Abstract
Objective
To assess the relative impact of clinical factors and medications on pain intensity, physical function, and health status in juvenile idiopathic arthritis (JIA).
Methods
We conducted a retrospective cross-sectional study of data from children with JIA enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. We tested whether clinical characteristics of JIA were associated with pain intensity, physical function, and health status using multivariable linear and ordinal logistic regression.
Results
During the study period, 2,571 JIA subjects enrolled in the CARRA Registry. Ratings of pain intensity, physical function, and health status differed significantly between JIA categories. In comparison to other categories of JIA, subjects with enthesitis-related arthritis (ERA) reported worse pain and function. In multivariable analyses, higher active joint count, and current NSAID, biologic, or corticosteroid use were associated with worse scores on all patient-reported measures. ERA and older age were significantly associated with higher pain intensity and poorer health status. Systemic JIA and uveitis were significantly associated with worse health status. Enthesitis, sacroiliac tenderness, and NSAID use were independently associated with increased pain intensity in ERA. The correlation between physician global assessment of disease activity and patient-reported pain intensity, physical function, and health status was low.
Conclusions
Significant differences of pain intensity, physical function, and health status exist among JIA categories. These results suggest that current treatments may not be equally effective for particular disease characteristics more common in specific JIA categories, such as enthesitis or sacroiliac tenderness in ERA.
Key Indexing terms: Juvenile Idiopathic Arthritis, pediatric rheumatic disease, pain, health status, epidemiology
Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatologic condition in childhood, affecting 1 to 4 per 1,000 children (1, 2). The term JIA describes a clinically heterogeneous group of diseases characterized by arthritis that begins before age 16, involves one or more joints, and lasts at least six weeks. Distinct clinical features characterize each of the JIA categories during the first 6 months of disease. The goal of treatment in JIA is to control inflammation and prevent morbidities such as vision loss, growth disturbances, joint contractures, joint destruction, and functional limitations. An increasing body of literature suggests that clinical examination and physician assessment of disease activity are insufficient for a complete understanding of the impact of disease on children with JIA. Therefore, a thorough assessment of treatment response includes not only physical examination components such as active joint count (AJC), but also patient-reported measures of pain, physical function, and health status. Differences in pain intensity, physical function, and health status in children across JIA categories have not been well described.
Several studies suggest that pain is highly prevalent in JIA, often persisting into adulthood (3–5). The results of several studies using various methodologies have shown that children with JIA have pain on most days in the mild to moderate range and that the amount of pain predicts activity limitations (6–8). Studies have demonstrated that pain in children with JIA is significantly associated with increased disease activity (9, 10) and reduced participation in school and social activities (8). Disease severity, however, only accounts for a small proportion of the variance in pain reported by children with JIA (6, 11–13). These reports suggest that there are many factors other than those identified by physical examination, physician global assessment (PGA), and disease activity scores that contribute to self-reported pain intensity in the JIA population.
Decreased physical function, like pain, is prevalent in JIA and not fully accounted for by physical examination findings. Previous studies show that only half of JIA patients have a Juvenile Arthritis Functionality Score of 0, indicating no disability (14) and that children with JIA have decreased physical function even in the absence of physical examination findings indicative of disease activity, including synovitis and joint contractures (15). Additionally, self-reported depression, anxiety, and behavioral problems are significantly associated with decreased physical function but not disease activity in children with polyarticular disease (16).
Health-related Quality of Life (HRQoL) and health status are also important components of the overall assessment of children with JIA (17). Several studies have reported that children with JIA have a poorer HRQoL in comparison to healthy children as well as children with other chronic diseases (7, 18–20). Significant predictors of HRQoL include pain, physical function, disease activity, school absences, and subjective burden of medication use (17, 20, 21).
For this study, we used a retrospective cross-sectional design to analyze data from the large Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry, which contains clinical and medication data from children with JIA enrolled at participating U.S. sites as well as several patient-reported outcomes. In our study, we aimed to: 1) characterize pain intensity, physical function, and health status in a large cross-sectional multicenter sample of children with JIA, 2) compare pain intensity, physical function, and health status across JIA categories, and 3) evaluate the relationship between PGA and pain intensity, physical function, and health status in JIA.
PATIENTS AND METHODS
This study was reviewed by The Children’s Hospital of Philadelphia institutional review board and was deemed “Not Human Subjects Research” because the data are retrospective and de-identified.
Patients
The source population for this study was subjects with JIA enrolled in the CARRA Registry between May 2010 and June 2011. The CARRA Registry is a multicenter observational database of U.S. children with rheumatic diseases. Sixty centers representing all major geographic regions of the U.S. contribute data to the registry. Subjects are a convenience sample enrolled without regard to disease duration, disease severity, current disease activity, or therapy. All subjects met International League Against Rheumatism (ILAR) criteria (22) for JIA according to the treating physician. The registry includes self- and physician-reported measures.
Measures
Pain intensity
At enrollment the subject or parent was asked, “How much pain have you [or your child] had because of your [his/her] rheumatic condition in the past week?” Pain intensity was reported using integers from 0 to 10, anchored by the words “No Pain” and “Very Severe Pain”. High correlation between the traditional 10-centimeter visual analogue scale (VAS) and the integer-based scale has been reported for the measurement of pain intensity (23–25).
Physical function
Subjects or parents rated the subject’s disease-related functional status during the past week using the Childhood Health Assessment Questionnaire (CHAQ) (26). The CHAQ is widely used in pediatric rheumatology research and clinical practice among children with JIA (27, 28). It comprises 30 questions, covering 8 functional ability domains related to daily living during the past week: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities. A disability index (DI) is calculated based on the mean of the 8 domains; the DI ranges from 0 to 3, with higher scores indicating worse disability (27).
Health status
Health status measures the parent/subject’s perception of his/her health taking into account personal values, cultural beliefs, goals, and concerns. At enrollment subjects or parents were asked, “How do you rate your [child’s] health?” Subjects and parents were given a 5-choice response scale with the following options: very poor, poor, good, very good, and excellent.
Disease characteristics and activity
Disease characteristics and activity were assessed at enrollment using a variety of sources including subject/parent report, physician assessment and physical examination, and chart abstraction. The duration of symptoms in years was reported by the subject/parent. The treating physician was responsible for determination of the following: 1) JIA ILAR category, 2) AJC, 3) presence of enthesitis, 4) presence of sacroiliac tenderness, and 5) PGA of disease severity. The AJC was defined as the number of joints with active arthritis; active arthritis was defined as a joint with 1) swelling or 2) tenderness with limited range of motion. The PGA of disease severity used integers from 0 to 10, anchored by the words “Not Active” and “Very Active”. The number of joints ever affected (<5 or ≥ 5), history of uveitis, and radiographic evidence of joint damage defined as joint narrowing, erosion, or ankylosis (present/absent) were abstracted from the subject’s medical record.
Medications
Past and current use of medications including daily NSAIDs, non-biologic disease modifying anti-rheumatic drugs (DMARD), biologics, and systemic corticosteroids were abstracted from the subject’s medical records. Medications in the non-biologic DMARD category include: sulfasalazine, methotrexate, leflunomide, cyclosporine, mycophenolate mofetil, cyclophosphamide, and azathioprine. Medications in the biologics category include: etanercept, adalimumab, abatacept, infliximab, rituximab, tocilizumab, rilonacept, golimumab, canakinumab, and anakinra.
Analysis
Differences in demographics and clinical characteristics between JIA categories were compared using the Kruskal-Wallis or chi-square test, as appropriate. We tested whether demographics and clinical characteristics of JIA were associated with pain intensity, physical function, and health status using multivariable linear (pain and function) and ordinal logistic (health status) regression. Predictor variables for pain intensity, physical function, and health status included the following: age at enrollment, sex, insurance status (uninsured or insured), physician-reported JIA ILAR category, duration of symptoms (years), AJC, number of joints ever affected (<5 or ≥ 5), radiographic evidence of joint damage (present/absent), current daily NSAID use (yes/no), current non-biologic DMARD use (yes/no), current biologic use (yes/no), and current systemic corticosteroid use (yes/no). Uveitis (past history, active, none) was also included as a variable in the model for health status. For all analyses the reference JIA category was oligoarticular-persistent. In the restricted analysis of subjects with enthesitis-related arthritis (ERA) additional variables included: current enthesitis (present/absent), current sacroiliac tenderness (present/absent), HLA-B27 positivity (yes/no), and inflammatory bowel disease (present/absent). Due to collinearity with AJC (correlation >0.5), PGA of disease was not used in any of the multivariable analyses.
Significant predictors in univariate analysis (p<0.05) were included in the initial multivariable models. The final multivariable models were built using a backward elimination, eliminating attributes with p values > 0.2 when the full and nested models were compared and found to have similar likelihood ratios. Standardized regression coefficients (betas) are reported for each analysis. For binary variables regression coefficients of 0.2, 0.5, and 0.8 are considered small, medium, and large associations, respectively (20, 29). For continuous predictors regression coefficients of 0.1, 0.3, and 0.5 are considered small, medium, and large associations, respectively (20, 29). For each regression, the explained variance (R square) was determined.
To test the association between PGA of disease activity and self-reported outcomes we performed pair-wise correlation tests between PGA of disease activity and self-reported arthritis-specific pain intensity, CHAQ, and health status. All analyses were performed using Stata 12 (StataCorp, College Station, TX, USA).
RESULTS
Subjects
During the 14-month study period there were 2,571 children with JIA enrolled in the CARRA Registry. The most common JIA categories were polyarticular RF-negative (N=761, 30%), oligoarticular-persistent (N= 697, 27%), and ERA (N=268, 10%) (Table 1). The median disease duration across JIA categories was 3.9 years (interquartile range (IQR): 1.9, 7.3). Seventy-three percent of subjects with JIA were female and 59% had a polyarticular course of disease (> 5 joints ever affected). Forty-seven percent had active arthritis and four percent had active enthesitis at the time of enrollment. Twenty-four and 42% of ERA patients had active enthesitis or a history of enthesitis, respectively. Enthesitis was most common in ERA, PsA, undifferentiated, and oligoarticular-extended JIA (Table 1); however, enthesitis was reported in all JIA categories. Three percent of subjects had active sacroiliac tenderness. Sacroiliac tenderness was most common in the ERA category. Eighteen and 39% of ERA patients had current or a history of sacroiliac tenderness on examination, respectively.
Table 1.
Patient characteristics by JIA category
| All | Systemic | Polyarticular RF-positive | Polyarticular RF-negative | Oligoarticular- persistent | Oligoarticular- extended | ERA | Undiff- erentiated | PsA | p- value* | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N (%) | 2571 | 232 (9) | 190 (7) | 761 (30) | 697 (27) | 210 (8) | 268 (10) | 52 (2) | 161 (6) | |
| Age of onset in years, median (IQR) | 5.4 (2.3, 9.9) | 4.4 (2.4, 8.5) | 11.1 (7.7, 13.7) | 5.2 (2.3, 9.4) | 3.6 (2.0, 6.9) | 2.8 (1.6, 6.0) | 10.4 (7.3, 12.8) | 6.6 (2.0,11.0) | 8.2 (2.8, 11.8) | <0.01 |
| Female, N (%) | 1879 (73) | 133 (57) | 167 (88) | 606 (80) | 536 (77) | 182 (87) | 111 (41) | 34 (65) | 110 (68) | <0.01 |
| Uninsured, N (%) | 40 (2) | 7 (3) | 12 (2) | 12 (2) | 7 (1) | 3 (1) | 5 (2) | 1 (2) | 1 (1) | 0.51 |
| Disease duration (years) | 3.9 (1.9, 7.3) | 4.2 (1.5, 8) | 3.5 (1.7, 5.8) | 4.5 (2.1, 7.9) | 3.2 (1.4, 6.2) | 5.8 (2.9, 9.5) | 3.3 (1.8, 6.0) | 3.3 (1.7, 6.7) | 4.0 (2.3, 7.7) | <0.01 |
| Active joint count, median (IQR) | 0 (0, 2) | 0 (0, 2) | 1 (0, 4) | 0 (0, 2) | 0 (0, 1) | 1 (0, 2) | 1 (0, 2) | 0 (0, 1) | 0 (0, 2) | <0.01 |
| ≥5 joints ever affected, median N(%) | 1,499 (59) | 158 (69) | 727 (96) | 177 (93) | 9 (1) | 182 (87) | 120 (45) | 27 (52) | 99 (62) | <0.01 |
| Radiographic evidence of joint damage, N (%) | 527 (21) | 62 (32) | 66 (40) | 151 (23) | 86 (15) | 52 (28) | 65 (27) | 9 (20) | 36 (26) | <0.01 |
| Enthesitis, N (%) | 98 (4) | 2 (1) | 2 (1) | 10 (1) | 2 (0.5) | 6 (3) | 62 (24) | 3 (6) | 11 (7) | <0.01 |
| Sacroiliac tenderness, N (%) | 73 (3) | 1 (<1) | 1 (1) | 12 (2) | 1 (1) | 2 (1) | 47 (18) | 0 (0) | 6 (4) | <0.01 |
| Uveitis | ||||||||||
| Active N (%) | 110 (4) | 0 (0) | 0 (0) | 26 (3) | 59 (9) | 12 (6) | 4 (2) | 2 (4) | 7 (4) | <0.01 |
| History N (%) | 175 (7) | 4 (2) | 2 (1) | 46 (6) | 67 (10) | 33 (16) | 12 (5) | 3 (6) | 8 (5) | |
RF= Rheumatoid factor, ERA= enthesitis-related arthritis, PsA= psoriatic arthritis, IQR= interquartile range.
Differences in demographics and clinical characteristics between the JIA categories were compared using the Kruskal-Wallis or chi-square test, as appropriate.
Pain intensity
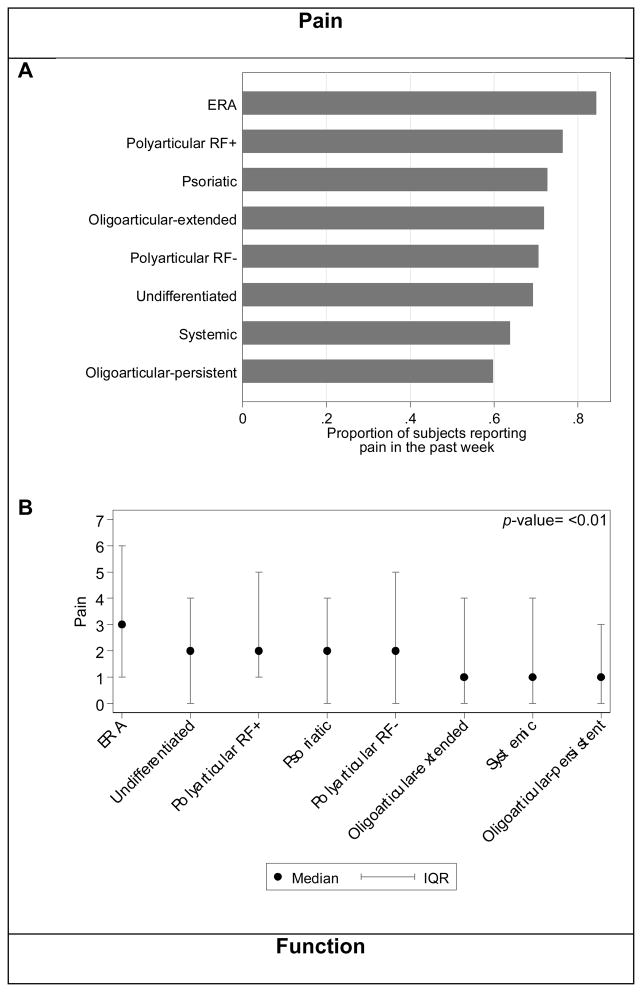
Sixty-nine percent of JIA subjects reported pain within the past week, defined as pain intensity greater than 0 on the numeric VAS scale. Figure 1A shows the proportion of children reporting pain within the past week by JIA category. The median pain intensity within the past week for all JIA subjects was 2 (IQR: 0, 4). This pain intensity is in accordance with previously published studies of children with JIA (6, 8, 30, 31). Pain intensity scores differed significantly between JIA categories (Figure 1B; p<0.001). In comparison to other categories of JIA, ERA subjects reported pain most frequently (N=226, 84%) and had the highest pain intensity (Figures 1A and B).
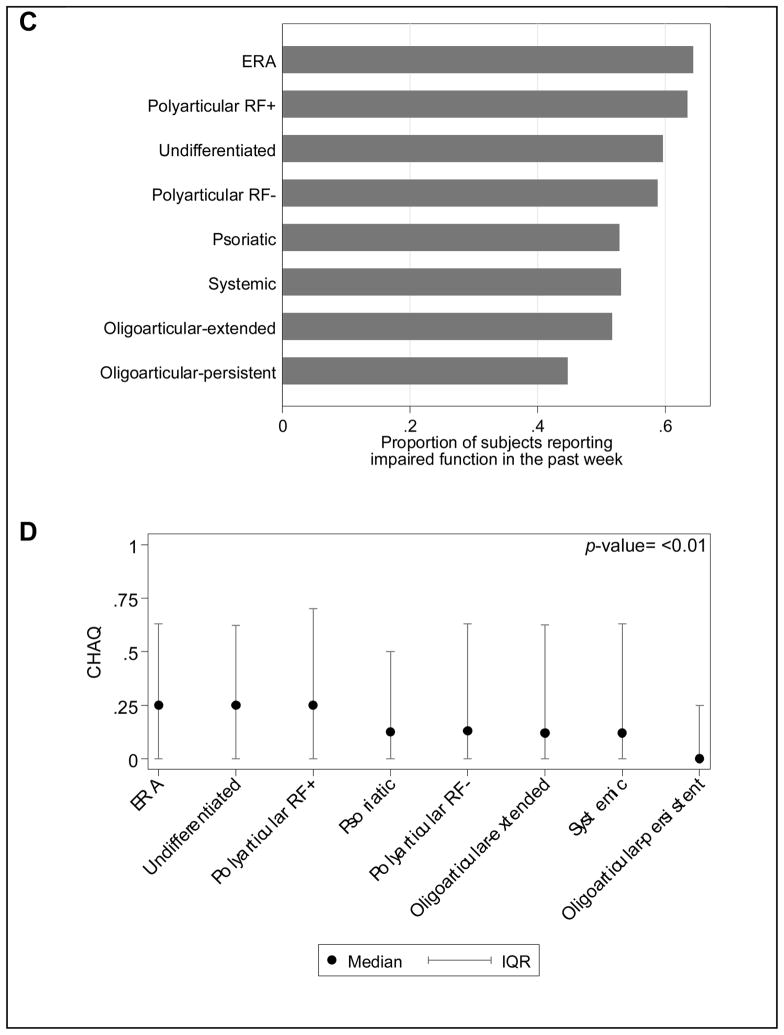
Figure 1. Pain and function by JIA category.
(A) Proportion of subjects reporting any pain within the past week. Pain was defined as a pain VAS >0. (B) Median and IQR of pain reported by VAS. Significant differences of pain existed across JIA categories (p-value <0.01). (C) Proportion of subjects reporting impaired function, as defined by CHAQ>0, within the past week. (D) Median and IQR of parent child function as reported by CHAQ. Significant differences of function exist across JIA categories using the Kruskal-Wallis test (p-value <0.01). Scores are not adjusted for subject characteristics or disease activity.
The results of univariate and multivariable analyses are shown in Table 2. After multivariable regression modeling significant clinical predictors of higher pain intensity were ERA disease category, older age, higher AJC, and current daily NSAID, biologic, and corticosteroid use. ERA had a medium strength association and current NSAID and corticosteroid use had a large association with increased pain intensity, whereby medium and large associations are defined by beta-coefficients greater than 0.5 and 0.8, respectively (20, 29). However, the multivariate model only accounted for 18% (R-square) of the variance in pain in accordance with previous studies demonstrating measurable clinical attributes such as disease activity and arthritis category only partially explain the distribution of self-reported pain intensity in JIA (6, 11).
Table 2.
Clinical variables associated with pain, function, and health status
| Pain Coefficient (95% CI) | Function Coefficient (95% CI) | Health status Coefficient (95% CI) | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | |
| ERA | 1.53 (1.17, 1.90) *** | 0.68 (0.21, 1.15) ** | 0.18 (0.11, 0.25) *** | 0.09 (−0.01, 0.19) | 0.93 (0.66, 1.19) *** | 0.41 (0.02, 0.79) * |
| Undifferentiated | 0.75 (0.02, 1.49) * | 0.06 (−0.89, 1.02) | 0.18 (0.04, 0.32) * | 0.09 (−0.10, 0.28) | 0.35 (−0.17, 0.87) | 0.24 (−0.48, 0.96) |
| Polyarticular RF-positive | 1.13 (0.71, 1.55) *** | 0.11 (−0.38, 0.59) | 0.24 (0.16, 0.32) *** | 0.00 (−0.12, 0.11) | 0.99 (0.69, 1.29) *** | 0.13 (−0.27, 0.54) |
| Psoriatic | 0.51 (0.06, 0.95) * | 0.42 (−0.14, 0.98) | 0.10 (0.01, 0.19) * | 0.05 (−0.07, 0.17) | 0.50 (0.19, 0.82) ** | 0.10 (−0.35, 0.55) |
| Polyarticular RF-negative | 0.66 (0.39, 0.93) *** | −0.02 (−0.35, 0.30) | 0.21 (0.15, 0.26) *** | 0.02 (−0.08, 0.11) | 0.61 (0.42, 0.80) *** | 0.24 (−0.02, 0.51) |
| Systemic | 0.54 (0.15, 0.93) ** | 0.11 (−0.30, 0.53) | 0.17 (0.10, 0.25) *** | 0.03 (−0.06, 0.13) | 0.72 (0.44, 1.00) *** | 0.46 (0.12, 0.80) ** |
| Oligoarticular - extended | 0.27 (−0.13, 0.67) | 0.15 (−0.28, 0.59) | 0.13 (0.05, 0.21) ** | 0.04 (−0.06, 0.15) | 0.21 (−0.07, 0.50) | 0.12 (−0.22, 0.47) |
| Older age (each additional year) | 0.10 (0.08, 0.12) *** | 0.04 (0.02, 0.07) *** | 0.01 (−0.00, 0.01) | --- | 0.07 (0.05, 0.08) *** | 0.05 (0.03, 0.07) *** |
| Symptom duration | 0.03 (0.01, 0.06) ** | --- | −0.01 (−0.01, 0.00) | --- | 0.02 (0.00, 0.04) | --- |
| Sex | 0.05 (−0.18, 0.28) | --- | 0.04 (−0.00, 0.09) | --- | 0.09 (−0.07, 0.25) | --- |
| Active joint count | 0.18 (0.15, 0.20) *** | 0.11 (0.08, 0.14) *** | 0.04 (0.04, 0.05) *** | 0.03 (0.03, 0.04) *** | 0.11 (0.09, 0.13) *** | 0.07 (0.05, 0.10) *** |
| ≥5 joints ever affected | 0.41 (0.20, 0.62) *** | --- | 0.17 (0.13, 0.21) *** | 0.06 (−0.01, 0.14) | 0.48 (0.33, 0.63) *** | --- |
| Imaging evidence of joint damage | 0.23 (−0.03, 0.49) | --- | 0.06 (0.01, 0.11) * | --- | 0.45 (0.27, 0.63) *** | --- |
| NSAIDs | 1.63 (1.44, 1.83) *** | 1.28 (1.04, 1.51) *** | 0.24 (0.20, 0.28) *** | 0.17 (0.13, 0.22) *** | 0.81 (0.66, 0.95) *** | 0.71 (0.52, 0.90) *** |
| DMARDs | 0.03 (−0.17, 0.24) | 0.39 (0.15, 0.64) ** | 0.06 (0.02, 0.10) ** | 0.09 (0.04, 0.14) *** | 0.30 (0.15, 0.44) *** | 0.45 (0.25, 0.65) *** |
| Biologics | 0.23 (0.02, 0.44) * | 0.94 (0.57, 1.31) *** | 0.08 (0.04, 0.12) *** | 0.22 (0.15, 0.30) *** | 0.48 (0.33, 0.63) *** | 0.70 (0.39, 1.01) *** |
| Corticosteroids | 1.71 (1.35, 2.08) *** | --- | 0.40 (0.33, 0.48) *** | 0.03 (−0.01, 0.08) | 1.23 (0.94, 1.51) *** | 0.14 (−0.05, 0.33) |
| Insured | −0.29 (−1.11, 0.53) | --- | −0.06 (−0.23, 0.10) | --- | 0.01 (−0.57, 0.60) | --- |
| Uveitis | ||||||
| Current | --- | --- | --- | --- | 0.01 (−0.34, 0.37) | 0.53 (0.09, 0.97) * |
| Past | --- | --- | --- | --- | −0.44 (−0.72, −0.15) ** | −0.09 (−0.44, 0.27) |
p<0.05,
p<0.01,
p<0.001. For all analyses the reference JIA category is oligoarticular-persistent JIA. Significant predictors in univariate analysis (p<0.05) were included in initial multivariable models. The final multivariable models were built using a backward elimination, eliminating attributes with p values > 0.2 when the full and nested models were compared. Only results for variables included in the final models are listed in the multivariable analyses columns. For binary variables regression coefficients of 0.2, 0.5, and 0.8 are considered small, medium, and large associations, respectively (16, 25). For continuous predictors regression coefficients of 0.1, 0.3, and 0.5 are considered small, medium, and large associations, respectively (16, 25).
Since ERA was associated with increased pain intensity in comparison to other JIA categories we investigated if particular disease characteristics more common in ERA, such as enthesitis, sacroiliac tenderness, HLA-B27 positivity, and concurrent inflammatory bowel disease, contributed to this association. Results of univariate and multivariable analyses are shown in Table 3. Active enthesitis, active sacroiliac tenderness, and current daily NSAID use were significantly and independently associated with increased pain intensity in subjects with ERA. Of these associations, enthesitis and sacroiliac tenderness were the strongest.
Table 3.
Clinical variables associated with pain in ERA
| Pain VAS Coefficient (95% CI) | ||
|---|---|---|
|
| ||
| Univariate | Multivariable | |
| Older age (years) | 0.11 (0.01, 0.21) * | 0.06 (−0.07, 0.20) |
| Symptom duration | 0.08 (−0.03, 0.18) | --- |
| Sex | 0.55 (−0.14, 1.23) | --- |
| Active joint count | 0.21 (0.07, 0.36) ** | 0.18 (−0.04, 0.40) |
| ≥5 joints ever affected | −0.19 (−0.87, 0.50) | --- |
| Imaging evidence of joint damage | −0.41 (−1.22, 0.40) | --- |
| NSAIDs | 1.27 (0.60, 1.93) *** | 1.77 (0.86, 2.69) *** |
| DMARDs | 0.23 (−0.45, 0.91) | --- |
| Biologics | 0.11 (−0.58, 0.80) | --- |
| Corticosteroids | 1.43 (0.06, 2.81) * | 0.60 (−0.83, 2.03) |
| Insured | 2.97 (0.50, 5.44) * | 2.54 (−0.16, 5.23) |
| Enthesitis | 1.72 (0.93, 2.50) *** | 1.49 (0.18, 2.79) * |
| Sacroiliac tenderness | 2.15 (1.30, 3.00) *** | 1.77 (0.39, 3.15) * |
| HLA-B27 positivity | −0.77 (−1.48, −0.06) * | 0.32 (−0.63, 1.26) |
| Inflammatory bowel disease | −0.57 (−1.56, 0.42) | --- |
p<0.05,
p<0.01,
p<0.001. Significant predictors in univariate analysis (p<0.05) were analyzed further in multivariable analyses. The final multivariable model was built using a backward elimination, eliminating attributes with p values > 0.2 when the full and nested models were compared. Only results for variables included in the final model are listed in the multivariable analysis column.
Physical function
Fifty-four percent of JIA subjects reported impaired function within the past week, defined as a CHAQ greater than 0. Figure 1C shows the proportion of children reporting impaired function by JIA category. The median CHAQ score for all JIA patients was 0.125 (IQR: 0, 0.75), but differed significantly between JIA categories (Figure 1D). The JIA category with the largest proportion of children reporting impaired function was ERA (N=172, 64%).
The results of univariate and multivariable analyses are shown in Table 2. After multivariable regression modeling and controlling for disease severity, significant clinical predictors of higher CHAQ scores (more impaired physical function) were a higher AJC, and current NSAID, biologic, and corticosteroid use. The multivariate model accounted for 19% (R-square) of the variance in function.
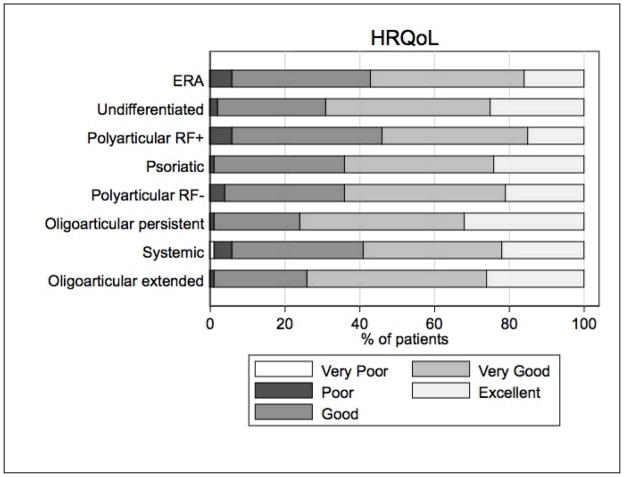
Health status
The median health status for all JIA patients was 2 or “very good” (IQR: 2, 3) (Figure 2). Scores on health status differed significantly between JIA categories (p<0.01) (Figure 2). The results of univariate and multivariable analyses are shown in Table 2. After multivariable regression modeling, significant clinical predictors of a higher (worse) health status score were ERA or systemic categories of JIA, older age, higher AJC, current uveitis, and current NSAID, biologic, or corticosteroid use. However, the multivariate model only accounted for 7% (R-square) of the variance in health status.
Figure 2. Health status by JIA category.
The health status question uses a 5-choice response scale, very poor, poor, good, very good, and excellent. Percent based on total number of patients in each JIA category.
Association of physician global assessment (PGA) of disease activity and parent/patient reported outcomes
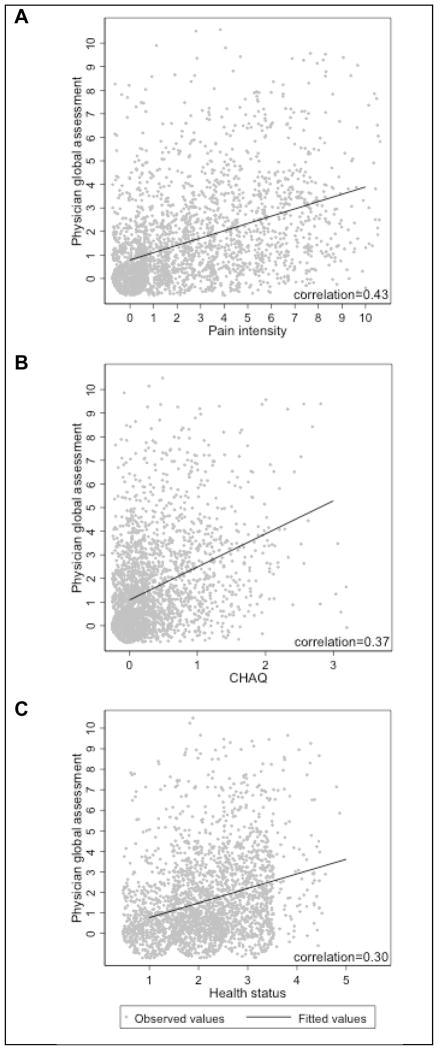
The median PGA for all JIA categories was 1 (IQR: 0,2). The pair-wise correlation between PGA of disease activity and pain, function, and health status scores were low (0.43, 0.37, and 0.30, respectively). The relationship between the PGA and pain, function, and health status are shown in Figure 3.
Figure 3. Correlation of PGA with self-reported outcomes.
Pair-wise correlation between PGA of disease activity and (A) pain intensity, (B) physical function as measured by the CHAQ, and (C) health status. The jitter function was used to randomly place observed values around their true value to avoid overlap with identical values, thus depicting the amount of data at a given value.
DISCUSSION
This retrospective cross-sectional study of enrollment data for children with JIA from a large national registry demonstrates significant differences in pain intensity, physical function, and health status across JIA categories. Despite low disease activity as measured by PGA, pain and decreased function were present in the majority of JIA subjects within the past week. A higher proportion of subjects with ERA reported pain and decreased function than subjects with other JIA categories. Additionally, subjects with ERA reported higher levels of pain intensity than subjects with other JIA categories. Multivariable regression modeling identified higher AJC, and current NSAID, biologic, or corticosteroid use as predictors of worse scores on all three self-reported measures – pain intensity, physical function, and health status. ERA and older age were significantly associated with increased pain intensity and decreased health status. Systemic JIA and uveitis were significantly associated with decreased health status. Active enthesitis, sacroiliac tenderness and current NSAID use were significantly and independently associated with pain intensity in subjects with ERA.
The correlation between PGA of disease activity and self-reported measurements of pain intensity, physical function, and health status were low. This finding highlights the importance of collecting patient-reported outcomes both in clinical practice and in registries as they provide different qualitative information than the PGA. Additionally, our models accounted for 18%, 19%, and 7% of the variance in pain, function, and health status, respectively, suggesting that clinical examination, PGA, and disease attributes are insufficient for a complete understanding of the impact of disease on children with JIA. Other factors that might impact these self-reported outcomes that were not studied include stress, mood, and emotion regulation.
Our findings should be interpreted in the context of several limitations. First, the CARRA Registry consists of a convenience sample of JIA patients; however, subjects are recruited without regard to disease duration, disease severity, current disease activity, or therapy. However, it is unknown if disease severity or treatments differed in children who were and were not enrolled in the registry at the same clinical site. However, selection bias was likely center-specific and diminished by the large number of contributing clinical centers. Second, treating physicians likely have made some errors with ILAR JIA category assignment. If the diagnosis of ERA relies on having tender entheses (and the child is actually tender everywhere due to pain amplification and not true ERA by ILAR criteria) there may be differential misclassification. Future studies should assess the validity of physician-assigned JIA categories in the CARRA registry. Third, the treating pediatric rheumatologist ascertained AJC and presence of enthesitis during routine clinical care. All pediatric rheumatologists contributing data to the CARRA Registry perform a standard joint examination to assess for arthritis and use standard measures to define active arthritis (22). However, currently there are no standardized methods of assessing or defining pediatric enthesitis. Therefore, the interpretation of enthesitis likely varies amongst physicians. Future development of a standardized enthesitis assessment either by physical examination or imaging is vital. Lastly, the presence of fibromyalgia tender points and diagnoses of amplified pain or fibromyalgia are not currently captured in the CARRA registry.
Despite these caveats, our study indicates that significant differences exist across JIA categories in the patient-reported outcomes of pain intensity, physical function, and health status. Further, a higher proportion of subjects with ERA reported increased pain and decreased function than subjects with other the JIA categories. These differences suggest that 1) our current treatments may not be equally effective for each of the JIA categories, 2) our current treatments may not be as effective for particular disease characteristics more common in specific JIA categories, such as enthesitis or limited back mobility in ERA (32), or 3) we are treating certain JIA categories more aggressively and earlier than others resulting in improved self-reported outcomes. Therefore, future comparative effectiveness and clinical trials should be powered to perform subgroup analyses rather than grouping all categories of JIA together.
Our study also demonstrates the ERA category of JIA is a significant predictor of increased pain intensity and poorer health status. Active enthesitis, sacroiliac tenderness and current NSAID use were significantly and independently associated with pain intensity in subjects with ERA. This finding is not unexpected as enthesitis has a major impact on function and quality of life in adults with ankylosing spondylitis (AS) (33). In comparison to adults with AS who do not have enthesitis, those with enthesitis show significantly worse scores on the Bath Ankylosing Spondylitis Functional Index, and the AS-Specific Quality of Life Index (33). Our results confirm these findings in children and demonstrate the compelling need for a standardized assessment of enthesitis as part of the routine evaluation of children with JIA.
We also found a low correlation between standardized scores of PGA of disease activity and self-reported measurements of pain intensity, physical function, and health status as previously reported in rheumatoid arthritis (34) and JIA (35). These findings underscore the importance of collecting self-reported outcomes in clinical practice, registries such as the CARRA registry, and clinical trials as they provide different qualitative information than the PGA.
In summary, this study demonstrates significant differences in self-reported outcomes between JIA categories. ERA subjects reported more frequent and more intense pain as well as more frequent decreased physical function than subjects with other JIA categories. Enthesitis and sacroiliac tenderness were significantly and independently associated with pain in subjects with ERA. Our findings suggest that current treatment strategies may not be equally aggressive or effective across JIA categories. Future comparative effectiveness studies and clinical trials should carefully account for JIA categorization and test therapeutic strategies to improve self-reported outcomes in JIA and ERA in particular in order to optimize the health status of children with JIA.
Acknowledgments
Grant support: The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry is supported by NIH grant RC2AR058934. CARRA is supported by grants from the Arthritis Foundation and Friends of CARRA. PF Weiss is supported by NIAMS NIH grant 1-K23-AR059749-01A1. LE Schanberg is supported by NIH grants RC2AR058934 and RO1 AR053845.
The authors thank Chris Feudtner, Andrew Klink, and Robert Ward for their critical review of this manuscript and the following CARRA Registry site principal investigators: L Abramson, B Adams, J Birmingham, P Blier, S Bowyer, E Chalom, F Dedeoglu, P Ferguson, B Gottlieb, T Graham, M Klein-Gitelman, D Goldsmith, G Higgins, JR Hollister, J Hsu, A Huttenlocher, N Ilowite, L Imundo, C Inman, T Jerath, L Jung, P Kahn, D Kingsbury, A Lasky, T Lehman, C Lindsley, J Lopez-Benitez, D McCurdy, N Moorthy, B Myones, K Nanda, J Olson, K O’Neil, K Onel, K Peterson, S Prahalad, M Punaro, A Quintero, C Rabinovich, A Reed, S Ringold, D Rothman, N Ruth, C Sandborg, E von Scheven, K Schikler, D Sherry, N Singer, S Spalding, R Syed, K Torok, R Vehe, C Wallace, J Weiss, A White, A Yalcindag, and L Zemel.
References
- 1.Bowyer S, Roettcher P. Pediatric rheumatology clinic populations in the United States: results of a 3 year survey. Pediatric Rheumatology Database Research Group. J Rheumatol. 1996;23(11):1968–74. [PubMed] [Google Scholar]
- 2.Manners PJ, Diepeveen DA. Prevalence of juvenile chronic arthritis in a population of 12-year-old children in urban Australia. Pediatrics. 1996;98(1):84–90. [PubMed] [Google Scholar]
- 3.Ostlie IL, Aasland A, Johansson I, Flato B, Moller A. A longitudinal follow-up study of physical and psychosocial health in young adults with chronic childhood arthritis. Clinical and experimental rheumatology. 2009;27(6):1039–46. [PubMed] [Google Scholar]
- 4.Packham JC, Hall MA, Pimm TJ. Long-term follow-up of 246 adults with juvenile idiopathic arthritis: predictive factors for mood and pain. Rheumatology. 2002;41(12):1444–9. doi: 10.1093/rheumatology/41.12.1444. [DOI] [PubMed] [Google Scholar]
- 5.Peterson LS, Mason T, Nelson AM, O’Fallon WM, Gabriel SE. Psychosocial outcomes and health status of adults who have had juvenile rheumatoid arthritis: a controlled, population-based study. Arthritis and rheumatism. 1997;40(12):2235–40. doi: 10.1002/art.1780401219. [DOI] [PubMed] [Google Scholar]
- 6.Schanberg LE, Lefebvre JC, Keefe FJ, Kredich DW, Gil KM. Pain coping and the pain experience in children with juvenile chronic arthritis. Pain. 1997;73(2):181–9. doi: 10.1016/S0304-3959(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez-Suarez R, Pistorio A, Cespedes Cruz A, Norambuena X, Flato B, Rumba I, et al. Health-related quality of life of patients with juvenile idiopathic arthritis coming from 3 different geographic areas. The PRINTO multinational quality of life cohort study. Rheumatology. 2007;46(2):314–20. doi: 10.1093/rheumatology/kel218. [DOI] [PubMed] [Google Scholar]
- 8.Schanberg LE, Anthony KK, Gil KM, Maurin EC. Daily pain and symptoms in children with polyarticular arthritis. Arthritis and rheumatism. 2003;48(5):1390–7. doi: 10.1002/art.10986. [DOI] [PubMed] [Google Scholar]
- 9.Varni J, Thompson K, Hanson V. The Varni/Thompson pediatric pain questionnaire. I. Chronic musculoskeletal pain in juvenile rheumatoid arthritis. Pain. 1987;28:27. doi: 10.1016/0304-3959(87)91056-6. [DOI] [PubMed] [Google Scholar]
- 10.Vandvik IH, Eckblad G. Relationship between pain, disease severity and psychosocial function in patients with juvenile chronic arthritis (JCA) Scandinavian journal of rheumatology. 1990;19(4):295–302. doi: 10.3109/03009749009102536. [DOI] [PubMed] [Google Scholar]
- 11.Ilowite NT, Walco GA, Pochaczevsky R. Assessment of pain in patients with juvenile rheumatoid arthritis: relation between pain intensity and degree of joint inflammation. Annals of the rheumatic diseases. 1992;51(3):343–6. doi: 10.1136/ard.51.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagglund KJ, Schopp LM, Alberts KR, Cassidy JT, Frank RG. Predicting pain among children with juvenile rheumatoid arthritis. Arthritis Care Res. 1995;8(1):36–42. doi: 10.1002/art.1790080109. [DOI] [PubMed] [Google Scholar]
- 13.Thompson KL, Varni JW, Hanson V. Comprehensive assessment of pain in juvenile rheumatoid arthritis: an empirical model. J Pediatr Psychol. 1987;12(2):241–55. doi: 10.1093/jpepsy/12.2.241. [DOI] [PubMed] [Google Scholar]
- 14.Filocamo G, Schiappapietra B, Bertamino M, Pistorio A, Ruperto N, Magni-Manzoni S, et al. A new short and simple health-related quality of life measurement for paediatric rheumatic diseases: initial validation in juvenile idiopathic arthritis. Rheumatology (Oxford) 2010;49(7):1272–80. doi: 10.1093/rheumatology/keq065. [DOI] [PubMed] [Google Scholar]
- 15.Miller ML, Kress AM, Berry CA. Decreased physical function in juvenile rheumatoid arthritis. Arthritis Care Res. 1999;12(5):309–13. doi: 10.1002/1529-0131(199910)12:5<309::aid-art1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Ding T, Hall A, Jacobs K, David J. Psychological functioning of children and adolescents with juvenile idiopathic arthritis is related to physical disability but not to disease status. Rheumatology. 2008;47(5):660–4. doi: 10.1093/rheumatology/ken095. [DOI] [PubMed] [Google Scholar]
- 17.Shaw KL, Southwood TR, Duffy CM, McDonagh JE. Health-related quality of life in adolescents with juvenile idiopathic arthritis. Arthritis and rheumatism. 2006;55(2):199–207. doi: 10.1002/art.21852. [DOI] [PubMed] [Google Scholar]
- 18.Willems DC, Joore MA, Nieman FH, Severens JL, Wouters EF, Hendriks JJ. Using EQ-5D in children with asthma, rheumatic disorders, diabetes, and speech/language and/or hearing disorders. Int J Technol Assess Health Care. 2009;25(3):391–9. doi: 10.1017/S0266462309990171. [DOI] [PubMed] [Google Scholar]
- 19.Norrby U, Nordholm L, Andersson-Gare B, Fasth A. Health-related quality of life in children diagnosed with asthma, diabetes, juvenile chronic arthritis or short stature. Acta Paediatr. 2006;95(4):450–6. doi: 10.1080/08035250500437499. [DOI] [PubMed] [Google Scholar]
- 20.Haverman L, Grootenhuis MA, van den Berg JM, van Veenendaal M, Dolman KM, Swart JF, et al. Predictors of health-related quality of life in children and adolescents with juvenile idiopathic arthritis: Results from a web-based survey. Arthritis Care Res (Hoboken) 2012 doi: 10.1002/acr.21609. [DOI] [PubMed] [Google Scholar]
- 21.Sawyer MG, Carbone JA, Whitham JN, Roberton DM, Taplin JE, Varni JW, et al. The relationship between health-related quality of life, pain, and coping strategies in juvenile arthritis--a one year prospective study. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2005;14(6):1585–98. doi: 10.1007/s11136-004-7710-3. [DOI] [PubMed] [Google Scholar]
- 22.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 23.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56(2):217–26. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 25.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10(4):390–2. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 26.Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37(12):1761–9. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 27.Ruperto N, Ravelli A, Pistorio A, Malattia C, Cavuto S, Gado-West L, et al. Cross-cultural adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries. Review of the general methodology. Clin Exp Rheumatol. 2001;19(4 Suppl 23):S1–9. [PubMed] [Google Scholar]
- 28.Pouchot J, Ecosse E, Coste J, Guillemin F. Validity of the childhood health assessment questionnaire is independent of age in juvenile idiopathic arthritis. Arthritis Rheum. 2004;51(4):519–26. doi: 10.1002/art.20529. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. The statistical power of abnormal-social psychological research: a review. J Abnorm Soc Psychol. 1962;65:145–53. doi: 10.1037/h0045186. [DOI] [PubMed] [Google Scholar]
- 30.Lovell DJ, Walco GA. Pain associated with juvenile rheumatoid arthritis. Pediatric clinics of North America. 1989;36(4):1015–27. doi: 10.1016/s0031-3955(16)36734-7. [DOI] [PubMed] [Google Scholar]
- 31.Sherry D, Bohnsack J, Salmonson K. Painless juvenile rheumatoid arthritis. J Pediatr. 1989;116:921. doi: 10.1016/s0022-3476(05)80652-3. [DOI] [PubMed] [Google Scholar]
- 32.Donnithorne KJ, Cron RQ, Beukelman T. Attainment of inactive disease status following initiation of TNF-alpha inhibitor therapy for juvenile idiopathic arthritis: enthesitis-related arthritis predicts persistent active disease. The Journal of rheumatology. 2011;38(12):2675–81. doi: 10.3899/jrheum.110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maksymowych WP, Mallon C, Morrow S, Shojania K, Olszynski WP, Wong RL, et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index. Ann Rheum Dis. 2009;68(6):948–53. doi: 10.1136/ard.2007.084244. [DOI] [PubMed] [Google Scholar]
- 34.Rohekar G, Pope J. Test-retest reliability of patient global assessment and physician global assessment in rheumatoid arthritis. The Journal of rheumatology. 2009;36(10):2178–82. doi: 10.3899/jrheum.090084. [DOI] [PubMed] [Google Scholar]
- 35.Papsdorf V, Horneff G. Complete control of disease activity and remission induced by treatment with etanercept in juvenile idiopathic arthritis. Rheumatology. 2011;50(1):214–21. doi: 10.1093/rheumatology/keq292. [DOI] [PubMed] [Google Scholar]