Abstract
Touch and temperature are recognized as important factors in food perception, but much remains to be learned about how they contribute to the perception of flavor. The present paper describes human psychophysical studies that investigated two recently discovered effects of mechanical and thermal stimulation on taste: (1) enhancement of the savory taste of MSG by active tongue and mouth movements, and (2) modulation of the rate of adaptation to sucrose sweetness by temperature. The first study provides evidence that for MSG but not other taste stimuli, movement of the tongue against the palate enhances taste intensity both by increasing spatial summation between opposing gustatory surfaces and by a hypothesized interaction with touch/kinesthesis. The second study shows that the rate of adaptation to sucrose sweetness (but not quinine bitterness) on the tongue tip is strongly influenced by temperature. It is hypothesized that warming slows adaptation to sucrose by increasing the sensitivity of an early stage of taste transduction. Together these results demonstrate that models of flavor perception must include somatosensory stimuli both as components of flavor perception and as modulators of taste.
Keywords: psychophysics, flavor, taste, temperature, touch, human
Introduction
Flavors are most often described in terms of the qualities of taste and smell that uniquely define specific foods. But flavor also comprises the chemesthetic, thermal, and tactile qualities that impart the feel of foods and beverages, which can be equally important determinants of their identity, liking, and consumption. For example, cooling mints, carbonated beverages, and hot peppers are all defined by their chemesthetic qualities, and the temperature and texture of foods like ice cream and peanut butter are essential to their flavor identities.
In addition, somatosensory stimuli have the potential to influence taste and modify flavor by direct effects on the gustatory system. Electrophysiological evidence that mechanical and thermal stimulation can modulate activity in the taste pathway has been available for decades. Early studies of the effect of temperature on the sensitivity of the chorda tympani nerve in rats and cats showed that the response to most taste stimuli is higher at moderate temperatures (between 20° to 35°C) than at colder or warmer temperatures [1, 2], and later studies supported these findings but also showed that the effect of temperature varies depending upon neuron type and adapting temperature [3, 4]. Studies of individual gustatory nerve fibers and central neurons have also demonstrated a high degree of mechanical as well as thermal sensitivity. In at least one species (cat), 82% of gustatory units have been reported to respond to mechanical stimulation and 36% to temperature [5]. In addition, an early study of the whole-nerve response of the human chorda tympani demonstrated a high sensitivity to both thermal and tactile stimuli [6].
But how these basic neural effects influence human gustatory perception has rarely been studied. A few investigations of the effect of temperature on taste thresholds confirmed that temperature matters, but they did not consistently find the u-shaped function evident in the early animal data [7, 8]. Suprathreshold studies of taste perception have shown some reductions in taste intensity at cold temperatures, but only for relatively weak stimuli, and consistently only for sucrose sweetness [9-12]. Although a perceptual correlate of the excitatory effects of temperature on the human chorda tympani nerve has been reported in psychophysical studies (Thermal Taste) [13-15], evidence of an excitatory effect of mechanical stimulation is less clear [13]. Specifically, Cruz and Green [15] discovered that dynamic local heating of the cooled tongue can stimulate sweetness, whereas cooling the tongue to ~15°C or ~10°C can evoke sourness or saltiness. However, for reasons that are still unclear [13-15], thermal tastes are not perceived by everyone. The evidence that touch can stimulate sensations of taste is essentially anecdotal: 2 of 4 subjects in a preliminary study of electrical stimulation of taste papillae reported experiencing tastes when papillae were touched by plastic or wire probes when no current was applied [16]. A much clearer and potentially functionally important interaction between taste and touch—but one that does not appear to arise from mechanical stimulation of the taste nerves—is the ability of touch to “capture” taste and cause it to be “referred” to the location of tactile stimulation [17-19]. It has been proposed that together with referral of retronasal odors to the mouth by taste [20], referral of taste to touch helps to fuse chemosensory and somatosensory stimulation into coherent perceptions of flavor [17, 18, 21].
The present paper reports two newly discovered effects of somatosensory stimulation on taste that also have the potential to affect flavor perception. The first, an enhancement of the taste of MSG (umami, or savory taste) produced by active tongue movements, suggests there is a selective effect of mechanical and/or kinesthetic stimulation on the savory component of flavor. The second, a difference in amount of measured adaptation across stimulus temperatures, implies that the primary effect of temperature on the ability to taste sucrose (and perhaps other taste stimuli) is to modulate the rate of adaptation—and even counteract its affects—rather than to alter sweet taste sensitivity per se.
Study 1: Active vs. Passive Tasting
The perception of foods in the mouth is an active process in which stimuli are acquired rather than passively received, and it has been proposed by Gibson [22] that the act of tasting is essential for normal taste perception. While this view can be appreciated in general terms as recognizing the role of voluntary motor actions in sensory perception, beyond the possible effects of no clear experimental evidence has been presented that demonstrates a perceptual advantage for “active tasting” over passive receipt of taste stimulation. We therefore set out to conduct a simple experiment in which stimuli that evoke sweet, salty or savory tastes would be either passively received or actively tasted. After a significant advantage of active tasting was found for the savory taste of MSG, a second experiment was run to rule out the possibility that the advantage was due to spread of MSG to more sensitive taste areas in the back of the mouth.
Experiment 1: The effect of tongue-palate contact on taste intensity
During normal eating and drinking the tongue is constantly in motion, creating opportunities during chewing and swallowing for taste buds to come in contact with the chemical stimulus. In addition to spreading the stimulus throughout the mouth and increasing sensation via spatial summation, this active process may also optimize delivery of taste stimuli into taste pores. Tasting, chewing and swallowing also generate efferent motor signals and correlated tactile feedback from the touched oral surface (reafference). While the former effects may lead to greater chemical stimulation of taste receptors, the latter might help differentiate chemical from mechanical stimulation of the gustatory nerves by inhibiting the tactile input and improving the signal-to-noise ratio [23-25]. With the exception of a study on the effect of mouth movements on taste adaptation [26], we could find no studies that investigated a possible advantage of active over passive tasting. We therefore designed an experiment to compare these two modes of tasting for different stimuli perceived on different gustatory fields of the tongue and palate.
Method
Subjects
24 adults (18 females, 6 males) under the age of 45 were recruited by fliers posted on the Yale University Medical School and College campuses. All were self-reported to be in good health, to have no taste or smell disorders, and had no oral or lingual body piercings. Each person gave informed consent and was paid for their participation in the experiment.
Stimuli
The stimuli were aqueous solutions of 0.32 M sucrose, 0.32 M NaCl, and 180 mM MSG. The stimuli were prepared weekly and kept refrigerated between testing sessions. Stimulus application of the room-temperature solutions was via saturated Q-tip swabs.
Procedure
Before data collection began, all subjects were given instruction and practice in the use of the general version [27] of the Labeled Magnitude Scale (gLMS) [28, 29], which was used to quantify taste sensations. To become comfortable with the concept and use of the scale subjects first rated the intensity of 15 common sensations (e.g., the sweetness of cotton candy; the tingling from a carbonated beverage) which they were asked to imagine. Subjects were then given practice rating the intensity of a variety of taste stimuli applied to the tongue tip. Finally, experience was given with the taste of monosodium glutamate (MSG), which most subjects had never tasted by itself. Samples of 180mM MSG were applied to the front and back of the tongue, with the resulting sensation described to subjects as “savory”.
The test stimuli were applied to four oral surfaces: the tongue tip (TT), the hard palate (HP; the alveolar ridge), the circumvallate region (CV), and the soft palate (SP). On some trials stimulus was applied to only one of these surfaces and on others it was applied to two opposing surfaces: the tongue tip and hard palate (TT + HP), or the circumvallate region and soft palate (CV +SP). When applied to opposing surfaces, the stimuli were swabbed onto opposite sides of the midline to allow the stimulus to contact unstimulated areas of the opposing surfaces during active tasting, thus providing an opportunity for spatial summation to occur. When the stimulus was applied to opposing surfaces the experimenter swabbed one site immediately after the other with a second fresh swab. Subjects were instructed to rate the intensity of sweet, sour, salty, bitter and savory tastes either (1) immediately after stimulus application with the tongue immobile (Passive Tasting), or (2) after touching the tongue to the roof of the mouth and swallowing once (Active Tasting). Subjects rinsed at least twice between each stimulus with 37°C deionized water. Six pseudorandom orders of stimulus applications were created in which the Active and Passive Tasting conditions were intermixed across trials. The order of testing was counterbalanced across subjects, and replicate ratings for each condition and stimulus were collected over 3 sessions.
Results and Discussion
Figure 1 summarizes the main results of the first experiment: the log-mean intensity ratings of the primary taste quality for each of the 3 stimuli under conditions of passive (gray bars) and active (black bars) tasting. The data for stimulus application to the HP alone were omitted from the analysis because taste responses there were nil, which skewed statistical calculations that included application site as a factor. It can be seen at a glance that mode of tasting had the greatest effect on the savory taste of MSG (middle graph). For every oral site and combination of sites the mean savory rating was higher for active tasting than for passive tasting [repeated-measures ANOVA, main effect of condition; F(1,23) = 36.96, P<0.0001]. However, this effect was not consistent across sites; there was a significant site × condition interaction [F(4,92) = 2.85, p<0.028], and Tukey HSD tests showed that the differences reached statistical significance (p<0.05) only for the TT + HP, the SP, and the CV + SP. The difference between modes of tasting was most striking for the TT + HP, where the difference in mean ratings reached 0.58 log10, meaning that on those test sites savory taste was rated 3.8 times stronger during active tasting than during passive tasting. This magnitude of increase is more than would be predicted by merely adding the same amount of MSG to the contralateral side of the tongue (i.e., doubling stimulus area), which is what occurred when the tongued was touched against the hard palate. Spatial summation would maximally predict a doubling of perceived intensity (0.3 log10), not the near quadrupling of intensity observed. Such a large increase in perceived intensity suggests that active tasting may have enhanced the perception of MSG by a second mechanism in addition to spatial summation.
Fig. 1.
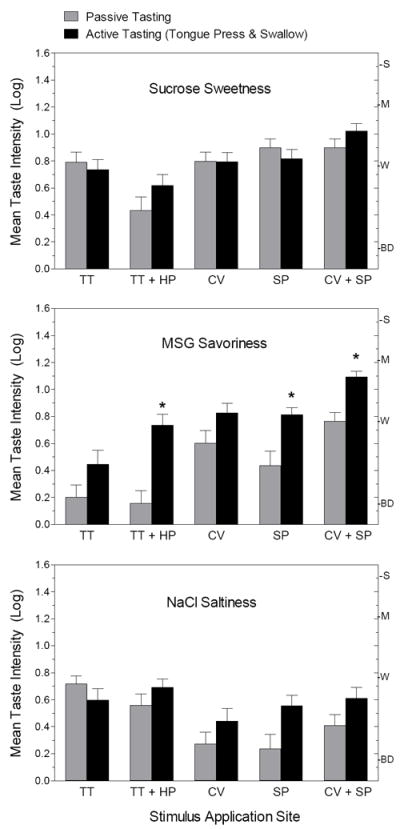
Log-mean intensity ratings for the dominant taste quality of each of the three stimuli tested in Exp.1 in the passive tasting (gray bars) and active tasting (black bars) conditions. Application sites: TT = tongue tip; HP = hard palate; CV = circumvallate papillae; SP = soft palate. Letters on the right Y-axis refer to intensity descriptors on the gLMS: BD = barely detectable; W = weak; M = moderate; S = strong. Vertical bars = SEMs. (* denotes a significant difference)
Surprisingly, mode of tasting had no effect on ratings of sucrose sweetness [F(1,23) = 1.63, p = 0.22], and although there was a main effect of condition for NaCl saltiness [F(1,23) = 12.66, p<0.005], ratings were not significantly higher during active tasting on any of the individual test sites. These results imply that spatial summation was weak or absent for both of these tastes. Also supporting this conclusion is the fact that perceived intensity was not significantly higher in either condition when the stimulus was applied to 2 sites rather than 1. When 2 sites were stimulated, sweetness and saltiness were not significantly higher than for the more sensitive of the 2 sites (Tukey HSD, p>0.05). In contrast, when MSG was the stimulus, in both instances stimulating 2 sites together produced significantly higher savory ratings than did stimulating the most sensitive of the 2 sites alone (Tukey HSD, p<0.05).
The absent or meager spatial summation for NaCl and sucrose conflicts with prior evidence of spatial summation of suprathreshold taste [30, 31]. However, in the present study spatial summation would have to occur between gustatory nerves (TT = chorda tympani; CV = glossopharyngeal; SP = greater superficial petrosal) and across the midline of the tongue within gustatory nerves. Previous studies that measured suprathreshold spatial summation of taste did so by increasing stimulus area across the midline of the tongue [30, 31], which created the opportunity for spatial summation to occur ipsilaterally. Spatial summation produced by adding a contralateral stimulus appears to have been measured only at threshold, where a small advantage for bilateral stimulation was attributed to probability summation rather than to spatial summation per se [32]. The present study appears to be the first to measure suprathreshold spatial summation across the midline and across gustatory nerves, and the results suggest that summation is far more robust for savory taste than for sweetness or saltiness.
Also noteworthy is the front-to-back gradient in responsiveness to MSG, with perception being stronger in the back of the mouth [33], particularly when the stimulus is passively received. The sucrose and NaCl stimuli produced similar taste intensity ratings on the tongue tip while MSG evoked ratings that were at least half a log-unit lower. In contrast, aided by the enhancement produced by actively tasting, MSG produced taste intensities on the CV and SP that were comparable to those produced by sucrose but stronger than those produced by NaCl. Indeed, one of the striking impressions of Fig. 1 is the lesser responses to NaCl on the posterior test sites compared to sucrose and MSG.
Experiment 2: Spatial summation and stimulus spread as factors in active tasting
Because in Exp. 1 the back of the mouth was so much more sensitive to MSG than the front of the mouth, it was possible that the enhancement of savory taste during active tasting resulted in part from MSG spreading rearward to more sensitive sites as subjects swallowed. While this explanation seems less likely to account for the significant effect of active tasting in the CV and SP regions, it was possible that it could account for some of the dramatic enhancement produced by active tasting when the stimulus was applied to the TT + HP. In the present experiment this potential confound was eliminated by instructing subjects to make a mouth movement that would tend to push the taste stimulus forward rather than backward. In addition, we explored whether the larger effect of active tasting on MSG taste at the tongue tip might have been partially due to its very weak perception there. Testing was also extended to sour and bitter stimuli to determine to what extent active tasting might enhance the sensitivity to those tastes as well.
Method
Subjects
A total of 27 subjects (20 females and 7 males) were recruited for this experiment in the same manner and with the same restrictions as Exp. 1. Two subjects were omitted from the final analysis after it was determined they had not reported savory taste from the lowest concentration of MSG under either mode of tasting. Each person gave informed consent and was paid for their participation.
Stimuli
Five taste stimuli were tested in 2 aqueous concentrations each: sucrose (0.56 M and 0.18 M), MSG (250 mM and 80 mM), NaCl (0.56 M and 0.18 M), citric acid (56 mM and 18 mM), and QSO4 (0.32 mM and 0.1 mM). Application was again via cotton swab.
Procedure
Subjects again used the gLMS to make intensity ratings, this time of sweetness, sourness, saltiness, bitterness, savory, burning/stinging. Burning/stinging was added because for some subjects the higher concentration of citric acid was perceived to produce one or more of these sensations in addition to sourness. Subjects were instructed to base their ratings on sensations experienced (1) as the stimulus was being applied to the tongue tip via the cotton swab (Stimulus Application); (2) 3 sec after the stimulus had been applied and the tongue was retracted into the mouth without touching the palate (Passive Tasting), and (3) during articulation of the words “taste-taste-taste” (Active Tasting). Of primary interest was the difference between ratings made under conditions (2) and (3), which tested the hypothesis that motor movements of the tongue against the hard palate would significantly enhance the savory taste of MSG but not other tastes. This mode of motor movement was chosen because articulation of the plosive consonant “t” and the fricative consonant “s” both require the tongue tip to touch the hard palate just behind the front teeth (the alveolar ridge) while air is being expelled from the mouth, thus working against any tendency for the stimulus to spread toward the back of the mouth. Condition (1) was included as a control for the effect of passive mechanical stimulation versus voluntary movement of the tongue tip against another oral surface. Subjects were instructed not to swallow in any of the rating conditions. An experimental session was comprised of 30 stimuli partitioned into blocks of 10 with 5-min rest periods between. Subjects again rinsed at least twice between trials with 37°C de-ionized water.
Results and Discussion
Figure 2 compares the results for active and passive tasting for the high and low concentrations of each of the 5 stimuli. Consistent with the results of experiment 1, active tongue and mouth movements significantly enhanced the savory taste of MSG but no other stimulus, including citric acid and QSO4. These data confirm that the advantage gained by touching the tongue to the hard palate was not an artifact of stimulus movement to more sensitive gustatory surfaces. Moreover, because the solutions were applied only to the tongue tip and not to the hard palate, the enhancement cannot be attributed solely to spatial summation. The data also show that the large effect on MSG’s savory taste on the tongue tip in Exp. 1 was not due to its relatively weak intensity; there was a trend toward enhancement at the lower concentration (80 mM) which only became significant (Tukey HSD, p<0.05) at the higher concentration (250 mM). Finally, the same pattern of results held for ratings of savory taste experienced at the time of stimulus application (with the cotton swab) versus during active tasting, with savory ratings being significantly higher during active tasting, but again only for the higher of the two concentrations (Tukey HSD, p>0.05; data not shown). The latter finding indicates that mere mechanical stimulation cannot account for the enhancement of MSG taste during articulation. The same “control” condition was present throughout experiment 1 as well, since mechanical stimulation was always produced as the stimuli were swabbed onto the gustatory areas.
Fig. 2.
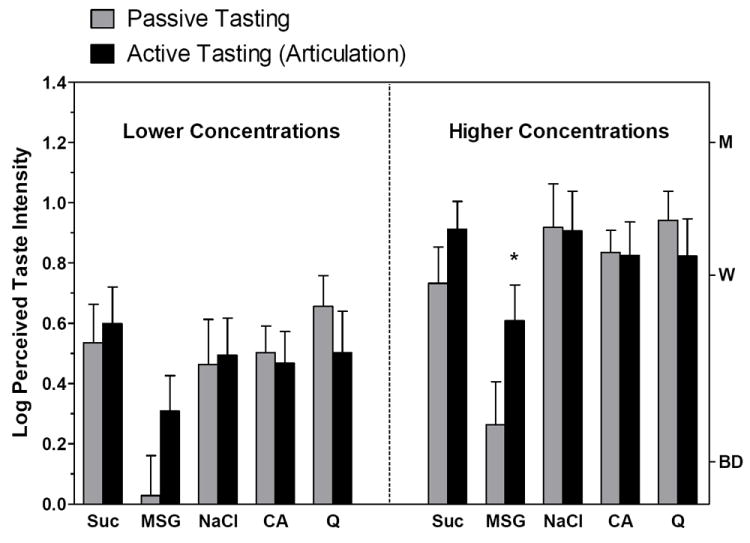
Log-mean intensity ratings for the dominant taste quality produced by 2 different concentrations of each of the 5 taste stimuli of Exp. 2 when the taste was passively received (gray bars) or actively perceived by articulating “taste-taste-taste” (black bars). Letters on the right Y-axis refer to intensity descriptors on the gLMS: BD = barely detectable; W = weak; M = moderate. Vertical bars = SEMs. (* denotes a significant difference)
Together these two experiments point to a unique effect of voluntarily-produced tongue movements on the perception of savory taste. The mechanisms responsible for the effect are unclear. In Exp. 1 it is possible that some of the advantage came from pressing the tongue against the palate, which may have heightened spatial summation by helping to integrate or fuse the taste stimulation on the two surfaces into a single sensation. That is, when the tongue is pressed against the palate the taste sensations evoked from the opposing surfaces are no longer perceived as spatially separate, but instead appear to arise from a common stimulus. Alternatively, the advantage may derive from a central interaction between the tactile/kinesthetic system and mechanical stimulation produced in the afferent pathway sensitive to MSG (i.e., a form of reafference may occur). Studies of the mechanical sensitivity of the TAS1R1-TAS1R3 heterodimer that mediates glutamate taste [34, 35] and its associated transduction cascade could establish whether the tactile information comes at least in part from the mechanical sensitivity of the gustatory system or only from mechanoreceptors in the trigeminal system. In addition, studies that search for convergence of stimulation from MSG and mechanical stimulation on central “gustatory” neurons [36-38] might reveal the nature of any interactions between tactile stimulation and savory taste.
With respect to Gibson’s overarching concept of tasting as an active process, the present results do demonstrate that taste perception cannot be fully understood by studying chemosensitivity alone. On the other hand, the absence of a consistent effect of tongue and mouth movements on all tastes indicates that the most significant effects of active tasting are likely to be related to the temporal and spatial unfolding of flavor as foods are taken into the mouth, chewed, and swallowed [e.g. 39, 40]. It is interesting to consider whether the selective effect of mouth movements on savory taste and the greater sensitivity to MSG toward the back of the mouth may be linked to mastication. The release of both unbound and bound glutamates from solid foods such as meats and vegetables is undoubtedly greatest as the foods are crushed and ground by the molar teeth.
Study 2: The effect of solution temperature on taste adaptation to sucrose
The motivation for this study grew out of a phenomenon that we first interpreted as possible evidence of another effect of mechanical stimulation on taste. During preliminary investigations of a potential role of tactile stimulation in the referral of retronasal olfaction to the mouth, we noticed that licking hard candy (a lollipop) outside the mouth failed to evoke a distinct sweet taste, whereas sweetness rapidly bloomed when the tongue was retracted back into the mouth. Preliminary testing with aqueous sucrose indicated that the difficulty in perceiving sweetness with only the tongue tip was not unique to licking hard candy: dipping the tongue into a room-temperature sucrose solution also gave only a weak and fleeting sweetness. This observation led us to conduct a formal test to quantify the phenomenon and test the hypothesis that differences in temperature between the two sampling conditions resulted in different rates of sweetness adaptation.
Experiment 1: Adaptation to sucrose outside vs. inside the mouth
The objective of this experiment was to measure sweetness intensity and adaptation when the tongue tip is immersed and kept motionless in a sucrose solution compared to when a small volume of the solution is sipped and held in the front of the mouth. After pilot tests indicated that the bitterness of QSO4 did not adapt as much outside the mouth as did the sweetness of sucrose, it was also included as a test stimulus to determine whether adaptation to bitterness and sweetness differs when sampled by the tongue tip outside versus inside the mouth.
Method
Subjects
A total of 35 subjects (18 females and 17 males) were recruited for this experiment in the same manner and with the same restrictions as in Study 1.
Stimuli
Three different adapting stimuli were used: 0.42M sucrose, 0.18mM QSO4, and deionized H2O as a control. The test stimuli were 2 concentrations each of sucrose (0.42M and 0.75M) and QSO4 (0.18mM and 0.32mM). The higher concentration of each stimulus was included to provide subjects with a wider range of taste sensations than the adapting stimuli alone, and to present stimuli that would be unlikely to adapt significantly after exposure to the weaker solutions. This strategy prevented subjects from anticipating the same or similar taste intensity on every trial. Because adaptation is significant only when the adapting stimulus is equal to or stronger than the post-test stimulus, the data for the higher concentrations of sucrose and QSO4 were not included in the analysis. The solutions were kept at room temperature (~21°C) prior to delivery.
Practice session
Prior to data collection all subjects attended a practice session in which they were instructed in how to use the general version of the Labeled Magnitude Scale [27, gLMS; 28, 29]. The session had 3 parts, each designed to give subjects experience rating the intensity of different types of sensations. After being introduced to the important features of the scale, subjects rated a list of 15 remembered or imagined sensations (e.g., the sweetness of cotton candy, the burn of cinnamon gum) then practiced rating the intensity of actual stimuli of various kinds (e.g., “The cold sensation from a penny placed on the wrist”). Next, subjects practiced rating the individual taste qualities by sampling 10 stimuli: 0.42M sucrose, 0.32M NaCl, 18mM citric acid, 29mM MSG, and 0.18mM QSO4, and 4 binary mixtures of these stimuli (sucrose + QSO4, citric acid + NaCl, NaCl + QSO4, and NaCl + MSG). For each stimulus subjects rated sweetness, saltiness, sourness, bitterness and savory on the gLMS.
Experimental Procedure
Subjects again used the gLMS to make intensity ratings, this time of sweetness or bitterness only. Omission of the other taste qualities simplified the rating task with the objective of speeding the subjects response, which if too slow could influence measured amounts of adaptation.
Subjects experienced the stimuli in two ways: By dipping the tongue tip into approximately 6 mL of solution pipetted into a 41mm × 41mm × 8mm polystyrene weigh boat (Fisher Scientific, Pittsburgh, PA) (Tongue Dip condition) or by sipping 5 mL of the solution into the mouth (Sip & Spit condition). The adaptation procedure required the subject to keep her or his tongue tip dipped into a solution or held in the mouth for 3, 10 or 20 sec before sampling a post-test solution. In the Tongue Dip condition the stimuli were presented on a wire rack in 2 adjacent weigh boats. The left weigh boat served as the adapting solution (0.42M sucrose, 0.18mM QSO4, or dH2O) and the right weight boat contained the post-test sucrose or QSO4 solution. The post-test solution was sampled by lifting the tongue tip from the adapting solution and immediately dipping the tongue into an adjacent weigh boat that contained the post-test stimulus. In the Sip & Spit condition the adapting stimulus was expectorated and the post-test stimulus was immediately sipped. In both conditions the post-test stimulus was sampled for 3 sec, after which the experimenter instructed the subjects to either lift the tongue from the solution or to expectorate it, then immediately rate the intensity of sweetness or bitterness experienced just prior to expectorating or lifting the tongue. As subjects made their ratings the tongue was kept outside the mouth in the Tongue Dip condition or immobile inside the mouth in the Sip & Spit condition. No ratings of the adapting stimulus were obtained. This procedure therefore provided an independent measure of the taste intensity of a “fresh” stimulus sample after exposure to an adapting stimulus for a controlled period of time. A deionized H2O control was used to obtain ratings of the perceived intensities of the test stimuli without prior exposure to an adapting taste stimulus. This was done by either sipping or dipping the tongue into the deionized H2O for 3 sec before sampling the post-test solution. The adapting and test stimuli were presented in 4 different pseudorandom sequences (21 stimuli each) with sucrose and QSO4 trials intermixed. Subjects rinsed at least 3 times with 37°C deionized water between trials. Each subject served in both conditions in separate testing sessions. The order in which the conditions were tested was counterbalanced across subjects.
Results and Discussion
Figure 3 contains log-mean sweetness and bitterness ratings for sucrose and QSO4 over time for the 2 conditions of the experiment. To highlight the similarities and differences in adaptation for the two stimuli, a constant was added to the data from the Tongue Dip condition to equalize the means in the unadapted state (i.e., H2O only; duration of exposure = 0). The most striking finding was that sucrose sweetness adapted to a much greater degree in the Tongue Dip condition than in the Sip & Spit condition (condition × time interaction; F3,102 = 5.90, p < 0.001). There was no interaction between condition and time for QSO4 bitterness (F3,102 = 0.28, p = 0.84), which adapted at nearly identical rates in both conditions. The rate and degree of adaptation of sucrose sweetness when the stimulus was sipped and expectorated was very similar to the amount of adaptation found for QSO4, regardless of condition.
Fig. 3.
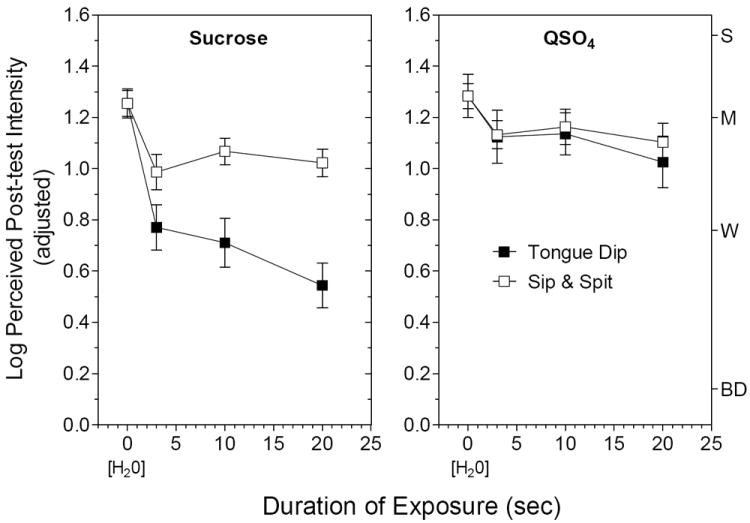
Log-mean intensity ratings for the dominant taste quality of the sucrose and QSO4 post-test solutions in the Tongue Dip condition (filled squares) and Sip & Spit condition (empty squares) as a function of duration of exposure to the same taste stimulus. Log-means were adjusted by adding a constant to the sucrose means to equalize the sucrose and QSO4 means for the H2O only (pre-exposure) condition. Letters on the right Y-axis refer to intensity descriptors on the gLMS: BD = barely detectable; W = weak; M = moderate; S = strong. Vertical bars = SEMs.
The first explanation we considered for the greater adaptation to sweetness in the Tongue Dip condition was that spreading sucrose over larger areas of the tongue may have counteracted adaptation in the Sip & Spit condition. However, it was difficult to explain why restricting the stimulus to the tongue tip would increase adaptation for sweetness but not for bitterness. Another difference between conditions is that outside the mouth the tongue tip cools as it is immersed in a room-temperature solution whereas in the Sip & Spit condition the solution begins to warm-up quickly once inside the mouth. This suggested that adaptation to sucrose sweetness might be modulated by temperature. A quick test with room temperature vs. 37°C aqueous solutions supported this possibility: Sucrose sweetness was fleeting when the tongue was dipped into the 21°C solution but persisted when dipped into the 37°C solution.
Experiment 2: Adaptation to sucrose sweetness at 21° versus 37°C
We therefore set out to measure sweetness intensity over time in the Tongue Dip condition for room-temperature and mouth temperature solutions. However, as we began testing we realized that simply comparing sweetness adaptation at room and mouth temperature does not mimic the pattern of temperature change that occurs when the tongue is removed from the cool adapting solution and retracted back into the mouth. Under those circumstances the tongue begins to re-warm as exposure to the sucrose that is still coating the tongue continues. Thus we hypothesized that warming the tongue counteracts or reverses sweetness adaptation that occurs in a cool solution. To test this hypothesis we designed an experiment in which subjects dipped the tongue tip into either room-temperature or warm sucrose solutions for varying durations and then immediately dipped the tongue into another sucrose solution having either the same or different temperature.
Method
Subjects
A total of 26 subjects (18 females and 8 males) between 18 and 45 years of age were recruited from public postings on the Yale University Medical School and Yale College campuses as for the prior experiments. Each person gave informed consent and was paid for their participation.
Stimuli
The adapting and test stimuli were aqueous solutions of 0.42 M sucrose prepared with deionized water. Deionized H2O was again used as a control condition to measure the sweetness of the sucrose solution prior to exposure to the adapting solution. During testing the solutions were kept in glass jars in circulated constant temperature baths that were set to room temperature (~21°C) or 37°C. The stimuli were sampled in the same square weigh boats that were used in The Tongue Dip condition of Exp.1. The stimulus was prepared daily in 250-mL aliquots and stored in airtight flasks.
Practice Session
Subjects who were new to the study participated in the practice session described above.
Experimental sessions
Tasting was conducted using only the tprocedure. During the test session subjects sampled a total of 20 pairs of stimuli in a pseudorandom order and rated their perceived sweetness using the gLMS. Subjects were informed to attend carefully to the sweetness of the solutions and that on some trials there might be no taste at all. The stimuli were presented on a wire rack in 2 adjacent weigh boats. As in Exp. 1, the left weigh boat contained the adapting solution (0.42 M sucrose) or dH2O at one of 2 temperatures: 21° or 37°C. The weigh boat on the right contained the post-test solution, which was 0.42 M sucrose at either 21° or 37°C. The adapting and post-test solutions were not always the same temperature. Subjects were instructed to seal off the tongue with the lips and to stick the tongue tip into the liquid in the left weigh boat (adapting solution) for intervals of 3, 7, or 15 sec timed by the experimenter. At the end of the interval the subject was instructed to lift the tongue tip from the adapting solution and immediately dip it into the post-test solution. After 3 sec the experimenter signaled the subject to lift the tongue from the post-test solution and to rate its sweetness. Importantly, the sweetness rating was made with the tongue tip still outside the mouth. The difference in perceived sweetness between the pre- and post-test conditions provided the measure of adaptation. During a 60-sec inter-trial interval subjects rinsed with deionized water heated in a circulated water bath to 37°C to render the rinses thermally neutral to the mouth.
Results and Discussion
The main results of Exp. 2 are displayed in Fig. 4. In the left graph are log-means ratings of the sweetness a 21° or 37°C post-test stimulus after exposure to either H2O for 3 sec or to sucrose for 3, 7 or 15 sec, all at 37°C. The right graph shows the results when the H2O and adapting solutions were 21°C. It is clear that the amount of adaptation measured depended more strongly on the post-test temperature than on the adapting temperature: more adaptation was found when the post-test was 21°C than when it was 37°C, regardless of the temperature of the adapting stimulus. Specifically, after 15 sec of exposure sweetness was rated to be less than “barely detectable” (dashed line) only when the post-test stimulus was 21°C. This effect were confirmed statistically by a repeated measures ANOVA of the ratings at 15 sec, which indicated there was a significant main effect of post-test temperature [F(1,25) = 26.8, p<0.0001] but not adapting temperature [F(1,25) = 0.87, p=0.36]. Nor was there an interaction between post-test and adapting temperature [F(1,25) = 3.35, p=0.08].
Fig. 4.
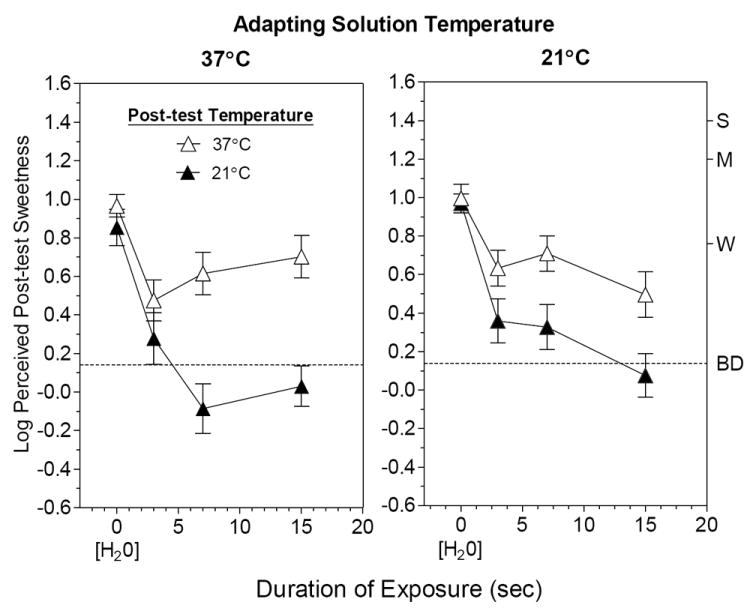
Log-mean sweetness intensity ratings of the post-test sucrose solution following exposure to sucrose at 37°C (left) or 21°C (right) when the post-test solution was 37°C (open triangles) or 21°C (filled triangles). The amount of adaptation measured was always significantly greater when the post-test solution was 21°C, irrespective of the temperature of the adapting stimulus. Letters on the right Y-axes refer to intensity descriptors on the gLMS: BD = barely detectable; W = weak; M = moderate, S = strong. Vertical bars = SEMs.
Separate ANOVAs on the data for the two post-test temperatures provided additional evidence of the importance of the temperature of the post-test solution. Although there was a main effect of temperature in the 21°C post-test condition [F(1,25) = 13.2, P<0.005], the effect was due to overall higher ratings in the 21°C adapting condition rather than to a difference in the rate or degree of measured adaptation, i.e., there was no significant interaction between adapting temperature and sweetness intensity over time [F(3,75) = 1.54, p=0.21]. The reverse statistical results were found for the 37°C post-test condition, but they also illustrate the importance of the post-test temperature: There was no main effect of adapting solution temperature [F(1,25) = 0.10, p=0.75], confirming that there was no consistent difference in sweetness across adapting temperatures. There was, however, a significant interaction between adapting temperature and time [F(3,75) = 2.80, p<0.05], which was attributable to a trend for sweetness to be higher for sucrose at 37°C after 15 sec of adaptation to the 21°C solution compared to the 37°C solution. In other words, brief exposure to a warm post-exposure solution more than offset the greater adaptation produced by a 21°C solution after 15 sec (Fig. 4, right). This implies that warming increases the sensitivity of one or more steps in the transduction cascade which significantly counteracts adaptation that is ongoing during exposure to a cool solution.
Another important finding was that solution temperature alone did not significantly affect initial sucrose sweetness. This is shown by the fact that for both post-test temperatures, the sweetness of the sucrose solution did not differ after a 3-sec exposure to H2O at either adapting temperature (Fig. 4, H2O only; Tukey HSD tests, p>0.05). In contrast, adaptation was already significant for both temperatures after 3-sec exposures to sucrose. This result raises the possibility that the effect of cold on the sweetness of low concentrations of sucrose reported in prior studies that used a sip-and-spit procedure [9-11] may have been due to more rapid and pronounced adaptation to a cool solution rather than to a reduction in baseline sensitivity to sucrose. The evidence that re-warming the solution (as normally happens when a cool solution is sipped) blunts or even reverses the effects of adaptation may also explain why the affects in previous sip-and-spit experiments were limited to low concentrations and were greatest when the mouth and were tongue cooled before the stimulus was sipped [10, 11, 41].
Summary and Conclusions
The experiments described here provide new evidence of additional ways in which somatosensory stimulation can influence the perception of taste and flavor. Study 1 revealed a specific enhancing effect of voluntary mouth and tongue movements on the savory taste of MSG. The fact that enhancement was limited to MSG rules out the possibility that the mechanical sensitivity of the gustatory nerves results in a general amplification of taste. In addition, enhancement appeared to result from two different mechanisms: an increase in spatial summation between opposing gustatory surfaces and an interaction among gustatory, tactile, and possibly centrifugal neural signals that amplifies the central neural response to glutamates. Study 2 uncovered a previously unknown effect of temperature on adaptation to sucrose sweetness. However, the failure to find a similar effect for QSO4 indicates that temperature does not influence adaptation to all tastes. In fact, preliminary data recently collected in our laboratory indicate that adaptation to the artificial sweetener saccharin is as rapid and pronounced at 37°C as it is at 21°C. This implies that temperature alters adaptation to sucrose via a peripheral mechanism that is not common to all sweet tasting stimuli. Specifically, we hypothesize that warming slows adaptation to sucrose sweetness by increasing the sensitivity of one or more stages of the transduction cascade that may be unique to sucrose and other sugars. We plan to test this hypothesis by measuring the effect of temperature on adaptation to several sugars and artificial sweeteners known or believed to bind to the sweet taste receptor T1R2-T1R3 [42, 43]. Of interest will be whether adaptation is differentially affected by temperature in a way that is broadly consistent with the molecular structures of the stimuli that may determine how they bind to the T1R2-T1R3 heterodimer [44], or possibly to other sweet taste transduction pathways [45-47]. Similarly, experiments are planned with other bitter compounds, including caffeine, whose bitterness was previously shown to be altered by temperature [10]. The effect of temperature on taste adaptation may therefore reveal new insights into the ways molecules interact with taste receptors, or in the case of bitter taste, the temperature sensitivity of different TAS2R receptors.
Taken together these psychophysical results raise new questions about the neural and molecular mechanisms that underlie interactions among thermal, mechanical, and gustatory stimuli at both the earliest stages of sensory transduction and in brain regions where the afferent pathways that encode these stimuli converge. Such knowledge will be essential for developing a comprehensive theory of flavor perception that not only includes somatosensory stimuli as factors, but also describes and explains how they interact with the chemical senses.
Highlights.
A brief overview of somatosensory-taste interactions is presented.
It is shown that “active tasting” selectively enhances the savory taste of MSG.
Spatial summation was also found to be significant only for MSG savory taste.
Adaptation to the sweet taste of sucrose is shown to be temperature-dependent.
Acknowledgments
The authors thank Lenka Urban for collecting the data reported in Study 1 and for carrying out the preliminary statistical analyses. This research was supported by a grant from the National Institutes of Health (RO1 DC005002).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Yamashita S, Sato M. The effects of temperature on gustatory response of rats. J Cell Physiol. 1965;66:1–17. doi: 10.1002/jcp.1030660102. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita S, Yamada K, Sato M. The effect of temperature on neural taste response of cats. Jpn J Physiol. 1964;14:505–514. doi: 10.2170/jjphysiol.14.505. [DOI] [PubMed] [Google Scholar]
- 3.Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol. 2006;95:674–685. doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]
- 4.Travers SP, Smith DV. Responsiveness of neurons in the hamster parabrachial nuclei to taste mixtures. J Gen Physiol. 1984;84:221–250. doi: 10.1085/jgp.84.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson PP. The characteristics and regional distribution of afferent fibres in the chorda tympani of the cat. J Physiol. 1988;406:345–357. doi: 10.1113/jphysiol.1988.sp017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oakley B. Taste responses of human chorda tympani nerve. Chem Senses. 1985;10:469–481. [Google Scholar]
- 7.McBurney DH, Collings VB, Glanz LM. Temperature dependence of human taste response. Physiol Behav. 1973;11:89–94. doi: 10.1016/0031-9384(73)90127-3. [DOI] [PubMed] [Google Scholar]
- 8.Pangborn RM, Chrisp RB, Bertolero LL. Gustatory, salivary, and oral thermal responses to solutions of sodium chloride at four temperatures. Percept Psychophys. 1970;8:69–75. [Google Scholar]
- 9.Green BG, Frankmann SP. The effect of cooling on the perception of carbohydrate and intensive sweeteners. Physiol Behav. 1988;43:515–519. doi: 10.1016/0031-9384(88)90127-8. [DOI] [PubMed] [Google Scholar]
- 10.Green BG, Frankmann SP. The effect of cooling the tongue on the perceived intensity of taste. Chem Senses. 1987;12:609–619. [Google Scholar]
- 11.Bartoshuk LM, Rennert K, Rodin H, Stevens JC. Effects of temperature on the perceived sweetness of sucrose. Physiol Behav. 1982;28:905–910. doi: 10.1016/0031-9384(82)90212-8. [DOI] [PubMed] [Google Scholar]
- 12.Moskowitz HR. Effect of solution temperature on taste intensity in humans. Physiol Behav. 1973;10:289–292. doi: 10.1016/0031-9384(73)90312-0. [DOI] [PubMed] [Google Scholar]
- 13.Bajec MR, Pickering GJ. Thermal taste, PROP responsiveness, and perception of oral sensations. Physiol Behav. 2008;95:581–590. doi: 10.1016/j.physbeh.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Green BG, George P. ‘Thermal taste’ predicts higher responsiveness to chemical taste and flavor. Chem Senses. 2004;29:617–628. doi: 10.1093/chemse/bjh065. [DOI] [PubMed] [Google Scholar]
- 15.Cruz A, Green BG. Thermal stimulation of taste. Nature. 2000;403:889–892. doi: 10.1038/35002581. [DOI] [PubMed] [Google Scholar]
- 16.Dzendolet E. Electrical-Stimulation of Single Human Taste Papillae. Perceptual and Motor Skills. 1962;14:303–317. [Google Scholar]
- 17.Lim J, Green BG. Tactile interaction with taste localization: influence of gustatory quality and intensity. Chem Senses. 2008;33:137–143. doi: 10.1093/chemse/bjm070. [DOI] [PubMed] [Google Scholar]
- 18.Green BG. Studying taste as a cutaneous sense. Food Quality and Preference. 2003;14:99–109. [Google Scholar]
- 19.Todrank J, Bartoshuk LM. A taste illusion: taste sensation localized by touch. Physiol Behav. 1991;50:1027–1031. doi: 10.1016/0031-9384(91)90432-n. [DOI] [PubMed] [Google Scholar]
- 20.Lim J, Johnson MB. Potential mechanisms of retronasal odor referral to the mouth. Chem Senses. 2011;36:283–289. doi: 10.1093/chemse/bjq125. [DOI] [PubMed] [Google Scholar]
- 21.Small DM, Green BG. A model of flavor perception. In: Murray MM, Wallace MT, editors. The neural bases of multisensory processes. New York: CRC Press; 2012. pp. 717–738. [PubMed] [Google Scholar]
- 22.Gibson JJ. The senses considered as perceptual systems. Westport, CT: Greenwood Press; 1966. [Google Scholar]
- 23.Seki K, Perlmutter SI, Fetz EE. Task-dependent modulation of primary afferent depolarization in cervical spinal cord of monkeys performing an instructed delay task. J Neurophysiol. 2009;102:85–99. doi: 10.1152/jn.91113.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman CE, Beauchamp E. Differential controls over tactile detection in humans by motor commands and peripheral reafference. J Neurophysiol. 2006;96:1664–1675. doi: 10.1152/jn.00214.2006. [DOI] [PubMed] [Google Scholar]
- 25.Williams SR, Chapman CE. Time course and magnitude of movement-related gating of tactile detection in humans III Effect of motor tasks. J Neurophysiol. 2002;88:1968–1979. doi: 10.1152/jn.2002.88.4.1968. [DOI] [PubMed] [Google Scholar]
- 26.Theunissen MJ, Kroeze JH, Schifferstein HN. Method of stimulation, mouth movements, concentration, and viscosity: effects on the degree of taste adaptation. Percept Psychophys. 2000;62:607–614. doi: 10.3758/bf03212112. [DOI] [PubMed] [Google Scholar]
- 27.Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ. Labeled scales (e.g., category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Quality and Preference. 2002;14:125–138. [Google Scholar]
- 28.Green BG, Dalton P, Cowart BJ, Shaffer GS, Rankin KM, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 29.Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18:683–702. [Google Scholar]
- 30.Delwiche JF, Buletic Z, Breslin PA. Relationship of papillae number to bitter intensity of quinine and PROP within and between individuals. Physiol Behav. 2001;74:329–337. doi: 10.1016/s0031-9384(01)00568-6. [DOI] [PubMed] [Google Scholar]
- 31.Smith DV. Taste intensity as a function of area and concentration: Differentiation between compounds. J Exp Psychol. 1971;87:163–171. doi: 10.1037/h0030519. [DOI] [PubMed] [Google Scholar]
- 32.Linschoten MR, Kroeze JH. Spatial summation in taste: NaCl thresholds and stimulated area on the anterior human tongue. Percept Psychophys. 1994;55:387–393. doi: 10.3758/bf03205296. [DOI] [PubMed] [Google Scholar]
- 33.Boudreau JC. Taste and the taste of foods. Naturwissenschaften. 1980;67:14–20. [Google Scholar]
- 34.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 35.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 36.de Araujo IE, Kringelbach ML, Rolls ET, Hobden P. Representation of umami taste in the human brain. J Neurophysiol. 2003;90:313–319. doi: 10.1152/jn.00669.2002. [DOI] [PubMed] [Google Scholar]
- 37.Rolls ET. The representation of umami taste in the taste cortex. J Nutr. 2000;130:960S–965S. doi: 10.1093/jn/130.4.960S. [DOI] [PubMed] [Google Scholar]
- 38.Baylis LL, Rolls ET. Responses of neurons in the primate taste cortex to glutamate. Physiol Behav. 1991;49:973–979. doi: 10.1016/0031-9384(91)90210-f. [DOI] [PubMed] [Google Scholar]
- 39.De Wijk RA, Engelen L, Prinz JF. The role of intra-oral manipulation in the perception of sensory attributes. Appetite. 2003;40:1–7. doi: 10.1016/s0195-6663(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 40.Wilson CE, Brown WE. Influence of food matric structure and oral breakdown during mastication on temporal perception of flavor. J Sens Stud. 1997;12:69–86. [Google Scholar]
- 41.Green BG, Frankmann SP. The effect of cooling on the perception of carbohydrate and intensive sweeteners. Physiol Behav. 1988;43:515–519. doi: 10.1016/0031-9384(88)90127-8. [DOI] [PubMed] [Google Scholar]
- 42.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 43.Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- 44.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol. 2005;15:1948–1952. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 45.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci U S A. 2011;108:5431–5436. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R960–R971. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang P, Ji Q, Liu Z, Snyder LA, Benard LM, Margolskee RF, Max M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J Biol Chem. 2004;279:45068–45075. doi: 10.1074/jbc.M406779200. [DOI] [PubMed] [Google Scholar]


