Abstract
Methoxychlor (MXC) is an organochlorine pesticide widely used in many countries against various species of insects that attack crops and domestic animals. MXC reduces fertility by increasing atresia (death) of antral follicles in vivo. MXC also induces atresia of antral follicles after 96 h in vitro. The current work tested the hypothesis that MXC induces morphological atresia at early time points (24 and 48 h) by altering pro-apoptotic (Bax, Bok, Casp3, and caspase activity) and anti-apoptotic (Bcl2 and Bcl-xL) factors in the follicles. The results indicate that at 24 h, MXC increased Bcl-xL and Bax mRNA levels and increased the ratio of Bax/Bcl2. At 48–96 h, MXC induced morphological atresia. At 24–96 h, MXC increased caspase activities. These data suggest that MXC may induce atresia by altering Bcl2 factors and inducing caspase activities in antral follicles.
Keywords: methoxychlor, antral follicles, ovary, mouse, atresia, caspase
1 Introduction
The ovaries of female mammals contain a fixed number of primordial follicles at the time of birth [1]. These primordial follicles grow into more mature follicle stages known as primary, pre-antral, and antral follicles. During the reproductive lifespan, a constant stream of follicles grows from the primordial to the antral stage. Antral follicles are the only type of follicles capable of releasing a fertilizable oocyte and they are the major sites of synthesis of steroid hormones such as estrogens. Although a few antral follicles ovulate and become fertilized, between 95–99% of all follicles die via an apoptotic process known as atresia [1].
The endocrine disrupting chemical 1,1,1-trichloro-2,2-bis(4-methoxyphenyl)ethane (methoxychlor; MXC) is an organochlorine pesticide, which is primarily used against various species of insects that attack field crops, trees, vegetables, fruits, gardens, stored grain, livestock, and domestic pets [2]. MXC was first registered for use in 1948. It replaced the most commonly used organochlorine pesticide dichlorodiphenyltrichloroethane (DDT) during 1970s and was used in the United States until 2004 [3]. However, MXC is still being used in many countries on agricultural products that are imported to the United States, resulting in human exposure in the United States.
MXC specifically targets antral follicles in the ovary and its exposure leads to adverse reproductive functions in adult female mice by causing persistent estrus, ovarian atrophy, and follicular atresia [4, 5]. In vivo studies in mice have shown that MXC causes antral follicle toxicity, characterized by an increased percentage of atretic follicles and a decreased number of healthy antral follicles compared to controls [6]. Many in vitro studies also have shown that MXC inhibits follicle growth and increases atresia of cultured mouse antral follicles [7, 8].
Despite the potential adverse reproductive abnormalities associated with MXC exposure, little is known about the mechanisms by which MXC induces atresia in antral follicles. The present study was designed to identify whether the mechanism by which MXC destroys antral follicles involves BCL2 family members and caspases (CASP). BCL2 family members include two distinct groups: pro-apoptotic factors that promote apoptosis (e.g., Bax, Bok) and anti-apoptotic factors that promote cell survival (e.g., Bcl2, Bcl-xL) [9, 10]. The fate of follicles depends on the balance between these different pro-apoptotic and anti-apoptotic factors. BCL2 and BAX in particular are major regulators of follicular atresia in the mammalian ovary [9, 11–13]. There are many functions of BCL2 family members, but their primary roles are to regulate mitochondrial membrane homeostasis and the release of cytochrome c from the mitochondrial intermembrane space, both of which are decisive steps in apoptosis [9]. The pro-apoptotic factor BAX dimerizes with the anti-apoptotic factor BCL2 and the pro-apoptotic factor BOK specifically heterodimerizes with the anti-apoptotic factors MCL-1 and BHRF [9, 14]. This relieves apoptotic protease activating factor-1 (APAF-1) from suppression by BCL2 proteins and promotes CASP activation [9]. Additionally, pro-apoptotic factors such as BAX alter mitochondrial membrane homeostasis and increase cytochrome c release by forming pores in the outer mitochondrial membrane and opening the permeability transition pore following mitochondrial permeability transition, while anti-apoptotic factors such as BCL2 counteract these actions [9, 10, 15, 16]. Cytochrome c interacts with APAF-1 and pro-CASP9 to form an apoptosome. Apoptosome formation results in activation of CASP9 and subsequent activation of downstream caspases, which in turn results in DNA fragmentation [9].
Previous studies have determined that MXC causes atresia of antral follicles after 96 h exposure in vitro and that MXC increases Bax mRNA levels between 48–96 h and decreases Bcl2 mRNA levels at 96 h [8]. However, they have not determined if short term MXC exposure causes atresia nor have they determined the time course of onset of atresia with MXC exposure. Also, studies have not measured whether MXC alters Bcl-xL, Bok, and Casp levels and CASP activity in antral follicles. Such information is critical for understanding the effects of MXC on ovarian follicles and for delineating the mechanism involved in MXC induced atresia. Thus, the current study examined both short term (24–48 h) and long term (96 h) exposure to MXC to determine the earliest time point at which MXC induces atresia. Given the roles of pro-apoptotic and anti-apoptotic factors in regulating atresia, the proposed work was also designed to test the hypothesis that MXC induces atresia by altering pro-apoptotic and anti-apoptotic factors in the antral follicles of the ovary. Specifically, we examined the effects of MXC on expression of Bcl2, Bcl-xL, Bax, Bok, and Casp levels and CASP activity after both short term and long term MXC exposure.
2 Materials and Methods
2.1 Reagents and Materials
2.1.1 Reagents
MXC (99% pure) was purchased from Chemservice (West Chester, PA). Stock solutions of MXC were prepared using vehicle control (diemthylsulfoxide; DMSO) (Sigma, St. Louis MO) as a solvent, and in various concentrations (2, 20, and 200 mg/ml) that permitted an equal volume of solvent to be added to individual culture wells for each treatment group. Thus, final concentrations of MXC in culture were 1, 10, and 100 μg/ml (ppm). The doses used in these experiments were selected based on previously published studies showing that this range of concentrations of MXC induces toxicity in antral follicles, granulosa cell culture models, gonadal cultures, and in uterine leiomyoma cells [7, 8, 17–20]. These selected concentrations may be relevant to occupational exposure levels. An occupational exposure study in farmers revealed that concentration of MXC can reach up to 5.16 μg/ml in serum [2]. Thus, the occupational exposure dose may be much higher than normal human exposure (4 ng/kg/day) and lies between the doses (1 and 10 μg/ml) used in the present experiments. For controls and MXC treatment groups, DMSO was used at 0.05%, which is able to solubilize MXC in aqueous media. DMSO, ITS (insulin, transferrin, selenium), penicillin, and streptomycin were obtained from Sigma-Aldrich (St. Louis, MO). Alpha-minimal essential media (α-MEM) was obtained from Invitrogen (Carlsbad, CA). Human recombinant follicle stimulating hormone (rFSH) was obtained from Dr. A.F. Parlow from the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA). Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Lawrenceville, GA).
2.1.2 Animals
Adult female cycling CD-1 mice were purchased from Charles River Laboratories (Charles River, CA) and housed in the core animal facility located at College of Veterinary Medicine, University of Illinois and maintained on 12L:12D cycles. Mice were housed in the animal facility for at least two days to relieve transportation stress, given ad libitum food and water, and temperature was maintained at 22±1 °C. Animals were euthanized at 35–39 days of age by carbon dioxide (CO2) inhalation followed by cervical dislocation. The ovaries were removed and antral follicles were isolated as explained below. The University of Illinois Institutional Animal Care and Use Committee approved all protocols involving animal care, euthanasia, and tissue collection.
2.2 Antral follicle culture
Ovaries were removed and antral follicles were isolated from the ovaries of mice between 35–39 days old because this time point was used in previous studies, and this is the age at which mice are cycling young adults [7, 8, 19–20]. Antral follicles were isolated mechanically from the ovaries based on relative size and interstitial tissue was removed using fine watchmaker forceps. About 3–4 mice were used per experiment and they yielded approximately 20–30 follicles per mouse. Once follicles were isolated, they were placed individually in wells of a 96-well culture plate with 150 μl of unsupplemented α-MEM medium prior to treatment. Vehicle controls and dose response regimens of MXC (1–100 μg/ml) were individually prepared in supplemented α-MEM. For treatment, unsupplemented α-MEM was removed from each well and replaced with 150 μl of supplemented α-MEM containing MXC or DMSO. The follicles were cultured in supplemented media according to previously described protocols [7, 8, 19–20]. Follicles then were incubated for 24, 48, and 96 hours (h). Non-treated controls (supplemented medium alone) were used in each experiment as a control for culture conditions. At the end of 24, 48, and 96 h follicle cultures, follicles were collected, snap frozen, and stored at −80 °C for later use. Follicle cultures were repeated three times to obtain enough power for statistical analysis.
2.3 Histological evaluation of atresia
At selected times in culture, follicles were collected and stored in Dietrick’s solution at least for 24 h. Then, follicles were processed through series of washes with ethanol: 70% for 10 min, 85% for 10 min, 95% for 7 min (2X), and 100% for 7 min (2X). The follicles were embedded in plastic blocks using Technovit 7100 kits from Heraeus Kulzer GmbH, Germany. The follicles were incubated in a pre-infiltration medium for one hour and then with infiltration medium overnight before they were embedded in plastic blocks using embedding medium. Pre-infiltration solution was prepared with one part 100% ethanol and one part base solution. Infiltration solution was prepared with 1 gm of hardener-1 with 100 ml of base solution. Embedding medium was prepared by adding 1 ml of hardener-2 to 15 ml of infiltration solution.
The follicles embedded in plastic blocks were randomly assigned numbers and thus, the observers were blinded with respect to treatment groups. These blocks were cut into 2 μm sections using a microtome. Follicle sections were stained with Lee’s methylene blue-basic fuchsin stain for 30 sec and washed with distilled water before they were cover slipped. Each follicle section was examined for level of atresia (follicle death) as evidenced by the presence of apoptotic bodies and reported at the highest atresia rating observed throughout the tissue. Follicles were rated on a scale of 1–4 for the presence of apoptotic bodies: 1= healthy, 2 = less than 10% apoptotic bodies (early atresia), 3 = 11–30% apoptotic bodies (mid atresia), 4 = greater than 30% apoptotic bodies (late atresia) as previously described by Miller et al. [8]. Each follicle section was examined and rated by two observers. Ratings were then averaged and plotted to compare the effect of chemical treatments on atresia levels.
2.4 Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was isolated from frozen follicles using the RNeasy Micro Kit (Qiagen, Inc., Valencia, CA) following the manufacturer’s protocol. RNA was treated with DNAse to remove any possible genomic DNA contamination. The concentration of RNA in each sample was measured at 260 nm using the Nanodrop ND1000 UV-Vis spectrophotometer (Nanodrop Technologies, Wilmington, DE). The cDNA was synthesized by reverse transcribing mRNA (50 ng) using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. The cDNA was diluted to 1:2 with nuclease free water.
qPCR was conducted using a CFX96 Real-time System C1000 Thermal Cycler (Bio-Rad). All samples were measured in triplicate, and each reaction contained 2 μL of diluted cDNA, 0.6 μL (300nM) of gene-specific primers (Integrated DNA Technologies, Inc, Coralville, IA, Table 1), 2.4 μL of nuclease-free water, and 5 μL of SsoFast EvaGreen Supermix (Bio-Rad) for a final volume of 10 μL. The qPCR program consisted of an enzyme activation step (95 °C for 1 min), an amplification and quantification program [40 cycles of 95 °C for 10 sec, 60 °C annealing/extension for 10 sec, single fluorescence reading], a 72 °C for 5 min step, a melt curve (65–95 °C heating 0.5 °C per second with continuous fluorescence readings) and a final step at 72 °C for 5 min.
Table 1.
Real-time qPCR primer accession numbers, abbreviations, and sequences
| Accession no | Gene name | Abbreviation | Forward | Reverse |
|---|---|---|---|---|
| NM_009741.3 | B cell leukemia/lymphoma 2 | Bcl2 | 5′-ATG CCT TTG TGG AAC TAT ATG GC-3′ | 5′-GGT ATG CAC CCA GAG TGA TGC-3′ |
| NM_007527.3 | Bcl2-associated X protein | Bax | 5′-TGA AGA CAG GGG CCT TTT TG-3′ | 5′-AAT TCG CCG GAG ACA CTC G-3′ |
| NM_016778.2 | Bcl2-related ovarian killer protein | Bok | 5′-CTG CCC CTG GAG GAC GCT TG-3′ | 5′-CCG TCA CCA CAG GCT CCG AC-3′ |
| NM_009810.2 | Caspase 3 | Casp3 | 5′-TGG TGA TGA AGG GGT TGA AGG GGT CAT TTA TG-3′ | 5′-TTC GGC TTT CCA GTC TTT CCA GTC AGA CTC-3′ |
| NM_009743.4 | Bcl2-like 1 | Bcl2l1/Bcl-xL | 5′-AAG CGT AGA CAA GGA GAT GCA GGT-3′ | 5′-CTG CTG CAT TGT TCC CGT AGA GAT-3′ |
Primer sequences are shown in Table 1. The specificity of each primer was tested using BLASTN2.218+ and by the presence of a single peak in the melt curve analysis. In addition, each product was run on 2% agarose gels to confirm the product size. A standard curve was generated from six serial dilutions of one of the samples (1:2, 1:4, 1:8, 1:16, 1:32, and 1:64); thus, allowing analysis of the amount of cDNA in the exponential phase. Actin b was used as a reference gene and its values did not differ across treatment groups. Final values were expressed as genomic equivalents and were calculated as the ratio of each gene to actin b. The reported data were obtained from the mean expression values of 3–4 separate culture experiments.
2.5 CASP 3/7 activity assay
CASP activity was measured using the Caspase-Glo 3/7 Assay Kit from Promega. The follicles were cultured for 24, 48, and 96 h and then transferred with 100 μl of medium to a white-walled 96 well plate. Caspase-Glo 3/7 assay reagent (100 μl) was added and the plate was incubated at room temperature for 150 min. The CASP3/7 present in the follicles cleaves the luminogenic substrate containing the DEVD sequence, releasing aminoluciferin, which in turn acts as a substrate for luciferase emitting luminescence. The amount of luminescence is a direct measurement of the CASP activity. The luminescence was captured using Synergy 2 Alpha Microplate Reader (Biotek).
2.6 Statistical analysis
All data were analyzed using SPSS statistical software (SPSS Inc., Chicago, IL). For all comparisons, statistical significance was assigned at p ≤ 0.05. Comparisons between DMSO and the different doses of MXC were conducted with data obtained from 3 to 4 separate experiments using one-way analysis of variance (ANOVA), Kruskal-Wallis test followed by Tukey’s post hoc test, or Mann-Whitney test or a test for linear regression when applicable.
3 Results
3.1 Effect of MXC on atresia
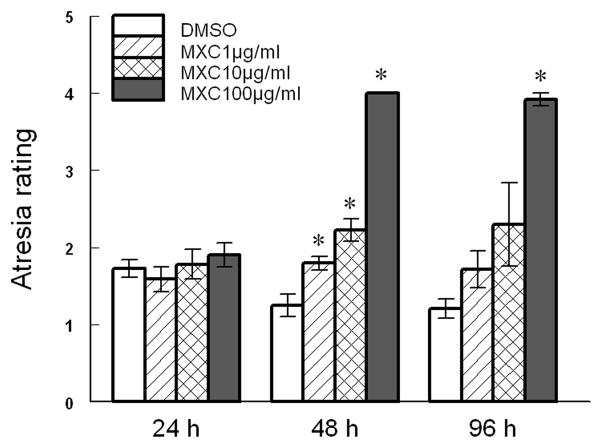
Previous studies have shown that MXC induces atresia of mouse antral follicles at 96 h of culture [7, 8, 20]. In the present study, we confirmed these previous findings by showing that MXC induces atresia at 96 h (MXC 100 μg/ml) (Fig 1). We also expanded previous studies by determining the earliest time at which MXC induces atresia. At 24 h, MXC did not induce atresia, but by 48 h, MXC (1, 10 and 100 μg/ml) increased follicle atresia ratings compared to vehicle control (DMSO) (Fig 1).
Fig 1. Effect of in vitro MXC exposure on antral follicular atresia.
Antral follicles isolated from CD-1 mice were exposed to vehicle control (DMSO) or MXC (1–100 μg/ml) for 24, 48, or 96 h. At the end of culture, follicles were subjected to histological analysis of atresia. Data represent means ± SEM from 3 separate experiments (* indicates significant difference from vehicle controls; n= 4–6 follicles per treatment; p ≤ 0.05).
3.2 Effect of MXC on expression of pro-apoptotic and anti-apoptotic factors
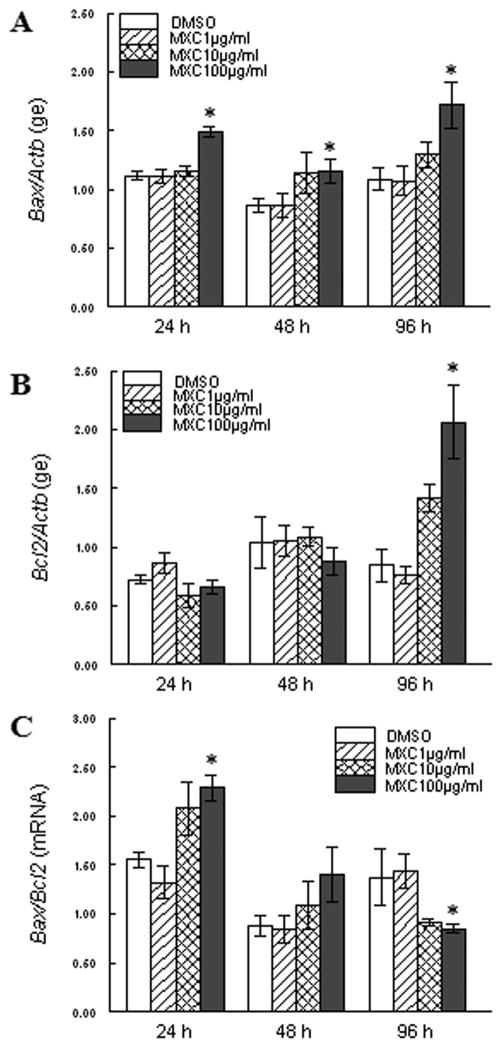
Since MXC induced atresia in antral follicles, we next examined whether MXC alters the mRNA expression of pro-apoptotic and anti-apoptotic factors. Upon treatment with MXC, the mRNA expression levels of the pro-apoptotic factor Bax were increased at all time points. Specifically, MXC (100 μg/ml) significantly increased expression of Bax levels at 24, 48, and 96 h compared to DMSO (Fig 2A). Interestingly, Bax levels were increased as early as 24 h, preceding the time of onset of morphological atresia (Fig 1).
Fig 2. Effect of MXC on expression of Bcl2 family members.
Antral follicles isolated from CD-1 mice were exposed to vehicle control (DMSO) or MXC (1–100 μg/ml) and cultured for 24, 48, or 96 h. Bax (panel A), Bcl2 (panel B), and Bax/Bcl2 (panel C) mRNA levels were measured in antral follicles using qPCR. Data represent means ± SEM from 3 separate experiments (* indicates significant difference from vehicle controls; n=12–14 follicles per treatment; p ≤ 0.05).
MXC did not affect Bcl2 levels at the 24 and 48 h time points, but MXC (100 μg/ml) significantly increased expression of Bcl2 at 96 h compared to DMSO (Fig 2B). Interestingly, there were no changes in this anti-apoptotic factor at 24 h (Fig 2B), the time point which precedes morphological atresia (Fig 1).
From the mRNA expression values of Bax and Bcl2, we calculated the Bax/Bcl2 ratio across all time points. On treatment with MXC (100 μg/ml), Bax/Bcl2 ratios were increased at 24 h, not altered at 48 h, and decreased by 96 h (Fig 2C). Thus, the Bax/Bcl2 ratio was increased as early as 24 h (Fig 2C), preceding the onset of morphological atresia (Fig 1).
Since MXC altered Bax, Bcl2, and their ratios, we measured the expression of other factors involved in induction of apoptosis in the antral follicles of the ovary. At 24 and 96 h, 10 μg/ml MXC significantly increased Bcl-xL levels and 100 μg/ml MXC increased Bcl-xL levels in a trend-dependent manner compared to DMSO (Fig 3). MXC (100 μg/ml) significantly decreased Bok levels at 24 h, but not at 48 and 96 h compared to DMSO (Fig 4).
Fig 3. Effect of MXC on expression of Bcl-xL.
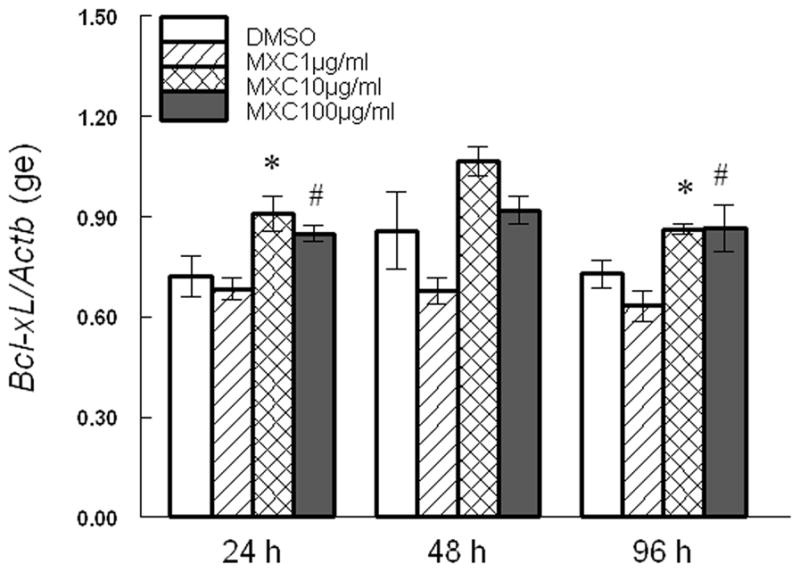
Antral follicles isolated from CD-1 mice were exposed to vehicle control (DMSO) or MXC (1–100 μg/ml) and cultured for 24, 48, or 96 h. Bcl-xL mRNA levels were measured in antral follicles using qPCR. Data represent means ± SEM from 3 separate experiments (* indicates significant difference from vehicle controls; # indicates significant trend using linear regression; n=12–14 follicles per treatment; p ≤ 0.05).
Fig 4. Effect of MXC on expression of Bok.
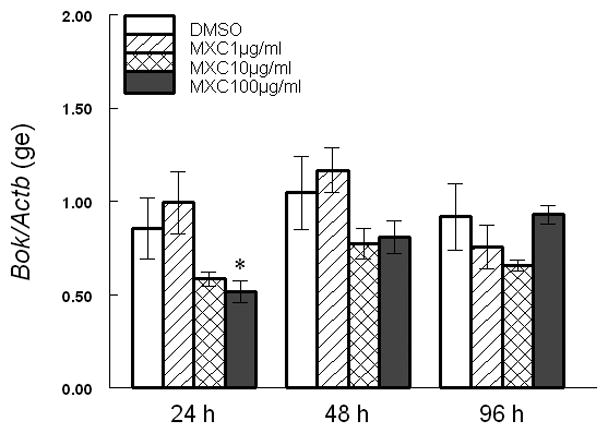
Antral follicles isolated from CD-1 mice were exposed to vehicle control (DMSO) or MXC (1–100 μg/ml) and cultured for 24, 48, or 96 h. Bok mRNA levels were measured in antral follicles using qPCR. Data represent means ± SEM from 3 separate experiments (* indicates significant difference from vehicle controls; n=12–14 follicles per treatment; p ≤ 0.05).
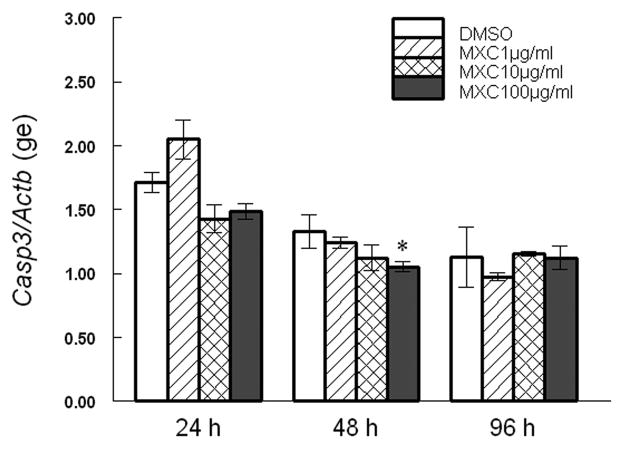
Since increased Bax/Bcl2 ratios are associated with induction of Casp expression [9], we examined the levels of Casp3 mRNA in antral follicles exposed to vehicle and MXC. We found that MXC (100 μg/ml) treatment significantly decreased Casp3 levels at 48 h compared to DMSO, but it did not affect Casp3 levels at 24 and 96 h. (Fig 5).
Fig 5. Effect of MXC on expression of Casp3.
Antral follicles isolated from CD-1 mice were exposed to vehicle control (DMSO) or MXC (1–100 μg/ml) and cultured for 24, 48, or 96 h. Casp3 mRNA levels were measured in antral follicles using qPCR. Data represent means ± SEM from 3 separate experiments (* indicates significant difference from vehicle controls; n=12–14 follicles per treatment; p ≤ 0.05 using linear regression).
3.3 Effect of MXC on CASP3/7 activity
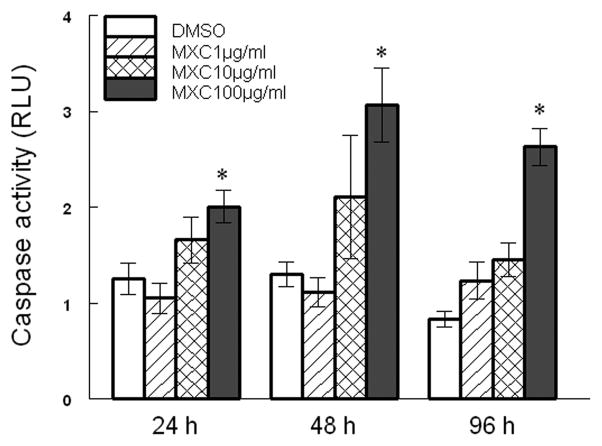
While MXC decreased mRNA expression levels of Casp3 at 48 h, it did not change Casp3 mRNA levels at a time that precedes the morphological onset of atresia. However, changes in CASP activities may be important for induction of atresia rather than changes in mRNA levels. Thus, we measured CASP3/7 activities in cultured antral follicles exposed to controls or MXC and found that MXC significantly increases CASP3/7 activities (Fig 6). Specifically, MXC (100 μg/ml) significantly increases CASP3/7 activities at 24, 48, and 96 h compared to DMSO (Fig 6).
Fig 6. Effect of MXC on CASP3/7 activities.
Antral follicles isolated from CD-1 mice were exposed to vehicle control (DMSO) or MXC (1–100 μg/ml) and cultured for 24, 48, or 96 h. At the end of culture, follicles were subjected to measurement of CASP3/7 activities. Data represent means ± SEM from 3 separate experiments (* indicates significant difference from vehicle control; n=12–14 follicles per treatment; p ≤ 0.05).
4 Discussion
The present studies were conducted to determine if short term exposure to MXC induces atresia of antral follicles and alters pro-apoptotic and anti-apoptotic factors. First, we examined the effects of both short term and long term exposure to MXC on antral follicles. We found that MXC induces atresia as early as 48 h and that it continues to do so for up to 96 h. These findings are consistent with previous studies indicating that MXC increases atresia of mouse antral follicles at 96 h of culture [7, 8, 20]. However, these studies also expand previous studies by showing that MXC induces atresia as early as 48 h. This suggests that short term exposure to MXC can quickly set into motion processes that result in death of antral follicles and that once the death process is initiated, it is not possible to stop it.
Given that MXC induces atresia in antral follicles, we next examined the effect of MXC on Bax and Bcl2 expression in follicles, as it is well known from previous studies that BAX and BCL2 are important regulators of apoptosis and atresia [21–30]. For example, previous studies have shown that Bax deficient mice contain fewer atretic follicles than WT mice [13]. Similarly, another study has shown that Bcl2 overexpression increases the number of primordial follicles at birth, possibly due to decreased apoptosis [11]. Further, mice deficient in Bcl2 have an increased number of unhealthy primordial follicles and an increased number of apoptotic oocytes [24]. In addition to being important regulators of natural atresia, BAX and BCL2 are involved in chemical induction of apoptosis [21, 25–27]. The polyaromatic hydrocarbon dimethylbenz(a)anthracene (DMBA) has been shown to increase Bax levels, inducing apoptosis in mouse oocytes [27]. DMBA also increases BAX and activated CASP3 immunostaining in granulosa cells of cultured rat antral follicles [28]. Another chemical, 4-vinylcyclohexene diepoxide, (VCD) also has been shown to induce atresia in primordial/primary follicles by altering expression of BCL2 family members [26]. Further, chromium has been shown to increase BAX and decrease BCL2 levels, inducing apoptosis in rat ovarian granulosa cells [25].
We found that MXC exposure increases Bax as early as 24 h, but it does not induce changes in Bcl2 expression at this time point. This increases the Bax/Bcl2 ratio at 24 h, which may lead to morphological atresia beginning at 48 h. We also found that MXC increases Bax (48 and 96 h), increases Bcl2 (96 h), and decreases Bax/Bcl2 ratios (96 h) at later time points. This may be a coordinated attempt by the live cells in the follicles in response to death of some of the cells in the follicles to rescue the entire follicle from MXC-induced atresia. However, our data indicate that once the higher dose of MXC (100 μg/ml) induces atresia at 48 h, the follicles cannot recover from atresia throughout the culture, but the follicles treated with lower doses of MXC (1 and 10 μg/ml) can recover from initial induction of atresia at 48 h. It is likely that any increase in anti-apoptotic factor Bcl2 cannot compensate for the pro-apoptotic effects of Bax. The changes we observed with Bax and Bcl2 in response to MXC in vitro are consistent with previous in vivo studies showing that exposure (20 days) to MXC induces atresia of antral follicles by increasing BAX and without altering BCL2 levels in the mouse ovary and that Bax deletion protects antral follicles from MXC-induced atresia [21]. The data are also consistent with previous in vitro studies showing that MXC increases Bax (48 h) and decreases Bcl2 (96 h) expression [8].
It is unclear whether MXC initiates atresia by increasing BAX expression in the granulosa cells, thecal cells, or the oocyte. Previous studies have shown that MXC induces atresia in the granulosa cells of the ovary [6, 8]. Further, studies have shown that other environmental chemicals (i.e., chromium and dimethylbenz(a)anthracene) alter BCL2 and BAX levels both in granulosa cells and oocytes [25, 27]. It is possible that chemicals initially induce atresia in the granulosa cells and that because granulosa cells are required for healthy oocytes and thecal cells [1], death of granulosa cells eventually leads to death in the oocytes and thecal cells.
Given that the pro-apoptotic factor Bax plays a decisive role in MXC-induced atresia, we wanted to expand our findings by determining whether other pro- and anti-apoptotic factors are involved in MXC-induced atresia. Previous studies have shown that Bcl-xL acts as anti-apoptotic factor by preventing apoptosis in the ovary [29]. Hence, the current studies measured Bcl-xL expression levels in response to MXC in the antral follicles of the ovary. Our data indicate that MXC increases Bcl-xL levels at 24 and 96 h. However, these changes in Bcl-xL expression are not consistent with the time course for onset of MXC-induced atresia. Thus, it is likely that Bcl-xL factors are not involved in MXC-induced atresia.
We also examined the effect of MXC on the pro-apoptotic factor Bok. This factor has been shown to induce apoptosis in reproductive tissues [14]. Hence, the current studies measured Bok expression in response to MXC in the ovary. Our data indicate that MXC decreased Bok levels at 24 h, but did not affect Bok expression at other time points. These changes in Bok at 24 h are not consistent with the morphological appearance of atresia that occurred at 48 h. Thus, it is possible that Bok is not involved in MXC-induced atresia and that the decreased Bok expression at 24 h was insufficient to rescue follicles from atresia that became evident at 48 h.
While changes in BCL2 family members help to regulate apoptosis, CASP3 is the critical rate limiting factor in the apoptotic pathway. In apoptotic cells, pro-CASP3 is cleaved and activated by CASP9, the activated CASP3 then autoregulates to increase the permeability of mitochondrial membrane by conversion of anti-apoptotic factors to pro-apoptotic factors, further amplifying CASP3 activity. Finally, CASP3 cleaves several proteins and DNA leading to formation of apoptotic bodies [30]. In the present study, we measured Casp3 mRNA and CASP3/7 activities in MXC treated follicles. Our data indicate that MXC decreases Casp3 mRNA levels compared to DMSO at 48 h in the follicles. In contrast, MXC increases CASP3/7 activities compared to DMSO at all the time points in the follicles. These data suggest that MXC-induced atresia is not regulated by changes in Casp3 mRNA expression. Instead, MXC-induced atresia may be regulated by increase in CASP3 activity. While, to our knowledge, no studies have examined the effects of MXC on CASP activities in the ovary, some studies have shown that other chemicals induce CASP3 activity or protein levels in the ovarian cells. Studies in granulosa cells have shown that acute exposure to molybdenum and chromium induce apoptosis by inducing CASP3 [25, 31]. Other studies have shown that exposure to VCD induces apoptosis in primordial/primary follicles by inducing CASP3 [32, 33]. Our data are consistent with these studies in that chemicals including MXC cause apoptosis by inducing CASP3 activity in follicles or granulosa cells.
In conclusion, the present studies provide evidence that short term exposure to MXC induces atresia as quickly as 48 h. The present studies also show that MXC exposure alters expression of key anti- and pro-apoptotic factors and increases CASP activity. At 24 h, MXC increases Bax levels and does not affect Bcl2 levels (Fig 7). This increases the Bax/Bcl2 ratio, which in turn may increase mitochondrial permeability, leading to activation of CASP3/7 activities. Thus, early changes in Bax expression and CASP3/7 activity may induce the onset of morphological atresia beginning at 48 h. Once MXC-induced atresia occurs, the follicles cannot recover and continue to undergo atresia at 96 h (Fig 7).
Fig 7. Mechanism of MXC-induced atresia in antral follicles.
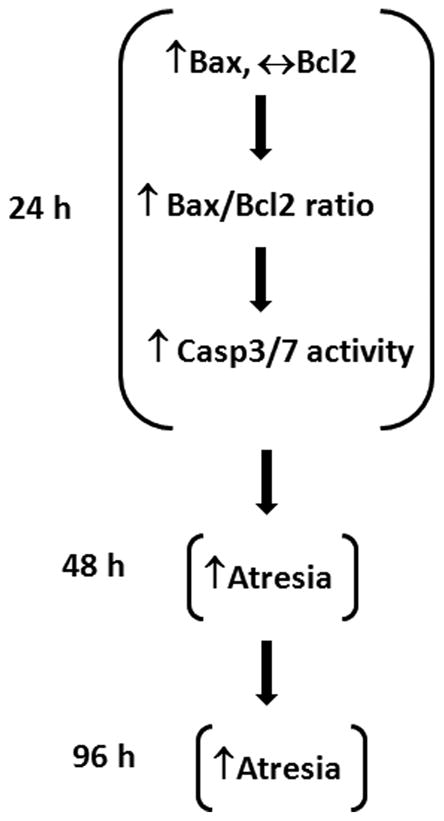
Schematic illustrates the proposed mechanism by which MXC induces atresia in mouse ovarian antral follicles.
Highlights.
Methoxychlor increases the Bax/Bcl2 ratio in antral follicles at 24 h.
Methoxychlor increases caspase activity in antral follicles at 24 h.
Methoxychlor-induced increases in the Bax/Bcl2 ratio and caspase activity may lead to atresia by 48 h.
Acknowledgments
Funding
This work was supported by National Institutes of Health (NIH) R01ES019178 (JAF), an Environmental Toxicology Fellowship from the Interdisciplinary Environmental Toxicology Program at UIUC (MSB), a Pre-doctoral Trainee Fellowship in Endocrine, Developmental, and Reproductive Toxicology from NIEHS (BNK), Post-doctoral Trainee Fellowships in Endocrine, Developmental, and Reproductive Toxicology from NIEHS (RKG and WW), and a Billie A Field Fellowship in Reproductive Biology (WW).
The authors thank Dr. Zelieann Craig, Dr. Tessie Paulose, Dr. Liying Gao, Jackye Peretz, and Sharon Meachum for technical help, support, and input throughout the project.
Footnotes
Conflict of interest
The authors declare that they do not have any conflicts of interest to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mallikarjuna S. Basavarajappa, Email: mbshivapur@gmail.com.
Bethany N. Karman, Email: bklement@illinois.edu.
Wei Wang, Email: weiwang2@illinois.edu.
Rupesh K. Gupta, Email: drrupesh@yahoo.com.
Jodi A. Flaws, Email: jflaws@illinois.edu.
References
- 1.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 2.ATSDR. Toxicological profile for methoxychlor. Atlanta, GA: Toxicological Profile for Methoxychlor; 2002. [Google Scholar]
- 3.Stuchal LD, Kleinow KM, Stegeman JJ, James MO. Demethylation of the pesticide methoxychlor in liver and intestine from untreated, methoxychlor-treated, and 3-methylcholanthrene-treated channel catfish (Ictalurus punctatus): Evidence for roles of CYP1 and CYP3A family isozymes. Drug Metab Dispos. 2006;34:932–8. doi: 10.1124/dmd.105.009068. [DOI] [PubMed] [Google Scholar]
- 4.Eroschenko VP, Swartz WJ, Ford LC. Decreased superovulation in adult mice following neonatal exposures to technical methoxychlor. Reprod Toxicol. 1997;11:807–14. doi: 10.1016/s0890-6238(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 5.Martinez EM, Swartz WJ. Effects of methoxychlor on the reproductive system of the adult female mouse 1. Gross and histologic observations. Reprod Toxicol. 1991;5:139–47. doi: 10.1016/0890-6238(91)90042-e. [DOI] [PubMed] [Google Scholar]
- 6.Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol Sci. 2002;68:473–8. doi: 10.1093/toxsci/68.2.473. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative Stress Pathway. Toxicol Sci. 2006;93:382–9. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- 8.Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and bax-mediated pathways. Toxicol Sci. 2005;88:213–21. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- 9.Hsu SY, Hsueh AJW. Tissue-specific Bcl-2 protein partners in apoptosis: an ovarian paradigm. Physiol Rev. 2000;80:593–614. doi: 10.1152/physrev.2000.80.2.593. [DOI] [PubMed] [Google Scholar]
- 10.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Flaws JA, Hirshfield AN, Hewitt JA, Babus JK, Furth PA. Effect of Bcl-2 on the primordial follicle endowment in the mouse ovary. Biol Reprod. 2001;64:1153–9. doi: 10.1095/biolreprod64.4.1153. [DOI] [PubMed] [Google Scholar]
- 12.Hsu SY, Lai RJ, Finegold M, Hsueh AJ. Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology. 1996;137:4837–43. doi: 10.1210/endo.137.11.8895354. [DOI] [PubMed] [Google Scholar]
- 13.Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–3. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- 14.Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJW. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc Natl Acad Sci U S A. 1997;94:12401–6. doi: 10.1073/pnas.94.23.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–85. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chedrese PJ, Feyles F. The diverse mechanism of action of dichlorodiphenyldichloroethylene (DDE) and methoxychlor in ovarian cells in vitro. Reprod Toxicol. 2001;15:693–8. doi: 10.1016/s0890-6238(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 18.Quigot N, Desmots S, Barouki R, Lemazurier A comparison of two human cell lines and two rat gonadal cell primary cultures as in vitro screening tools for aromatase modulation. Toxicol In Vitro. 2012;26:107–18. doi: 10.1016/j.tiv.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Basavarajappa MS, Craig ZR, Hernàndez-Ochoa I, Paulose T, Leslie TC, Flaws JA. Methoxychlor reduces estradiol levels by altering steroidogenesis and metabolism in mouse antral follicles in vitro. Toxicol Appl Pharmacol. 2011;253:161–9. doi: 10.1016/j.taap.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basavarajappa MS, Hernandez-Ochoa I, Wang W, Flaws JA. Methoxychlor inhibits growth and induces atresia through the aryl hydrocarbon receptor pathway in mouse ovarian antral follicles. Reprod Toxicol. 2012;34:16–21. doi: 10.1016/j.reprotox.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borgeest C, Miller KP, Gupta R, Greenfeld C, Hruska KS, Hoyer P, et al. Methoxychlor-induced atresia in the mouse involves Bcl-2 family members, but not gonadotropins or estradiol. Biol Reprod. 2004;70:1828–35. doi: 10.1095/biolreprod.103.022889. [DOI] [PubMed] [Google Scholar]
- 22.Basu A, Haldar S. The relationship between Bcl2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4:1099–109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- 23.Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–52. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 24.Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995;136:3665–8. doi: 10.1210/endo.136.8.7628407. [DOI] [PubMed] [Google Scholar]
- 25.Banu SK, Stanley JA, Lee J, Stephen SD, Arosh JA, Hoyer PB, Burghardt RC. Hexavalent chromium-induced apoptosis of granulosa cells involves selective sub-cellular translocation of Bcl-2 members, ERK1/2 and p53. Toxicol Appl Pharmacol. 2011;251:253–66. doi: 10.1016/j.taap.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu X, Christian P, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-xL protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001;65:1489–95. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- 27.Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu H-Y, et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–60. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 28.Tsai-Turton M, Nakamura BN, Luderer U. Induction of apoptosis by 9,10-dimethyl-1,2-benzanthracene in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione. Biol Reprod. 2007;77:442–51. doi: 10.1095/biolreprod.107.060368. [DOI] [PubMed] [Google Scholar]
- 29.Tilly JL, Tilly KI, Kenton ML, Johnson AL. Expression of members of the bcl-2 gene family in the immature rat ovary: equine chorionic gonadotropin-mediated inhibition of granulosa cell apoptosis is associated with decreased bax and constitutive bcl-2 and bcl-xlong messenger ribonucleic acid levels. Endocrinology. 1995;136:232–41. doi: 10.1210/endo.136.1.7828536. [DOI] [PubMed] [Google Scholar]
- 30.Cheng EHY, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–8. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 31.Kolesarova A, Capcarova M, Sirotkin AV, Medvedova M, Kalafova A, Filipejova T, et al. In vitro assessment of molybdenum-induced secretory activity, proliferation and apoptosis of porcine ovarian granulosa cells. J Environ Sci Health, Part A: Toxic/Hazard Subst Environ Eng. 2011;46:170–5. doi: 10.1080/10934529.2011.532430. [DOI] [PubMed] [Google Scholar]
- 32.Devine PJ, Sipes IG, Skinner MK, Hoyer PB. Characterization of a rat in vitro ovarian culture system to study the ovarian toxicant 4-vinylcyclohexene diepoxide. Toxicol Appl Pharmacol. 2002;184:107–15. [PubMed] [Google Scholar]
- 33.Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, et al. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol Appl Pharmacol. 2005;203:114–23. doi: 10.1016/j.taap.2004.07.010. [DOI] [PubMed] [Google Scholar]