Abstract
Connexin 43 (Cx43) is the most abundant gap junction protein expressed in bone cells and plays a central role in cell-to-cell communication in the skeleton. Findings of the last decade uncovered functions of Cx43 hemichannels expressed on unopposed plasma cell membranes as mediators of the communication between bone cells and their extracellular milieu. Additionally, through its cytoplastmic C-terminus domain, Cx43 serves as a scaffolding protein that associates with structural and signaling molecules leading to regulation of intracellular signaling, independently of channel activity. This perspective discusses the evidence demonstrating that via these diverse mechanisms Cx43 is a key component of the intracellular machinery responsible for signal transduction in bone in response to pharmacologic, hormonal and mechanical stimuli. This advance in the knowledge of the role of connexins increases our understanding of the pathophysiological mechanisms that regulate bone cell function and provides new opportunities to treat bone diseases.
Keywords: connexin43, osteoblast, osteocyte, apoptosis, bisphosphonates, mechanotransduction
Gap junction channels, hemichannels, and other potential functions of connexins
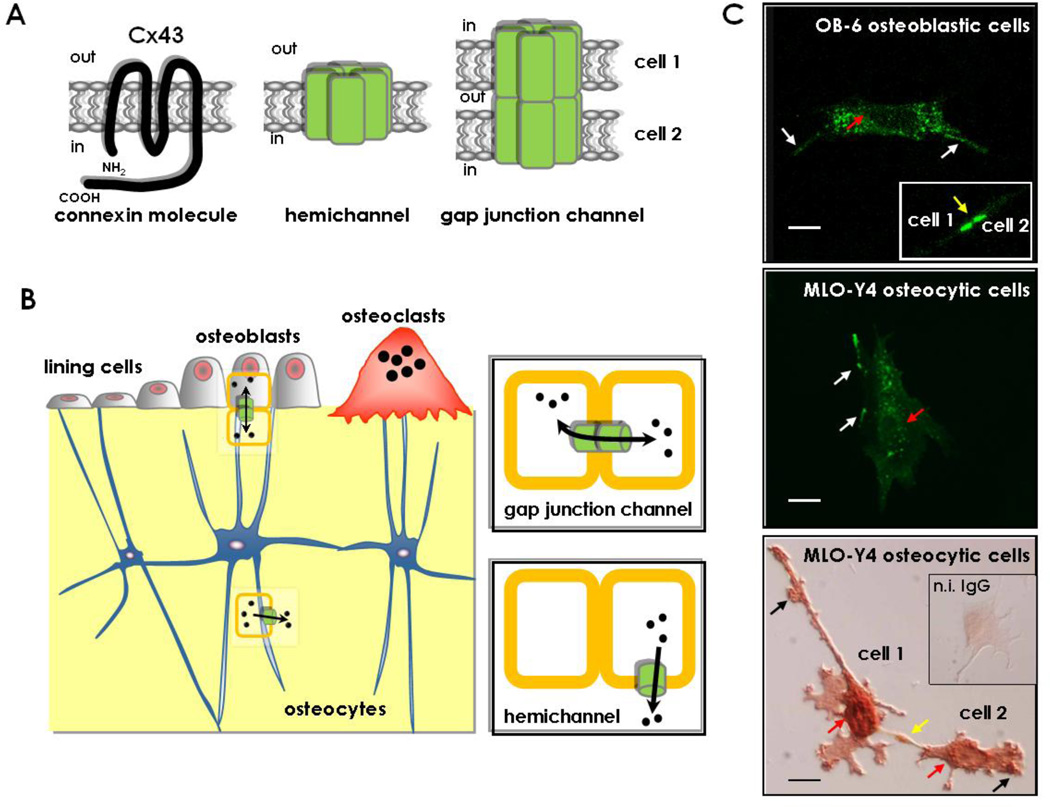
Connexins are structurally-conserved proteins that consist of four transmembrane domains, one intracellular and two extracellular loops, with both the C-terminus and the N-terminus domains facing the cytoplasm [1]. Six molecules of connexin assemble in the Golgi apparatus to form a hemichannel or connexon that is transported to the plasma membrane [2]. Hemichannels from neighboring cells align to form gap junction channels that allow intercellular communication (Figure 1A and B). Gap junction channels and hemichannels are closed under normal conditions but can open, allowing the passage of molecules directly from one cell to the other or from cells to the extracellular milieu. Only molecules smaller than 1,000 daltons pass through connexin channels, including inorganic ions, sugars, aminoacids, nucleotides and vitamins. Channel opening and closure is tightly regulated by mechanisms still not completely understood. Changes in cytosolic pH, voltage, intracellular or extracellular Ca2+, as well as oncogenes and growth factors have been shown to open or close connexin channels, likely resulting from changes in the state of phosphorylation of the connexin molecules [3].
Figure 1. Connexin43, hemichannels and gap junction channels.
(A) Schematic representation of a Cx43 molecule, showing the amino (NH2) and carboxy (COOH) domains facing the cytoplasm and the 4 transmembrane domains. Six molecules of Cx43 associate to form a connexon or hemichannel that might be expressed in unopposed cell membranes; and 2 connexons expressed in neighboring cells align to form a gap junction channel. (B) Cx43 expressed in osteoblasts, osteocytes and osteoclasts, forms gap junction channels that allow inter-cellular communication and hemichannels that establish communication between cells and the extracellular milieu. (C) Representative images of OB-6 osteoblastic cells (confocal microscopy, upper panel) and MLO-Y4 osteocytic cells (fluorescence microcopy, middle panel) transfected with Cx43 tagged with green fluorescent protein; and MLO-Y4 osteocytic cells stained with anti-Cx43 antibody (bright field microscopy, lower panel). Localization of Cx43 in areas of cell-cell contact (yellow arrows), unopposed membranes (white and black arrows) and in the perinuclear area (red arrows) is shown. Bars represent 20 µm. The inset in the lower panel corresponds to an MLO-Y4 osteocytic cell stained with non-immune IgG as a negative control to demonstrate the specificity of the anti-Cx43 antibody.
The relative contribution of junctional versus extra-junctional connexin channels to cell communication or to a particular cellular function has been difficult to establish due to the fact that deletion or overexpression of particular connexin gene modifies both gap junctions and hemichannels. Permeability of both types of channels is also identical and their opening and closure are regulated by the same factors. Therefore, specific pharmacological inhibition of hemichannel or gap junction channel activity is not possible. Moreover, some inhibitors of connexin channels also inhibit channels formed by pannexins [4], molecules that form plasma membrane channels but that are not involved in cell-to-cell communication, suggesting that some of the functions ascribed to connexin hemichannels might be mediated by pannexins. Recent advances in this regard were made by developing tools that distinguish between half and full channels, in particular for connexin 43 (Cx43), one of the most studied members of the connexin family of proteins. An antibody directed against the extracellular loop of Cx43 (antibody E2) has been shown to block hemichannel activity induced by mechanical stimulation, without affecting gap junction communication in MLO-Y4 osteocytic cells [5], astrocytes and specialized ependymal-glial cells (tanycytes) [6]. In addition, Gap26 and Gap27, Cx43 mimetic peptides comprising amino acids 64–76 and 201–211 of the protein, respectively, were originally designed to inhibit the docking of two Cx43 molecules from opposing cells. However, more recent evidence demonstrated that they also block hemichannel opening within minutes, and that gap junctions are only inhibited at longer times of exposure [7]. Thus, the decreased osteoclast formation and resorption observed by prolonged exposure of osteoclast precursors to Gap27 cannot be attributed to either type of Cx43 channel [8]. The plasma membrane-permeable TAT-Cx43L2 peptide, corresponding to amino acids 119–144 in the Cx43 cytoplasmic loop, was shown to inhibit hemichannel responses, without affecting gap junctions in several cell types; and to block fear conditioning memory when microinfused in the basolateral amygdala [9, 10]. However, the effect of TAT-Cx43L2 in bone cells has not been tested.
Mutated forms of Cx43 have been also used to inhibit gap junction communication without affecting hemichannel activity. In particular, a Cx43 mutant that lacks six cysteines in the extracellular domain required for docking of juxtaposed channels (Cx43cys-less) is able to form active hemichannels, but not gap junctions [11]. This mutant was found to confer responsiveness to PTH in osteoblastic cells in which Cx43 was silenced, consistent with an anti-apoptotic effect of PTH independent of gap junction communication (see below) [12]. Other Cx43 variants with impaired gap junctional communication but normal or even enhanced hemichannel activity are the Cx43G138R mutant, associated with oculodentodigital dysplasia (ODDD), and the Cx43-R76W mutant. Mice expressing G138R-Cx43 ubiquitously [13] or in osteochondroprogenitors [14], or R76W-Cx43 in osteocytes [15] exhibit reduced bone mineral density. These findings suggest that functional hemichannels cannot compensate for the absence of gap junctions during bone development. However, the role of hemichannels for bone homeostasis in the adult remains to be determined.
In addition to their role in the formation of membrane channels, connexins, and in particular Cx43, have been shown to interact with intracellular structural and signaling molecules and to affect cellular functions [16]. The majority of these interactions are exerted through the cytoplasmic C-terminal tail of Cx43, which does not participate in channel formation but contains domains that regulate channel closure [17]. As it will be discussed later, hemichannel opening induced by the bone-active drugs bisphosphonates triggers activation of the Src/ERK pathway by promoting interaction of SH2 and SH3 binding domains present in the C-terminus of Cx43 with the kinase Src, leading to survival of osteoblasts and osteocytes [18]. This study was the first demonstration in bone cells not only of hemichannel opening but also of modulation by Cx43 of intracellular signaling pathways that affect osteoblastic cell function [2]. In addition, interaction of the cytoplasmic C-tail of Cx43 with arrestins dictates the intracellular localization of activated ERKs [19]. Association between Cx43 and β-arrestins is also a requirement for PTH receptor/cAMP signaling in osteoblasts [12].
Remarkably, the truncated protein generated by the sequence encoding only the C-terminus portion of Cx43 inhibits cell proliferation when transfected into cells [20, 21], and also increases cell migration [21]. These findings suggest that at least part of the regulation of cell behavior by Cx43 is exerted through functions independent of the ability of the protein to form membrane channels. Moreover, knock-in mice expressing a truncated Cx43 mutant lacking the C-terminal tail (Cx43K258stop) exhibit defective epidermal barrier leading to perinatal death due to dehydration in homozygous mice, impairment of cardiac function, female infertility, and enhanced cerebral injury after stroke [22, 23]. It is likely that these phenotypic features are not solely due to lack of the scaffolding property of the Cx43 cytoplasmic domain, since cells from Cx43K258stop mice also exhibit reduced ability of establishing gap junctions and altered hemichannel activity [22].
Connexin43 and osteoblast differentiation and function
Cx43 is expressed in osteoblasts, osteocytes, and osteoclasts and it is the most abundant connexin in bone cells [24–26]. Ultra microscopy studies showed that gap junction channels are present in the dendritic processes of adjacent osteocytes and in osteocytic projections that reach cells of the bone surface, including bone lining cells, osteoblasts and osteoclasts [27, 28]. This evidence is consistent with the notion that gap junctions are involved in inter-cellular coupling among the different bone cell types within the osteocyte network (Figure 1B). In addition, the expression of Cx43 in unopposed membranes of osteocytic cells and the functionality of the hemichannels have been demonstrated using cell surface biotinylation followed by immunoprecipitation, and dye uptake, respectively [18, 29]. Accordingly, Cx43 is detected also in non-junctional plasma membranes of osteoblasts and osteocytes, although the primary site of expression is surrounding the cell nucleus [18, 29–31] and (Figure 1C). This subcellular localization supports a potential role of Cx43 in functions beyond its role in gap junction communication in bone.
Regardless of the specific mechanism of action, it has been established that Cx43 expression is required for normal osteoblastic gene expression and function in primary calvaria cells and cultured osteoblastic cell lines [32–34]. This is supported by studies showing that mineralization is impaired in osteoblastic cells isolated from calvaria of mice with global deletion of Cx43 (Cx43−/− mice) or with specific deletion of Cx43 from osteoblast precursors (Cx43fl/−;Col1a.1–2.3kb-Cre mice) [33–35]. Impaired osteoblast differentiation is consistent with accumulation of mesenchymal progenitors in bones from Cx43fl/−;Col1a.1–2.3kb-Cre and of Cx43fl/−;Dermo1-Cre mice lacking Cx43 in osteochondreoprogenitors, as evidenced by increased colony forming units (CFU) for fibroblasts and for osteoblasts in ex vivo bone marrow cell cultures, and by reduced levels of Sost and DMP1, genes preferentially expressed in terminally differentiated osteocytes [14, 36]. In addition, osteoblast markers including osteocalcin, collagen 1α1, osteopontin and Runx2 are decreased in osteoblastic cells isolated from Cx43−/− mice or osteoblastic cell lines overexpressing Cx45, which acts as a functional dominant negative for Cx43 [32, 34]. The regulation of the osteocalcin and collagen 1α1 genes by Cx43 occurs via modulation of binding of the Sp1/Sp3 transcription factors to Cx43 responsive domains found in the promoters of these genes [37]. Disruption of Cx43 channels by overexpression of Cx45 or by pharmacological inhibitors in ROS17/2.8 rat osteosarcoma cells reduces activation of the extracellular signal-regulated kinases (ERKs). In turn, decreased ERK activity leads to reduced phosphorylation and DNA binding of the stimulator of transcription Sp1 and recruitment of the inhibitor of transcription Sp3 [37, 38]; resulting in reduced transcription of the osteocalcin and collagen 1α1 genes. Cx43 also potentiates the induction of osteoblast differentiation by fibroblast growth factor 2 (FGF2) [39]. In the presence of FGF2, the C-terminus tail of Cx43 interacts with protein kinase C (PKC) δ and this phenomenon, together with activation of ERKs, increases Runx2 activity and, ultimately, osteocalcin gene transcription [39, 40]. Overall, this evidence suggests that Cx43 has a central role in the regulation of intracellular signaling pathways that are required for osteoblast differentiation and function.
Recent studies have shown that pannexins 1 and 3 are also expressed in osteoblastic cells [41, 42]. Pannexin1 might mediate the effects of mechanical stimulation in osteoblastic cells (see below) whereas pannexin3 is a target of Runx2 signaling. Whether pannexins are involved in bone development or homeostasis remains unknown.
Table 1 summarizes the skeletal phenotypes of the Cx43 mutant mice reported to date. Although Cx43 deletion from the mouse genome renders mice that die within hours after birth due to cardiac malformations precluding the study of the adult skeleton [43], neonatal bones from these mice exhibit delayed intramembranous ossification and a less pronounced delay of endochondral ossification, supporting the involvement of Cx43 in osteoblast differentiation also in vivo [33, 44]. In addition, mice globally expressing a Cx43 mutant associated with oculodentodigital dysplasia (ODDD), which does not form gap junctions and acts as dominant negative for endogenous Cx43, exhibit low bone mass and decreased bone strength [13]. Moreover, Cx43fl/fl;Dermo1-Cre mice lacking Cx43 in osteochondroprogenitors show a severe skeletal phenotype, with decreased whole body mineral density and cortical thickness [14]. In contrast, deletion of Cx43 from more mature cells in the lineage exhibit less pronounced skeletal defects. Cx43 deletion from early osteoblastic cells (Cx43fl/−;Col1a.1–2.3kb-Cre mice) exhibit only mild reduction in bone volume, osteoblast number and bone mass [34]. Furthermore, deletion of Cx43 from mature osteoblasts and osteocytes (Cx43fl/−;OCN-Cre mice) exhibit indistinguishable BMD measured by Dexa [45] or only a small decrease in cortical femoral BMD measured by µCT [46]; and deletion of Cx43 from osteocytes (Cx43fl/fl;DMP1–8kb-cre mice) does not affect bone mass [30]. Consistent with a role of Cx43 in the differentiation of early osteoblast precursors, but not in more mature cells, a Cx43 ODDD mutant only prevents osteoblast differentiation when present as a germline mutation (and therefore, in all undifferentiated progenitors) and not when it is expressed after osteoblast commitment [47]. Moreover, a recent study reported that Cx43fl/−;OCN-Cre mice exhibit defective fracture healing, with reduced bone formation and resorption at the site of the fracture, and decreased biomechanical properties [48]. Overall, these findings indicate that Cx43 expression in osteoblast precursors, but not in mature osteoblasts or osteocytes, is required for full skeletal development, and that Cx43 expression is also needed for proper function of mature osteoblasts and osteocytes.
Table 1.
Phenotype of mice with deletions or mutations of Cx43
| Mouse model | Deletion/mutation | Challenge | Phenotype |
|---|---|---|---|
| Cx43−/− | global | delayed skeletal mineralization altered osteoblast gene expression |
|
| Cx43+/G138R (ODDD) | global | craniofacial abnormalities reduced cancellous bone volume |
|
| Dermo1-cre;Cx43fl/fl | osteochondro progenitors | low bone mass delayed mineralization increased endocortical resorption and femoral cortical thinning reduced mechanical strength increased periosteal and decrease endocortical bone formation reduced expression of osteoblastic and osteocytic genes, and OPG increased osteoclast formation |
|
| Dermo1-cre;Cx43+/fl(G138R) (ODDD) | osteochondro progenitors | low bone mass | |
| Col1a1–2.3kb-cre;Cx43fl/− | osteoblast precursors | reduced body weight decreased BMD reduced cancellous bone volume delayed osteoblast differentiation reduced expression of osteoblastic and osteocytic genes reduced homing and engraftment of hematopoietic stem cells |
|
| intermittent PTH | attenuated response | ||
| tibia 3-point bending | attenuated endocortical response | ||
| OCN-cre;Cx43fl/fl | mature osteoblasts | no overt changes in BMD increased endocortical resorption without femoral cortical thinning reduced bone material strength increased endocortical resorption and periosteal apposition increased bone resorption markers in young mice and reduced bone formation markers in adult mice increased osteocyte apoptosis in cortical bone |
|
| bisphosphonates | lack of anti-apoptotic effect in osteoblasts and osteocytes | ||
| tibia loading | enhanced periosteal bone formation | ||
| femoral fracture | impaired bone formation and resorption | ||
| DMP1–8kb-cre;Cx43fl/fl | osteocytes | no change in BMD increased endocortical resorption without femoral cortical thinning increased bone formation markers increased RANKL/OPG ratio and reduced expression of sclerostin increased osteocyte apoptosis in cortical bone |
|
| ulna loading | enhanced periosteal bone formation |
Connexin43 and differentiation and function of osteoclasts and hematopoietic cells
Although less explored than its role on cells of the osteoblastic lineage, Cx43 is also required for generation and function of osteoclasts. Expression of Cx43 in osteoclast precursors and mature osteoclasts has been demonstrated in vitro and in vivo [25, 49, 50]. Gap junction communication might be involved in the process of osteoclast differentiation as studies in intact rat calvarial bone showed the presence of Cx43-containing gap junctions between osteoclasts and overlying mononuclear cells at sites of active resorption [49]. Consistent with this notion, blockade of connexin channel function using pharmacologic inhibitors or the Gap27 connexin mimetic peptide leads to impaired fusion of osteoclast precursors and bone resorption in vitro [8, 50]. However, considering that these reagents block both full and half connexin channels, further research using specific channel blockers is needed to discriminate whether gap junctions or hemichannels are required for osteoclastogenesis.
In addition to these direct actions of Cx43 on osteoclasts, recent evidence indicates that Cx43 regulates osteoclast formation by modulating the expression of RANKL and OPG in cells of the osteoblastic lineage that support osteoclast formation. Thus, deletion of Cx43 from osteochondroprogenitors (and their progeny), from mature osteoblasts/osteocytes, or from osteocytes in the DMP1–8kb-Cre;Cx43fl/fl mice, results in increased osteoclast formation, elevated endocortical resorption and high RANKL/OPG ratio in cortical bone [14, 30, 46]. When Cx43 is deleted from more mature cells the increase in osteoclasts and resorption is localized to the endocortical surface of the long bones, changing their geometry but not affecting BMD. These findings suggest that removal of Cx43 from osteocytes is sufficient to control osteoclast generation.
Cx43 expression in osteoblasts and osteocytes also regulates the hematopoietic stem cell niche, as demonstrated by reduced homing and engraftment of hematopoietic progenitors transplanted into irradiated col1a1–2.3kb-cre;Cx43fl/fl mice [36]. However, transplanted hematopoietic stem cells are better retained in the bone marrow of non-irradiated col1a1–2.3kb-cre;Cx43fl/fl mice associated with increased expression of the chemoattractant Cxcl12 in mesenchymal cells and osteoprogenitors. Therefore, Cx43 expression in cells of the osteoblastic lineage differentially regulates hematopoietic stem cell homing/mobilization in normal versus myeloablated mice.
Connexin43 as transducer of survival signals elicited by bisphosphonates
The search for effects of the anti-osteoporotic drugs bisphosphonates on osteoblastic cells showed that these compounds protect osteocytes and osteoblasts from apoptosis [18, 45, 51–56], via a novel mechanism that activates ERKs and requires Cx43 in vitro and in vivo (Figure 2A) [18, 45, 51, 57]. In contrast to the survival effect of bisphosphonates at low concentrations, high concentrations of bisphosphonates induce apoptosis of cells of the osteoblastic lineage (similar to osteoclasts) [58–62]. Moreover, bisphosphonates regulate apoptosis of osteoblasts/osteocytes and osteoclasts by different mechanisms (reviewed in [63]). Thus, the inhibitory actions of the drugs on osteoclasts are due to inhibition of enzymes of the mevalonate pathway or to generation of toxic nucleotide analogs [64].
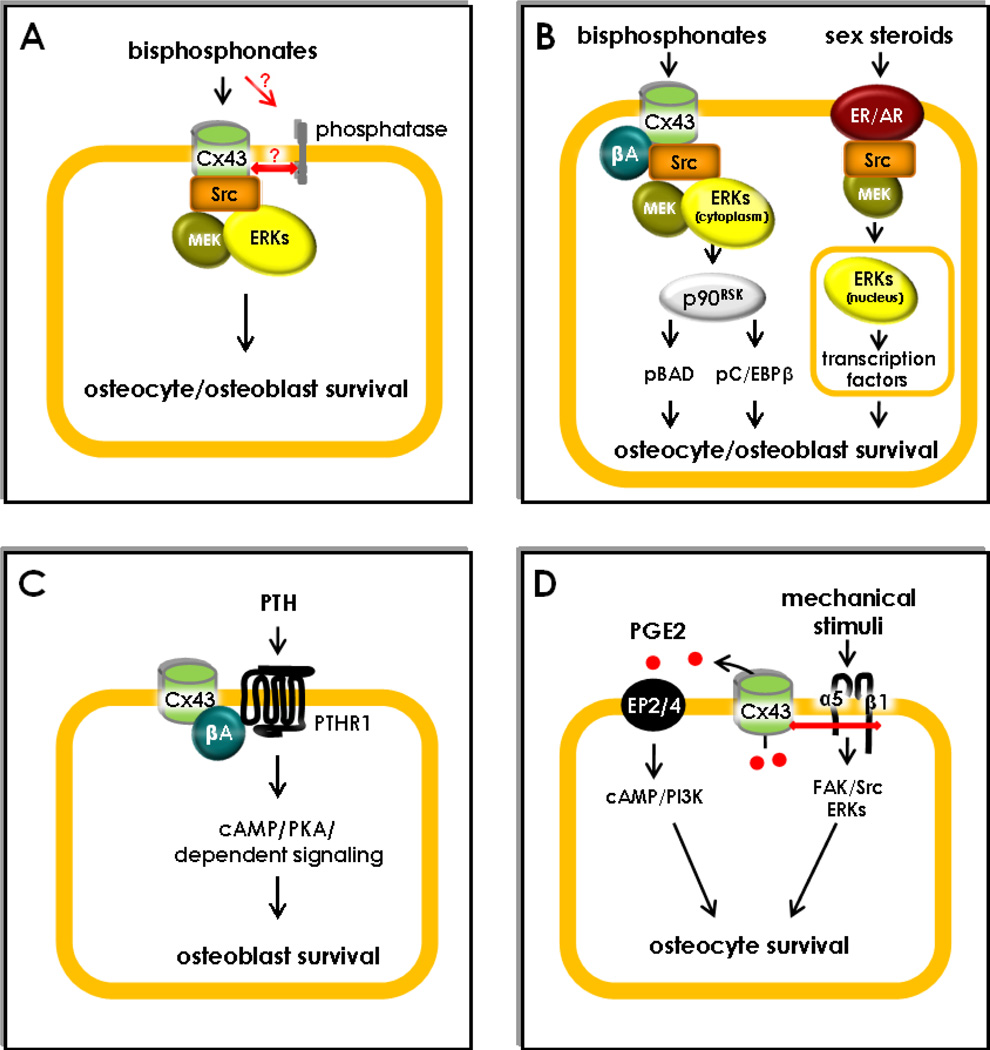
Figure 2. Scaffolding function of connexin43 as a regulator of osteoblast and osteocyte survival.
(A) Bisphosphonates induce the transient opening of non-junctional Cx43 hemichannels, likely indirectly by binding to a phosphatase that interact with Cx43. Hemichannel opening results in Src activation, followed by MEK and ERK phosphorylation, which in turn activates survival signaling in osteocytes and osteoblasts. (B) Opening of Cx43 hemichannels by bisphosphonates results in the retention of activated ERKs in the cytoplasm through Cx43/β-arrestin (βA) interactions. This leads to phosphorylation of the cytoplasmic ERK targets p90RSK, BAD and C/EBPβ. In contrast, ERKs activated by estrogens translocate to the nucleus and activate transcription factors that promote survival. (C) Cx43 sequesters β-arrestin (βA) away from the PTHR1, facilitating cAMP/PKA-mediated downstream signaling promoting osteoblast survival. (D) Mechanical stimulation induces α5β1integrin engagement, followed by FAK/Src/ERK activation. Association of integrins with Cx43 opens the hemichannels. Release of PGE2 through the hemichannels induces autocrine/paracrine signaling through the EP2/4 prostaglandin receptor, cAMP/PI3K activation and inhibition of apoptosis.
Only Cx43 mediates the survival pathway activated by bisphosphonates. This strict requirement of Cx43 for anti-apoptosis by bisphosphonates raised the possibility that the connexin would be a receptor for the drugs. However, recent evidence suggests the possibility that bisphosphonates bind to a phosphatase, and not to Cx43, on the cell surface [65, 66]. This phosphatase might in turn interact with Cx43 and activate downstream signaling (Figure 2A).
Despite the requirement of Cx43 for bisphosphonate anti-apoptotic action, remarkably, bisphosphonates do not stimulate gap junction communication, but instead open hemichannels in osteoblastic cells [18]. This phenomenon was surprising at a time at which Cx43-mediated events were synonymous of gap junction channels, as functional hemichannels had not been described in bone cells [2]. It was then hypothesized that half Cx43 channels may serve as transducers of extracellular signals for endogenously produced molecules or other stimuli. Indeed, as it will be discussed below, Cx43 hemichannels are opened by mechanical stimulation and likely participate in prostaglandin release and survival of osteocytic cells induced by strain [29, 67].
The specific requirement of Cx43 for bisphosphonate anti-apoptotic action is due to domains located in the cytoplasmic C-terminal region of the protein, which differs considerably among members of the connexin family. This is demonstrated by the inability of a Cx43 mutant lacking the cytoplasmic tail to confer survival responsiveness to bisphosphonates. This portion of the molecule contains sites that bind to and are phosphorylated by intracellular kinases, including Src and ERKs, known to protect cells from apoptosis (Figure 2A).
A notable feature of ERKs activated by bisphosphonates through the Cx43/Src pathway is that instead of undergoing nuclear translocation like ERKs activated by most stimuli, including sex steroids (estrogens and androgens), bisphosphonate-activated ERKs are retained in the cytoplasm (Figure 2B) [68]. The cytoplasmic versus nuclear location of activated ERKs determines that different molecular targets mediate the biological response to bisphosphonates versus estrogens in osteoblastic cells. The cytoplasmic retention of ERKs activated by bisphosphonates results also from the scaffolding function of Cx43 by which its C-terminus tail interacts with β-arrestins [12, 19].
Nevertheless, bisphosphonates still prevent bone loss induced by glucocorticoids [45] or ovariectomy [69] in mice lacking Cx43 in osteoblasts/osteocytes, demonstrating that the anti-apoptotic effect of bisphosphonates does not explain the effect of these agents on bone mass. This is due to the potent inhibition of resorption exerted by bisphosphonates that might mask the potential contribution of prevention of osteoblast and osteocyte apoptosis to the overall effects of the drugs. Indeed, when a bisphosphonate that does not affect osteoclasts but prevents osteoblast and osteocyte apoptosis is administered to mice, the decrease in bone mass and strength induced by glucocorticoids is prevented, even though bone remodeling is not reduced [57, 70, 71]. Bisphosphonate analogs that do not inhibit osteoclasts might represent an alternative tool to increase bone strength in condition in which a decrease in bone remodeling is not advised. However, a potential stimulatory effect on osteoblasts through the Cx43 pathway with the bisphosphonates currently used in the clinic has minimal, if any, contribution to the overall pharmacologic action of the drugs.
Connexin43/β-arrestin interaction and PTH-induced osteoblast survival
Mice lacking Cx43 (Cx43fl/−; Col1a1–2.3kb-cre mice) do not exhibit a full anabolic response to PTH, as evidenced by decreased mineral appositional rate (MAR) and bone mineral content (Table 1) [34]. The inability of the hormone to induce full anabolism might be explained by defective cAMP production [72] and survival signaling [12] induced by PTH in osteoblasts lacking Cx43. Mechanistic studies showed that Cx43 interacts with β-arrestin, thereby removing the inhibitory effect of β-arrestin on cAMP downstream signaling (Figure 2C). Association of Cx43 and β-arrestin occurs through phosphorylated serine 368 in the C-terminus tail of Cx43 and leads to decreased binding of β-arrestin to the PTH receptor. This is another case in which the ability of Cx43 to interact with other molecules, through specific phosphorylation sites in its cytoplasmic tail, regulates intracellular signaling in osteoblastic cells.
Connexin43 and mechanotransduction
Cx43 is highly expressed in osteocytes, the cells ideally positioned to sense and transmit signals induced by mechanical forces in the skeleton, as well as in osteoblasts. Mechanical stimulation has marked effects on Cx43-related functions in these two cell types. First, Cx43 expression is enhanced by loading in bones in vivo as well as in cultured osteoblasts and osteocytes [73–76]. Moreover, pulsatile fluid flow or substrate stretching increase gap junction communication among osteocytic and osteoblastic cells and this phenomenon has been proposed as a major way of transmission of signals among cells within the osteocyte network [73, 74] (Figure 2D). More recent studies demonstrated that mechanical strain also induces opening of Cx43 hemichannels in osteocytic cells, which is required for the release of prostaglandin PGE2 [5, 29]. However, channels formed by P2x7 receptors and/or pannexin1 might are also involved in the release of PGE2 by mechanical stimulation [41, 77].
Regardless of the mechanism by which PGE2 is released by mechanical stimulation, fluid flow inhibits glucocorticoid-induced apoptosis of osteocytic cells through activation of the PGE2 receptors EP2/4 and activation of the cAMP/PI3K pathway [67]. More recent evidence suggests that physical interaction of the C-terminus domain of Cx43 with α5 and β1 integrins leads to activation of PI3K, which in turn is responsible for hemichannel opening induced by mechanical stimulation [78]. This evidence is consistent with our earlier studies showing that mechanical stimulation leads to engagement of integrins α5 and β1, which in turn activate the kinases FAK/Src and the ERK pathway, promoting osteocyte survival [79] (Figure 2D). Taken together with earlier studies demonstrating that unloading by tail suspension leads to increased prevalence of osteocyte apoptosis [80], this evidence suggests that mechanical forces prevent osteocyte apoptosis via PGE2 release through Cx43 hemichannels. Moreover, lack of survival effect of mechanical stimulation during normal ambulatory conditions might explain the increased osteocyte apoptosis observed in mice lacking Cx43 in osteoblasts/osteocytes or in osteocytes [30], as will be discussed below.
These in vitro findings suggest that Cx43 is a critical component of the mechanotransduction machinery by which the skeleton responds to mechanical forces. Consistent with this, bone formation on the endocortical surface of the tibia is attenuated in mice in which Cx43 was deleted from preosteoblasts, osteoblasts and osteocytes (Col1a1–2.3kb), resulting from reduced number of osteoblasts laying matrix (MS/BS) as well as activity of osteoblasts (MAR) [81]. However, three different research groups demonstrated that mice lacking Cx43 from osteochondroprogenitors (Dermo1), osteoblasts and osteocytes (OCN), or osteocytes (DMP1–8kb) exhibit enhanced bone formation on the periosteal surface of long bones instead of the anticipated decreased response to loading [46, 82] (Table 1). Periosteal bone formation rate is higher in Cx43 deficient mice compared to the respective controls, mainly due to an increased MS/BS. These findings are consistent with a differential regulation by Cx43 function of the number and/or synthetic activity of osteoblasts depending on whether they are located on the periosteal versus the endosteal surface of bone. The reasons for the apparent discrepancy between the studies showing a requirement of Cx43 expression for PGE2 release induced by mechanical stimulation in vitro versus the enhanced periosteal response to loading of mice lacking Cx43 are not known. However, Cox2 deficient mice exhibit similar increased periosteal bone formation rate in response to loading than wild type animals [83], demonstrating that PGE2 synthesis is not a prerequisite for anabolism and that other molecules are involved at the periosteal surface. Among the potential mediators, the inhibitor of bone formation sclerostin is downregulated by loading [84]; and its decrease is essential for the anabolic response [76]. Mice lacking Cx43 indeed exhibit lower levels of Sost/sclerostin [14, 30], opening then the possibility that activation of Wnt signaling under basal conditions is the cause of the exaggerated response of these animals to mechanical loading at the periosteal surface.
Deletion of Cx43 from osteoblastic cells also alters the response of the bone to reduced mechanical forces. The decrease in cortical thickness due to endosteal resorption observed in wild type mice subjected to muscle paralysis is abolished in mice lacking Cx43 in preosteoblasts, osteoblasts and osteocytes (Col1a1–2.3kb), whereas both type of mice exhibit similar cancellous bone loss in the femur [85]. Hindlimb unloading also results in differential effects in wild type versus osteoblast/osteocyte-specific Cx43 deletion (OCN) [86], although in this case the attenuated response occurs in cancellous bone. The apparent contradiction between the two studies might be due to the different means to reduce loading of the skeleton (muscle paralysis versus hindlimb unloading), to the fact that preosteoblasts lack Cx43 in one animal model and not in the other (Col1a1–2.3kb versus OCN) or to differences in the genetic background of the mice (Cx43fl/− mixed C57BL/6-C129/J for Col1a1–2.3kb mice versus Cx43fl/fl backcrossed to C57BL/6 for three generations for OCN).
Osteocyte intrinsic actions of Cx43 regulating osteoclast and osteoblast activity
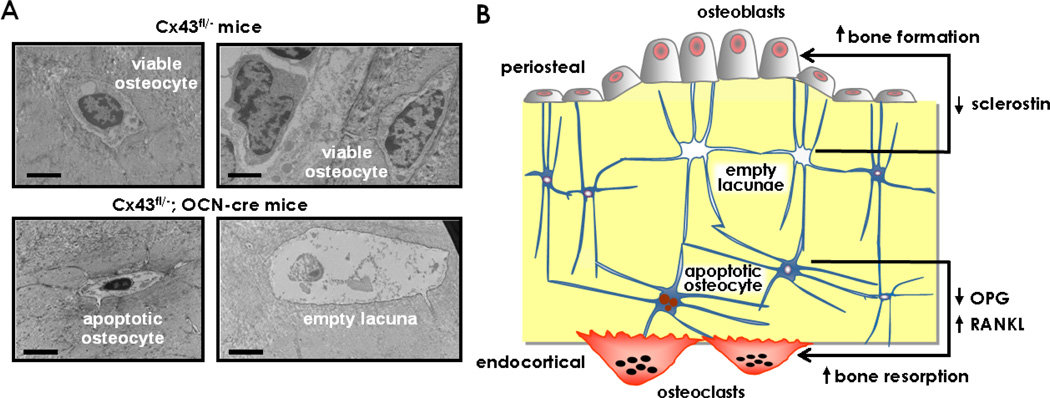
A feature of mice in which Cx43 is deleted from osteocytes is the elevated prevalence of apoptotic osteocytes and accumulation of empty lacunae in cortical bone, without changes in cancellous bone (Figure 3A) [30]. Whether this differential effect on osteocyte apoptosis is due to higher expression of Cx43 in murine cortical versus cancellous bone, as previously suggested [14, 30], remains to be investigated. Increased osteocyte apoptosis is associated with increased endocortical resorption and high periosteal bone formation in the femur, with the consequent higher marrow cavity and total tissue areas measured in femoral mid-diaphysis. Remarkably, similar geometrical changes are exhibited by mice lacking Cx43 in osteochoprogenitors (Dermo1), or in preosteoblasts, osteoblasts and osteocytes (Col1a.1–2.3kb) [14, 81], or in mature osteoblasts and osteocytes [30, 46]. Thus, removal of Cx43 from osteocytes [30] is sufficient to recapitulate the cortical bone phenotype observed in all the models of Cx43 deletion in osteoblastic cells and their progenitors.
Figure 3. Cell Autonomous requirement of Connexin43 for osteocyte survival and control of osteoblast and osteoclast activity.
(A) Transmission electron micrographs showing apoptotic and empty lacunae in bones from Cx43fl/−;OCN-cre mice lacking Cx43 in osteoblasts and osteocytes. Scale bars indicate 2 µm. (B) Increased osteocyte apoptosis in mice lacking Cx43 from osteocytes regulates periosteal apposition and endocortical resorption in cortical bone, by separate mechanisms. Accumulation of empty lacunae adjacent to periosteal surfaces results in decreased sclerostin expression and enhanced bone formation. Apoptotic osteocytes trigger osteoclast recruitment and decreased expression of osteocytic OPG is a permissive event, leading to enhanced resorption on the endocortical surface.
Blockade of endocortical resorption with bisphosphonates reversed the increased marrow cavity and increased cortical bone area in mice lacking Cx43 in osteoblasts and osteocytes. However, bisphosphonate treatment did not affect total tissue area (bone plus bone marrow). These findings demonstrate that endocortical resorption and periosteal apposition are independently regulated by Cx43 [30]. Indeed, anatomical mapping of apoptotic osteocytes, osteocytic proteins and formation/resorption, revealed that Cx43 controls osteoclast and osteoblast activity in separate areas of cortical bone and by different mechanisms (Figure 3B). Reduced expression of the inhibitor of bone formation sclerostin due to loss of osteocytes was found close to periosteal surfaces exhibiting elevated bone formation. Similarly, Sost expression is reduced in mice lacking Cx43 in osteochondroprogenitors [14]. On the other hand, the number of osteocytes expressing osteoprotegerin (OPG) was decreased throughout cortical bone; and apoptotic osteocytes were preferentially located adjacent to endocortical areas containing osteoclasts. These findings suggest that whereas reduced OPG expression is a permissive event, osteoclast recruitment might require active signaling from dying osteocytes. Cx43 deletion in cultured osteocytic cells resulted in increased apoptosis, and increased RANKL and decreased OPG expression resulting in a marked increase in RANKL/OPG ratio demonstrating a cell autonomous role of Cx43 in osteocytes (Figure 3B) [30, 46].
In conclusion, although tissue/cell-restricted promoters rarely achieve absolute specificity and efficiency, the current available mouse models of Cx43 deletion strongly suggest distinct roles of Cx43 in osteoblastic cells at different stages of differentiation. Cx43 expression in osteoblast precursors is indispensable for bone development and bone mass acquisition; Cx43 expression in osteoblast precursors, osteoblasts and osteocytes regulates osteoclast development; and Cx43 expression in osteocytes maintains their viability. Development of novel models to target more specifically cells of the osteoblastic lineage, as well as to directly remove Cx43 from osteoclasts, are required to increase our understanding of the biology of Cx43 in bone cells. Moreover, inducible deletion of Cx43 is needed to establish the role of the protein on the adult skeleton, independent of its influence on bone development.
Connexin43 signaling and aging bone
Bones from Cx43-deficient mice exhibit features of bones from aging rodents and humans, including elevated osteocyte apoptosis, exacerbated endosteal resorption and periosteal expansion of long bones [87, 88]. Although Cx43 expression in bones or cells from old rodents appears unaltered [89, 90]. However, PTH-induced gap junction communication is reduced in old animals, suggesting defective Cx43 function. Osteoblastic cells from old mice are also resistant to insulin-like growth factor-1 (IGF-1)-induced survival [91], even when receptor expression and its binding ability are not decreased, suggesting defective downstream signaling. Recent studies found that osteocytes lacking Cx43 are also refractory to the survival effect of IGF-1 [92]. Cx43-deficient osteocytes also exhibit altered expression of microRNA (miRs) miR21 and miR218, which are associated with regulation of apoptosis in several cancer cells [93, 94]. These miRs control activation of Akt [95] and NFκB [96], known mediators of IGF-1 survival signaling [97, 98]. This evidence raises the possibility that resistance of osteoblasts/osteocytes to IGF-1 and/ or PTH signaling is due to altered Cx43 function in aging. More research is warranted to confirm this hypothesis and to determine whether miRs regulate osteocyte survival and/or play a role in skeletal aging downstream of Cx43.
Other connexins and bone homeostasis
While Cx43 has received the most attention of all the skeletal connexins, other members of this family of proteins are also expressed in bone cells. Cx46 and Cx45 are detected in osteoblasts and osteocytes [44, 99, 100]. Cx46 localizes in the perinuclear region in osteoblastic cells as monomers and does not form membrane channels [101]. Whether Cx46 has any function in osteoblast biology is not known. On the other hand, Cx45 functions as a dominant negative for Cx43 actions, likely due to its ability to form heterochannels with Cx43. This results in blockade of Cx43 actions, including gap junction communication, osteoblast differentiation, and osteoblast and osteocyte survival [18, 32, 102]. Global Cx45 deletion is embryonically lethal due to defective vascular development and some Cx45+/− embryos exhibit growth retardation [99]. However, the role of Cx45 per se on the skeleton or the skeletal phenotype of the global Cx45+/− mice or that of mice with bone specific deletion of Cx45 have not been reported.
Polymorphisms in the Cx37 gene have been associated with bone mineral density in men [103]. Recent evidence demonstrate that Cx37 is expressed in osteocytes and osteoblasts, although its expression is higher in osteocytes [104]. Future studies are warranted to address the potential role of Cx37 on bone homeostasis.
Conclusions and future directions
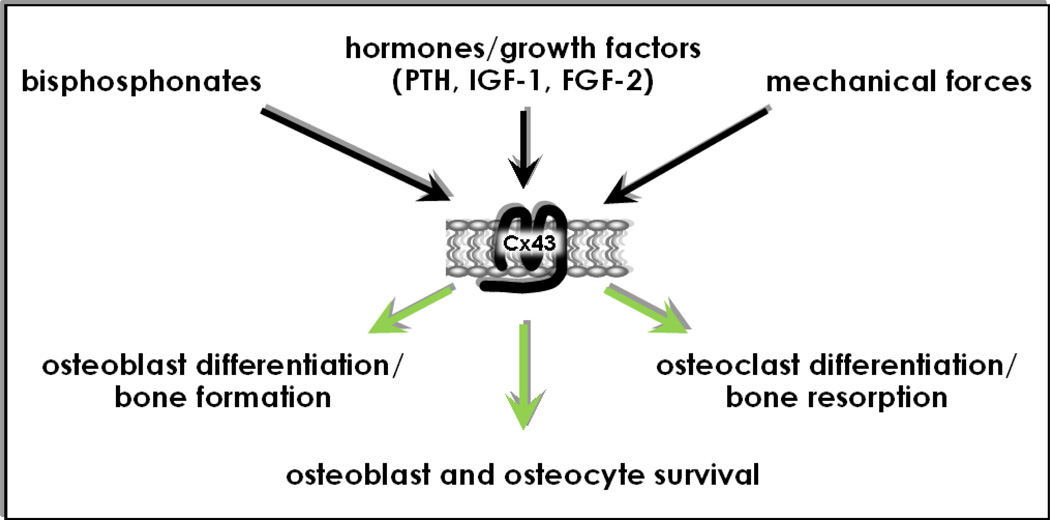
In conclusion, work of several investigators during the last fifteen years points towards a role of Cx43 in bone cells that not only depends on its function as a gap junction protein. Thus, Cx43 also forms hemichannels that open in response to pharmacological and mechanical cues and, likely, to endogenous stimuli yet to be discovered. Opening of Cx43 hemichannels appears to be triggered by the interaction of Cx43 with other proteins, such as phosphatases in the case of bisphosphonates, or integrins in the case of mechanical stimulation. Moreover, the ability of Cx43 to act as a docking platform for signaling molecules, such as the Src kinase and β-arrestin, results in activation of the ERK/MAPK pathway as well as in crosstalk with the PTH receptor/cAMP signaling pathway, leading to modulation of biological outcomes in osteoblastic cells. In summary, Cx43 controls the differentiation, activity, and survival of bone cells, impacting bone mass and bone mechanical properties and geometry (Figure 4).
Figure 4. Connexin43 as a mediator of pharmacologic, hormonal and mechanical stimuli.
Cx43 is a key component of intracellular machinery responsible for signal transduction in bone in response to pharmacologic, hormonal, and mechanical stimuli.
The current understanding of the pathophysiologic role of Cx43 in bone cells serves as the foundation for developing new strategies to manipulate bone cell function with the final therapeutic goal of improving bone mass and strength. It is expected that future approaches will take advantage of function-specific tools to trigger gap junctions, hemichannels, or scaffolding functions of Cx43 to achieve the desired outcomes. Understanding the mechanism that mediates the control of osteoblast and osteoclast function by osteocytes could provide new molecular targets to modulate bone formation and resorption. These strategies will also shed light on the crosstalk between Cx43 and bone acting stimuli such as PTH or IGF-1, and on the potential impact of Cx43 and associated pathways on the process of skeletal aging. It is also anticipated that revealing the mechanisms that regulate the balance between Cx43 and other connexins in bone cells will provide new means to control bone cell viability and function, with the final goal of improving bone mass and strength.
Highlights.
Cx43 is the most abundant gap junction protein expressed in bone cells.
Cx43 forms gap junctions, hemichannels and serves as a scaffold that regulates intracellular signaling in bone cells.
Through these diverse mechanisms, Cx43 regulates the response of bone to pharmacologic, hormonal and mechanical stimuli.
Understanding the role of Cx43 in bone cell function provides new opportunities to treat bone diseases.
ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health (R01-AR053643, KO2-AR02127, R03 TW006919, R01-DK076007, and P01-AG13918).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb. Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a002576. a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat. Rev. Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 3.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem. J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cea LA, Riquelme MA, Cisterna BA, Puebla C, Vega JL, Rovegno M, Saez JC. Connexin- and Pannexin-Based Channels in Normal Skeletal Muscles and Their Possible Role in Muscle Atrophy. J. Membr. Biol. 2012 doi: 10.1007/s00232-012-9485-8. [DOI] [PubMed] [Google Scholar]
- 5.Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, Jiang JX. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J. Biol. Chem. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orellana JA, Saez PJ, Cortes-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, Figueroa V, Velarde V, Jiang JX, Nualart F, Saez JC, Garcia MA. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. 2012;60:53–68. doi: 10.1002/glia.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans WH, Leybaert L. Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun. Adhes. 2007;14:265–273. doi: 10.1080/15419060801891034. [DOI] [PubMed] [Google Scholar]
- 8.Ilvesaro J, Tavi P, Tuukkanen J. Connexin-mimetic peptide Gap 27 decreases osteoclastic activity. BMC. Musculoskelet. Disord. 2001;2:10. doi: 10.1186/1471-2474-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponsaerts R, De VE, Retamal M, D'hondt C, Vermeire D, Wang N, De SH, Zimmermann P, Himpens B, Vereecke J, Leybaert L, Bultynck G. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J. 2010;24:4378–4395. doi: 10.1096/fj.09-153007. [DOI] [PubMed] [Google Scholar]
- 10.Stehberg J, Moraga-Amaro R, Salazar C, Becerra A, Echeverria C, Orellana JA, Bultynck G, Ponsaerts R, Leybaert L, Simon F, Saez JC, Retamal MA. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 2012 doi: 10.1096/fj.11-198416. [DOI] [PubMed] [Google Scholar]
- 11.Tong D, Li TY, Naus KE, Bai D, Kidder GM. In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J. Cell Sci. 2007;120:4016–4024. doi: 10.1242/jcs.011775. [DOI] [PubMed] [Google Scholar]
- 12.Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with arrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J. Cell. Biochem. 2011;112:2920–2930. doi: 10.1002/jcb.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, Bukauskas FF, Civitelli R, Lewalter T, Fleischmann BK, Willecke K. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum. Mol. Genet. 2008;17:539–554. doi: 10.1093/hmg/ddm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E, Civitelli R. Osteoblast Connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol. Biol. Cell. 2011;22:1240–1251. doi: 10.1091/mbc.E10-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang J, Burra S, Harris S, Zhao H, Johnson M, Bonewald L. Inhibiting connexin 43 gap junction function in osteocytes, but not connexin 43 hemmichannel function results in defects in skeletal structure and bone mass. J. Bone Miner. Res. 2010;25:S12. [Google Scholar]
- 16.Giepmans BN. Role of connexin43-interacting proteins at gap junctions. Adv. Cardiol. 2006;42:41–56. doi: 10.1159/000092561. [DOI] [PubMed] [Google Scholar]
- 17.Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun. Signal. 2009;7:4. doi: 10.1186/1478-811X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 19.Plotkin LI, Vyas K, Gortazar AR, Manolagas SC, Bellido T. βarrestin complexes with connexin (Cx) 43 and anchors ERKs outside the nucleus: a requirement for the Cx43/ERK-mediated anti-apoptotic effect of bisphosphonates in osteocytes. J. Bone Miner. Res. 2006;21:S65. [Google Scholar]
- 20.Dang X, Jeyaraman M, Kardami E. Regulation of connexin-43-mediated growth inhibition by a phosphorylatable amino-acid is independent of gap junction-forming ability. Mol. Cell Biochem. 2006;289:201–207. doi: 10.1007/s11010-006-9162-2. [DOI] [PubMed] [Google Scholar]
- 21.Crespin S, Bechberger J, Mesnil M, Naus CC, Sin WC. The carboxy-terminal tail of connexin43 gap junction protein is sufficient to mediate cytoskeleton changes in human glioma cells. J. Cell Biochem. 2010;110:589–597. doi: 10.1002/jcb.22554. [DOI] [PubMed] [Google Scholar]
- 22.Kozoriz MG, Bechberger JF, Bechberger GR, Suen MW, Moreno AP, Maass K, Willecke K, Naus CC. The connexin43 C-terminal region mediates neuroprotection during stroke. J Neuropathol. Exp. Neurol. 2010;69:196–206. doi: 10.1097/NEN.0b013e3181cd44df. [DOI] [PubMed] [Google Scholar]
- 23.Maass K, Shibayama J, Chase SE, Willecke K, Delmar M. C-terminal truncation of connexin43 changes number, size, and localization of cardiac gap junction plaques. Circ. Res. 2007;101:1283–1291. doi: 10.1161/CIRCRESAHA.107.162818. [DOI] [PubMed] [Google Scholar]
- 24.Civitelli R, Beyer EC, Warlow PM, Robertson AJ, Geist ST, Steinberg TH. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J. Clin. Invest. 1993;91:1888–1896. doi: 10.1172/JCI116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilvesaro J, Väänänen K, Tuukkanen J. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J. Bone Min. Res. 2000;15:919–926. doi: 10.1359/jbmr.2000.15.5.919. [DOI] [PubMed] [Google Scholar]
- 26.Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. J. Bone Miner. Res. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 27.Doty SB. Morphological evidence of gap junctions between bone cells. Calcif. Tissue Int. 1981;33:509–512. doi: 10.1007/BF02409482. [DOI] [PubMed] [Google Scholar]
- 28.Marotti G, Ferretti M, Muglia MA, Palumbo C, Palazzini S. A quantitative evaluation of osteoblast-osteocyte relationships on growing endosteal surface of rabbit tibiae. Bone. 1992;13:363–368. doi: 10.1016/8756-3282(92)90452-3. [DOI] [PubMed] [Google Scholar]
- 29.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol. Biol. Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun L, Rhee Y, Bellido T, Plotkin LI. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J. Bone Min. Res. 2012;27:374–389. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan K, Solan JL, Dominguez M, Sia M, Hand A, Lampe PD, Laird DW. Trafficking, assembly, and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol. Biol. Cell. 1999;10:2033–2050. doi: 10.1091/mbc.10.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecanda F, Towler DA, Ziambaras K, Cheng SL, Koval M, Steinberg TH, Civitelli R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol. Biol. Cell. 1998;9:2249–2258. doi: 10.1091/mbc.9.8.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung D, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J. Cell Sci. 2006;119:4187–4198. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 35.Thi MM, Urban-Maldonado M, Spray DC, Suadicani SO. Characterization of human telomerase reverse transcriptase (hTERT) immortalized osteoblast cell lines generated from wildtype and connexin43-null mouse calvaria. Am. J Physiol Cell Physiol. 2010;299:C994–C1006. doi: 10.1152/ajpcell.00544.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Nieto D, Li L, Kohler A, Ghiaur G, Ishikawa E, Sengupta A, Madhu M, Arnett J, Santho R, Dunn SK, Fishman GI, Gutstein DE, Civitelli R, Barrio LC, Gunzer M, Cancelas JA. Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood. 2012;119:5144–5154. doi: 10.1182/blood-2011-07-368506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin - response elements in osteoblast promoters. J. Biol. Chem. 2003;278:24377–24387. doi: 10.1074/jbc.M212554200. [DOI] [PubMed] [Google Scholar]
- 38.Stains JP, Civitelli R. Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol. Biol. Cell. 2005;16:64–72. doi: 10.1091/mbc.E04-04-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima F, Niger C, Hebert C, Stains JP. Connexin43 Potentiates Osteoblast Responsiveness to Fibroblast Growth Factor 2 via a Protein Kinase C-Delta/Runx2-dependent Mechanism. Mol. Biol. Cell. 2009;20:2697–2708. doi: 10.1091/mbc.E08-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niger C, Buo AM, Hebert C, Duggan BT, Williams MS, Stains JP. ERK Acts in Parallel to PKC delta to Mediate the Connexin43-dependent Potentiation of Runx2 Activity by FGF2 in MC3T3 Osteoblasts. Am. J. Physiol Cell Physiol. 2012;302:C1035–C1044. doi: 10.1152/ajpcell.00262.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thi MM, Islam S, Suadicani SO, Spray DC. Connexin43 and pannexin1 channels in osteoblasts: who is the "hemichannel"? J. Membr. Biol. 2012;245:401–409. doi: 10.1007/s00232-012-9462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bond SR, Lau A, Penuela S, Sampaio AV, Underhill TM, Laird DW, Naus CC. Pannexin 3 is a novel target for Runx2, expressed by osteoblasts and mature growth plate chondrocytes. J. Bone Miner. Res. 2011;26:2911–2922. doi: 10.1002/jbmr.509. [DOI] [PubMed] [Google Scholar]
- 43.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 44.Chaible LM, Sanches DS, Cogliati B, Mennecier G, Dagli ML. Delayed Osteoblastic Differentiation and Bone Development in Cx43 Knockout Mice. Toxicol. Pathol. 2011;39:1046–1055. doi: 10.1177/0192623311422075. [DOI] [PubMed] [Google Scholar]
- 45.Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J. Bone Miner. Res. 2008;23:1712–1721. doi: 10.1359/JBMR.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Paul EM, Sathyendra V, Davidson A, Bronson S, Srinivasan S, Gross TS, Donahue HJ. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE. 2011;6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLachlan E, Plante I, Shao Q, Tong D, Kidder GM, Bernier SM, Laird DW. ODDD-Linked Cx43 Mutants Reduce Endogenous Cx43 Expression and Function in Osteoblasts and Inhibit Late Stage Differentiation. J. Bone Miner. Res. 2008;23:928–938. doi: 10.1359/jbmr.080217. [DOI] [PubMed] [Google Scholar]
- 48.Loiselle AE, Paul EM, Lewis GS, Donahue HJ. Osteoblast and osteocyte-specific loss of Connexin43 results in delayed bone formation and healing during murine fracture healing. J. Orthop. Res. 2012 doi: 10.1002/jor.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones SJ, Gray C, Sakamaki H, Arora M, Boyde A, Gourdie R, Green C. The incidence and size of gap junctions between the bone cells in rat calvaria. Anat. Embryol. (Berl.) 1993;187:343–352. doi: 10.1007/BF00185892. [DOI] [PubMed] [Google Scholar]
- 50.Schilling AF, Filke S, Lange T, Gebauer M, Brink S, Baranowsky A, Zustin J, Amling M. Gap junctional communication in human osteoclasts in vitro and in vivo. J. Cell Mol. Med. 2008;12:2497–2504. doi: 10.1111/j.1582-4934.2008.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kogianni G, Mann V, Ebetino F, Nuttall M, Nijweide P, Simpson H, Noble B. Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci. 2004;75:2879–2895. doi: 10.1016/j.lfs.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 53.Bivi N, Bereszczak JZ, Romanello M, Zeef LA, Delneri D, Quadrifoglio F, Moro L, Brancia FL, Tell G. Transcriptome and proteome analysis of osteocytes treated with nitrogen-containing bisphosphonates. J. Proteome. Res. 2009;8:1131–1142. doi: 10.1021/pr8005606. [DOI] [PubMed] [Google Scholar]
- 54.Gangoiti MV, Cortizo AM, Arnol V, Felice JI, McCarthy AD. Opposing effects of bisphosphonates and advanced glycation end-products on osteoblastic cells. Eur. J. Pharmacol. 2008;600:140–147. doi: 10.1016/j.ejphar.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 55.Abe Y, Kawakami A, Nakashima T, Ejima E, Fujiyama K, Kiriyama T, Ide A, Sera N, Usa T, Tominaga T, Ashizawa K, Yokoyama N, Eguchi K. Etidronate inhibits human osteoblast apoptosis by inhibition of pro-apoptotic factor(s) produced by activated T cells. J. Lab. Clin. Med. 2000;136:344–354. doi: 10.1067/mlc.2000.109757. [DOI] [PubMed] [Google Scholar]
- 56.Follet H, Li J, Phipps RJ, Hui S, Condon K, Burr DB. Risedronate and alendronate suppress osteocyte apoptosis following cyclic fatigue loading. Bone. 2007;40:1172–1177. doi: 10.1016/j.bone.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 57.Plotkin LI, Bivi N, Bellido T. A bisphosphonate that does not affect osteoclasts prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids in mice. Bone. 2011;49:122–127. doi: 10.1016/j.bone.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frediani B, Spreafico A, Capperucci C, Chellini F, Gambera D, Ferrata P, Baldi F, Falsetti P, Santucci A, Bocchi L, Marcolongo R. Long-term effects of neridronate on human osteoblastic cell cultures. Bone. 2004;35:859–869. doi: 10.1016/j.bone.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Pan B, To LB, Farrugia AN, Findlay DM, Green J, Gronthos S, Evdokiou A, Lynch K, Atkins GJ, Zannettino AC. The nitrogen-containing bisphosphonate, zoledronic acid, increases mineralisation of human bone-derived cells in vitro. Bone. 2004;34:112–123. doi: 10.1016/j.bone.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 60.Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000;60:6001–6007. [PubMed] [Google Scholar]
- 61.Pozzi S, Vallet S, Mukherjee S, Cirstea D, Vaghela N, Santo L, Rosen E, Ikeda H, Okawa Y, Kiziltepe T, Schoonmaker J, Xie W, Hideshima T, Weller E, Bouxsein ML, Munshi NC, Anderson KC, Raje N. High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin. Cancer Res. 2009;15:5829–5839. doi: 10.1158/1078-0432.CCR-09-0426. [DOI] [PubMed] [Google Scholar]
- 62.Idris AI, Rojas J, Greig IR, van't Hof RJ, Ralston SH. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif. Tissue Int. 2008;82:191–201. doi: 10.1007/s00223-008-9104-y. [DOI] [PubMed] [Google Scholar]
- 63.Bellido T, Plotkin LI. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone. 2011;49:50–55. doi: 10.1016/j.bone.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 65.Morelli S, Bilbao PS, Katz S, Lezcano V, Roldan E, Boland R, Santillan G. Protein phosphatases: possible bisphosphonate binding sites mediating stimulation of osteoblast proliferation. Arch. Biochem. Biophys. 2011;507:248–253. doi: 10.1016/j.abb.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 66.Lezcano V, Bellido T, Plotkin LI, Boland R, Morelli S. Role of connexin 43 in the mechanism of action of alendronate: Dissociation of anti-apoptotic and proliferative signaling pathways. Arch. Biochem. Biophys. 2012;518:95–102. doi: 10.1016/j.abb.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitase Y, Barragan L, Jiang JX, Johnson ML, Bonewald LF. Mechanical induction of PGE(2) in osteocytes blocks glucocorticoid induced apoptosis through both the beta-catenin and PKA pathways. J. Bone Miner. Res. 2010;25:2381–2392. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of ERK activation. J. Biol. Chem. 2005;280:7317–7325. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- 69.Watkins MP, Norris JY, Grimston SK, Zhang X, Phipps RJ, Ebetino FH, Civitelli R. Bisphosphonates improve trabecular bone mass and normalize cortical thickness in ovariectomized, osteoblast connexin43 deficient mice. Bone. 2012;51:787–794. doi: 10.1016/j.bone.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown RJ, Van Beek E, Watts DJ, Lowik CW, Papapoulos SE. Differential effects of aminosubstituted analogs of hydroxy bisphosphonates on the growth of Dictyostelium discoideum. J. Bone Min. Res. 1998;13:253–258. doi: 10.1359/jbmr.1998.13.2.253. [DOI] [PubMed] [Google Scholar]
- 71.Plotkin LI, Manolagas SC, Bellido T. Dissociation of the pro-apoptotic effects of bisphosphonates on osteoclasts from their anti-apoptotic effects on osteoblasts/osteocytes with novel analogs. Bone. 2006;39:443–452. doi: 10.1016/j.bone.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 72.Vander Molen MA, Rubin CT, McLeod KJ, McCauley LK, Donahue HJ. Gap junctional intercellular communication contributes to hormonal responsiveness in osteoblastic networks. J. Biol. Chem. 1996;271:12165–12171. doi: 10.1074/jbc.271.21.12165. [DOI] [PubMed] [Google Scholar]
- 73.Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J. Bone. Miner. Res. 2001;16:249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 74.Ziambaras K, Lecanda F, Steinberg TH, Civitelli R. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J. Bone Min. Res. 1998;13:218–228. doi: 10.1359/jbmr.1998.13.2.218. [DOI] [PubMed] [Google Scholar]
- 75.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. WNT/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 76.Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J. Biol. Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 78.Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, Desimone D, Bonewald LF, Lafer EM, Sprague E, Schwartz MA, Jiang JX. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3359–3364. doi: 10.1073/pnas.1115967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases and ERKs. Am. J. Physiol. Cell Physiol. 2005;289:C633–C643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 80.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Min. Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 81.Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the Connexin43 gene (Gja1) J. Bone Miner. Res. 2008;23:879–886. doi: 10.1359/JBMR.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bivi N, Farlow N, Brun L, Benson JD, Condon KW, Robling AG, Bellido T, Plotkin LI. Unexpected enhanced response to mechanical loading of mice lacking Cx43 exclusively in osteocytes. J. Bone Miner. Res. 2011;25:S11. [Google Scholar]
- 83.Alam I, Warden SJ, Robling AG, Turner CH. Mechanotransduction in bone does not require a functional cyclooxygenase-2 (COX-2) gene. J. Bone Miner. Res. 2005;20:438–446. doi: 10.1359/JBMR.041124. [DOI] [PubMed] [Google Scholar]
- 84.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MJ, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido T, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 85.Grimston SK, Goldberg DB, Watkins M, Brodt MD, Silva MJ, Civitelli R. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. J. Bone Miner. Res. 2011;26:2151–2160. doi: 10.1002/jbmr.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lloyd SA, Lewis GS, Zhang Y, Paul EM, Donahue HJ. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J. Bone Miner. Res. 2012 doi: 10.1002/jbmr.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qiu S, Rao DS, Palnitkar S, Parfitt AM. Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone. 2002;31:313–318. doi: 10.1016/s8756-3282(02)00819-0. [DOI] [PubMed] [Google Scholar]
- 88.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC. Decreased oxidative stress and greater bone anabolism in the aged, as compared to the young, murine skeleton by parathyroid hormone. Aging Cell. 2010;9:851–867. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Genetos DC, Zhou Z, Li Z, Donahue HJ. Age-related changes in gap junctional intercellular communication in osteoblastic cells. J. Orthop. Res. 2012 doi: 10.1002/jor.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao JJ, Kurimoto P, Boudignon B, Rosen C, Lima F, Halloran BP. Aging impairs IGF-I receptor activation and induces skeletal resistance to IGF-I. J. Bone Miner. Res. 2007;22:1271–1279. doi: 10.1359/jbmr.070506. [DOI] [PubMed] [Google Scholar]
- 92.Pacheco-Costa R, Brun L, Southern D, Reginato RD, Bivi N, Bellido T, Plotkin LI. Altered expression of apoptosis-associated miRNAs that regulate IGF-1 survival signaling underlies the cell autonomous requirement of Cx43 for osteocyte survival. J. Bone Miner. Res. 2012;27 in press. [Google Scholar]
- 93.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu. Rev. Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 94.Tatarano S, Chiyomaru T, Kawakami K, Enokida H, Yoshino H, Hidaka H, Yamasaki T, Kawahara K, Nishiyama K, Seki N, Nakagawa M. miR-218 on the genomic loss region of chromosome 4p15.31 functions as a tumor suppressor in bladder cancer. Int. J. Oncol. 2011;39:13–21. doi: 10.3892/ijo.2011.1012. [DOI] [PubMed] [Google Scholar]
- 95.Ma X, Kumar M, Choudhury SN, Becker Buscaglia LE, Barker JR, Kanakamedala K, Liu MF, Li Y. Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10144–10149. doi: 10.1073/pnas.1103735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 2011;3:159–166. doi: 10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Novack DV. Role of NF-kappaB in the skeleton. Cell Res. 2011;21:169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grey A, Chen Q, Xu X, Callon K, Cornish J. Parallel phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways subserve the mitogenic and antiapoptotic actions of insulin-like growth factor I in osteoblastic cells. Endocrinology. 2003;144:4886–4893. doi: 10.1210/en.2003-0350. [DOI] [PubMed] [Google Scholar]
- 99.Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- 100.Stains JP, Civitelli R. Gap junctions in skeletal development and function. Biochim. Biophys. Acta. 2005;1719:69–81. doi: 10.1016/j.bbamem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 101.Koval M, Harley JE, Hick E, Steinberg TH. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J. Cell Biol. 1997;137:847–857. doi: 10.1083/jcb.137.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koval M, Geist ST, Westphale EM, Kemendy AE, Civitelli R, Beyer EC, Steinberg TH. Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43. J Cell Biol. 1995;130:987–995. doi: 10.1083/jcb.130.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamada Y, Ando F, Shimokata H. Association of candidate gene polymorphisms with bone mineral density in community-dwelling Japanese women and men. Int. J. Mol. Med. 2007;19:791–801. [PubMed] [Google Scholar]
- 104.Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, Kuo L, Shin DG, Rowe DW, Harris SE, Kalajzic I. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45:682–692. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]