decrease in TRH levels in the PVN. Although these data indicate a potential problem with TH feedback on the PVN, the expression of thyroid receptor-β (THRβ), which is a key receptor involved in TH feedback on the PVN, was not observed to be altered (data not shown). Reports have also indicated that isoflurane anesthesia may decrease peripheral T4 levels in adult male rats [37], but given the short span of time between anesthesia and blood collection in the adult rats in our study, it is unlikely that this explains the inconsistent TH results between juvenile and adult rats. Finally, peripheral TH levels may not reflect the non-neuroendocrine effects mediated by TRH, suggesting that local signaling by this peptide within the brain may be influencing CBT and other metabolic processes.
In summary, our data indicate that exposure to DEX during GD18–21 in rats results in decreased CBT in female, but not male offspring. A possible explanation for this phenomenon is that decreased TRH signaling exists in these animals, but a lack of peripheral TH differences in adulthood suggest that any involvement of TRH in the observed decrease in CBT is due to non-neuroendocrine effects within the brain. Our data also suggest that the fetal DEX paradigm employed by these studies results in abnormal corticosterone rhythms in the female offspring, based on the expression of GR in the PVN. Given the role for decreased TRH in multiple metabolic and psychiatric disorders, our observations suggest a potential mechanism by which fetal GC overexposure potentiates adult disease.
Highlights.
Exposure to DEX during late gestation reduces CBT in adult female offspring.
Fetal DEX exposure reduces Trh expression in female offspring in the PVN.
TRH-immunoreactive nerves and fibers are reduced by fetal DEX exposure.
Reductions in T3 and T4 following fetal DEX are not maintained into adulthood.
Acknowledgements
We thank Drs. Stuart Tobet and Jill M. Goldstein for their editorial assistance in the preparation of this manuscript.
Funding: This work was supported by the National Institutes of Health NS039951 (R.J.H.) and MH082679 (R.J.H).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Summary: D.L.C., D.G.Z., A.F.L, R.F.M., and R.J.H. have nothing to disclose.
References
- 1.Norberg H, Stalnacke J, Heijtz RD, Smedler AC, Nyman M, Forssberg H, et al. Antenatal corticosteroids for preterm birth: dose-dependent reduction in birthweight, length and head circumference. Acta Paediatr. 2010 doi: 10.1111/j.1651-2227.2010.02074.x. [DOI] [PubMed] [Google Scholar]
- 2.Reinisch JM, Simon NG, Karow WG, Gandelman R. Prenatal exposure to prednisone in humans and animals retards intrauterine growth. Science. 1978;202:436–438. doi: 10.1126/science.705336. [DOI] [PubMed] [Google Scholar]
- 3.Watterberg KL. Policy statement--postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics. 2010;126:800–808. doi: 10.1542/peds.2010-1534. [DOI] [PubMed] [Google Scholar]
- 4.de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, et al. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest. 2007;117:1058–1067. doi: 10.1172/JCI30982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake AJ, Raubenheimer PJ, Kerrigan D, McInnes KJ, Seckl JR, Walker BR. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology. 2010;151:1581–1587. doi: 10.1210/en.2009-1088. [DOI] [PubMed] [Google Scholar]
- 6.Hossain A, Hajman K, Charitidi K, Erhardt S, Zimmermann U, Knipper M, et al. Prenatal dexamethasone impairs behavior and the activation of the BDNF exon IV promoter in the paraventricular nucleus in adult offspring. Endocrinology. 2008;149:6356–6365. doi: 10.1210/en.2008-0388. [DOI] [PubMed] [Google Scholar]
- 7.Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 8.Carbone DL, Zuloaga DG, Hiroi R, Foradori CD, Legare ME, Handa RJ. Prenatal Dexamethasone Exposure Potentiates Diet-Induced Hepatosteatosis and Decreases Plasma IGF-I in a Sex-Specific Fashion. Endocrinology. 2011 doi: 10.1210/en.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westerterp KR, Speakman JR. Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int J Obes (Lond) 2008;32:1256–1263. doi: 10.1038/ijo.2008.74. [DOI] [PubMed] [Google Scholar]
- 10.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–235. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- 11.Segerson TP, Kauer J, Wolfe HC, Mobtaker H, Wu P, Jackson IM, et al. Thyroid hormone regulates TRH biosynthesis in the paraventricular nucleus of the rat hypothalamus. Science. 1987;238:78–80. doi: 10.1126/science.3116669. [DOI] [PubMed] [Google Scholar]
- 12.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duksal F, Kilic I, Tufan AC, Akdogan I. Effects of different corticosteroids on the brain weight and hippocampal neuronal loss in rats. Brain Res. 2009;1250:75–80. doi: 10.1016/j.brainres.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 14.Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity, and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets, and sex selectivity. Neuropsychopharmacology. 2006;31:12–35. doi: 10.1038/sj.npp.1300783. [DOI] [PubMed] [Google Scholar]
- 15.Nyirenda MJ, Welberg LA, Seckl JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence? J Endocrinol. 2001;170:653–660. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- 16.Carbone DL, Zuloaga DG, Hiroi R, Foradori CD, Legare ME, Handa RJ. Prenatal dexamethasone exposure potentiates diet-induced hepatosteatosis and decreases plasma IGF-I in a sex-specific fashion. Endocrinology. 2012;153:295–306. doi: 10.1210/en.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handa RJ, Zuloaga DG, McGivern RF. Prenatal ethanol exposure alters core body temperature and corticosterone rhythms in adult male rats. Alcohol. 2007;41:567–575. doi: 10.1016/j.alcohol.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The rat brain in stereotaxic coordinates / George Paxinos, Charles Watson. Amsterdam: Elsevier; 2007. [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki S, Solberg LC, Redei EE, Handa RJ. Prepro-thyrotropin releasing hormone 178–199 immunoreactivity is altered in the hypothalamus of the Wistar-Kyoto strain of rat. Brain Res. 2001;913:224–233. doi: 10.1016/s0006-8993(01)02853-0. [DOI] [PubMed] [Google Scholar]
- 21.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 22.Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Mericq V, Medina P, Kakarieka E, Marquez L, Johnson MC, Iniguez G. Differences in expression and activity of 11beta-hydroxysteroid dehydrogenase type 1 and 2 in human placentas of term pregnancies according to birth weight and gender. Eur J Endocrinol. 2009;161:419–425. doi: 10.1530/EJE-09-0308. [DOI] [PubMed] [Google Scholar]
- 24.Cleasby ME, Kelly PA, Walker BR, Seckl JR. Programming of rat muscle and fat metabolism by in utero overexposure to glucocorticoids. Endocrinology. 2003;144:999–1007. doi: 10.1210/en.2002-220559. [DOI] [PubMed] [Google Scholar]
- 25.Coe CL, Lubach GR. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci Biobehav Rev. 2005;29:227–235. doi: 10.1016/j.neubiorev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 26.O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone 'programmes' hypotension, but stress-induced hypertension in adult offspring. J Endocrinol. 2008;196:343–352. doi: 10.1677/JOE-07-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi YH, Hartzell D, Azain MJ, Baile CA. TRH decreases food intake and increases water intake and body temperature in rats. Physiol Behav. 2002;77:1–4. doi: 10.1016/s0031-9384(02)00784-9. [DOI] [PubMed] [Google Scholar]
- 28.Schuhler S, Warner A, Finney N, Bennett GW, Ebling FJ, Brameld JM. Thyrotrophin-releasing hormone decreases feeding and increases body temperature, activity and oxygen consumption in Siberian hamsters. J Neuroendocrinol. 2007;19:239–249. doi: 10.1111/j.1365-2826.2006.01524.x. [DOI] [PubMed] [Google Scholar]
- 29.Sharp T, Bennett GW, Marsden CA, Tulloch IF. A comparison of the locomotor effects induced by centrally injected TRH and TRH analogues. Regul Pept. 1984;9:305–315. doi: 10.1016/0167-0115(84)90083-1. [DOI] [PubMed] [Google Scholar]
- 30.Pekary AE, Stevens SA, Sattin A. Rapid modulation of TRH and TRH-like peptide levels in rat brain and peripheral tissues by corticosterone. Neurochem Int. 2006;48:208–217. doi: 10.1016/j.neuint.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Muneoka K, Mikuni M, Ogawa T, Kitera K, Kamei K, Takigawa M, et al. Prenatal dexamethasone exposure alters brain monoamine metabolism and adrenocortical response in rat offspring. Am J Physiol. 1997;273:R1669–R1675. doi: 10.1152/ajpregu.1997.273.5.R1669. [DOI] [PubMed] [Google Scholar]
- 32.Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 33.Menger Y, Bettscheider M, Murgatroyd C, Spengler D. Sex differences in brain epigenetics. Epigenomics. 2010;2:807–821. doi: 10.2217/epi.10.60. [DOI] [PubMed] [Google Scholar]
- 34.Fukumoto K, Morita T, Mayanagi T, Tanokashira D, Yoshida T, Sakai A, et al. Detrimental effects of glucocorticoids on neuronal migration during brain development. Mol Psychiatry. 2009;14:1119–1131. doi: 10.1038/mp.2009.60. [DOI] [PubMed] [Google Scholar]
- 35.Guerra-Crespo M, Perez-Monter C, Janga SC, Castillo-Ramirez S, Gutierrez-Rios RM, Joseph-Bravo P, et al. Transcriptional profiling of fetal hypothalamic TRH neurons. BMC Genomics. 2011;12:222. doi: 10.1186/1471-2164-12-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethasone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desaulniers D, Yagminas A, Chu I, Nakai J. Effects of anesthetics and terminal procedures on biochemical and hormonal measurements in polychlorinated biphenyl treated rats. Int J Toxicol. 2011;30:334–347. doi: 10.1177/1091581810397774. [DOI] [PubMed] [Google Scholar]