Abstract
The obese Zucker rat carries two recessive fa alleles that result in the expression of an obese phenotype. Obese Zuckers have higher food intake than lean controls in free-feed studies in which rats have ready access to a large amount of one type of food. The present study examined differences in obese and lean Zucker rats using concurrent schedules of reinforcement, which more ecologically models food selection using two food choices that have limited, but generally predictable, availability. Lever-pressing of ten lean (Fa/Fa or Fa/fa) and ten obese (fa/fa) Zucker rats was placed under three concurrent variable interval variable interval (conc VI VI) schedules of sucrose and carrot reinforcement, in which the reinforcer ratios for 45-mg food pellets were 5:1, 1:1, and 1:5. Allocation of responses to the two food alternatives was characterized using the generalized matching equation, which allows sensitivity to reinforcer rates (a) and bias toward one alternative (log k) to be quantified. All rats showed a bias to sucrose, though there were no differences between lean and obese Zucker rats. In addition, obese Zucker rats exhibited higher sensitivity to reinforcement rates than lean rats. This efficient pattern of responding was related to overall higher deliveries of food pellets. Effective matching for food, then, may be another behavioral pattern that contributes to an obese phenotype.
Keywords: concurrent schedules of reinforcement, generalized matching, obesity, palatability, sensitivity, Zucker rats (fa/fa)
1.1 Obese Zucker rats and Free Food Intake
The obese Zucker rat, which has impaired leptin signaling, is a genetic model of obesity that has historically been used to determine behavioral and physiological mechanisms that contribute to obesity-related health problems (see [1, 2] for reviews). However, much of the behavioral research on the Zucker strain is based on free food intake studies, in which obese Zuckers are found to have significantly higher caloric intake than their lean counterparts (e.g., [3–6]. In free food intake studies, a large amount of food is readily available with minimal effort required to gain access to it, and because obese Zuckers have a higher free-food intake, it is concluded that food is more rewarding to them.
When effort is required to procure food, however, the differences between lean and obese rats in food consumption patterns becomes smaller. Rasmussen and Huskinson [7], for example, placed lever-pressing for sucrose under a progressive ratio schedule of reinforcement, a well-established measure of food reinforcement [8, 9]. The differences in breakpoints (the point at which lever-pressing ceases) between lean and obese rats was not statistically significant, thus providing no evidence of a difference in the value of sucrose between the two groups. In addition, larger differences in food consumption are found between lean and obese Zucker rats when the effort to obtain food is small (e.g., 1–50 lever-presses), but when the effort is larger (90–300 lever-presses), the differences become negligible [10, 11]. Therefore, the large differences in food consumption observed between lean and obese Zucker rats may be limited to the free-feed environment.
Because small changes in the environmental arrangement of food leads to strong differences in food consumption patterns, an argument can be made for expanding the study of food intake beyond the free-feeding environment. For example, the use of a procedure that models choice, in which two or more food options vary in certain properties such as quality and availability, may extend our understanding of food consumption differences between lean and obese rats.
1.2 Concurrent Schedules as a Model of Choice
A paradigm that more ecologically models environmental features of food procurement in the natural world is the concurrent schedule of reinforcement, a well-established model of choice (see [12–15] for reviews). Typically, reinforcers are made available for responding on two levers, and are delivered under independent variable interval (VI) schedules. In a single VI schedule, a reinforcer is delivered following the first response after a variable interval of time passes. A VI 12-s schedule, for example, delivers reinforcers after the first response is made after an average of 12-s elapses. Thus, it is possible to earn five reinforcers per minute at an overall predictable rate, but it is not predictable from moment to moment when those deliveries will occur.
Allocation of behavior to the two VI schedules is a measure of choice. Humans and non-humans have shown predictable patterns of responding under concurrent VI VI (conc VI VI) schedules, such that the allocation of their responses to each alternative is a linear function of the ratio of reinforcers earned on each side. This relationship is illustrated by Equation 1, known as the generalized matching equation [16]:
in which B1 and B2 are the total number of responses to two alternatives (e.g., a left lever and a right lever, respectively), r1 and r2 are the total number of reinforcers earned on each respective alternative, and a and log k are free parameters that represent the slope and intercept of the line, respectively. This equation suggests that the log ratio of responses to the left lever (with respect to the number of responses made on the right lever) will equal, or “match” the log ratio of reinforcers earned on the left lever (with respect to the number of reinforcers earned on the right lever).
The free parameters, a and log k, are of interest in examining deviations from matching. The parameter, a, or slope of the matching line, refers to sensitivity of behavior (e.g., responses) to the differing reinforcer densities. A value of 1 means “perfect” matching, meaning that the organism is allocating behavior solely based on relative reinforcer rates. However, a pattern of responding that is more commonly found across species responding under conc VI VI schedules is “undermatching,” and results in a value of a < 1. This means that allocation of responses to the two food alternatives is less sensitive to richer sources of reinforcement. For example, if food is delivered at a ratio of 1:5, an organism that is less sensitive to the richer source of reinforcement will allocate its responses such that fewer responses are made to the richer alternative (the right lever in this case), resulting in a response ratio such as 1:4 or 1:3. A value of a > 1 represents “overmatching,” which suggests hypersensitivity to differences in reinforcer rates and results in more responses allocated to the richer alternative than would be predicted by perfect matching.
The log k parameter represents bias toward one alternative. When log k = 0, no bias is evident. A log k value > 0 suggests a bias toward the alternative represented in the numerator, here, the left alternative, and < 0 suggests a bias toward the right alternative. Baum [16] asserted that bias can result from previous experience with one lever more than the other, or when one side offers a different amount or quality of reinforcers, such as when the reinforcers differ in palatability. One study [17], for example, assessed preference for hemp, buckwheat, and wheat in varying pairs using the bias parameter and found that buckwheat was preferred over hemp, wheat was preferred over buckwheat, and predicted wheat would be preferred over hemp. Another study by Matthews and Temple (1979) also found, using the bias parameter, that dairy cows had a small preference for hay over dairy meal, though others had a small preference for dairy meal [18].
1.3 The Present Study
Sensitivities to amount of food or preference for different kinds of food (e.g., those high in fat or sugar content) may be behavioral patterns involved in obesity; therefore, it is important to understand these patterns in food selection. There have been no studies published (to our knowledge) in which obese Zucker rats respond for food in a choice procedure using concurrent schedules. In the present study, we exposed lean and obese Zuckers to three different concurrent schedules that differed in terms of programmed reinforcer ratios. Rats chose between allocating responses to two levers that differed only in relative amount of reinforcers delivered from each lever (Phase 1). Then (in Phase 2) a second pellet type was introduced on one of the levers that had a differing percentage of sucrose content, such that features of palatability could be examined in the context of choice. We hypothesized that all rats would display a bias toward the higher sucrose alternative, though obese Zuckers may have a stronger bias toward sucrose. We were also interested in whether lean and obese Zuckers would show differences in sensitivity to relative rates (amount) of reinforcement.
2.1 Materials and Method
2.1.1 Subjects
Twenty experimentally naïve male Zucker rats (lean, n = 10; obese, n = 10) were acquired from a commercial breeder (Harlan; Livermore, CA) at approximately 21 days of age. They were housed in individual cages in a climate-controlled room, with a 12-h light/dark cycle (light beginning at 6:00 a.m.). Rats were given ad libitum access to standard rat chow and water until they were 10 weeks old, at which time they began lever-press training for operant sessions. At this time, lean rats weighed between 245–286 g and obese weighed 320–390 g. After this point, access to food was restricted to a daily 2-hr free-feeding session, immediately following experimental sessions. This food restriction procedure has been shown to allow food to function as a reinforcer, but also keep deprivation levels similar between lean and obese rats while allowing minimal weight gain [10, 11].
2.1.2 Apparatus
Seven Coulbourn ® Habitest standard rat operant chambers were used for training and experimental sessions. Each chamber was placed inside a sound-attenuating cubicle, and contained a fan for air circulation and a speaker which provided white noise to reduce extra-chamber noise. Each chamber contained two levers, one on each side of a feeding trough, with a cue light above each lever. The depression of each lever controlled one of two pellet feeders on the outside of the chamber, which delivered either a single 45-mg sucrose (3.4 kcal/g) or carrot-flavored (3.4 kcal/g) pellet (TestDiet®). Pellets were redirected from either side of the chamber to the feeding trough via a “Y” shaped tube. Following completion of the response criteria (as specified by the schedule), a light in the feeder area as well as a house light located 13 cm above the food dispenser was illuminated for 3 seconds during delivery of the pellet. During this interval, lever-presses had no programmed consequences, though the individual VI timers continued to run. A Windows-based computer with Graphic State® software controlled reinforcer contingencies and collected data. All sessions were conducted in the morning at the same time (±15 min) from Monday to Friday.
2.1.3 Procedure
Training
Rats were randomly assigned to train to lever-press with one type of pellet (sucrose or carrot-flavored) available from both levers (SUC/SUC or CAR/CAR). These pellet types were chosen as they were calorically similar but different in terms of palatability from the chow that was used during extra-experimental feedings. A pilot experiment determined that carrot flavored pellets were preferred less than chow flavored pellets, which were preferred less than sucrose pellets.
Pellet type was counterbalanced within groups. Each rat was trained to lever-press by placing a conc FR1 FR1 schedule in effect for a one-hour session. Here, a single lever-press on either lever resulted in a pellet delivery. A rat was considered lever-press trained to both levers if more than 70 reinforcers were earned on each lever in a session. If a rat had emitted more than 70 responses on one lever, but not the other during any session, it was considered trained for that lever. On subsequent sessions, the presses on the trained lever were placed on extinction (no programmed consequences) and only the other lever was programmed for food reinforcement under an FR1 schedule until the rat emitted more than 70 responses on that lever. If no levers had been trained within five sessions, handshaping was used to train responding to both levers. When a rat had been trained to press both levers consistently, experimental sessions began.
Phase 1: Conc VI VI schedules with Same Reinforcers (CAR/CAR or SUC/SUC)
After all rats responded reliably to both levers on FR1 schedules, conc VI VI schedules were arranged with the same pellet type that was used for training (SUC/SUC or CAR/CAR) on both levers. Pellets were programmed on two levers (i.e., left vs. right) such that each VI timer for each reinforcer ran independently of the other. The concurrent VI VI schedules (and programmed ratios of reinforcement) that were used were conc VI 20-s VI 20-s (1:1), conc VI 12-s VI 60-s (5:1) and conc VI 60-s VI 12-s (1:5). For example, under the conc VI 20-s VI 20-s schedule, for each alternative (i.e., lever), the first lever-press after an average of 20-s had passed produced a single pellet, though the moment-to-moment deliveries were unpredictable. These VI schedules were chosen so that an average of six reinforcers per minute may be earned on each schedule. We subsequently will refer to these schedules by their programmed ratios of reinforcement. A changeover delay (COD) of 3-s was implemented in each conc VI VI schedule, so that switching from one lever to the other would not be adventitiously reinforced [19, 20]. Sessions lasted for 30-min. VI schedule values were determined by constant probability distributions using the Fleshler and Hoffman [21] progression, resulting in 20 intervals for each VI schedule.
Each concurrent schedule was implemented until stability was observed, which was defined as the last three consecutive sessions in which response ratios for both levers did not show an increasing or decreasing trend and did not vary by more than 10% from the mean of the last 3 sessions. Also, total responses could not show an increasing or decreasing trend. All rats experienced the 1:1 ratio schedule first, but the order of the schedules with 5:1 and 1:5 ratios was counterbalanced across groups and pellet types.
Phase 2: Concurrent Schedules with Different Reinforcers (SUC/CAR or CAR/SUC)
After Phase 1 was completed, the second type of food pellet was introduced into the left or right feeder, such that two different reinforcers were available (SUC/CAR)—one from each lever. Location of the pellet (left vs. right lever) was counterbalanced across groups. Concurrent sessions were run in a manner that was identical to Phase 1, except the types of pellets from which to choose were different. All procedures were approved by the Idaho State University Institutional Animal Care and Use Committee.
2.1.4 Data Analysis
The total number of lever-press responses and reinforcers obtained from each lever for each pellet alternative in Phase 1 (SUC/SUC or CAR/CAR) and Phase 2 (SUC/CAR or CAR/SUC) was recorded. Data from the last three stable sessions of each concurrent schedule were used in the analysis.
The data from Phase 1 provided a baseline for how each rat allocated its behavior when each of the two concurrent VI schedules provided the same type of pellet. Responses and reinforcers earned on the left side were recorded as B1 and r1, respectively, and responses and reinforcers earned on the right side were recorded as B2 and r2, respectively. The ratios of responses on B1 to the responses on B2 were calculated, log-transformed, and plotted against the log ratio of reinforcers obtained (r1/r2). Data were fit to Equation 1 using linear regression. The slope of the resulting lines of fit represented a, the measure of response sensitivity, and the intercept represented log k, the measure of bias, from the generalized matching equation. Here, a positive bias value would represent a bias toward the left alternative; a negative value would represent a bias toward the right alternative.
The data from Phase 2 represent matching when two types of food pellets that differ in terms of sucrose content (i.e., palatability) were available. In Phase 2, though the position of SUC and CAR was counterbalanced between left and right levers, responses made on the SUC lever and total number of SUC pellets earned are presented here as B1 and r1, respectively, and responses made on the CAR lever and total number of CAR pellets earned were recorded as B2 and r2, respectively, for ease of interpretation. In this case, a positive bias would represent a bias toward the SUC lever, and a negative bias would represent a bias toward the CAR lever.
We determined bias parameters from Phase 2 by subtracting the bias value from Phase 1. For example, if a rat has a bias of 0.03 in Phase 1, this would represent a slight baseline position bias toward the left lever. If in Phase 2, a bias of 0.18 was found, this would represent a sucrose bias of 0.15 (0.18–0.03 = 0.15). Positive values represent bias toward SUC and negative values represent a bias toward CAR.
In Phase 1, bias and sensitivity values were compared between lean rats and obese rats and compared using a 2-way ANOVA with phenotype (obese vs. lean) and pellet type (SUC/SUC vs. CAR/CAR) as factors. It was hypothesized that bias values for all animals would be near 0 and sensitivity values near or < 1 for baseline conditions. We were interested in whether obese rats would show differences in the sensitivity of their behavior to the differing schedules of reinforcement, but we were uncertain in what manner their sensitivity parameters would differ.
Responses and reinforcers per session were analyzed in two ways. First, the total responses and reinforcers made under each concurrent schedule (left + right) were compared to determine whether there were differences in behavior across the three concurrent schedules. In this case, the total numbers of responses (and obtained reinforcers) for the last three stable sessions under each of the three concurrent schedules were averaged, resulting in a single datum for each rat representing responses and reinforcers for each concurrent schedule. These data were analyzed using a three-way ANOVA with phenotype and pellet type as between-subjects factors, and concurrent schedule as a within-subjects factor. Second, responses and reinforcers on each lever (left vs. right) were analyzed and compared. For example, means were calculated for each rat for responses from the last three stable sessions made to the VI 12-s lever (left) and the VI 60-s lever (right) of the conc VI 12-s VI 60-s (5:1) schedule. This was repeated for all three schedules to determine differences in behavior in schedule component. These data were analyzed using a three-way ANOVA with phenotype, VI component (VI 12-s vs. VI 20-s vs. VI 60-s), and position (left vs. right) as factors. For all ANOVAs, Mauchly's W was observed to check for the assumption of sphericity. When applicable, Greenhouse-Geisser corrections for degrees of freedom was applied if epsilon was less than 0.75, and the Huynh-Feldt correction was applied if epsilon was greater than 0.75.
3.1 Results
Note: One rat (L29) completed Phase 1 after completing Phase 2, but there were no systematic differences in his data compared to other rats' data. Phase 1 data were analyzed with and without his data, but the results were unchanged. Therefore, the data reported for Phase 1 includes all rats (lean n=10, obese n=10). There were no group differences in the number of sessions required for stability across any concurrent schedule.
3.1.1 Phase 1. SUC/SUC and CAR/CAR
Generalized matching
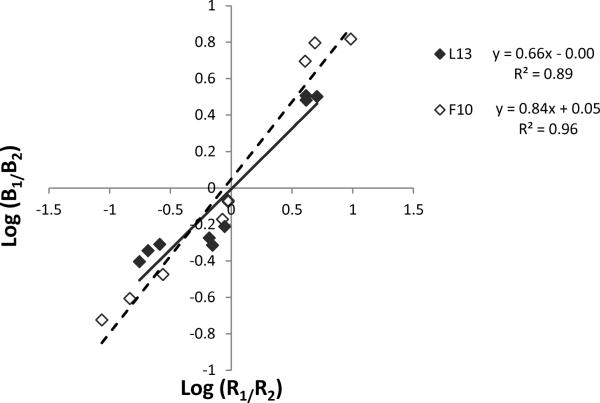
Figure 1 is an illustrative graph that shows generalized matching data from two rats whose data each represent the means of the groups. One lean and one obese rat were selected from the CAR/CAR conditions (rats from the SUC/SUC group are not shown, as they did not have significantly different bias or sensitivity parameters). Of these two rats, both undermatched, illustrated as a slope of less than 1 (sensitivity for the lean and obese rat were 0.66 and 0.84, respectively). Both also had bias values around 0, illustrated as a y-intercept near the origin (bias for the lean and obese rat were 0.00 and 0.05, respectively). In addition, the R2 goodness of fit values of the model were greater than 0.89. Parameters from the matching equation for all rats are described and compared in subsequent figures.
Figure 1.
Two illustrative matching lines from Phase 1. Regression lines were fit using individual data from two rats-- one lean (closed symbols, dashed line) and one obese (open symbols) from the CAR/CAR condition. Fitted lines to the matching equation are also shown: The generalized matching equations for each respective rat are shown to the right, along with R2 values.
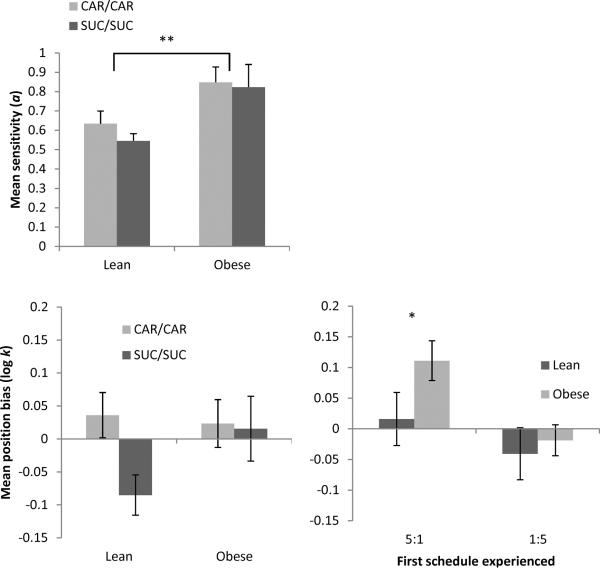
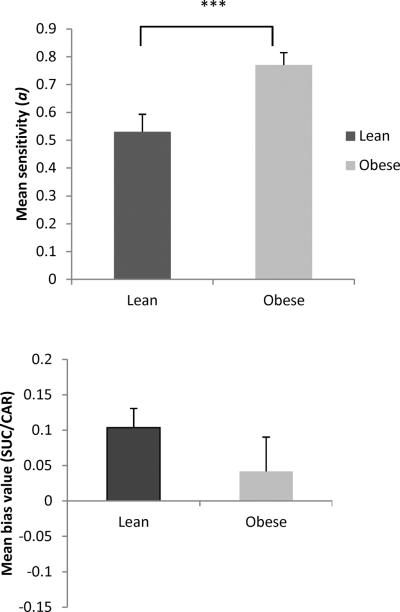
The top of Figure 2 shows that obese Zuckers exhibited higher sensitivity (a) values than leans [F(1,16) = 9.43, p = 0.007, ηp2 = 0.37], and there were no differences between SUC/SUC and CAR/CAR conditions. Bias values (bottom left) ranged from −0.12 to 0.17 for lean rats, and from −0.11 to 0.16 for obese rats. There were no main effects of phenotype (p = 0.27) or pellet type (p = 0.12) on bias values, or an interaction.
Figure 2.
Mean sensitivity (top) and bias (bottom) parameter values for lean and obese Zuckers in the CAR/CAR (gray) and SUC/SUC pellet (black) condition in Phase 1. Bottom right shows mean bias values as a function of which schedule with an uneven ratio was experienced first (5:1 or 1:5 ratio). Lean rats are shown in black; obese in gray. Error bars = 1 SEM.
Mean position bias values (bottom right) were also analyzed as a function of whether the schedule with a 5:1 ratio (i.e., conc VI 12-s VI 60-s) or 1:5 ratio (i.e., conc VI 60-s VI 12-s) was experienced first. There was a main effect of first schedule experienced [F(1,16) = 5.96, p = 0.027, ηp2 = 0.27], such that rats that received the 5:1 ratio schedule first (left side is richer) showed an average position bias to the left, and rats that experienced the 1:5 schedule first (right side is richer) showed an average position bias to the right. There was no main effect of phenotype (p = 0.15).
R2 values, which represent the goodness of fit, were computed for each subject. Values ranged from 0.69 to 0.99 for lean Zuckers and from 0.91 to 0.99 for obese Zuckers, but were not significantly different.
Responses and reinforcers
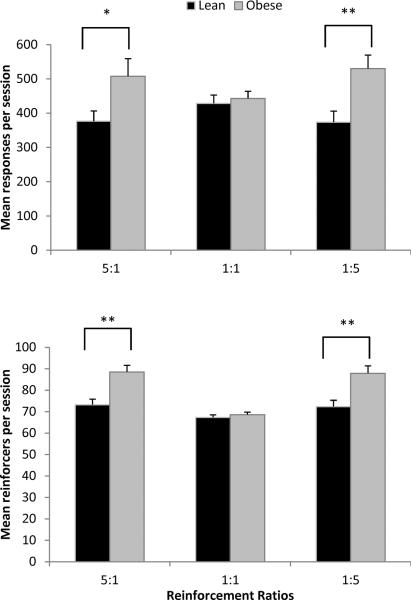
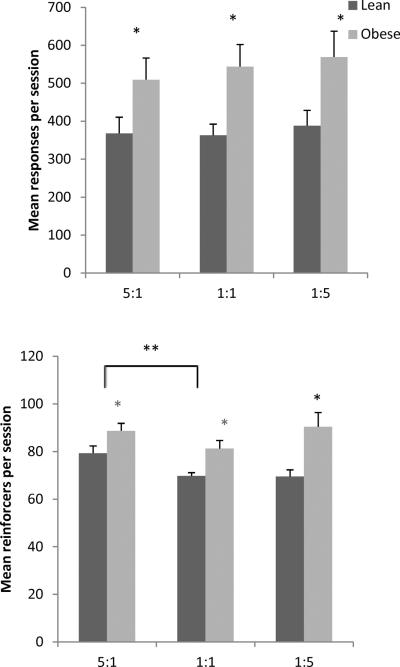
The top of Figure 3 shows a main effect of phenotype on responses per session across concurrent schedules, with obese Zuckers emitting significantly more responses than lean Zuckers [F(1,16) = 6.65, p = 0.02, ηp2 = 0.29]. There were no main effects of conc VI VI schedule, but there was a significant phenotype X schedule interaction [F(2,32) = 4.12, p = 0.026, ηp2 = 0.21]. Separate one-way ANOVAs performed between groups showed that obese rats emitted more responses than lean rats in the 5:1 [F(1,18) = 4.94, p = 0.039, ηp2 = 0.22] and 1:5 schedules [F(1,18) = 9.66, p = 0.006, ηp2 = 0.35], but not the 1:1 schedule. Pellet type was excluded for simplicity, as there were no main effects of pellet.
Figure 3.
Mean total responses (left + right) emitted per session (top) and mean total reinforcers (left + right) earned per session (bottom) in Phase 1 by reinforcement ratios. Lean rats = black; obese rats = gray. Error bars = 1 SEM, * p < 0.05, ** p< 0.01
The bottom of Figure 3 shows a main effect of phenotype on reinforcers, with obese Zuckers earning significantly more than lean Zuckers [F(1,16) = 19.19, p < 0.001, ηp2 = 0.55]. There was also a main effect of schedule [F(2,32) = 20.54, p < 0.001, ηp2 = 0.562]. Post hoc contrasts show that fewer reinforcers were earned under the 1:1 schedule compared to the 5:1 [F(1,16) = 44.57, p < 0.001, ηp2 = 0.74], and the 1:5 schedule [F(1,16) = 23.68, p < 0.001, ηp2 = 0.60]. There was a significant phenotype X schedule interaction [F(2,32) = 7.87, p = 0.002, ηp2 = 0.33]. Separate one-way ANOVAs conducted to examine schedule-dependent differences between lean and obese Zucker rats showed that obese rats earned more reinforcers than lean rats for the 5:1 [F(1,18) = 15.06, p = 0.001, ηp2 = 0.46] and the 1:5 schedules [F(1,18) = 12.09, p = 0.003, ηp2 = 0.40], but not 1:1.
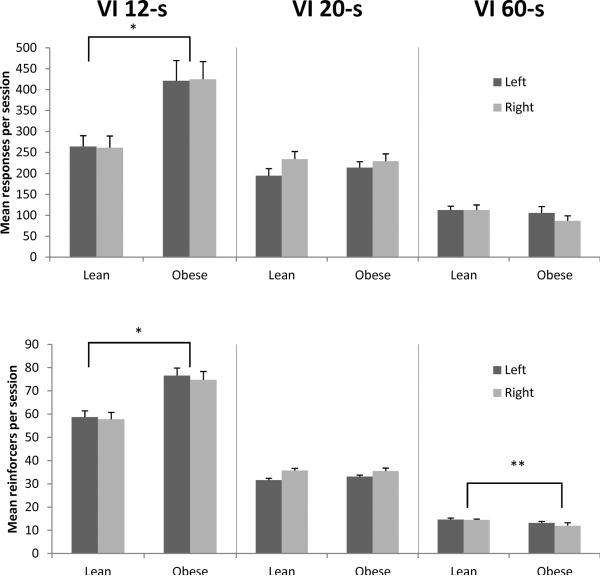
The top of Figure 4 shows a main effect of phenotype on responses within each schedule component [F(1,18) = 7.43, p = 0.014, ηp2 = 0.29], with obese Zuckers emitting more responses on each component compared to lean rats. There was also a main effect of VI component [F(1.32,23.69) = 73.47, p < 0.001, ηp2 = 0.80]. Post hoc contrasts revealed that responses from the VI 12-s components were significantly higher than VI 20-s [F(1,18) = 30.66, p < 0.001, ηp2 = 0.63], and VI 20-s was higher than VI 60-s [F(1,18) = 118.5, p < 0.001, ηp2 = 0.87]. Lever position (left vs. right) was not significant (p = 0.62), and neither were any interactions with position. There was a significant phenotype X component interaction [F(1.32,23.69) = 11.84, p < 0.001, ηp2 = 0.40]. Separate one-way ANOVAs were performed to examine schedule-dependent differences between lean and obese Zucker rats. Obese Zucker rats emitted more responses than lean rats under VI 12-s [F(1,18) = 12.15, p = 0.003, ηp2 = 0.40], but not VI 20-s or VI 60-s.
Figure 4.
Mean number of responses (top) and reinforcers (bottom) per session in Phase 1 by VI component for lean and obese rats. The left lever is shown in black; right lever in gray. Error bars = 1 SEM. *p< 0.01, **p < 0.05
The bottom of Figure 4 shows main effects of phenotype on reinforcers per session [F(1,18) = 19.02, p < 0.001, ηp2 = 0.51], with obese rats earning more reinforcers than lean rats. There was also a main effect of VI component [F(1.17,21.06) = 552.76, p < 0.001, ηp2 = 0.97] with more reinforcers in the VI 12-s components compared to the VI 20-s components [F(1,18) = 324.23, p < 0.001, ηp2 = 0.95], which were significantly higher than the VI 60-s components [F(1,18) = 919.36, p < 0.001, ηp2 = 0.98]. There was no effect of position (p = 0.65) or any position interactions. There was also a significant phenotype X component interaction, [F(1.17,21.06) = 21.01, p < 0.001, ηp2 = 0.54]. Separate one-way ANOVAs on each schedule component showed that obese rats earned significantly more reinforcers than leans under VI 12-s [F(1,18) = 22.41, p < 0.001, ηp2 = 0.56] and VI 60-s [F(1,18) = 4.41, p = 0.050, ηp2 = 0.2], but not VI 20-s.
3.1.2 Phase 2: SUC/CAR and CAR/SUC
Between Phase 1 and Phase 2, three obese rats were euthanized for various medical reasons. Of the seven remaining, one rat (F3) only completed two of the three conc VI VI schedules of Phase 2, so it was excluded from analyses (lean n=10, obese n=6).
Generalized matching
The top of Figure 5 shows mean sensitivity (a) parameters for lean and obese rats for Phase 2. All rats, regardless of phenotype, exhibited sensitivity parameters < 1. There was a main effect of phenotype, with higher sensitivity values overall for obese Zuckers [F(1,14) = 16.32, p < 0.001, ηp2 = 0.34]. The bottom of Figure 5 shows bias (computed by subtracting from Phase 1 bias values). There was a significant bias toward sucrose [F(1,14) = 7.18, p = 0.018, ηp2 = 0.34]. There was no significant effect of phenotype, p = 0.95, or an interaction. R2 values for goodness of fit ranged from 0.69 to 0.99 for lean Zuckers and from 0.88 to 0.998 for obese Zuckers, though the means were not significantly different.
Figure 5.
Mean sensitivity (top) and bias (bottom) values in Phase 2 (CAR/SUC and SUC/CAR) for lean and obese Zuckers. Values > 0 indicate a bias toward sucrose. Lean rats are shown in black; obese in gray. Error bars = 1 SEM.
Responses and reinforcers
The top of Figure 6 shows that in Phase 2 obese Zuckers emitted significantly more responses per session than lean Zuckers across conc VI VI schedules [F(1,14) = 6.83, p = 0.02, ηp2 = 0.33]. There was no main effect of schedule, p = 0.11, or an interaction. Obese Zuckers also earned more reinforcers (bottom) than lean Zuckers [F(1,14) = 17.46, p < 0.001, ηp2 = 0.56]. There was also a significant main effect of schedule [F(2,28) = 4.69, p = 0.018, ηp2 = 0.25]; post hoc contrasts confirmed that more reinforcers were earned on the 5:1 schedule compared to the 1:1 schedule [F(1,14) = 13.24, p = 0.003, ηp2 = 0.49].
Figure 6.
Mean number of responses (top) and reinforcers (bottom) per session in Phase 2 by concurrent schedule for lean and obese rats. Error bars = 1 SEM. *p < 0.05, **p < 0.01
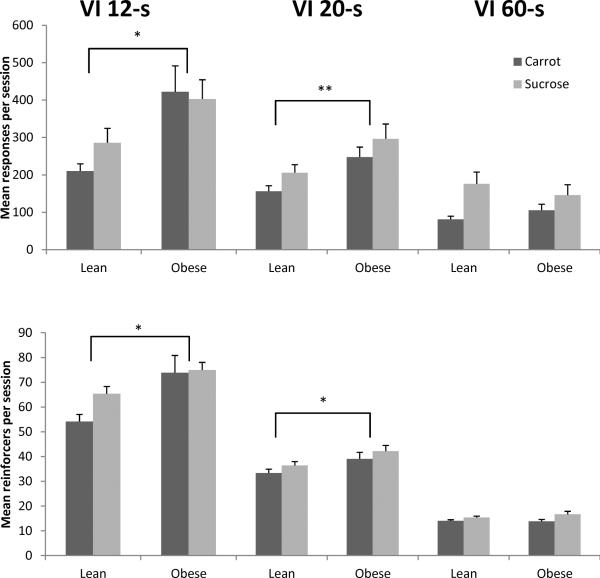
The top of Figure 7 shows that obese Zuckers emitted more responses compared to lean rats across schedule components [F(1,14) = 6.83, p = 0.02, ηp2 = 0.33], and all rats emitted more responses to components delivering sucrose compared to carrot [F(1,14) = 5.44, p = 0.035, ηp2 = 0.28]. There was a main effect of VI component [F(1.52,21.33) = 77.71, p < 0.001, ηp2 = 0.85]. Post hoc contrasts revealed significantly more responses were made to the VI 12-s component than the VI 20-s [F(1,14) = 42.37, p < 0.001, ηp2 = 0.75], which had more than the VI 60-s component [F(1,14) = 91.12, p < 0.001, ηp2 = 0.87]. No interactions with pellet type were found. A phenotype X component interaction was found [F(1.52,21.33) = 13.33, p < 0.001, ηp2 = 0.49]. Separate one-way ANOVAs conducted for each component showed that obese rats emitted more responses than lean rats under VI 12-s [F(1,14) = 9.86, p = 0.007, ηp2 = 0.41] and VI 20-s [F(1,15) = 4.64, p = 0.048, ηp2 = 0.24], but not VI 60-s.
Figure 7.
Mean responses (top) and reinforcers (bottom) per session, for VI 12-s levers (left), VI 20-s levers (center) and VI 60-s levers (right) in Phase 2. The carrot lever is shown in dark gray; the sucrose lever is in light gray. Error bars = 1 SEM. * p< 0.01, **p< 0.05
The bottom of Figure 7 shows obese rats earned more reinforcers than lean rats [F(1,14) = 17.46, p < 0.001, ηp2 = 0.56]. There were no differences in the number of sucrose vs. carrot reinforcers earned (p = 0.084). There was also a main effect of component [F(1.19,16.63) = 505.72, p < 0.001, ηp2 = 0.97] with contrasts revealing more reinforcers in the VI 12-s component than the VI 20-s [F(1,14) = 268.64, p < 0.001, ηp2 = 0.95], and more reinforcers in the VI 20-s component compared to VI 60-s [F(1,14) = 918.78, p < 0.001, ηp2 = 0.99]. In addition, there was a phenotype X component interaction [F(1.19,16.63) = 9.43, p = 0.005, ηp2 = 0.40]. Separate one-way ANOVAs showed that obese Zucker rats earned more reinforcers under VI 12-s [F(1,14) = 13.08, p = 0.003, ηp2 = 0.48] and VI 20-s [F(1,15) = 10.50, p = 0.005, ηp2 = 0.41], but not VI 60-s.
4.1 Discussion
4.1.1 Phase 1: SUC/SUC or CAR/CAR
The sucrose and carrot-flavored pellets functioned as reinforcers for all rats. Moreover, behavioral allocation was sensitive to the differences in the relative rates of reinforcement across the three concurrent schedules. More behavior was allocated and more reinforcers were earned in the richer component (i.e., VI 12-s) of the concurrent schedules with 5:1 and 1:5 reinforcer ratios compared to the leaner (VI 60-s) component. Under the 1:1 schedule, behavior was equally distributed to the two VI 20-s components. This finding replicates other studies that demonstrate matching in standard laboratory rat strains with food, alcohol, and exercise-based reinforcement [22–25] and is a novel finding for the obese and lean Zucker strain.
The average sensitivity parameters seen here (0.6 and 0.84) approximate those reported for various rat strains responding on concurrent VI VI schedules between sessions (e.g., [22–23]). Average bias parameters also appeared to be within the normal range when the pellets alternatives were the same. In addition, R2 values ranged from 0.69 to 0.99 for all rats, meaning that, at the lowest, 69% of the variance in the data was accounted for by the generalized matching equation, which is also within the normal range for the generalized matching equation.
Obese Zuckers had significantly higher sensitivity values than lean Zuckers. This suggests that the relative responding by obese Zuckers was more sensitive to the relative reinforcement rates of each concurrent schedule, and matched their allocation of responses more closely to reinforcement rates than lean Zuckers. Bias values, which would indicate position bias in Phase 1 of the experiment, did not differ between obese and lean Zuckers. However, rats that experienced the richer schedule on the left (i.e., 5:1) following completion of the schedule with equal ratios of reinforcement showed a bias toward the left, and rats that experienced the richer schedule on the right first (i.e., 1:5) showed a bias toward the right. These values provided us with a baseline position bias that was able to be controlled for when examining matching in Phase 2, when allocation of behavior was compared between two qualitatively different reinforcers.
Obese Zuckers emitted significantly more responses and earned more reinforcers per session, regardless of pellet type, than lean Zuckers. This pattern replicates differences seen in responding for food under progressive ratio schedules for food in one study [7] as well as fixed ratios varying from FR1 to FR 50 [11], and from FR1 to FR30 [10]. Further analyses showed that obese rats had more responses and reinforcers in the conc VI VI schedules with uneven programmed reinforcement rates (i.e., 5:1 and 1:5) as opposed to when the programmed reinforcer rates were equal (1:1). Moreover, when behavior in each of the three components was examined separately, obese rats demonstrated the highest responses and reinforcers in the schedule with the richest programmed rate of reinforcement (VI 12-s) compared to lean rats.
Sensitivity can manifest as allocating more behavior to richer sources of reinforcement, less behavior to leaner sources of reinforcement, or both. The sensitivity differences observed between lean and obese rats in Phase 1 likely came from the obese rats allocating more behavior toward the richest source of reinforcement (and thereby earning more reinforcement), compared to the leans. Lean rats, on the other hand, allocated relatively less behavior to the richer lever and relatively more to the leaner lever (VI 60-s).
4.1.2 Phase 2: SUC/CAR or CAR/SUC
In Phase 2, allocation to two pellets types that were calorically similar, but different in palatability, were compared. Sensitivity values were again higher for obese Zuckers than lean Zuckers. No differences in sensitivity were found between Phase 1 and Phase 2. There was a bias toward sucrose for both groups, but level of bias did not differ between lean and obese rats, suggesting that obese Zucker rats do not prefer sucrose more than leans; rather, they simply exhibit more sensitivity in responding to larger sources of reinforcement. These observations are also supported from data on responses and reinforcers, which showed no differences for carrot vs. sucrose, but did show that obese rats made more responses and reinforcers compared to lean rats across all concurrent schedules and schedule components. The strongest differences between groups (i.e., effect size), though, appeared in the richest schedule component (VI 12-s).
4.1.3 General Discussion
The present experiment extends free-feeding research on Zucker rats by identifying behavioral patterns underlying eating behavior in a choice situation. The results of this experiment contribute to the literature by demonstrating robust and stable differences in sensitivity to reinforcement rates in obese vs. lean Zuckers. In both phases of the experiment, obese Zucker rats exhibited higher sensitivity to relative reinforcement rates than leans, which coincided with earning more reinforcers, especially when richer schedules were available. The lean rats exhibited sensitivity parameters than were in the lower range of what is typically reported with rats, which may be related to their phenotype. A study that directly compares strains using the same procedure would be required to determine whether lean Zuckers' behavior is less sensitive to larger reinforcer ratios than more typical strains of rats.
When seeking food in any choice situation, an effective strategy is to exhibit high sensitivity to overall rates of reinforcement, such that the amount of food that is obtained is maximized. If behavior is not sensitive to rates of reinforcement, either by allocating too many responses to a lean source of reinforcement or too few to a rich source of reinforcement, few reinforcers overall will be earned. The lean Zuckers demonstrated greater undermatching in both phases of the study, meaning their responding was less sensitive to the richer sources of reinforcement than obese rats. Their pattern of emitting proportionately more responses on the lean side and too few on the rich side likely resulted in earning fewer reinforcers. On the other hand, because the obese Zuckers matched better, they were able to obtain more of the reinforcers from the rich side, and thus increased the total amount of food earned in the session.
Higher reinforcement rates could have also contributed to the higher sensitivity values seen in the obese Zuckers. In five of six schedules (two in Phase 1 and all three in Phase 2), obese rats earned more reinforcers. Alsop and Elliffe [26] found that as reinforcer rates increased, sensitivity values systematically increased. However, the range of reinforcer rates used in this study was much larger than the range in reinforcer rates between the lean and obese rats used in the present study, so it is unclear whether the differing reinforcer rates were related to the different sensitivity parameters seen here. Future studies that manipulate the rate of reinforcement could determine the extent to which this factor affects differences in sensitivity to reinforcement in lean and obese Zucker rats.
Though a relation between obesity and sensitivity to reinforcement is evident from this study, no causal statements, in terms of direction, can be made. One possibility is that being obese makes one more likely to be more sensitive to differences in food densities. Alternatively, having more experience with food, because of a heightened sensitivity to reinforcement rates, can facilitate overeating and therefore obesity. Another possibility is that other conditions which contribute to obesity may also contribute to increasing sensitivity to changes in relative reinforcement. More research that examines the direction of causality may help elucidate this question.
There are some limitations to this study. First, in order to obtain the most representative matching function, about five different conc VI VI schedules that vary in reinforcer ratios are typically used. Because of the amount of time that was required to obtain stability on each schedule, the length of the study was long in relation to the lifespan of the obese Zucker rat, which can be significantly shorter than lean rat (~450 days for obese Zuckers, compared to about 660 for leans; [27]). In addition, obese Zucker rats experience obesity-related health issues similar to those of humans, including hypertension [2] and insulin resistance [28], which may further reduce their lifespan, making three concurrent schedules a necessity. Indeed, in the present study, three obese rats developed health problems and were euthanized—the majority occurred toward the end of the experiment. The three concurrent schedules, however, included one in which the relative number of reinforcers was identical (1:1) and the other two (1:5 and 5:1) allowed for discrimination between the differing rates of reinforcement. Further, the 1:5 and 5:1 schedules allow for a symmetrical experience on both levers. If obese Zuckers had longer life-spans, we would have incorporated two additional concurrent schedules with larger ratios of reinforcement (e.g., 1:10 and 10:1). Another option would be to replace the 1:5 and 5:1 ratios with these larger ratio schedules. While it is possible that further differences in matching could be detected in the obese and lean Zucker rats with additional concurrent schedules, we still observed order in their behavior in a manner that matching would predict.
A second potential limitation of the study may be the level of deprivation used. Both groups of rats were food deprived an equivalent amount of time before each experimental session. They were allowed to free-feed for an equal amount of time after experimental sessions, and during this time they ate about 2.3% of their body weights in food. While this deprivation method has been used in other studies with Zuckers [7,10,11], it is unclear whether hours of deprivation affects food reinforcer efficacy differently in lean and obese Zucker rats. A study that manipulates level of deprivation could help elucidate this relation.
For future studies, it would be interesting to expand the range of reinforcers to foods that vary in both caloric content and palatability, to further examine the differences between food choices in the obese Zucker. If obesity in general is related to higher overall sensitivity to reinforcement rates regardless of palatability or caloric content, then perhaps the most effective intervention would be to ensure that food choices are as healthy as possible, and low in calories. This research could clarify some of the underlying behavioral (and potentially physiological) mechanisms involved in obesity that would produce possible interventions.
Highlights
Behavioral allocation to different densities and types of food reinforcement was compared in obese and lean Zucker rats.
Allocation was characterized using the generalized matching equation.
Obese Zucker rats were more sensitive to differing densities of food than lean Zucker rats.
Obese Zucker rats did not demonstrate stronger preference for high-sucrose food options.
Acknowledgements
This manuscript is part of the first author's Master's thesis at Idaho State University and was supported by funding from the WeLEAD project, which was funded by the National Science Foundation SBE-0620073, the Idaho INBRE Program, NIH Grant Nos. P20 RR016454 (National Center for Research Resources) and P20 GM103408 (National Institute of General Medical Sciences), and grants from the Humanities and Social Sciences Research and Graduate Research Committees at Idaho State University. We thank Steven Boomhower, Megan Brinton, Brent Call, Tiffany Doherty, Kory Farley, Vanessa Hanson, Conrad Hillman, Casey Johnson, Mathew Luras, Zachary Schumacher, Sasha Scott, Jennifer Stoll, and Jennifer White for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trivedi PS, Barouch LA. Cardiomyocyte apoptosis in animal models of obesity. Curr Hypertens Rep. 2008;10(6):454–60. doi: 10.1007/s11906-008-0085-z. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz TW, Morris RC, Pershadsingh HA. The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension. 1989;13(6 Pt 2):896–901. doi: 10.1161/01.hyp.13.6.896. [DOI] [PubMed] [Google Scholar]
- 3.Drewnowski A, Grinker JA. Food and water intake, meal patterns and activity of obese and lean Zucker rats following chronic and acute treatment with delta-9-tetrahydrocannabinol. Pharm Biochem Behavr. 1978;9(5):619–630. doi: 10.1016/0091-3057(78)90213-7. [DOI] [PubMed] [Google Scholar]
- 4.Vickers SP, et al. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharm. 2003;167(1):103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- 5.Bjenning CA, Rimvall K. oCRF and CRF (6–33) depress food but not water intake in the obese Zucker rat. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S140–1. doi: 10.1038/sj.ijo.0801304. [DOI] [PubMed] [Google Scholar]
- 6.Davis LM, et al. Bromocriptine administration reduces hyperphagia and adiposity and differentially affects dopamine D2 receptor and transporter binding in leptin-receptor-deficient Zucker rats and rats with diet-induced obesity. Neuroendocrinology. 2009;89(2):152–62. doi: 10.1159/000170586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen EB, Huskinson SL. Effects of rimonabant on behavior maintained by progressive ratio schedules of sucrose reinforcement in obese Zucker (fa/fa) rats. Behav Pharmacolog. 2008;19(7):735–742. doi: 10.1097/FBP.0b013e3283123cc2. [DOI] [PubMed] [Google Scholar]
- 8.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;269:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 9.Stafford DMG, LeSage M, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139(3):169–84. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen EB, Reilly W, Buckley J, Boomhower S. Rimonabant reduces the essential value of sucrose: An exponential demand analysis. Physiol Behav. 2012;105:734–741. doi: 10.1016/j.physbeh.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen EB, Reilly W, Hillman C. Demand for sucrose in lean and obese Zuckers. Behav Processes. 2010;85:191–7. doi: 10.1016/j.beproc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Pierce WD, Epling WF. The applied importance of research on the matching law. J Appl Behav Anal. 1995;28(2):237–41. doi: 10.1901/jaba.1995.28-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shull RL. The sensitivity of response rate to the rate of variable-interval reinforcement for pigeons and rats: A review. J Exper Anal Beh. 2005;84:99–110. doi: 10.1901/jeab.2005.03-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce WD, Epling WF. Choice, matching, and human behavior: A review of the literature. The Behavior Analyst. 1983;6:57–76. doi: 10.1007/BF03391874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davison M, McCarthy D. The matching law. L. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 16.Baum W. On two types of deviation from the matching law: Bias and undermatching. J Exper Anal of Behav. 1974;22:231–242. doi: 10.1901/jeab.1974.22-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller HL. Matching-based hedonic scaling in the pigeon. J Exper Anal Behav. 1976;26:335–347. doi: 10.1901/jeab.1976.26-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews LR, Temple W. Concurrent schedule assessment of food preference in cows. J Exper Anal Behav. 1979;32:245–254. doi: 10.1901/jeab.1979.32-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrnstein RJ. Relative and absolute strength of response as a function of frequency of reinforcement. J Exper Anal of Behav 196. 4:267–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shull RL, Pliskoff SS. Changeover delay and concurrent schedules: Some effects on relative performance measures. J Exper Anal of Behav. 1967;10:517–527. doi: 10.1901/jeab.1967.10-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. J Exp Anal Behav. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banna KM, Newland MC. Within-session transitions in choice: a structural and quantitative analysis. J Exp Anal Behav. 2009;91:319–35. doi: 10.1901/jeab.2009.91-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belke TW, Belliveau J. The general matching law describes choice on concurrent variable-interval schedules of wheel-running reinforcement. J Exp Anal Behav. 2001;75:299–310. doi: 10.1901/jeab.2001.75-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jimenez-Gomez C, Shahan TA. Matching law analysis of rats' alcohol self-administration in a free-operant choice procedure. Behav Pharmacol. 2008;19:353–6. doi: 10.1097/FBP.0b013e328308f1c5. [DOI] [PubMed] [Google Scholar]
- 25.MacDonall JS. The stay/switch model of concurrent choice. J Exp Anal Behav. 2009;91:21–39. doi: 10.1901/jeab.2009.91-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsop B, Elliffe D. Concurrent choice: Effects of overall reinforcer rate and the temporal distribution of reinforcers. J Exp Anal Behav. 1996;65:445–463. doi: 10.1901/jeab.1996.65-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azain MJ, Broderson JR, Martin RJ. Effect of long-term somatotropin treatment on body composition and life span in aging obese Zucker rats. Exp Biol Md. 2006;231(1):76–83. doi: 10.1177/153537020623100109. [DOI] [PubMed] [Google Scholar]
- 28.Kasiske BL, O'Donnell BP, Keane WF. The Zucker rat model of obesity, insulin resistance, hyperlipidemia, and renal injury. Hypertension. 1992;19:110–5. doi: 10.1161/01.hyp.19.1_suppl.i110. [DOI] [PubMed] [Google Scholar]