Abstract
Light-induced damage to photosystem I (PSI) was studied during low-light illumination of barley (Hordeum vulgare L.) at chilling temperatures. A 4-h illumination period induced a significant inactivation of PSI electron transport activity. Flash-induced P700 absorption decay measurements revealed progressive damage to (a) the iron-sulfur clusters FA and FB, (b) the iron-sulfur clusters FA, FB, and FX, and (c) the phylloquinone A1 and the chlorophyll A0 or P700 of the PSI electron acceptor chain. Light-induced PSI damage was also evidenced by partial degradation of the PSI-A and PSI-B proteins and was correlated with the appearance of smaller proteins. Aggravated photodamage was observed upon illumination of barley leaves infiltrated with KCN, which inhibits Cu,Zn-superoxide dismutase and ascorbate peroxidase. This indicates that the photodamage of PSI in barley observed during low-light illumination at chilling temperatures arises because the defense against active oxygen species by active oxygen-scavenging enzymes is insufficient at these specific conditions. The data obtained demonstrate that photoinhibition of PSI at chilling temperatures is an important phenomenon in a cold-tolerant plant species.
Light is the ultimate energy source for photosynthesis. However, if the absorbed light energy that is transferred to the reaction centers exceeds the consumption, this may cause damage. Initially, mechanisms such as nonphotochemical thermal dissipation of excess energy (Demmig-Adams and Adams, 1992; Horton et al., 1994) and short- and long-term dynamic regulation of the antenna size (Anderson and Andersson, 1988; Aro et al., 1993) serve to protect the photosynthetic machinery. At later stages, when the protective capacities are exceeded, photoinhibition takes place. This damage results in a net decrease in photosynthetic efficiency (Powles, 1984; Aro et al., 1993) and has been estimated to cause losses of carbon assimilation of approximately 10% in willow fields (Ögren and Rosenqvist, 1992). A combination of high light with other stress factors such as chilling or heat, drought, or low carbon dioxide supply greatly increases the inhibition process (Powles, 1984; Demmig-Adams and Adams, 1992; Aro et al., 1993).
PSII has long been considered the primary target for photoinhibition (Andersson and Styring, 1991; Barber and Andersson, 1992; Prasil et al., 1992; Aro et al., 1993) because PSI is more stable than PSII during strong light treatments (Powles, 1984) and because its inactivation has rarely been observed in vivo (Havaux and Eyletters, 1991). However, in isolated thylakoid membranes, PSI is as susceptible as PSII to light stress (Satoh, 1970a; Inoue et al., 1986, 1989; Tjus, 1995). The primary target for photoinhibition of PSI upon illumination of thylakoid membranes was shown to be FX, FB, and FA (Inoue et al., 1986). Also, it has been shown that oxygen is required for light-induced inactivation of PSI to take place (Satoh, 1970a; Inoue et al., 1989; Tjus and Andersson, 1993), which suggests that the damage is caused by active oxygen species.
The increased sensitivity of PSI to light stress in isolated thylakoid membranes compared with whole plants demonstrates that the PSI complex is effectively protected by mechanisms residing within the chloroplast. The site of superoxide production in chloroplasts is near to PSI (Asada and Takahashi, 1987; Asada, 1994). A Cu,Zn-superoxide dismutase, which converts superoxide to hydrogen peroxide, is present at a high concentration in the chloroplast stroma, specifically around PSI (Ogawa et al., 1995). Hydrogen peroxide is reduced to water by the action of ascorbate peroxidase, which is bound to the stromal thylakoids near PSI (Miyake and Asada, 1992). Most likely, the oxygen-scavenging enzymes of the chloroplast may serve to protect PSI in vivo.
In the cold-sensitive plants cucumber and potato, light treatments at low temperature implicate PSI, rather than PSII, as the primary target for photoinhibition in vivo (Havaux and Davaud, 1994; Sonoike and Terashima, 1994; Terashima et al., 1994; Sonoike, 1995, 1996a; Sonoike et al., 1995; for review, see Sonoike, 1996b). The damage was suggested to be caused by superoxide and/or singlet oxygen produced by PSI. It was speculated that the low temperature caused a decrease in the rate of carbon dioxide fixation that would result in an accumulation of reducing power on the acceptor side of PSI. Furthermore, it was proposed that the chilling temperature introduces lesions into the lipid bilayer or inactivates the oxygen-scavenging enzymes such as superoxide dismutase.
It has been shown in wheat that high levels of active oxygen, as produced under severe stress conditions, directly inactivate and degrade chloroplastic superoxide dismutase (Casano et al., 1997). When the acceptor side of PSI is fully reduced, recombination between the radical pairs P700+/A0− or P700+/A1− can generate the triplet state of P700 (Shuvalov et al., 1986; Golbeck, 1987; Golbeck and Bryant, 1991). Chlorophyll triplets can react with molecular oxygen to create very toxic singlet oxygen that could cause photoinhibitory damage to PSI.
One reason that photoinhibition of PSI has so far largely been neglected is the use of relatively high light intensities in most earlier investigations. High-light stress may actually protect PSI by causing rapid photoinhibition of PSII and thereby depleting electron donation to P700+. In agreement with this idea, it is known that photoinhibition of PSI is prevented when isolated thylakoids are illuminated in the presence of DCMU to block electron transport from PSII (Satoh, 1970b). Under these conditions, P700 will be oxidized into P700+, which is able to dissipate excess excitation energy as heat (Nuijs et al., 1986) and thereby to effectively quench deleterious effects otherwise caused by excess light. Accordingly, it would be predicted that photoinhibition of PSI in vivo may occur when the light intensity is sufficiently low not to cause a direct photoinhibition of PSII. In the end, however, inactivation of PSI will inevitably cause overreduction of the acceptor side of PSII and, consequently, will induce damage to PSII.
In the present study, we demonstrated that low-light illumination at chilling temperatures of barley (Hordeum vulgare L.), a cold-tolerant plant, results in significant photoinhibition of PSI. Furthermore, we show that the observed light-stress damage of barley PSI is increased when superoxide dismutase and ascorbate peroxidase are inhibited in the leaves prior to illumination.
MATERIALS AND METHODS
Barley (Hordeum vulgare L. cv Svalöfs Bonus) was sown indoors in vermiculite. After 8 to 10 d of growth in continuous low light (30 μmol photons m−2 s−1) at room temperature, the green parts of primary leaves were cut off above the white base.
Uptake and Illumination Experiments
Detached leaves were kept at room temperature for 1 h to reduce the turgor pressure, increasing their ability to absorb treatment medium. The leaves were subsequently transferred to a beaker containing 50 mm Tricine, pH 7.5, and 100 mm sorbitol, which in some experiments was supplemented with 4 or 10 mm KCN, and were allowed 30 min to take up the medium and regain their turgor. To further facilitate the uptake of medium, the leaves were placed in an air flow supplied by a fan for 2 h to increase the transpiration rate. These pretreatments were performed at very low light intensities (approximately 2–5 μmol photons m−2 s−1). At the end of the uptake period (time 0 controls) and after different periods of subsequent illumination, samples consisting of 50 leaves were taken from each experiment. Thylakoid membranes were immediately prepared from the leaves.
During illumination, the leaves were floated on the respective uptake media contained in plastic trays. The illumination was performed at 2 to 4°C in a 600-L cold bench at a PPFD of 100 μmol photons m−2 s−1 provided by a sodium lamp (SON/T AGRO 400W, Philips, Eindhoven, The Netherlands). The heat from the lamp was shielded by heat-absorbing glass plates covering the cold bench, while the temperature inside was kept uniform by fans. Control leaves (dark) were placed adjacent to the illuminated leaves but were protected from the light.
Isolation of Thylakoid Membranes
Leaf samples (50 leaves, approximately 10 g) were homogenized in a blender (Braun, Allentown, PA) equipped with exchangeable razor blades (Kannangara et al., 1977) in 90 mL of 50 mm Tricine, pH 7.5, 100 mm Suc, and 5 mm MgCl2. Chloroplasts were pelleted by centrifugation at 1000g for 5 min, resuspended in the same buffer, and repelleted. Osmotic rupture of the chloroplasts was achieved by incubation in 5 mm MgCl2 for 5 min, after which the thylakoid membranes were obtained by centrifugation at 2000g for 5 min. The thylakoid membranes were washed twice in 10 mm Tricine, pH 7.5, repelleted as above, and finally resuspended in 20 mm Tricine, pH 7.5, 400 mm Suc, 10 mm NaCl, and 5 mm MgCl2. Aliquots were frozen in liquid nitrogen and stored at −80°C until assayed for photosynthetic activities. Chlorophyll content was determined according to the method of Arnon (1949).
Electrophoresis
SDS-PAGE was carried out in 8 to 25% linear gradient gels according to the method of Fling and Gregerson (1986) using Mini-Protean electrophoresis chambers (Bio-Rad). To efficiently prevent the deposition of protein aggregates in the sample wells, the samples were solubilized for 20 min at 75°C using 1.7% SDS, 34 mm DTT, and 34 mm Na2CO3.
Immunoblot Analyses
To identify and quantify the PSI polypeptides, immunoblotting was carried out essentially as described by Towbin et al. (1979). Proteins were transferred by electrophoresis to nitrocellulose membranes in a semidry blotting device. The membrane was incubated with polyclonal antibodies raised in rabbits against the isolated barley PSI complex. Subsequently, secondary antibodies ligated to alkaline phosphatase were applied. Bromo-chloro-indolyl-phosphate and tetrazolium blue were used for the coloring reaction. The developed membranes were analyzed with a scanner (model JX330, Sharp Electronic, Hamburg, Germany) combined with Image-Master software (Pharmacia).
Electron Transport Measurements
PSI electron transport was measured using a Clark-type oxygen electrode and a 3-mL reaction mixture composed of 40 mm Tricine, pH 7.5, 10 mm Suc, 167 μm MV, 0.1 mm DCIP, 1 mm sodium ascorbate, 10 mm NH4Cl, 10 μm DCMU, 5 mm sodium azide, and thylakoids corresponding to 15 μg of chlorophyll. Similarly, PSII electron transport was assayed in a 3-mL reaction mixture composed of 50 mm Tricine, pH 7.5, 20 mm NaCl, 5 mm MgCl2, 100 mm Suc, 1 mm phenyl-p-benzoquinone, and thylakoids corresponding to 15 μg of chlorophyll.
P700 Absorption Decay
Flash-induced P700 absorption changes were measured at 834 nm in a 300-μL reaction mixture containing thylakoid membranes corresponding to 8 μg of chlorophyll dissolved in 20 mm Tricine, pH 7.5, 0.065% (w/v) n-decyl-β-d-maltopyranoside, 670 μm sodium ascorbate, and 20 μm DCIP. The measuring beam and detection system were as previously described (Naver et al., 1996). Actinic light pulses (532 nm, 6 ns) at full width of half-maximum pulse amplitude were provided by a Nd:YAG laser (Quanta Ray model GCR-100, Spectra Physics Lasers, Mountain View, CA) at a frequency of 1 Hz. A total of 20 to 64 flash-induced decay curves were collected and averaged for each sample. The recorded absorption changes were resolved into exponential decay components by a Levenberg-Marquardt nonlinear regression procedure (Press et al., 1989).
RESULTS
Low-Light Illumination of Barley at Chilling Temperatures
Barley leaves were illuminated at 2 to 4°C with low light (100 μmol photons m−2 s−1). Thylakoid membranes were isolated from the illuminated leaves, and their photochemical activities were determined.
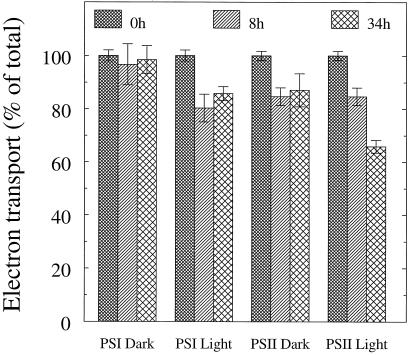
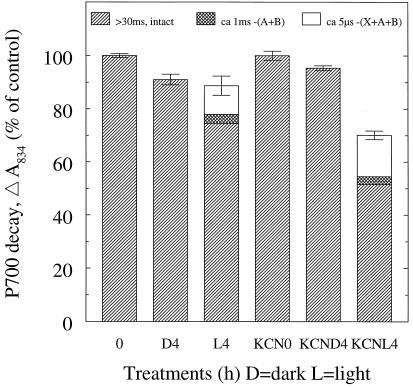
A small yet clear decrease in PSI activity (DCIP → MV) was induced by the light treatment (Fig. 1). After illumination for 8 h, the PSI activity was lowered by approximately 20%, whereas the dark control was stable. At the same time, PSII (H2O → phenyl-p-benzoquinone) was slightly inactivated, but to the same degree in both the light and dark, pointing toward a light-independent effect of chilling. With 34 h of illumination, PSI was not further inhibited, whereas the PSII activity had decreased 34%.
Figure 1.
Electron transport in thylakoid membranes isolated from barley leaves subjected to illumination (100 μmol photons m−2 s−1) at 4°C for 8 or 34 h. Electron transport rates were assayed using an oxygen electrode. Error bars indicate sds (n = 2–3). The 100% activity was −229 and 178 μmol O2 (mg chlorophyll)−1 h−1 for PSI and PSII, respectively.
To further corroborate photodamage of PSI and to specifically reveal the lesion sites, flash-induced P700 absorption decay kinetics were investigated. Photooxidation of P700 leads to an 834-nm absorption change that rapidly relaxes. The total P700 absorption change, similarly to the oxygraph measurements above, can be used to quantify the remaining activity. In addition, the deconvolution of the decay into different time components can reveal specific damages in the electron acceptor chain. When isolated thylakoid membranes are used without the addition of Fd, the terminal electron acceptors with an intact PSI were FA and FB.
The time constant (τ) for charge recombination between (FA/FB)− and P700+ is known to be >30 ms. With damage to FA/FB, the charge recombination will proceed from FX− with a time constant of approximately 1 ms. With further damage to the electron transport chain, the charge recombination from either A1− or A0−, via the P700 triplet state, takes place in 3 to 5 μs. Direct back-reaction from A0− to P700+, with a τ of approximately 30 ns, is below the 1-μs time resolution of our experimental system (for reviews of charge recombination, see Golbeck, 1987; Golbeck and Bryant, 1991). In this context it should be stressed that the decyl maltoside concentration was carefully chosen to effectively dissolve the thylakoid membranes while still retaining all of the bound antenna pigments. This is important because excited free chlorophyll molecules decay via chlorophyll triplet states with microsecond kinetics.
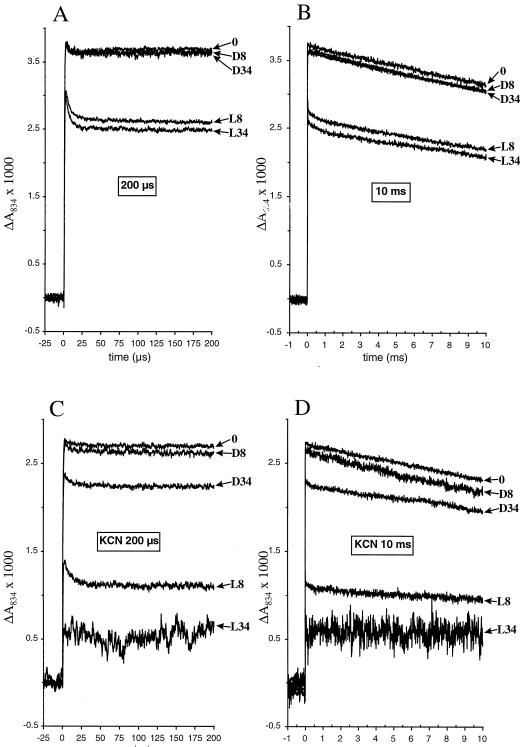
To achieve optimal deconvolution of the P700 decay kinetics, two time windows were analyzed. On a wide, 10-ms time scale (Fig. 2B), the 1-ms back reaction from FX−, indicative of damaged FB and FA centers, is resolved (Golbeck 1987; Golbeck and Bryant 1991). In a narrow 200-μs window (Fig. 2A), rapid, microsecond back reactions are unveiled, representing damaged FX, FB, and FA (Golbeck, 1987; Golbeck and Bryant, 1991) and the total amplitude can be resolved. To illustrate the results calculated from the decay curves, bar graphs were constructed in which the amplitudes belonging to different decay components within a sample are stacked in the same bar (Fig. 3).
Figure 2.
Flash-induced absorption change of P700 in isolated thylakoid membranes. A and B represent 200-μs and 10-ms time windows, respectively, of an illumination experiment carried out as described in Figure 1. C and D represent 200-μs and 10-ms time windows, respectively, of an illumination experiment carried out as described in Figure 4 using leaves infiltrated with 10 mm KCN. Arrows refer to different treatments in hours. D, Dark-treated; L, light-treated.
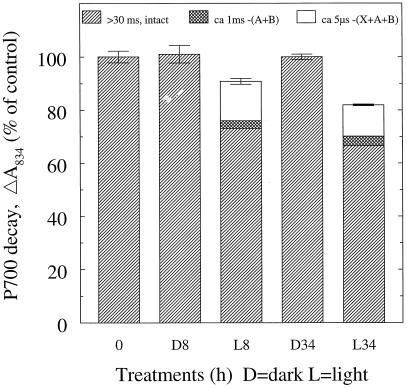
Figure 3.
Resolution of flash-induced absorption changes of P700 into decay components with different time constants. The data shown in Figure 2, A and B, are a subset of the data used to produce this figure. A, B, and X refer to the Fe-S clusters FA, FB and FX, respectively. The >30-ms time component represents an intact PSI reaction center with recombination from (FA/FB)−. The 1-ms time component reflects recombination from FX− after loss of FA/FB activity. The 5-μs time component represents recombination from either A1− or A0− when FA/FB and FX are all inactivated. Error bars indicate sds for the total absorption amplitudes (n = 2–8).
In both windows, the 0-time controls and the 8- and 34-h dark samples show decay kinetics (Figs. 2, A and B, and 3) calculated to be >30 ms, demonstrating the presence of an intact PSI and antenna system. In the 200-μs window, thylakoids from the 8-h illuminated leaves demonstrated (Figs. 2A and 3) a reduction to 91 ± 1% of the total absorption amplitude and the appearance of a 5-μs decay component, amounting to 15 ± 1% of the total control amplitude. Furthermore, the 10-ms window (Figs. 2B and 3) resolved a 1-ms decay component, representing 2.9 ± 0.8% of the 0 control amplitude, and a main fraction showing intact PSI with an amplitude of 73 ± 1%. The 34-h illuminated sample showed the same pattern of damage as at 8 h but was slightly more pronounced (Figs. 2, A and B, and 3).
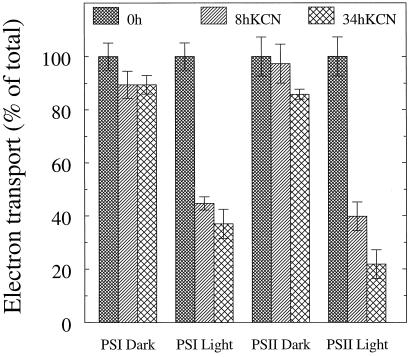
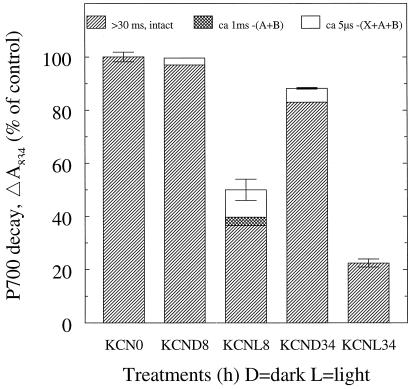
Cu,Zn-superoxide dismutase and ascorbate peroxidase are effective scavengers of active oxygen species. Both enzymes are inhibited by CN. To test the importance of these two oxygen-scavenging systems in the protection against light stress, leaves were infiltrated with 10 mm KCN prior to illumination. Otherwise, the light treatment was as above. In the 8-h dark control, PSI activity as determined with the oxygen electrode was decreased by approximately 10%. The 8-h light sample was inactivated by as much as 55% (Fig. 4). When illuminating for 34 h, the dark control showed no additional decrease, whereas the light sample was inactivated by approximately 63%. The same pattern was seen in PSII, with 60 and 78% decreases of oxygen evolution at 8 and 34 h of light treatment, respectively (Fig. 4). Accordingly, the prolonged light treatment did not proportionally increase the damage to either photosystem but, instead, the inactivation seemed to level out.
Figure 4.
Electron transport in thylakoid membranes isolated from barley leaves infiltrated with 10 mm KCN and subjected to illumination (100 μmol photons m−2 s−1) at 4°C for 8 and 34 h. Electron transport rates were assayed using an oxygen electrode. Error bars indicate sds (n = 2–3). The 100% activity was −234 and 163 μmol O2 (mg chlorophyll)−1 h−1 for PSI and PSII, respectively.
Flash-induced P700 decay measurements verified the PSI inactivation (Figs. 2, C and D, and 5). After 8 h of treatment, the dark control exhibited a minor microsecond decay with an amplitude of about 3%. With 8 h of light, damage in PSI was demonstrated in the 200-μs window (Figs. 2C and 5) as a decrease to 50 ± 4% in total absorption amplitude and in the appearance of a 5-μs decay component, contributing to 10 ± 2% of the amplitude. Furthermore, the 10-ms window (Figs. 2D and 5) resolved a 1-ms decay component with 3.2 ± 0.4% of the P700 amplitude and an intact fraction of 37 ± 1%. At 34 h, the dark control showed reduction to 88% in total amplitude and demonstrated a microsecond decay with an amplitude of 5.2%. The 34-h light sample was heavily deteriorated and thylakoid isolation resulted in aggregated material. The combination of aggregation and pronounced damage led to a low signal-to-noise ratio that prevented the resolution of the decay into time components. However, the total loss of amplitude was shown to be approximately 78%.
Figure 5.
Resolution of flash-induced absorption changes of P700 into decay components with different time constants. The data shown in Figure 2, C and D, are a subset of the data used to produce this figure. Error bars indicate sds for the total absorption amplitudes (n = 2–7). For further explanation, see legend to Figure 3.
Light-Stress Effects on the PSI Reaction Center Polypeptides
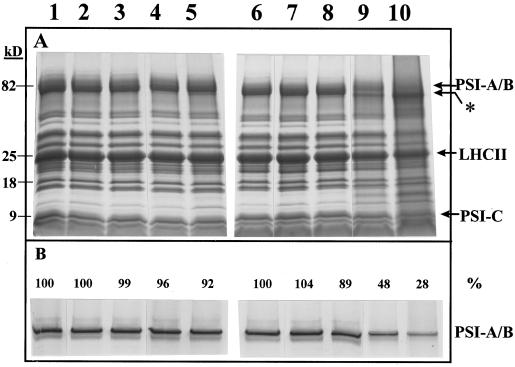
Thylakoid membranes isolated from the light-stressed barley leaves were further analyzed by SDS-PAGE (Fig. 6A). The barley PSI-A and PSI-B proteins are very difficult to resolve from each other during electrophoresis, and we could not discriminate between them. In the material illuminated without KCN, no pronounced changes in the polypeptide pattern were detectable. However, the appearance of an additional protein band migrating at 78 kD and slightly faster than the PSI-A/B band was noticeable in the illuminated samples (Fig. 6A, lanes 4 and 5, marked with an asterisk) but not in the dark controls (Fig. 6A, lanes 1–3). In the illuminated and KCN-treated leaves, a marked decrease in the content of the reaction center proteins was already evident at 8 h, and this reduction was even more pronounced at 34 h (Fig. 6A, lanes 9 and 10). As observed in the samples without KCN, an additional, faster-migrating band was observed at 78 kD. Quantitatively, the appearance of the 78-kD band correlated with the decrease of PSI-A/B and we therefore suggest that it is derived from degradation or modification of the reaction center proteins. In addition, a polypeptide migrating at 15 kD also appeared. It should be noted that the dark controls (Fig. 6A, lanes 7 and 8) also showed minor amounts of the 78-kD band. This correlates well with the observed nonspecific inactivation of PSI induced in the 34-h dark sample of approximately 10% (Figs. 4 and 5).
Figure 6.
A, SDS-PAGE of thylakoid membranes isolated after illumination of barley leaves with or without KCN, as specified in Figures 1 and 4. Each sample corresponds to 8 μg of chlorophyll. Lane 1, 0 h; lane 2, 8 h of dark; lane 3, 34 h of dark; lane 4, 8 h of illumination; lane 5, 34 h of illumination; lane 6, KCN plus 0 h; lane 7, KCN plus 8 h dark; lane 8, KCN plus 34 h of dark; lane 9, KCN plus 8 h of illumination; and lane 10, KCN plus 34 h of illumination. B, Immunoblot of the same samples in A, each corresponding to 0.5 μg of chlorophyll, using an antibody directed against the PSI complex. The blot was densitometrically scanned and the relative intensities of coloring are specified above the separate lanes.
A more quantitative analysis of the PSI-A/B decrease (Fig. 6B) was obtained by immunoblot analysis using antibodies raised against barley PSI. The antibodies recognize two closely migrating bands in the PSI-A/B region. Prolonged heat treatment during solubilization before SDS-PAGE results in more protein migrating in the lower band, and, accordingly, both bands are interpreted as representing PSI-A/B but with different degrees of denaturation. The antibodies did not recognize the new polypeptide at 78 kD, possibly because of lack of or denaturation-induced changes of binding epitopes for the antibodies on this tentative PSI-A/B product. A number of new polypeptide bands appeared below 20 kD after photoinhibition (data not shown). However, because the PSI antibodies also recognize the low-molecular-mass PSI subunits, we were not able to determine the specific origin of the new polypeptides. Illumination in the absence of KCN resulted in a small but distinct PSI-A/B decrease of 4 and 8% in the 8- and 34-h light samples, respectively (Fig. 6B, lanes 4 and 5). With KCN present during illumination, the PSI-A/B was degraded by 52 and 72% in the 8- and 34-h light samples, respectively (Fig. 6B, lanes 9 and 10). The dark control thylakoids showed no decrease in PSI-A/B except for the 34-h KCN sample, in which an 11% decrease was observed.
Illumination at 4 mm KCN Specifying the Origin of the Microsecond Decay
In the experiments described above, photodamage was demonstrated after an 8-h period of light stress. In a series of experiments, the period of light treatment at 4°C was reduced to 4 h, whereas the KCN concentration was lowered to 4 mm to minimize the non-light-induced damage from active oxygen and to accurately assess PSI damage by flash-induced P700 decay kinetics (Fig. 7). Without KCN present during illumination, the PSI activity after 4 h of illumination was damaged, as shown by (a) a reduction to 89 ± 4% in total amplitude; (b) the appearance of a 5-μs decay component with a 11 ± 2% amplitude, indicating damaged FA/FB and FX centers; and (c) a millisecond component with 3.4 ± 0.8% of the amplitude, representing destroyed FA/FB centers (Fig. 7). The same pattern was observed upon illumination of leaves infiltrated with KCN, but the damage was more pronounced (Fig. 7). The 4-h light sample was decreased to 70 ± 2% in total amplitude, showed a 5-μs component with 16 ± 1% amplitude, and a millisecond decay with 3.0 ± 0.4% amplitude.
Figure 7.
Flash-induced absorption changes of P700 in thylakoid membranes isolated from barley leaves infiltrated with 4 mm KCN and subjected to illumination (100 μmol photons m−2 s−1) at 4°C for 4 h. ΔA834 was resolved into decay components with different time constants. Error bars indicate sds for the total absorption amplitudes (n = 4–8). For further explanation, see legend to Figure 3.
Independently of the presence or absence of KCN, the control samples showed no microsecond decay component (Fig. 7), documenting that neither the isolation procedure nor the presence of detergent resulted in the generation of free chlorophyll molecules. Upon excitation by the laser flashes, detached chlorophyll molecules would have decayed via chlorophyll triplets with lifetimes in the microsecond range. However, it can be argued that the light treatment could detach pigments and that the 5-μs component observed after light stress would have a contribution originating from such a pool of free chlorophyll. The P700 reaction center chlorophyll receives light energy from 200 to 300 antenna chlorophyll molecules, whereas a “free” chlorophyll molecule serves only itself. Thus, absorption decay events originating from the reaction center of PSI should have a much lower light saturation level than decay reactions taking place in a free pigment pool.
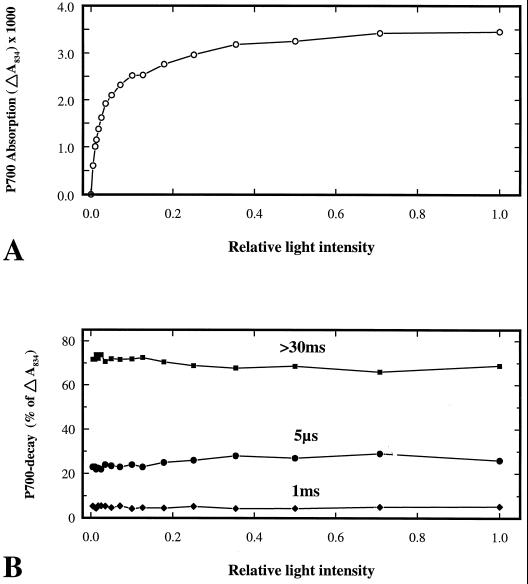
To substantiate that the observed microsecond decay does indeed originate from the reaction center and not from free antenna chlorophyll molecules, a light saturation experiment regarding P700 absorption decay was performed using a sample treated with 4 h of light and 4 mm KCN. Such thylakoids (Fig. 7) showed a distinct damage that is also easily resolved from the noise level at low excitation light intensities. The excitation laser flash was initially set at saturating intensity to ensure quantitative excitation of all P700, and the intensity was then successively lowered with the use of gray filters. At each light intensity the amplitude of P700 absorption change was monitored (Fig. 8A) and resolved into constituting decay components (Fig. 8B). It is evident from this experiment that the relative contribution from the different decay components to the total absorption amplitude is stable throughout the saturation curve. When the P700 absorption change increases, the amplitudes of the decay components increase to the same degree. Therefore, the contribution to ΔA834 from free pigment molecules is concluded to be negligible.
Figure 8.
Light-saturation analysis of P700 decay components. Isolated thylakoid membranes from 4 mm KCN-infiltrated leaves illuminated for 4 h, as specified in Figure 7, were subjected to analysis of P700 absorption changes at different excitation light intensities from 0 to the saturation level. A, Total signal amplitude. B, The amplitude resolved into relative contribution of three charge recombination components of >30 ms, 1 ms, and 5 μs. For an explanation of the charge recombination times, see legend to Figure 3.
DISCUSSION
Low-light illumination of barley leaves at chilling temperatures induced a clear damage of PSI electron transport, quantitatively similar to that of PSII. The PSI damage appears to stabilize at a degree of inhibition of approximately 15 to 20% (Fig. 1). This indicates that, after a first damaging hit to PSI, a level is subsequently reached where protection and potential repair mechanisms keep the inhibition at a relatively constant level. Contrary to this stabilizing damage to PSI, the damage to PSII continues. In the context of PSI inhibition, the progressing damage to PSII in the present study can be viewed as a mechanism that serves to protect PSI from further inhibition.
The combination of low temperature and low light used in this study is typical of conditions experienced in the spring and autumn in temperate climates such as in Nordic countries, where barley is an important crop. Thus, it is likely that the observed photodamage of PSI would also take place under field conditions and lower the overall photosynthetic productivity of the plants.
The light-induced inactivation of PSI was relatively small when assayed as oxygen consumption using MV as an electron acceptor. The site at which MV accepts electrons may vary from one electron acceptor to the other, depending on the concentration used (Sonoike and Terashima, 1994). With a concentration of 167 μm, as used in the present study, MV would serve as an efficient electron acceptor only from the iron-sulfur centers. A more clear-cut and diagnostic measure of PSI damage was obtained by monitoring the flash-induced P700 absorption changes. These measurements provide quantification of the activity decrease as well as specific identification of the lesion sites (Figs. 2, A and B, and 3).
The damage sequence includes: (a) a small damage to the terminal acceptors FA/FB represented by the millisecond decay, (b) a larger fraction showing additional destruction of FX that leads to the microsecond decay, and (c) a fraction in which detectable absorption by P700 is completely lost because of direct destruction of P700 or due to a very rapid back-reaction from A0 as a result of damaged A1. This sequential damage corroborates the results of Sonoike and Terashima (1994), who concluded that lesions are primarily localized to FX or A1 in photoinhibited cucumber leaves. This was evidenced by a close correlation between inactivation of NADP+ reduction and loss of P700 absorption with MV as the electron acceptor. In our studies the small contribution to the total damage from the millisecond decay phase compared with a large contribution from the microsecond decay phase indicates that once FA/FB is damaged Fx becomes unstable and quickly deteriorates. Alternatively, FX could be the initial target. However, since damage appears to involve active oxygen, we find it more likely that FA or FB is the initial target, because they are more exposed. FB is known to be particularly sensitive to chemical inactivation (Golbeck and Warden, 1982; Malkin, 1984; Sakurai et al., 1991).
The PSI photodamage detected by activity measurements correlates well with an actual decrease in the PSI-A/B reaction center protein content. The concurrent appearance of a new polypeptide migrating slightly faster than PSI-A/B and of several smaller polypeptides below 20 kD possibly represents modified and/or degraded PSI-A/B. Terashima et al. (1994) documented selective photoinhibition of PSI in cucumber leaves under conditions similar to those used in the present study (for review, see Sonoike, 1996b). Immunoblots using site-specific, peptide-derived antibodies (Sonoike, 1996a) revealed selective degradation of PSI-B in cucumber. In our study only 22% of the total PSI-A/B content was retained in the most severely inhibited sample, demonstrating that PSI-B as well as PSI-A were degraded. Additional experiments will be necessary to determine the individual fates of the PSI-A and PSI-B subunits during photoinhibition of barley.
In leaves illuminated in the absence of KCN, the remaining amount of undegraded reaction center proteins was slightly higher than the remaining electron transport activity, as measured both by oxygraph and by flash photolysis. In the KCN-infiltrated leaves, there was no significant difference between the remaining protein and activity levels. Thus, the data suggest that protein degradation is not the primary effect of photodamage in PSI but follows rapidly the initial damage to the electron transport cofactors. A key topic of future experiments will be whether the observed primary inhibition of the PSI electron acceptors necessitates a subsequent degradation and resynthesis of the PSI-A/B proteins in a fashion similar to the D1 protein after photoinhibition of PSII.
In thylakoid membranes, PSI is sensitive to photoinhibition even at normal room temperature. This suggests that PSI itself is not resistant to light damage, but effective protection mechanisms reside within the chloroplast that are able to counteract the active oxygen species. The higher resistance to photoinhibition at low temperatures seen in barley compared with cucumber may be due to either a higher content of oxygen-scavenging enzymes (e.g. superoxide dismutase, ascorbate peroxidases, glutathione reductases, and peroxidases) or a higher endurance of these enzymes at cold temperatures. The enzymes may be present constitutively at high levels in barley or may be induced by the cold-light treatment to circumvent or minimize radiation damage.
Acclimation of spinach and Scots pine to cold (Schöner et al., 1990; Krivosheeva et al., 1996) and of potato and wheat to high-light stress (Martinez and Maestri, 1995; Mishra et al., 1995) induces higher contents of oxygen-scavenging enzymes. The barley plants used in the present study were nonhardened and grown in low light. Therefore, it is possible that acclimated barley under natural field conditions may display higher resistance of PSI to photoinhibition. In the present study treatment of the barley leaves with KCN, which inhibits Cu,Zn-superoxide dismutase and ascorbate peroxidase (Halliwell and Gutteridge, 1989), resulted in dramatically increased photodamage to PSI. This demonstrates the importance of these detoxifying enzymes for the photoinhibitory protection. The CN-mediated pattern of photoinactivation closely followed that seen without the addition of CN but was more pronounced and thus more easily studied.
The inactivation of both photosystems seemed to level out after prolonged illumination. This can be explained in terms of an interplay between damaging and protective mechanisms. An initial damage to PSI may subsequently cause acceptor-side-induced photodamage to PSII and degradation of the D1 protein. The progressive damage to PSII reduces its function as an electron donor and may thereby protect PSI by increasing thermal dissipation of excess energy by P700+. Under normal conditions, degraded D1 is rapidly replaced by de novo synthesis (Aro et al., 1993). A similar repair of PSI would appear to be much slower because of its lower rate of turnover. However, under chilling conditions, proteolysis and de novo protein synthesis are slowed down, leading to an accumulation of damaged PSI and PSII reaction centers that may then act as energy sinks. Low-temperature-induced photoinhibition has also been shown to produce photochemically down-regulated PSII reaction centers that are able to convert excitation energy to heat, thus protecting other reaction centers from further photodamage (Aro et al., 1990; Tyystjärvi and Aro, 1990; Ottander et al., 1993; Krause, 1994; Schnettger et al., 1994). Altogether, this complex regulatory scheme acts to sequentially lower the photoinhibitory effects on both photosystems. As a further shield against photoinhibition, new protective proteins may be synthesized during the cold treatment.
In experiments in which KCN was present, the dark control showed a slightly decreased PSI activity. Most probably this reflects a direct damage to P700 induced by nonspecific active oxygen attack. Havaux and Davaud (1994) reported an increased photodamage of PSI in potato leaves infiltrated with 100 mm DDC, which is known to inhibit Cu,Zn-superoxide dismutase. In barley, experiments with 100 mm DDC caused severe damage of the dark controls (data not shown). When the DDC concentration was reduced to 25 mm, results similar to those with 10 mm KCN were obtained, although the damage of the dark control samples was increased (data not shown). The damage induced by inhibiting superoxide dismutase and ascorbate peroxidase would be expected to be found in PSI, where superoxide is mainly produced. During light treatment using KCN, however, PSII followed closely the photoinactivation curve of PSI. This could mean that the increased production of superoxide and other active oxygen species from PSI also migrate and severely damage PSII.
The results obtained show that photoinhibition of PSI may be expected to cause a loss in biomass yields, especially in winter cereals grown in cold climates and in other species grown in climates with fluctuating temperatures that touch their lower temperature-endurance limits. In the long term, detailed knowledge of the molecular mechanisms behind this photodamage may be a tool to transfer light-stress tolerance traits between plant species or varieties using genetic modification.
ACKNOWLEDGMENTS
We thank Prof. Bertil Andersson and Harry Teicher for valuable discussions.
Abbreviations:
- A0 and A1
primary and secondary electron acceptors of PSI
- DCIP
dichlorophenol indophenol
- DDC
diethyl dithiocarbamate
- FA
FB and FX, [4Fe-4S] iron-sulfur clusters of the PSI electron acceptor chain
- MV
methyl viologen
Footnotes
This work was financed by a grant to S.E.T. from the Energy Research Program of the Nordic Council of Ministers, and by the Danish Center for Plant Biotechnology.
LITERATURE CITED
- Anderson JM, Andersson B. The dynamic photosynthetic membrane and regulation of solar energy conversion. Trends Biochem Sci. 1988;13:351–355. doi: 10.1016/0968-0004(88)90106-5. [DOI] [PubMed] [Google Scholar]
- Andersson B, Styring S. Photosystem II: molecular organisation, function, and acclimation. Curr Top Bioenerg. 1991;16:1–81. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–14. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro EM, Hundal T, Carlberg I, Andersson B. In vitro studies on light-induced inhibition of photosystem II and D1-protein degradation at low temperatures. Biochim Biophys Acta. 1990;1019:269–275. [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Asada K. Production and action of active oxygen species in photosynthetic tissues. In: Foyer CF, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defence Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 77–104. [Google Scholar]
- Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ, editors. Photoinhibition. Amsterdam, The Netherlands: Elsevier Science Publishers; 1987. pp. 227–287. [Google Scholar]
- Barber J, Andersson B. Too much of a good thing can be bad for photosynthesis. Trends Biochem Sci. 1992;17:61–66. doi: 10.1016/0968-0004(92)90503-2. [DOI] [PubMed] [Google Scholar]
- Casano LM, Gómez LD, Lascano HR, González CA, Trippi VS. Inactivation and degradation of CuZn-SOD by active oxygen species in wheat chloroplasts exposed to photooxidative stress. Plant Cell Physiol. 1997;38:433–440. doi: 10.1093/oxfordjournals.pcp.a029186. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- Fling SP, Gregerson DS. Peptide and protein molecular weight determination by electrophoresis using a high molarity Tris buffer system without urea. Anal Biochem. 1986;155:83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Golbeck JH. Structure, function and organization of the photosystem I reaction center complex. Biochim Biophys Acta. 1987;895:167–204. doi: 10.1016/s0304-4173(87)80002-2. [DOI] [PubMed] [Google Scholar]
- Golbeck JH, Bryant DA. Photosystem I. Curr Top Bioenerg. 1991;16:3–177. [Google Scholar]
- Golbeck JH, Warden JT. Electron spin resonance studies of the bound iron-sulfur centers in photosystem I. Photoreduction of center A occurs in the absence of center B. Biochim Biophys Acta. 1982;681:77–84. doi: 10.1016/0005-2728(87)90223-4. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, Ed 2. Oxford, UK: Clarendon Press; 1989. [Google Scholar]
- Havaux M, Davaud A. Photoinhibition of photosynthesis in chilled potato leaves is not correlated with a loss of photosystem II activity. Preferential inactivation of photosystem I. Photosynth Res. 1994;40:75–92. doi: 10.1007/BF00019047. [DOI] [PubMed] [Google Scholar]
- Havaux M, Eyletters M. Is the in vivo photosystem I function resistant to photoinhibition? An answer from photo-acoustic and far-red absorbance measurements in intact leaves. Z Naturforsch. 1991;46c:1038–1044. [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Plant Physiol. 1994;106:415–420. doi: 10.1104/pp.106.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Fujii Y, Yokoyama E, Matsuura K, Hiyama K, Sakurai H. The photoinhibition site of photosystem I in isolated chloroplasts under extremely reducing conditions. Plant Cell Physiol. 1989;30:65–71. [Google Scholar]
- Inoue K, Sakurai H, Hiyama T. Photoinactivation of photosystem I in isolated chloroplasts. Plant Cell Physiol. 1986;27:961–968. [Google Scholar]
- Kannangara CG, Gough SP, Hansen B, Rasmussen JN, Simpson DJ. A homogenizer with replaceable razor blades for bulk isolation of active barley plastids. Carlsberg Res Commun. 1977;42:431–439. [Google Scholar]
- Krause GH (1994) Photoinhibition induced by low temperatures. In NR Baker, JR Bowyer, eds, Photoinhibition of Photosynthesis, From Molecular Mechanisms to the Field. BIOS Scientific Publishers, Oxford, UK, pp 331–348
- Krivosheeva A, Tao D-L, Ottander C, Wingsle G, Sylvain LD, Öquist G. Cold acclimation and photoinhibition of photosynthesis in Scots pine. Planta. 1996;200:296–305. [Google Scholar]
- Malkin R. Diazonium modification of Photosystem I. A specific effect on iron-sulfur center B. Biochim Biophys Acta. 1984;764:63–69. [Google Scholar]
- Martinez CA, Maestri M. Photoinhibition of photosynthesis in Andean potato (Solanum spp.) species differing in frost resistance. In: Mathis P, editor. Photosynthesis: From Light to Biosphere, Vol 4. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 179–182. [Google Scholar]
- Mishra NP, Fatma T, Singhal GS. Development of antioxidative defence system of wheat seedlings in response to high light. Physiol Plant. 1995;95:77–82. [Google Scholar]
- Miyake C, Asada K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 1992;33:541–553. [Google Scholar]
- Naver H, Scott MP, Golbeck JH, Møller BL, Scheller HV. Reconstitution of barley photosystem I with modified PSI-C allows identification of domains interacting with PSI-D and PSI-A/B. J Biol Chem. 1996;271:8996–9001. doi: 10.1074/jbc.271.15.8996. [DOI] [PubMed] [Google Scholar]
- Nuijs AM, Shuvalov A, van Gorkom HJ, Plijter JJ, Duysens LNM. Picosecond absorbance difference spectroscopy on the primary reactions and the antenna-excited states in photosystem I particles. Biochim Biophys Acta. 1986;850:310–318. [Google Scholar]
- Ogawa K, Kanematsu S, Takabe K, Asada K. Attachment of CuZn-superoxide dismutase to thylakoid membranes at the site of superoxide generation (PSI) in spinach chloroplasts: detection by immuno-gold labelling after rapid freezing and substitution method. Plant Cell Physiol. 1995;36:565–573. [Google Scholar]
- Ögren E, Rosenqvist E. On the significance of photoinhibition of photosynthesis in the field and its generality among species. Photosynth Res. 1992;33:63–71. doi: 10.1007/BF00032983. [DOI] [PubMed] [Google Scholar]
- Ottander C, Hundal T, Andersson B, Huner NPA, Öquist G. Photosystem II reaction centres stay intact during low temperature photoinhibition. Photosynth Res. 1993;35:191–200. doi: 10.1007/BF00014750. [DOI] [PubMed] [Google Scholar]
- Powles SB. Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol. 1984;35:15–44. [Google Scholar]
- Prasil O, Adir N, Ohad I. Dynamics of photosystem II: mechanism of photoinhibition and recovery process. Top Photosynth. 1992;11:295–348. [Google Scholar]
- Press WH, Flannery BP, Teukolsky SA, Vetterling W. Numerical Recipes in Pascal. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- Sakurai H, Inoue K, Fujii T, Mathis P. Effects of selective destruction of iron-sulfur center B on electron transfer and charge recombination in Photosystem I. Photosynth Res. 1991;27:65–71. doi: 10.1007/BF00029977. [DOI] [PubMed] [Google Scholar]
- Satoh K. Mechanism of photoinactivation in photosynthetic systems I. The dark reaction in photoinactivation. Plant Cell Physiol. 1970a;11:15–27. [Google Scholar]
- Satoh K. Mechanism of photoinactivation in photosynthetic systems II. The occurrence and properties of two different types of photoinactivation. Plant Cell Physiol. 1970b;11:29–38. [Google Scholar]
- Schnettger B, Critchley C, Santore UJ, Graf M, Krause GH. Relationship between photoinhibition of photosynthesis, D1 protein turnover and chloroplast structure: effects of protein synthesis inhibitors. Plant Cell Environ. 1994;17:55–64. [Google Scholar]
- Schöner S, Foyer C, Lelandais M, Krause HK. Increase in activities of scavengers for active oxygen in spinach related to cold acclimation in excess light. In: Baltscheffsky M, editor. Current Research in Photosynthesis, Vol 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 483–486. [Google Scholar]
- Shuvalov VA, Nuijs AM, van Gorkom HJ, Smit HWJ, Duysens LNM. Picosecond absorbance changes upon selective excitation of the primary electron donor P-700 in photosystem I. Biochim Biophys Acta. 1986;850:319–323. [Google Scholar]
- Sonoike K. Selective photoinhibition of photosystem I in isolated thylakoid membranes from cucumber and spinach. Plant Cell Physiol. 1995;36:825–830. [Google Scholar]
- Sonoike K. Degradation of PsaB gene product, the reaction center subunit of photosystem I, is caused during photoinhibition of photosystem I: possible involvement of active oxygen species. Plant Sci. 1996a;115:157–164. [Google Scholar]
- Sonoike K. Photoinhibition of photosystem I: its physiological significance in the chilling sensitivity of plants. Plant Cell Physiol. 1996b;37:239–247. [Google Scholar]
- Sonoike K, Terashima I. Mechanism of photosystem-I photoinhibition in leaves of Cucumis sativus L. Planta. 1994;194:287–293. [Google Scholar]
- Sonoike K, Terashima I, Iwaki M, Itoh S. Destruction of photosystem I iron-sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett. 1995;362:235–238. doi: 10.1016/0014-5793(95)00254-7. [DOI] [PubMed] [Google Scholar]
- Terashima I, Funayama S, Sonoike K. The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II. Planta. 1994;193:300–306. [Google Scholar]
- Tjus SE (1995) Photosystem I, Organisational and Functional Aspects. PhD thesis. Stockholm University, Sweden
- Tjus SE, Andersson B. Loss of the trans-thylakoid proton gradient is an early event during photoinhibitory illumination of chloroplast preparations. Biochim Biophys Acta. 1993;1183:315–322. [Google Scholar]
- Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitro-cellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyystjärvi E, Aro EM. Temperature-dependent changes in photosystem II heterogeneity support a cycle of photosystem II during photoinhibition. Photosynth Res. 1990;26:109–117. doi: 10.1007/BF00047082. [DOI] [PubMed] [Google Scholar]