Abstract
Studies on well characterized, large populations of estrogen receptor (ER)/progesterone receptor (PgR)/HER2-negative [triple-negative (TN)] breast cancer (BC) patients with long-term follow-up are lacking. In this study, we analyze clinical outcomes of TN BC and implications of epidermal growth factor receptor (EGFR) expression. Clinical and biologic features, time to first recurrence (TTFR), and overall survival (OS) were compared in 253 TN versus 1,036 ER positive, PgR positive, HER2-negative [estrogen-driven (ED)] BC. Compared to ED, TN tumors were larger (p = 0.02), more proliferative (high S-phase 54 vs. 17 %, p < 0.0001), more aneuploid (64 vs. 43 %, p < 0.0001) and more likely EGFR positive (≥10 fmol/mg by radioligand-binding assay, 49 vs. 7 %, p < 0.0001). Among TN, EGFR-positive BC were larger (p = 0.0018), more proliferative (p < 0.0001), and more aneuploid, (p < 0.0001) than EGFR-negative BC. Adjuvant-treated TN patients had shorter TTFR (p = 0.0003), and OS (p = 0.0017), than ED patients. However, in untreated patients, no differences in TTFR and OS were observed at 8 years median follow-up. Among TN patients, EGFR expression was not associated with worse outcome. TN tumors have a worse outcome in systemically treated patients but not in untreated patients. EGFR expression, does not predict for worse long-term survival.
Keywords: Basal-like breast cancer, EGFR, Estrogen receptor, HER2, Progesterone receptor, Triple-negative breast cancer
Introduction
Assessment of estrogen receptor (ER), progesterone receptor (PgR), and HER2 expression is a central part of the pathological work-up for breast cancer (BC) patients. ER, PgR, and HER2 expression allows patients allocation in three main groups: (i) ER/PgR positive (estrogen-driven ED tumors) accounting for 75–80 % of BC, who will receive endocrine treatment; (ii) HER2 positive (HER2-driven tumors) accounting for 15–20 % of BC, who will receive HER2 target therapy such as trastuzumab or lapatinib and (iii) a group accounting for 10–15 % of BC not expressing either ER/PgR or HER2 (the so-called “triple-negative tumors” TN) for whom no target therapy is available and chemotherapy remains the systemic treatment of choice.
Gene expression studies using DNA microarrays have identified at least five molecular subclasses of BC with distinct features: luminal A and B (ER+/PgR+/HER2−), HER2 overexpressing (ER−/PgR−/HER2+), basal-like (ER−/PgR−/HER2− and basal cytokeratins+), and normal-like BC [1, 2]. The TN and the basal-like subgroups share common features (i.e., lack of ER, PgR, and HER2 expression) and, consequently, have been often referred as synonymous [3, 4]. Although there is extensive overlap between the TN and basal-like BC, it is not complete. Basal-like BC is a more homogeneous group of tumors, while TN group can comprise also other subtypes of BC with distinct biologic features [5–7]. Recently an IHC surrogate to define basal cancers has been suggested by Nielsen et al. [8] using ER, PgR, HER2, EGFR, and cytokeratins (CK) 5/6. Despite this, patients are routinely assessed as TN exclusively on the basis of ER, PgR, and HER2 determination. Numerous studies have shown that TN tumors and basal-like tumors display features of tumor aggressiveness and poor outcome compared to other subtypes [9]. However, most of these studies rely either on limited cohorts of patients or are derived from patient populations treated with chemotherapy and/or endocrine therapy and therefore cannot derive pure prognostic information.
We sought to determine the clinical and biologic features of TN and ED BC in a well-characterized cohort of BC patients comprising a large group of systemically untreated patients. Full information about ER/PgR and HER2 status along with other biological characteristics were available.
Methods
Patient data and specimens
The Lester and Sue Smith Breast Center at Baylor College of Medicine maintains a database of BC patients whose tissue specimens were originally sent to a central reference laboratory for steroid receptor assays at the Nichols Institute in California. Follow-up information and pathologic characteristics were obtained from tumor registries, medical records, or by data collection forms completed by the referring physicians. This database contains information on 47,286 patients diagnosed between 1984 and 1999 with early breast cancer (stage I–IIIA). This repository has been reviewed by Institutional Review Boards at the University of Texas Health Science Center at San Antonio and at Baylor College of Medicine and both boards provided a waiver of informed consent. No patient identifiers were provided to the authors.
Statistical methods
Of 2,567 patients with complete ER and PgR information in our database, 2,200 had complete data on ER, PgR, and HER2. Among those, 253 were TN (ER/PgR/HER2 negative) and 1,036 were ED (ER/PgR+HER2 negative). One thousand two-hundred seventy-eight subjects in the data set did not fall into one of these categories (Table 1).
Table 1.
Distributions of ER, PgR, and HER2 status
| N | (%) | |
|---|---|---|
| ER+/PgR+/HER2+ | 176 | 8.0 |
| ER+/PgR+/HER2− | 1,036 | 47.1 |
| ER+/PgR−/HER2+ | 130 | 5.9 |
| ER+/PgR−/HER2− | 481 | 21.9 |
| ER−/PgR+/HER2+ | 10 | 0.5 |
| ER−/PgR+/HER2− | 41 | 1.9 |
| ER−/PgR−/HER2+ | 73 | 3.3 |
| ER−/PgR−/HER2− | 253 | 11.5 |
| Total | 2,200 | 100 |
N number of patients, ER estrogen receptor, PgR progesterone receptor, HER2 human epidermal growth factor receptor
The patient and tumor characteristics were summarized for 1,289 patients with TN and ED tumors, and the relationships between these characteristics and TN or ED status were examined using descriptive statistics and the Chi-squared test (Table 2). Next, the variables of interest were compared in TN patients by EGFR, ploidy status and S-phase status using descriptive statistics and the Chi-squared test.
Table 2.
Patient and tumor characteristics
| TN N = 253 | ED N = 1,036 | p Value | |
|---|---|---|---|
| Age | |||
| N. Tested | 251 | 1,030 | |
| Median age | 52 | 63 | |
| <50 years | 112 (44.6 %) | 250 (24.3 %) | <0.0001 |
| ≥50 years | 139 (55.4 %) | 780 (75.7 %) | |
| Race | |||
| N. Tested | 209 | 890 | |
| White | 178 (85.2 %) | 841 (94.5 %) | <0.0001 |
| Black | 26 (12.4 %) | 36 (4.0 %) | |
| Other | 5 (2.4 %) | 13 (1.5 %) | |
| Menopausal status | |||
| N.Tested | 156 | 709 | |
| Pre | 42 (26.9 %) | 63 (8.9 %) | <0.0001 |
| Post | 114 (73.1 %) | 646 (91.1 %) | |
| Histology | |||
| N. Tested | 253 | 1,036 | |
| IDC | 219 (86.6 %) | 844 (81.5 %) | 0.0187 |
| ILC | 10 (4.0 %) | 94 (9.1 %) | |
| Other | 24 (9.5 %) | 98 (9.5 %) | |
| Tumor size | |||
| N. Tested | 233 | 982 | |
| ≤2 cm | 105 (45.1 %) | 524 (53.4 %) | 0.0227 |
| >2 cm | 128 (54.9 %) | 458 (46.6 %) | |
| Nodal status | |||
| N. Tested | 222 | 919 | |
| 0 | 144 (64.9 %) | 568 (61.8 %) | 0.6251 |
| 1–3 | 49 (22.1 %) | 210 (22.9 %) | |
| >3 | 29 (13.1 %) | 141 (15.3 %) | |
| DNA ploidy | |||
| N. Tested | 247 | 1,026 | |
| Diploid | 88 (35.6 %) | 588 (57.3 %) | <0.0001 |
| Aneuploid | 159 (64.4 %) | 438 (42.7 %) | |
| S-phase | |||
| N. Tested | 204 | 943 | |
| Low | 52 (25.5 %) | 599 (63.5 %) | <0.0001 |
| Intermediate | 42 (20.6 %) | 186 (19.7 %) | |
| High | 110 (53.9 %) | 158 (16.8 %) | |
| EGFR | |||
| N.Tested | 253 | 1,036 | <0.0001 |
| EGFR+ | 124 (49.0 %) | 77 (7.4 %) | |
| EGFR− | 129 (51.0 %) | 959 (92.6 %) |
N number of patients, TN triple negative, ED estrogen driven, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, EGFR epidermal growth factor receptor
Time to first recurrence (TTFR) was calculated from the diagnostic biopsy date and a first recurrence was scored as an event while patients without an event were censored at the time of death or last follow-up. Overall survival (OS) was calculated from the diagnostic biopsy date and death was scored as an event whereas patients who were alive at the time of last follow-up were censored.
The effects of ED versus TN status on TTFR and OS were examined using Kaplan–Meier curves, and differences in survival were evaluated with the log-rank test in all subjects and by treatment status. In addition, time-varying covariates were employed to test the proportional hazards assumption of ED versus TN status in the survival analysis. Univariable Cox regression was also used to model the effects of EGFR status, ploidy and S-phase on TTFR and OS in TN subjects, and hazard ratios with 95 % confidence intervals and p values were calculated for these models. Multivariable analysis assessed the simultaneous importance of ED versus TN status, tumor size, nodal status, and S-phase on OS and time to first recurrence in all subjects and by treatment group and among TN subjects by EGFR status ploidy status and S-phase Cox regression was used to model the relationships, and adjusted hazards ratios, 95 % confidence intervals (CI), and p values were calculated for the multivariable models. All analyses were performed using SAS 9.2 (SAS, Cary, NC).
Prognostic factors
Estrogen receptor levels were measured by the dextran-coated charcoal method as previously described [10]. From 1970 to 1984, [3H]-estradiol was used as labeled ligand. During the same time period, PgR levels were measured by sucrose density gradient [10, 11]. In 1985, the standard multipoint dextran-coated charcoal assay was modified to incorporate [125I]-estradiol and [3H]-R5020 in a single assay, allowing the simultaneous determination of both ER and PgR [12, 13]. Levels ≥3 fmol/mg protein were considered positive for ER and levels ≥5 fmol/mg protein were considered positive for PgR. DNA ploidy and S-phase fraction were evaluated by flow cytometry as previously described [14–16]. S-phase fractions <6 % were considered low, 6–10 % intermediate, and >10 % high. HER-2 status was determined by Western blotting [17]. The cut off value between low and high HER-2 expression was 1 U/µg protein.
EGFR levels were measured by radioligand binding assay, using fixed concentrations of radiolabeled epidermal growth factor (EGF) and varying concentrations of unlabeled EGF. Levels ≥10 fmol/mg were considered positive. This cutoff has been in use at the Nichols Institute since 1992 and is in agreement with the majority of published studies [18].
Results
Demographic, clinical, biological characteristics
We identified 253 patients with TN and 1,036 patients with ED tumor. Table 2 summarizes the clinical and biological tumor characteristics according to tumor type.
Patients with TN BC were more likely to be younger (median age: 52 years in TN patients vs. 63 years in ED; p < 0.0001), to be pre-menopausal (26.9 % of TN vs. 8.9 % of ED; p < 0.0001) and African–American (12.4 % of TN vs. 4 % of ED; p < 0.0001).
TN tumors were slightly larger on average (54.9 % larger than 2 cm) than ED tumors (46.6 % larger than 2 cm), (p = 0.0227). No difference in the frequency of axillary node involvement was observed.
Compared with ED tumors, TN tumors were much more likely to be aneuploid (64.4 % in TN vs. 42.7 % in ED; p < 0.0001) and to have an higher proliferation rate (high S-phase fraction 53.9 % in TN vs. 16.8 % in ED; p < 0.0001). Interestingly, positive EGFR expression was 6.5 times more likely to occur in TN tumors than in ED tumors (EGFR high expression: 49 % in TN and 7 % in ED tumors; p < 0.0001).
Triple-negative phenotype and EGFR expression
Patients with TN BC (n = 253) could be further divided according to EGFR status with 124 women being EGFR-positive and 129 being EGFR-negative (Table 3). Of note, among TN patients, women with EGFR-positive disease were younger and 2.5 times more likely pre-menopausal compared to patients with EGFR-negative BC. Median age was 47 years in patient with EGFR-positive and 61 years in patients with EGFR-negative disease, respectively (p < 0.0001) and 40 % of women with EGFR-positive breast cancer were premenopausal compared to 16.7 % of patients with an EGFR-negative disease (p = 0.0007).
Table 3.
Clinical and tumor characteristics of TN subjects
| Patient and tumor characteristics |
EGFR+ N = 124 | EGFR− N = 129 | p Value | Aneuploid N = 159 | Diploid N = 88 | p Value | High/Intermediate S-phase N = 152 |
Low S-phase N = 52 | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| N. Tested | 123 | 128 | 157 | 88 | 151 | 52 | |||
| Median age | 47 | 61 | 50 | 57 | 50 | 60 | |||
| <50 years | 71 (57.7 %) | 41 (32.0 %) | <0.0001 | 78 (49.7 %) | 32 (36.4 %) | 0.0444 | 75 (49.7 %) | 15 (28.9 %) | 0.0091 |
| ≥50 years | 52 (42.3 %) | 87 (68.0 %) | 79 (50.3 %) | 56 (63.6 %) | 76 (50.3 %) | 37 (71.2 %) | |||
| Menopausal status | |||||||||
| N. Tested | 66 | 90 | 89 | 64 | 91 | 39 | |||
| Pre | 27 (40.9 %) | 15 (16.7 %) | 0.0007 | 26 (29.2 %) | 16 (25.0 %) | 0.5646 | 27 (29.7 %) | 8 (20.5 %) | 0.2807 |
| Post | 39 (59.1 %) | 75 (83.3 %) | 63 (70.8 %) | 48 (75.0 %) | 64 (70.3 %) | 31 (79.5 %) | |||
| Histology | |||||||||
| N. Tested | 124 | 129 | 159 | 88 | 152 | 52 | |||
| IDC | 110 (88.7 %) | 109 (84.5 %) | 0.0051 | 146 (91.8 %) | 69 (78.4 %) | 0.0009 | 140 (92.1 %) | 42 (80.8 %) | 0.0281 |
| ILC | 0 (0.0 %) | 10 (7.8 %) | 1 (0.6 %) | 8 (9.1 %) | 3 (2.0 %) | 5 (9.6 %) | |||
| Other | 14 (11.3 %) | 10 (7.8 %) | 12 (7.6 %) | 8 (12.5 %) | 9 (5.9 %) | 5 (9.6 %) | |||
| Tumor size | |||||||||
| N. Tested | 115 | 118 | 145 | 83 | 138 | 50 | |||
| ≤2 cm | 40 (34.8 %) | 65 (55.1 %) | 0.0018 | 52 (35.9 %) | 52 (62.7 %) | <0.0001 | 50 (36.2 %) | 34 (68.0 %) | 0.0001 |
| >2 cm | 75 (65.2 %) | 53 (44.9 %) | 93 (64.1 %) | 31 (37.4 %) | 88 (63.8 %) | 16 (32.0 %) | |||
| Nodal status | |||||||||
| N. Tested | 114 | 108 | 139 | 77 | 133 | 47 | |||
| 0 | 72 (63.2 %) | 72 (66.7 %) | 0.8470 | 82 (59.0 %) | 56 (72.7 %) | 0.1220 | 76 (57.1 %) | 36 (76.6 %) | 0.0494 |
| 1–3 | 26 (22.8 %) | 23 (21.3 %) | 35 (25.2 %) | 14 (18.2 %) | 33 (24.8 %) | 8 (17.0 %) | |||
| >3 | 16 (14.0 %) | 13 (12.0 %) | 22 (15.8 %) | 7 (9.1 %) | 24 (18.1 %) | 3 (6.4 %) | |||
| EGFR | |||||||||
| N. Tested | – | – | 159 | 88 | 152 | 52 | |||
| Positive | – | – | 97 (61.0 %) | 26 (29.6 %) | <0.0001 | 82 (54.0 %) | 12 (23.1 %) | 0.0001 | |
| Negative | – | – | 62 (39.0 %) | 62 (70.5 %) | 70 (46.1 %) | 40 (76.9 %) | |||
| DNA ploidy | |||||||||
| N. Tested | 123 | 124 | – | – | 152 | 52 | |||
| Diploid | 26 (21.1 %) | 62 (50.0 %) | <0.0001 | – | – | 38 (25.0 %) | 41 (78.9 %) | <0.0001 | |
| Aneuploid | 97 (78.9 %) | 62 (50.0 %) | – | – | 114 (75.0 %) | 11 (21.2 %) | |||
| S-phase | |||||||||
| N. Tested | 94 | 110 | 125 | 79 | – | – | |||
| Low | 12 (12.8 %) | 40 (36.4 %) | <0.0001 | 11 (8.8 %) | 41 (51.9 %) | <0.0001 | – | – | |
| Intermediate | 17 (18.1 %) | 25 (22.7 %) | 15 (12.0 %) | 27 (34.2 %) | – | – | |||
| High | 65 (69.2 %) | 45 (40.9 %) | 99 (79.2 %) | 11 (13.9 %) | – | – |
N number of patients, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, EGFR epidermal growth factor receptor
In addition, tumors expressing EGFR were larger (65.2 % EGFR-positive vs. 44.9 %; EGFR-negative tumor were larger than 2 cm; p = 0.0018), more likely to be aneuploid (78.9 % of EGFR-positive vs. 50 % of EGFR-negative tumor; p < 0.0001) and displayed an higher proliferation rate (69.2 % EGFR-positive vs. 40.9 % EGFR-negative had high S-phase fraction; p < 0.0001).
The vast majority (69.4 %) of patients with EGFR-positive disease received systemic adjuvant treatment compared to EGFR-negative patients (52.2 %) (p = 0.0014).
Triple-negative phenotype and ploidy status
Subjects with aneuploid TN tumor were more likely to be younger (median age was 50 years in aneuploid TN patients vs. 57 years in diploid TN patients; p = 0.04) and were slightly larger on average (64.1 % larger than 2 cm) than diploid TN tumors (37.4 % larger than 2 cm), (p < 0.0001). Aneuploid TN BC was more likely to be invasive ductal carcinoma (IDC) (91.8 %) compared to diploid TN tumors (78.4 %) (p = 0.0009). No difference in race, menopausal status or in the frequency of axillary node involvement was observed.
Interestingly, compared with diploid-, aneuploid-TN tumors were much more likely to have an higher proliferation rate (79.2 % in aneuploid TN vs. 13.9 % of diploid TN tumors; p < 0.0001). Moreover, aneuploid TN tumors more frequently expressed high levels of EGFR compared to diploid TN tumors (61.0 % in aneuploid vs. 29.6 % in diploid TN tumors; p < 0.0001) (Table 3).
Triple-negative phenotype and proliferation
Subjects with high/intermediate S-phase TN tumors were more likely to be younger (median age was 50 years in high/intermediate S-phase TN patients vs. 60 years in low S-phase TN patients; p = 0.0091). High/intermediate S-phase TN tumors were slightly larger on average (63.8 % larger than 2 cm) than low S-phase TN tumors (32.0 % larger than 2 cm), (p = 0.0001) and more likely to be IDC (92.1 % of high/intermediate S-phase TN tumors vs. 80.8 % of low S-phase TN tumors; p = 0.0281). High/intermediate S-phase TN patients were modestly more likely to be lymph node-positive compared to low S-phase TN tumors (p = 0.049). However, there were no differences in race and in menopausal status of patients.
Compared with low S-phase TN tumors, high/intermediate S-phase TN tumors were much more likely to have an aneuploid status (75 % in high/intermediate S-phase TN vs. 21.2 % in low S-phase TN tumors; p < 0.0001) and more frequently expressed high levels of EGFR (54.0 % in high/intermediate S-phase TN tumors vs. 23.1 % in low S-phase TN tumors; p < 0.0001) (Table 3).
Clinical outcomes
Patients with TN BC were more frequently treated with adjuvant systemic therapy compared to patients with ED tumors. Indeed, 47.8 % of patients with ED breast cancer and 39.5 % of patients with TN disease did not receive any type of adjuvant systemic treatment (p < 0.0001). Chemotherapy was the treatment of choice in patients with TN BC. Fiftythree percent of TN patients received adjuvant chemotherapy with or without endocrine therapy compared to 26.5 % of ED patients (p < 0.0001). On the other hand, given the absence of ER/PgR, adjuvant endocrine therapy was given less frequently to patients with TN tumor (7.2 %), compared to patients with ED tumor (25.7 %) (p < 0.0001) (Table 4).
Table 4.
Adjuvant therapy
| TN N = 253 | ED N = 1,036 | p Value | |
|---|---|---|---|
| Adjuvant therapy | |||
| Endo + Chemo | 16 (7.2 %) | 98 (10.4 %) | <0.0001 |
| Chemo | 103 (46.2 %) | 152 (16.1 %) | |
| Endo | 16 (7.2 %) | 242 (25.7 %) | |
| Untreated | 88 (39.5 %) | 451 (47.8 %) |
TN triple negative, ED estrogen driven
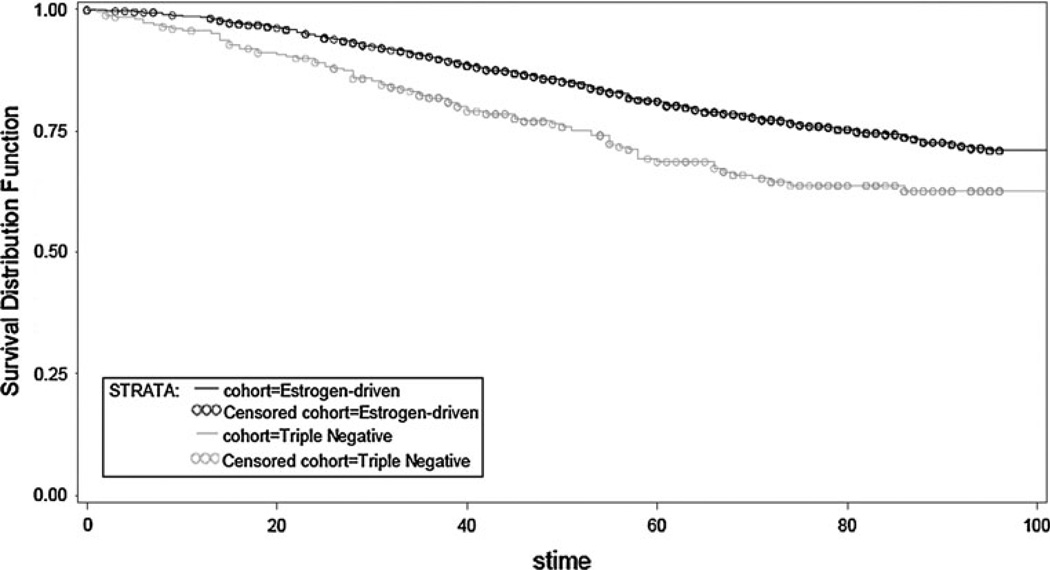
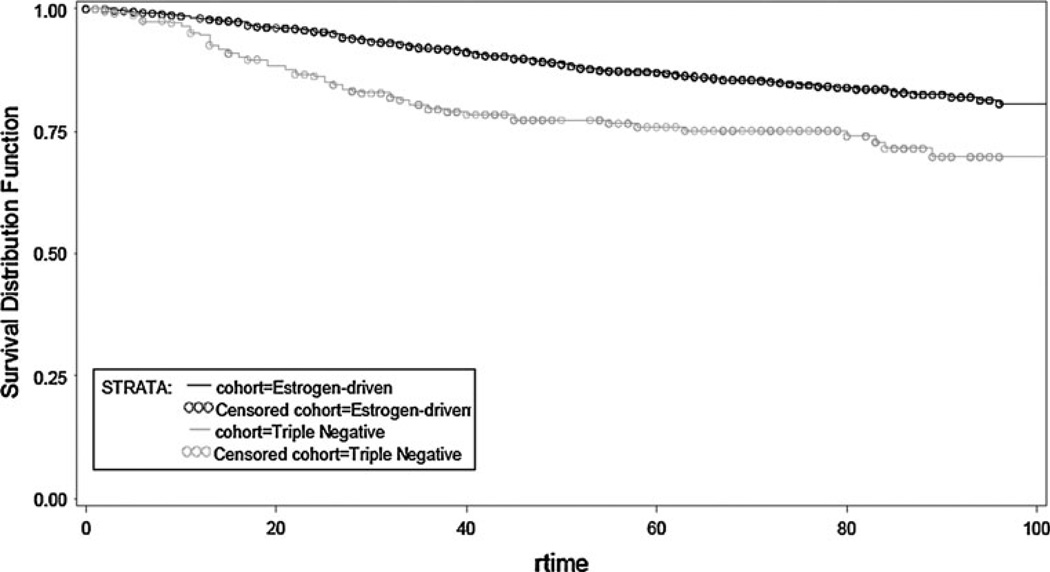
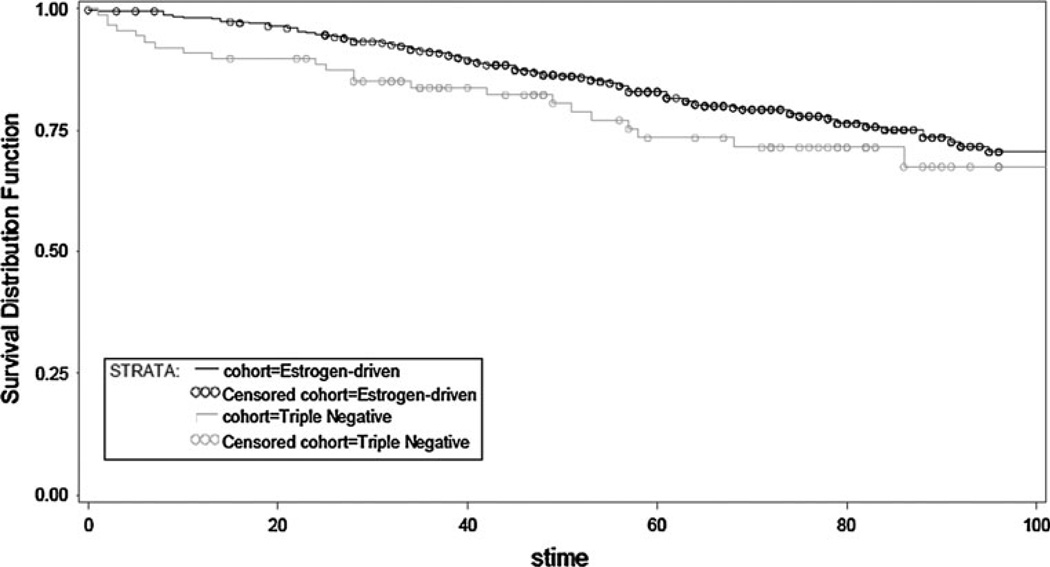
Overall, TN patients had a shorter time to first recurrence (TTFR) (OR, 0.503; 95 % CI, 0.371–0.682; p < 0.0001) and OS (OR, 0.624; 95 % CI, 0.481–0.810; p = 0.0004) compared to patients with an ED cancer (Figs. 1, 2). Median TTFR in ED patients was achieved at 67 months compared to 25 months in TN patients (Figs. 1, 2).
Fig. 1.
Overall survival by receptor group status in TN and ED subjects
Fig. 2.
Time to first recurrence by receptor group status in TN and ED subjects
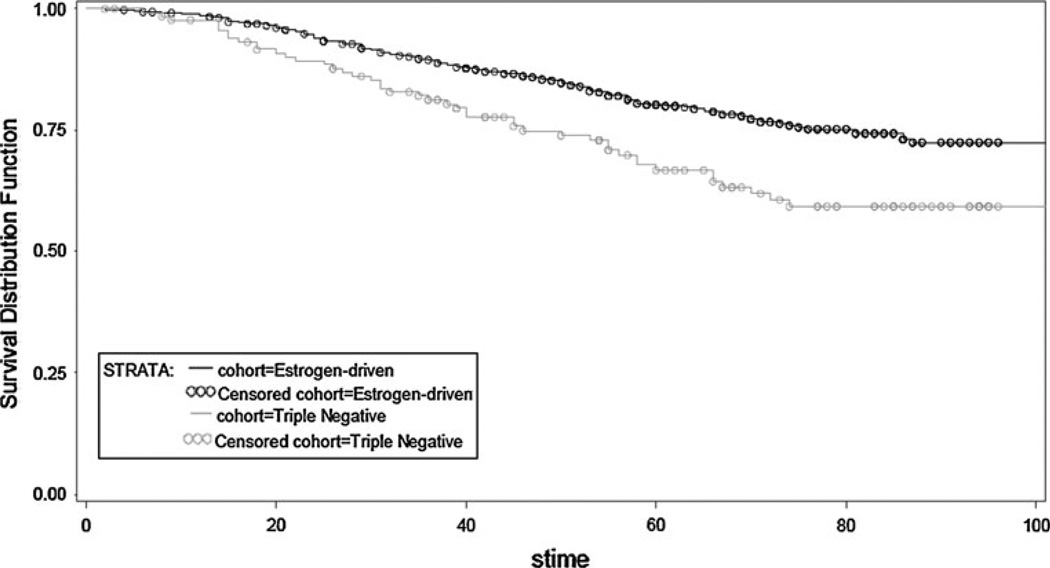
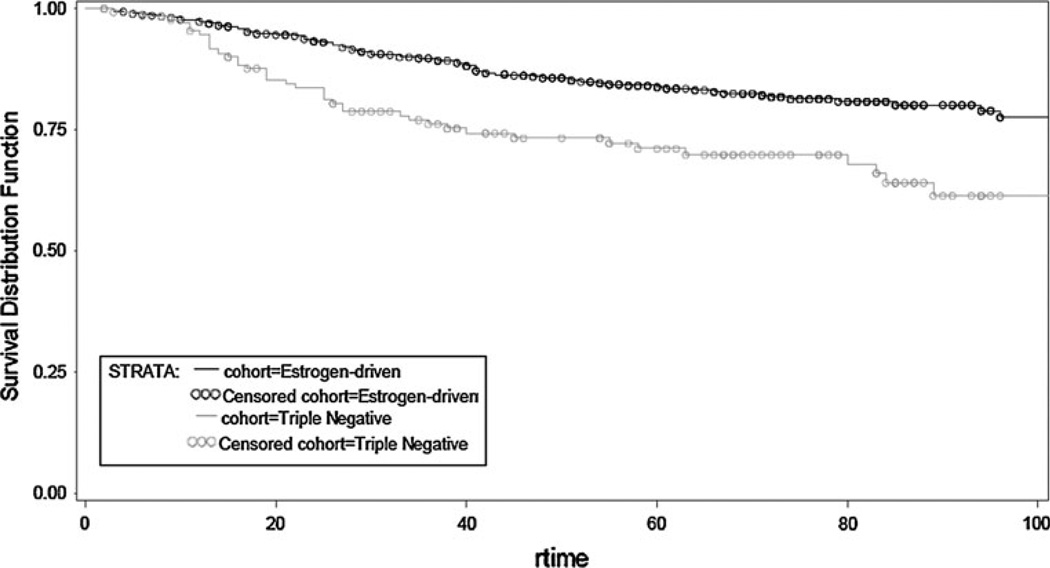
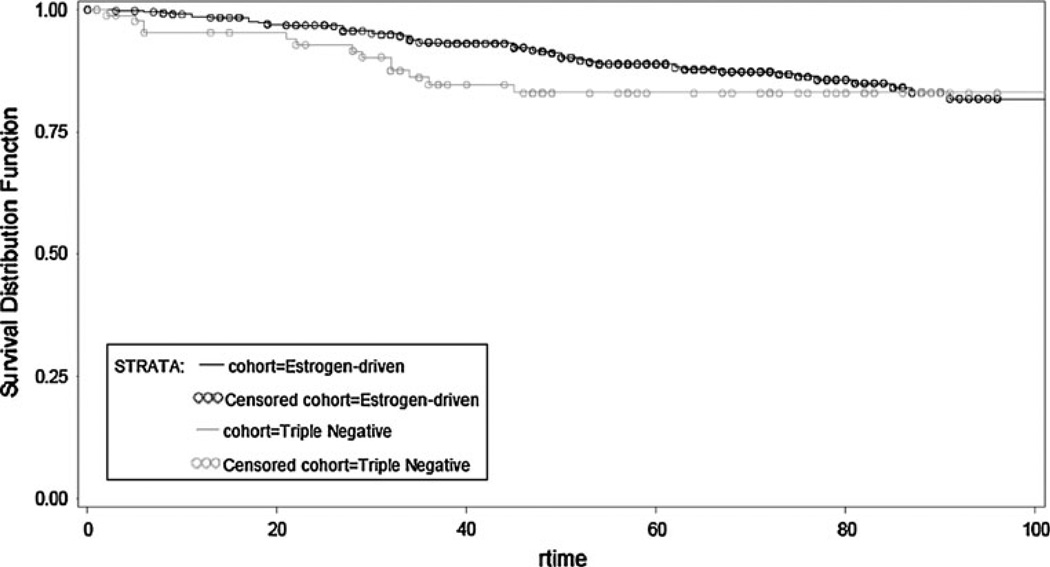
To evaluate the impact of therapy on tumor natural history, data were further analyzed first in patients receiving adjuvant therapy and next in systemically untreated women (Table 5). In the adjuvant (chemo, endo or both) therapy treatment group, patients with TN disease experienced worse TTFR (OR, 0.495; 95 % CI, 0.339–0.722; p = 0.0003) and worse OS (OR, 0.574; 95 % CI, 0.406–0.812; p = 0.0017) compared to patients with ED BC (Figs. 3, 4). This is not surprising given the wide use of endocrine therapy in the ED group. In contrast, no statistical significant difference in TTFR or OS was observed in patients not receiving any systemic adjuvant therapy (Figs. 5, 6).
Table 5.
Univariate and multivariate models (accounting for tumor size, nodes, and S-phase)
| Variable | TTFR OR (95 % CI) | p Value | OS OR (95 % CI) | p Value |
|---|---|---|---|---|
| ED vs. TN, All Subjects | ||||
| Uni | 0.503 (0.371, 0.682) | <0.0001 | 0.624 (0.481, 0.810) | 0.0004 |
| Multi | 0.598 (0.405, 0.883) | 0.0098 | 0.621 (0.442, 0.873) | 0.0062 |
| ED vs. TN, Treated Subjects | ||||
| Uni | 0.495 (0.339, 0.722) | 0.0003 | 0.574 (0.406, 0.812) | 0.0017 |
| Multi | 0.619 (0.385, 0.997) | 0.0485 | 0.596 (0.380, 0.936) | 0.0244 |
| ED vs. TN, Untreated Subjects | ||||
| Uni | 0.699 (0.381, 1.282) | 0.2470 | 0.721 (0.453, 1.147) | 0.1667 |
| Multi | 0.738 (0.332, 1.643) | 0.4575 | 0.651 (0.365, 1.162) | 0.1465 |
TN triple negative, ED estrogen driven, TTFR time to first recurrence, OS overall survival, OR odds ratio
Fig. 3.
Overall survival by receptor group status in TN and ED-treated subjects (adjuvant chemotherapy, endocrine therapy, or both)
Fig. 4.
Time to first recurrence (TTFR) by receptor group status in TN and ED-treated subjects (adjuvant chemotherapy, endocrine therapy, or both)
Fig. 5.
Overall survival by receptor group status in TN and ED untreated subjects
Fig. 6.
Time to first recurrence by receptor group status in TN and ED untreated subjects
Among TN patients only, regardless of the treatment, at univariable analyses EGFR expression, aneuploidy or higher proliferation were not significantly associated to worse outcome.
Multivariable analyses
The role of TN subtype as a prognostic factor on TTFR and OS was further evaluated by multivariable analyses in all subjects and by treatment group (Table 5). Potential confounders included in the model were tumor size, nodal status and S-phase. In untreated patients, TN was not associated with TTFR and OS. However, in patients treated with systemic adjuvant therapy, TN subtype was independently associated with worse TTFR (OR, 0.619; 95 % CI, 0.385–0.997; p = 0.0485) and OS (OR, 0.596; 95 % CI, 0.380–0.936; p = 0.0244).
In order to define the prognostic value of EGFR, ploidy, and proliferation rate in patients with TN tumors, multivariate analyses were performed. Potential confounders included in the model were tumor size, nodal status, and S-phase. Surprisingly, neither EGFR status nor aneuploidy was associated with outcome in this group of women.
Discussion
Targeted therapy has changed the natural history of BC expressing either ER, PgR and/or HER2. Unfortunately, there is a 10–15 % of BC not expressing either of these targets. For these tumors, often referred as TN, chemotherapy remains the only therapeutic tool. TN tumors are commonly believed to be more aggressive and related to a worse survival compared to non-TN tumors [19].
In this study we have analyzed a well-characterized database comprising BC patients with extensive data regarding tumor biology, treatment information and outcomes. We compared two cohorts of patients using very stringent clinical characteristics to limit confounding factors. Therefore, ED tumors were defined as ER and PgR positive excluding ER negative/PgR positive and ER positive/PgR negative patients who carry a more aggressive, less sensitive to hormonal treatment phenotype [20]. For the same reasons, all patients expressing HER2 were excluded from the ED group. On the other hand, the TN cohort is defined by the lack of ER, PgR, and HER2 expression similarly to the routine clinical practice of medical oncologists worldwide [21].
We show that TN tumors are larger, more proliferative and more aneuploid compared to ED tumors. We also confirm previous data in the literature of TN tumors being more frequent in young, African–American patients compared to non-TN tumors [22, 23]. Not surprisingly, when we studied TN tumors according to either ploidy or proliferative status, we found that TN tumors displaying higher aneuploidy or proliferation show also a more aggressive biology.
Several studies have investigated the role of EGFR as predictive or prognostic factor in BC [24–29]. We have recently shown that, EGFR expression, more common in BC of younger and black women, is associated with lower hormone receptor levels, higher proliferation, genomic instability, and HER2 overexpression [30]. In the same paper, EGFR status is correlated with higher risk of relapse in patients receiving adjuvant treatment despite tumor histotypes and ER, PgR, and HER2 levels.
Upon looking further into the TN EGFR+group, we also found that among TN patients, those with EGFR positivity display a distinct, biologically more aggressive phenotype. However, these features do not significantly affect long-term survival. EGFR status, in particular, does not confer a worse prognosis to TN patients. In the light of recent data showing disappointing results for the anti-EGFR-specific antibody cetuximab in metastatic TN breast cancer treatment [31], our data suggest that EGFR is not a strong biological determinant of TN tumor biology, at least not at this discrete time point in the evolution of BC. Our data are in contrast with other findings showing that EGFR-positive basal-like tumors have a poorer prognosis compared to EGFR-negative tumors [32]. This might be in part explained by the different method used for EGFR evaluation in our work and by the different criteria used for allocating patients to sub-groups (basal-/non-basal-like vs. TN/ED in our work).
Tumor ploidy is a surrogate marker of genomic instability [33]. We show in this work that TN tumors, compared to non-TN tumors are more frequently aneuploid. Therefore, TN tumors might be more sensitive to agents targeting genomic instability such as anti-DNA repair targeted agents.
Recent data have shown that TN tumors are a heterogeneous group of diseases that differ substantially in terms of outcome and response to treatment [34]. We were not able to find in our database any independent determinant of such heterogeneity since all of the analyzed factors did not significantly affect survival in multivariate analysis. This is suggestive of a complex biology underlying such heterogeneity requiring detailed molecular analyses to be dissected.
Most of the available data in the literature about the prognostic value of the TN phenotype are based on analyses of patients populations that are largely chemo and/or hormonal treated [8, 32, 35–39]. Since TN patients do not receive treatments that are as effective as endocrine treatment for ED patients, this makes prognostic conclusions not easily dissectible from treatment response predictions. The unique availability to our database of survival data for an untreated BC population fully characterized for ER/PgR and HER2 status gave us the possibility of exploring the true prognostic significance of the TN phenotype. Surprisingly, while in the chemo- and/or hormone-treated population the TN phenotype was significantly associated with a worse outcome compared to the ED group, such association was lost in the untreated population both at univariate and at multivariate analyses. This observation might be explained in two ways. First, the untreated population of patients in our data set has an excellent long-term prognosis both in the ED and in the TN subgroups. Therefore, a much larger sample size would be required to show a significant difference in outcome between such good prognostic cohorts. On the other hand, it is well known that both ER and PgR have very week prognostic value on long-term patient outcome [40], therefore we cannot exclude that, in the absence of an efficacious targeted treatment such as endocrine therapy, ED tumors would behave similarly to TN tumors for which no specific treatment exists to date. If this hypothesis is true, TN and ED tumors would not differ in their natural history but mostly in their exquisite sensitivity to a specific targeted agent. On this regard, it has been recently shown that HER2-positive patients, who are known to have a poor prognosis in the absence of treatment compared to HER2 negative patients, when treated with the HER2-specific monoclonal antibody trastuzumab achieve a similar survival as compared to HER2 negative patients [41].
In conclusion, our work displays an analysis of a unique, large database with well-characterized tumors and patients information that largely confirms data which are already been reported in the literature and gives novel and surprising view-angles for the interpretation of the TN phenotype in BC.
Acknowledgments
This study was funded in part by a NIH grant P50-CA58183 (SPORE) and a Susan Komen for the Cure Foundation Post-doctoral Fellowship Award.
Footnotes
The Authors have no financial disclosures to declare.
Contributor Information
L. Malorni, Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston, TX, USA “Sandro Pitigliani” Oncology Unit, Hospital of Prato, Prato, Italy.
P. B. Shetty, Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston, TX, USA Dun L. Duncan Cancer Center at Baylor College of Medicine, Houston, TX, USA.
C. De Angelis, Email: carmine.deangelis83@gmail.com, Department of Molecular and Clinical Endocrinology and Oncology, University of Naples Federico II, Naples, Italy.
S. Hilsenbeck, Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston, TX, USA Dun L. Duncan Cancer Center at Baylor College of Medicine, Houston, TX, USA.
M. F. Rimawi, Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston, TX, USA Dun L. Duncan Cancer Center at Baylor College of Medicine, Houston, TX, USA.
R. Elledge, Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston, TX, USA Dun L. Duncan Cancer Center at Baylor College of Medicine, Houston, TX, USA.
C. K. Osborne, Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston, TX, USA Dun L. Duncan Cancer Center at Baylor College of Medicine, Houston, TX, USA.
S. De Placido, Department of Molecular and Clinical Endocrinology and Oncology, University of Naples Federico II, Naples, Italy
G. Arpino, Department of Molecular and Clinical Endocrinology and Oncology, University of Naples Federico II, Naples, Italy
References
- 1.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartog H, Horlings HM, van der Vegt B, et al. Divergent effects of insulin-like growth factor-1 receptor expression on prognosis of estrogen receptor positive versus triple negative invasive ductal breast carcinoma. Breast Cancer Res Treat. 2011;129:725–736. doi: 10.1007/s10549-010-1256-6. [DOI] [PubMed] [Google Scholar]
- 6.Kreike B, van de Vijver MJ. Are triple-negative tumours and basal-like breast cancer synonymous? Authors’ response. Breast Cancer Res. 2007;9(6):405. doi: 10.1186/bcr1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertucci F, Finetti P, Cervera N, et al. How basal are triple-negative breast cancers? Int J Cancer. 2008;123:236–240. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- 8.Cheang MCU, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value then triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 9.Dent R, Trudeau M, Sun P, Narod S. Patterns of metastatic spread in triple negative breast cancer. Breast Cancer Res Treat. 2007;106:S41–S83. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 10.McGuire WL, et al. Evaluation of estrogen receptor assays in human breast cancer tissue. Cancer Res. 1977;37:637–639. [PubMed] [Google Scholar]
- 11.Powell B, et al. Measurement of progesterone receptor in human breast cancer biopsies. Cancer Res. 1979;39:1678–1682. [PubMed] [Google Scholar]
- 12.Clark GM, Dressler LG, Owens MA, Mcguire WL. Flow-cytometry identifies a group of node-negative breast-cancer patients with low-risk of recurrence. Breast Cancer Res Treat. 1988;12:132. [Google Scholar]
- 13.Dressler LG, Seamer LC, Owens MA, et al. DNA flow-cytometry and prognostic factors in 1331 frozen breast-cancer specimens. Cancer. 1988;61:420–427. doi: 10.1002/1097-0142(19880201)61:3<420::aid-cncr2820610303>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Clark GM, Mathieu MC, Owens MA, et al. Prognostic-significance of s-phase fraction in good-risk, node-negative breast-cancer patients. J Clin Oncol. 1992;10:428–432. doi: 10.1200/JCO.1992.10.3.428. [DOI] [PubMed] [Google Scholar]
- 15.Clark GM, Dressler LG, Owens MA, et al. Prediction of relapse or survival in patients with node-negative breast-cancer by DNA flow-cytometry. N Engl J Med. 1989;320:627–633. doi: 10.1056/NEJM198903093201003. [DOI] [PubMed] [Google Scholar]
- 16.Wenger CR, Beardslee S, Owens MA, et al. DNA-ploidy, S-phase, and steroid-receptors in more than 127, 000 breast-cancer patients. Breast Cancer Res Treat. 1993;28:9–20. doi: 10.1007/BF00666351. [DOI] [PubMed] [Google Scholar]
- 17.Ciocca DR, Fujimura FK, Tandon AK, et al. Correlation of Her-2/Neu amplification with expression and with other prognostic factors in 1103 breast cancers. J Natl Cancer Inst. 1992;84:1279–1282. doi: 10.1093/jnci/84.16.1279. [DOI] [PubMed] [Google Scholar]
- 18.Klijn JGM, Berns PMJJ, Bontenbal M, Foekens JA. Growth-factors: clinical implications in breast-cancer. Breast Cancer: from Biology to Therapy. 2006 [Google Scholar]
- 19.Curigliano G, Goldhirsch A. The triple-negative subtype: new ideas for the poorest prognosis breast cancer. J Natl Cancer Inst Monogr. 2011;43:108–110. doi: 10.1093/jncimonographs/lgr038. [DOI] [PubMed] [Google Scholar]
- 20.Arpino G, Weiss H, Lee AV, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann M, Pusztai L. Use of standard markers and incorporation of molecular markers into breast cancer therapy consensus recommendations from an international expert panel. Cancer. 2011;117:1575–1582. doi: 10.1002/cncr.25660. [DOI] [PubMed] [Google Scholar]
- 22.Huo DZ, Senie RT, Daly M, et al. Prediction of BRCA mutations using the BRCAPRO model in clinic-based African American, Hispanic, and other minority families in the United States (vol 27, p 1184, 2009) J Clin Oncol. 2009;27:3262. doi: 10.1200/JCO.2008.17.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stead LA, Lash TL, Sobieraj JE, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009;11:R18. doi: 10.1186/bcr2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowsett M, Houghton J, Iden C, et al. Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol. 2006;17:818–826. doi: 10.1093/annonc/mdl016. [DOI] [PubMed] [Google Scholar]
- 25.Leitzel K, Souder C, Ali SM, et al. Serum EGFR/HER-2 combination predicts poor survival in metastatic breast cancer. J Clin Oncol Meeting Abstracts. 2005;23(16):9613. [Google Scholar]
- 26.Lu CH, Speers C, Zhang Y, et al. Effect of epidermal growth factor receptor inhibitor on development of estrogen receptor-negative mammary tumors. J Natl Cancer Inst. 2003;95:1825–1833. doi: 10.1093/jnci/djg117. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 28.Press MF, Finn RS, Cameron D, et al. HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res. 2008;14:7861–7870. doi: 10.1158/1078-0432.CCR-08-1056. [DOI] [PubMed] [Google Scholar]
- 29.Rimawi MF, Weiss HL, Bhatia P, et al. EGFR expression in breast cancer: association with biologic phenotype, prognosis, and resistance to adjuvant therapy. J Clin Oncol (Meeting Abstracts) 2006;24(18):513. [Google Scholar]
- 30.Rimawi MF, Shetty PB, Weiss HL, et al. Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer. 2010;116:1234–1242. doi: 10.1002/cncr.24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey L, Mayer E, Marcom P, et al. TBCRC 001: EGFR inhibition with cetuximab in metastatic triple negative (basal-like) breast cancer (abstract 307) Breast Cancer Res Treat. 2007;106(suppl 1):S32. [Google Scholar]
- 32.Viale G, Rotmensz N, Maisonneuve P, et al. Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat. 2009;116:317–328. doi: 10.1007/s10549-008-0206-z. [DOI] [PubMed] [Google Scholar]
- 33.Kolodner RD, Cleveland DW, Putnam CD. Aneuploidy drives a mutator phenotype in cancer. Science. 2011;333:942–943. doi: 10.1126/science.1211154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irshad S, Ellis P, Tutt A. Molecular heterogeneity of triple-negative breast cancer and its clinical implications. Curr Opin Oncol. 2011;23:566–577. doi: 10.1097/CCO.0b013e32834bf8ae. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee S, Reis JS, Ashley S, et al. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J Clin Pathol. 2006;59:729–735. doi: 10.1136/jcp.2005.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 37.Dent R, Trudeau M, Pritchard K, et al. Patterns of recurrence and prognosis in women with basal-like breast cancer. Breast. 2007;16:S25. [Google Scholar]
- 38.Kreike B, van de Vijver MJ. Are triple-negative tumours and basal-like breast cancer synonymous? Breast Cancer Res. 2007;9(6):405. doi: 10.1186/bcr1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreike B, van Kouwenhove M, Horlings H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9(5):R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilsenbeck SG, Ravdin PM, de Moor CA, et al. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat. 1998;52:227–237. doi: 10.1023/a:1006133418245. [DOI] [PubMed] [Google Scholar]
- 41.Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]